Decreased Urinary Levels of SIRT1 as Non-Invasive Biomarker of Early Renal Damage in Hypertension
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Study Patients
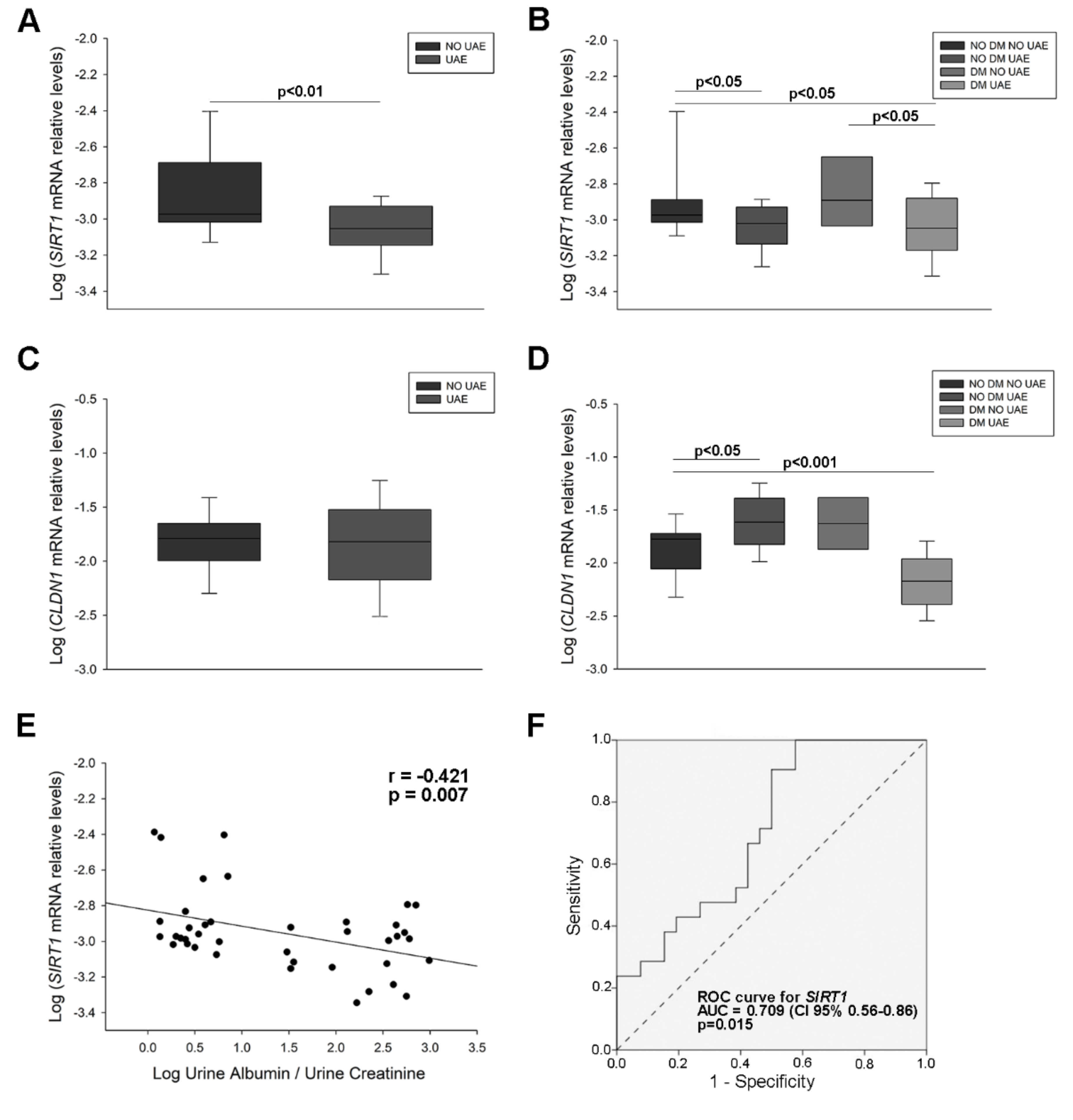
2.2. Sirtuin 1 (SIRT1) and Claudin 1 (CLDN1) Levels in Urine from Hypertensive Patients
2.3. SIRT1 and CLDN1 Levels in Podocyte Cultures Subjected to Stress
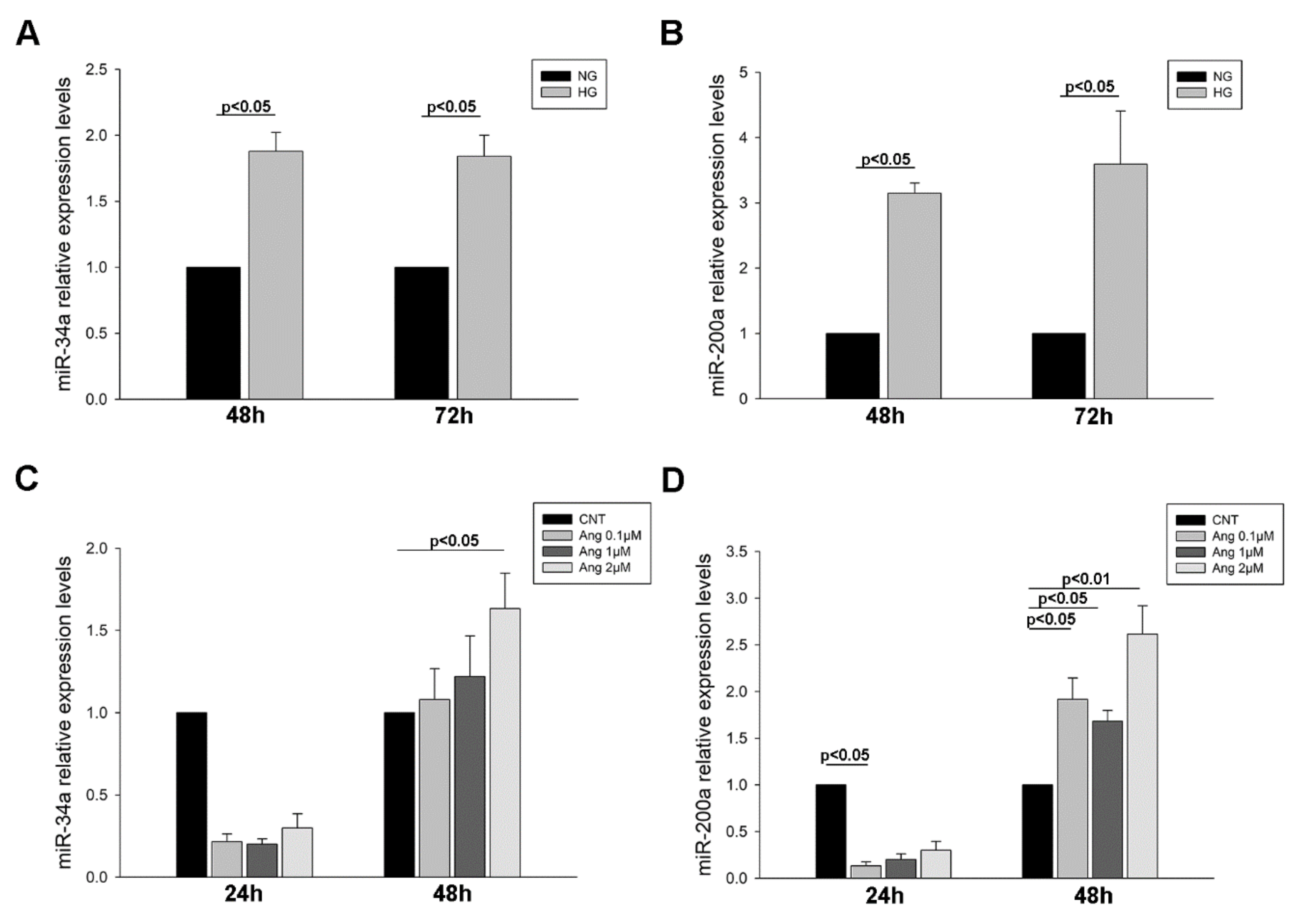
2.4. MiR-34a-and miR-200a Levels in Urinary Sediment and Podocyte Culture Pellets
2.4.1. MiRNA Levels in Urinary Sediment from Hypertensive Patients
2.4.2. MiRNA Levels in Treated Podocyte Cultures
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Urine Samples Processing and Urinary Albumin Excretion (UAE) Data Measurements
4.3. Human Podocytes Culture and Treatment
4.4. RNA Isolation and cDNA Synthesis
4.5. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.6. Homogenization of Samples, Electrophoresis and Western Blot
4.7. Immunofluorescence Analyses
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ang II | Angiotensin II |
| AUC | Area under the curve |
| CLDN1 | Claudin 1 |
| DM | Diabetes mellitus |
| GFR | Glomerular Filtration Rate |
| HG | High Glucose |
| NG | Normal Glucose |
| ROC | Receiver operating characteristic |
| SIRT1 | Sirtuin 1 |
| UAE | Urinary Albumin Excretion |
References
- Segura, J.; Garcia-Donaire, J.A.; Praga, M.; Ruilope, L.M. Chronic kidney disease as a situation of high added risk in hypertensive patients. J. Am. Soc. Nephrol. 2006, 17, S136–S140. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.M.; Bose, M.; Cooper, M.E. Glucose and Blood Pressure-Dependent Pathways-The Progression of Diabetic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckardt, K.U.; Berns, J.S.; Rocco, M.V.; Kasiske, B.L. Definition and classification of CKD: The debate should be about patient prognosis—A position statement from KDOQI and KDIGO. Am. J. Kidney Dis. 2009, 53, 915–920. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Viazzi, F.; Cappadona, F.; Pontremoli, R. Microalbuminuria in primary hypertension: A guide to optimal patient management? J. Nephrol. 2016, 29, 747–753. [Google Scholar] [CrossRef]
