Silencing PROK2 Inhibits Invasion of Human Cervical Cancer Cells by Targeting MMP15 Expression
Abstract
1. Introduction
2. Results
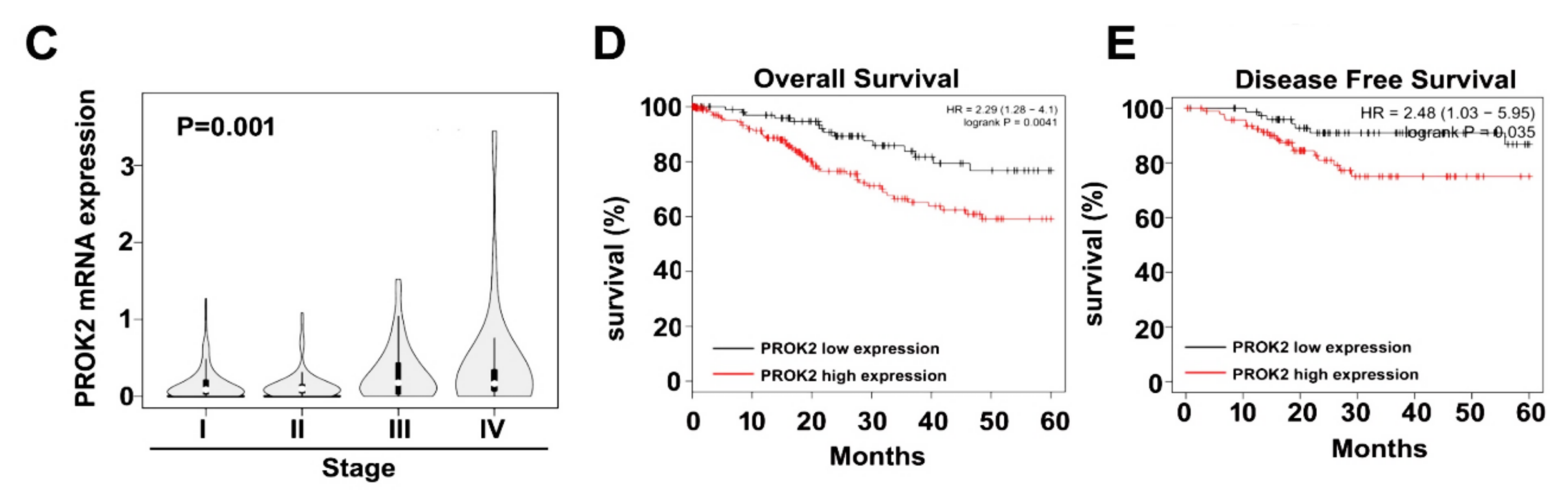
2.1. PROK2 Is Overexpressed in Cervical Cancer and Is Associated with Poor Survival