- Pedrinelli, R.; Dell’Omo, G.; Di Bello, V.; Pontremoli, R.; Mariani, M. Microalbuminuria, an integrated marker of cardiovascular risk in essential hypertension. J. Hum. Hypertens. 2002, 16, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Lambers Heerspink, H.J.; de Zeeuw, D. Debate: PRO position. Should microalbuminuria ever be considered as a renal endpoint in any clinical trial? Am. J. Nephrol 2010, 31, 458–461, discussion 468. [Google Scholar] [CrossRef]
- Glassock, R.J. Debate: CON position. Should microalbuminuria ever be considered as a renal endpoint in any clinical trial? Am. J. Nephrol. 2010, 31, 462–465, discussion 466–467. [Google Scholar] [CrossRef]
- Pavenstadt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [Green Version]
- Nagata, M. Podocyte injury and its consequences. Kidney Int. 2016, 89, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Lai, K.N. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol. Dial. Transplant. 2012, 27, 3049–3056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assady, S.; Wanner, N.; Skorecki, K.L.; Huber, T.B. New Insights into Podocyte Biology in Glomerular Health and Disease. J. Am. Soc. Nephrol. 2017, 28, 1707–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, R.E.; Cooper, M.E. The tubulointerstitium in progressive diabetic kidney disease: More than an aftermath of glomerular injury? Kidney Int. 1999, 56, 1627–1637. [Google Scholar] [CrossRef] [Green Version]
- Guzzi, F.; Cirillo, L.; Roperto, R.M.; Romagnani, P.; Lazzeri, E. Molecular Mechanisms of the Acute Kidney Injury to Chronic Kidney Disease Transition: An Updated View. Int. J. Mol. Sci. 2019, 20, 4941. [Google Scholar] [CrossRef] [Green Version]
- Fiseha, T.; Tamir, Z. Urinary Markers of Tubular Injury in Early Diabetic Nephropathy. Int. J. Nephrol. 2016, 2016, 4647685. [Google Scholar] [CrossRef] [Green Version]
- Perez-Hernandez, J.; Olivares, M.D.; Solaz, E.; Martinez, F.; Martinez-Hervas, S.; Pichler, G.; Chaves, F.J.; Redon, J.; Cortes, R. Urinary podocyte-associated molecules and albuminuria in hypertension. J. Hypertens. 2018, 36, 1712–1718. [Google Scholar] [CrossRef]
- Hagiyama, M.; Nakatani, Y.; Takashima, Y.; Kato, T.; Inoue, T.; Kimura, R.; Otani, T.; Sato, Y.; Mori, H.; Arima, S.; et al. Urinary Cell Adhesion Molecule 1 Is a Novel Biomarker That Links Tubulointerstitial Damage to Glomerular Filtration Rates in Chronic Kidney Disease. Front. Cell Dev. Biol. 2019, 7, 111. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Benigni, A. Sirtuins in Renal Health and Disease. J. Am. Soc. Nephrol. 2018, 29, 1799–1809. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef] [Green Version]
- Bazyluk, A.; Malyszko, J.; Hryszko, T.; Zbroch, E. State of the art-sirtuin 1 in kidney pathology-clinical relevance. Adv. Med. Sci. 2019, 64, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Liao, M.T.; Hou, Y.C.; Fang, Y.W.; Zheng, C.M.; Liu, W.C.; Chao, C.T.; Lu, K.C.; Ng, Y.Y. Sirtuin-1 and Its Relevance in Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, K.; Wakino, S.; Simic, P.; Sakamaki, Y.; Minakuchi, H.; Fujimura, K.; Hosoya, K.; Komatsu, M.; Kaneko, Y.; Kanda, T.; et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat. Med. 2013, 19, 1496–1504. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zhong, Y.; Li, X.; Chen, H.; Jim, B.; Zhou, M.M.; Chuang, P.Y.; He, J.C. Role of transcription factor acetylation in diabetic kidney disease. Diabetes 2014, 63, 2440–2453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motonishi, S.; Nangaku, M.; Wada, T.; Ishimoto, Y.; Ohse, T.; Matsusaka, T.; Kubota, N.; Shimizu, A.; Kadowaki, T.; Tobe, K.; et al. Sirtuin1 Maintains Actin Cytoskeleton by Deacetylation of Cortactin in Injured Podocytes. J. Am. Soc. Nephrol. 2015, 26, 1939–1959. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Zhang, L.; Das, B.; Li, Z.; Liu, B.; Cai, G.; Chen, X.; Chuang, P.Y.; He, J.C.; Lee, K. Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int. 2018, 93, 1330–1343. [Google Scholar] [CrossRef] [PubMed]
- Olivares, D.; Perez-Hernandez, J.; Forner, M.J.; Perez-Soriano, C.; Tormos, M.C.; Saez, G.T.; Chaves, F.J.; Redon, J.; Cortes, R. Urinary levels of sirtuin-1 associated with disease activity in lupus nephritis. Clin. Sci. (Lond.) 2018, 132, 569–579. [Google Scholar] [CrossRef]
- Nihalani, D.; Susztak, K. Sirt1-Claudin-1 crosstalk regulates renal function. Nat. Med. 2013, 19, 1371–1372. [Google Scholar] [CrossRef]
- Hou, J.; Rajagopal, M.; Yu, A.S. Claudins and the kidney. Annu. Rev. Physiol. 2013, 75, 479–501. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Hou, J. Claudins in barrier and transport function-the kidney. Pflug. Arch. 2017, 469, 105–113. [Google Scholar] [CrossRef]
- Yamakuchi, M. MicroRNA Regulation of SIRT1. Front. Physiol. 2012, 3, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Li, R.; Wei, W. Sirt1 activation prevents anti-Thy 1.1 mesangial proliferative glomerulonephritis in the rat through the Nrf2/ARE pathway. Eur. J. Pharmacol. 2018, 832, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, W.; Cheng, Y.; Xu, Z.; Cai, L. Role of sirtuin-1 in diabetic nephropathy. J. Mol. Med. (Berl.) 2019, 97, 291–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekaran, K.; Choi, J.; Arvas, M.I.; Salimian, M.; Singh, S.; Xu, S.; Gullapalli, R.P.; Kristian, T.; Russell, J.W. Nicotinamide Mononucleotide Administration Prevents Experimental Diabetes-Induced Cognitive Impairment and Loss of Hippocampal Neurons. Int. J. Mol. Sci. 2020, 21, 3756. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Sueyasu, K.; Washida, N.; Tokuyama, H.; Tzukerman, M.; et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 2010, 285, 13045–13056. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Baek, C.H.; Lee, R.B.; Chang, J.W.; Yang, W.S.; Lee, S.K. Anti-Fibrotic Effect of Losartan, an Angiotensin II Receptor Blocker, Is Mediated through Inhibition of ER Stress via Up-Regulation of SIRT1, Followed by Induction of HO-1 and Thioredoxin. Int. J. Mol. Sci. 2017, 18, 305. [Google Scholar] [CrossRef]
- Dong, Y.J.; Liu, N.; Xiao, Z.; Sun, T.; Wu, S.H.; Sun, W.X.; Xu, Z.G.; Yuan, H. Renal protective effect of sirtuin 1. J. Diabetes Res. 2014, 2014, 843786. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Connelly, K.A.; Thai, K.; Wu, X.; Kapus, A.; Kepecs, D.; Gilbert, R.E. Sirtuin 1 Activation Reduces Transforming Growth Factor-beta1-Induced Fibrogenesis and Affords Organ Protection in a Model of Progressive, Experimental Kidney and Associated Cardiac Disease. Am. J. Pathol. 2017, 187, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Lee, K.; He, J.C. SIRT1 Is a Potential Drug Target for Treatment of Diabetic Kidney Disease. Front. Endocrinol. (Lausanne) 2018, 9, 624. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Tian, N.; Wang, T.; Shi, Y.; Xu, J.; Wu, B. Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT-NF-kappaB p65 axis. Sci. Rep. 2019, 9, 323. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Chi, Y.; Ren, Y.; Du, C.; Shi, Y.; Li, Y. Resveratrol Reduces Oxidative Stress and Apoptosis in Podocytes via Sir2-Related Enzymes, Sirtuins1 (SIRT1)/Peroxisome Proliferator-Activated Receptor gamma Co-Activator 1alpha (PGC-1alpha) Axis. Med. Sci. Monit. 2019, 25, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Yang, M.; Qi, N.; Mei, S.; Chen, J.; Song, S.; Jing, Y.; Chen, M.; He, L.; Sun, L.; et al. Olmesartan Prevents Microalbuminuria in db/db Diabetic Mice Through Inhibition of Angiotensin II/p38/SIRT1-Induced Podocyte Apoptosis. Kidney Blood Press. Res. 2016, 41, 848–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Sunq, A.; Roth, R.A.; Hou, J. Inducible Expression of Claudin-1 in Glomerular Podocytes Generates Aberrant Tight Junctions and Proteinuria through Slit Diaphragm Destabilization. J. Am. Soc. Nephrol. 2017, 28, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K. Novel tubular-glomerular interplay in diabetic kidney disease mediated by sirtuin 1, nicotinamide mononucleotide, and nicotinamide adenine dinucleotide Oshima Award Address 2017. Clin. Exp. Nephrol. 2019, 23, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; He, S.; Guo, S.; Xie, W.; Xin, R.; Yu, H.; Yang, F.; Qiu, J.; Zhang, D.; Zhou, S.; et al. Down-regulation of miR-34a alleviates mesangial proliferation in vitro and glomerular hypertrophy in early diabetic nephropathy mice by targeting GAS1. J. Diabetes Complicat. 2014, 28, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Li, Y.; Hu, F.; Jia, Y.J.; Zheng, Z.J.; Wang, L.; Xue, Y.M. High glucose up-regulates microRNA-34a-5p to aggravate fibrosis by targeting SIRT1 in HK-2cells. Biochem. Biophys. Res. Commun. 2018, 498, 38–44. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, R.; Li, A.; Zhang, L.; Liu, H.; Peng, H.; Zhang, Z. Sequence variation in microRNA-34a is associated with diabetes mellitus susceptibility in a southwest Chinese Han population. Int. J. Clin. Exp. Pathol. 2018, 11, 1637–1644. [Google Scholar]
- Liu, S.; Yi, F.; Cheng, W.; Qu, X.; Wang, C. Molecular mechanisms in vascular injury induced by hypertension: Expression and role of microRNA-34a. Exp. Ther. Med. 2017, 14, 5497–5502. [Google Scholar] [CrossRef]
- Liu, R.; Yang, L.; Wei, Q. miR-34a targets PAI-1 to regulate urinary microalbumin and renal function in hypertensive mice. Eur. J. Med. Res. 2020, 25, 3. [Google Scholar] [CrossRef]
- Kito, N.; Endo, K.; Ikesue, M.; Weng, H.; Iwai, N. miRNA Profiles of Tubular Cells: Diagnosis of Kidney Injury. Biomed. Res. Int. 2015, 2015, 465479. [Google Scholar] [CrossRef]
- Sonoda, H.; Lee, B.R.; Park, K.H.; Nihalani, D.; Yoon, J.H.; Ikeda, M.; Kwon, S.H. miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci. Rep. 2019, 9, 4692. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Jiang, L.; Zhou, Y.; Qiu, W.; Fang, L.; Tan, R.; Wen, P.; Yang, J. The miR-200 family regulates TGF-beta1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am. J. Physiol. Renal Physiol. 2012, 302, F369–F379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Stavast, C.J.; Erkeland, S.J. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care 2015, 38 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Xing, C.Y.; Ni, L.; Mathieson, P.W.; Mundel, P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002, 13, 630–638. [Google Scholar]
- Martinez-Arroyo, O.; Ortega, A.; Perez-Hernandez, J.; Chaves, F.J.; Redon, J.; Cortes, R. The Rab-Rabphilin system in injured human podocytes stressed by glucose overload and angiotensin II. Am. J. Physiol. Renal Physiol. 2020, 319, F178–F191. [Google Scholar] [CrossRef]


| Non-Diabetic | Diabetic | |||
|---|---|---|---|---|
| Variables | Increased UAE (n = 13) | No UAE (n = 17) | Increased UAE (n = 18) | No UAE (n = 7) |
| Age (Years) | 50.38 ± 9.52 †† | 54.94 ± 5.89 | 60.00 ± 7.69 ‡‡ | 55.00 ± 4.73 |
| Gender (Male) | 61.5% | 58.8% | 72.2% | 71.4% § |
| BMI (kg/m2) | 29.47 ± 5.21 | 30.33 ± 5.80 | 34.16 ± 7.41 | 28.11 ± 4.30 |
| SBP (mmHg) | 130.08 ± 10.67 | 132.88 ± 18.08 | 140.89 ± 25.77 | 143.71 ± 38.75 |
| DBP (mmHg) | 81.85 ± 8.86 | 87.47 ± 11.65 | 82.00 ± 12.89 | 92.14 ± 16.89 |
| Glucose (mg/dL) | 97.77 ± 18.12 ††† | 103.18 ± 9.93 | 151.33 ± 56.22 ‡‡ | 154.14 ± 67.59 § |
| Glycated Hb (%) | 5.77 ± 0.07 ††† | 5.63 ± 0.21 | 6.99 ± 1.09 ‡‡‡ | 6.46 ± 1.17 |
| T Cholesterol (mg/dL) | 207.38 ± 36.14 † | 185.05 ± 24.34 | 185.17 ± 32.66 * | 154.29 ± 20.87 §§ |
| LDL (mg/dL) | 135.31 ± 30.49 † | 117.88 ± 20.01 | 115.28 ± 28.46 * | 90.71 ± 18.9 §§ |
| HDL (mg/dL) | 57.00 ± 13.82 †† | 51.76 ± 10.82 | 43.56 ± 11.02 ‡‡ | 46.00 ± 8.32 |
| TG (mg/dL) | 111.38 ± 44.90 †† | 118.71 ± 59.64 | 212.33 ± 142.66 ‡‡ | 140.43 ± 49.61 |
| Plasma Cr (mg/dL) | 0.92 ± 0.35 | 0.86 ± 0.18 | 0.95 ± 0.31 | 0.92 ± 0.25 |
| GFR (mL/min/1.73 m2) | 91.69 ± 30.44 | 88.01 ± 17.06 | 90.02 ± 25.96 | 87.61 ± 23.85 |
| Ratio UAE/Cr (mg/g) | 131.44 ± 210.89 ††† | 4.72 ± 6.78 | 343.18 ± 259.06 **‡‡ | 3.40 ± 1.36 |
| Target (Gene Name) | Primer | Sequence 5′→3′ | Size, bp |
|---|---|---|---|
| SIRT1 | Std-curve-F | agctgatgaaccgcttgctat | 300 |
| Std-curve-R | ttggcatattcaccacctaacc | ||
| qPCR-F | ttgttattgggtcttccctcaaa | 112 | |
| qPCR-R | aaatgcagatgaggcaaaggtt | ||
| CLDN1 | Std-curve-F | agcacattgcaagcaacccgtgcct | 320 |
| Std-curve-R | agggcacctcccagaaggcagaga | ||
| qPCR-F | ccgttggcatgaagtgtatg | 101 | |
| qPCR-R | agccagacctgcaagaagaa | ||
| ACTB | Std-curve-F | gaggcatcctcaccctgaagta | 232 |
| Std-curve-R | acagcctggatagcaacgtaca | ||
| qPCR-F | tggagaaaatctggcaccac | 125 | |
| qPCR-R | catgatctgggtcatcttctcg | ||
| B2M | Std-curve-F | ctactctctctttctggcctggag | 511 |
| Std-curve-R | aaacatggagacagcactcaaagt | ||
| qPCR-F | tccagcgtactccaaagattc | 113 | |
| qPCR-R | gtcaacttcaatgtcggatgg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Arroyo, O.; Ortega, A.; Galera, M.; Solaz, E.; Martinez-Hervas, S.; Redon, J.; Cortes, R. Decreased Urinary Levels of SIRT1 as Non-Invasive Biomarker of Early Renal Damage in Hypertension. Int. J. Mol. Sci. 2020, 21, 6390. https://doi.org/10.3390/ijms21176390
Martinez-Arroyo O, Ortega A, Galera M, Solaz E, Martinez-Hervas S, Redon J, Cortes R. Decreased Urinary Levels of SIRT1 as Non-Invasive Biomarker of Early Renal Damage in Hypertension. International Journal of Molecular Sciences. 2020; 21(17):6390. https://doi.org/10.3390/ijms21176390
Chicago/Turabian StyleMartinez-Arroyo, Olga, Ana Ortega, Miriam Galera, Elena Solaz, Sergio Martinez-Hervas, Josep Redon, and Raquel Cortes. 2020. "Decreased Urinary Levels of SIRT1 as Non-Invasive Biomarker of Early Renal Damage in Hypertension" International Journal of Molecular Sciences 21, no. 17: 6390. https://doi.org/10.3390/ijms21176390





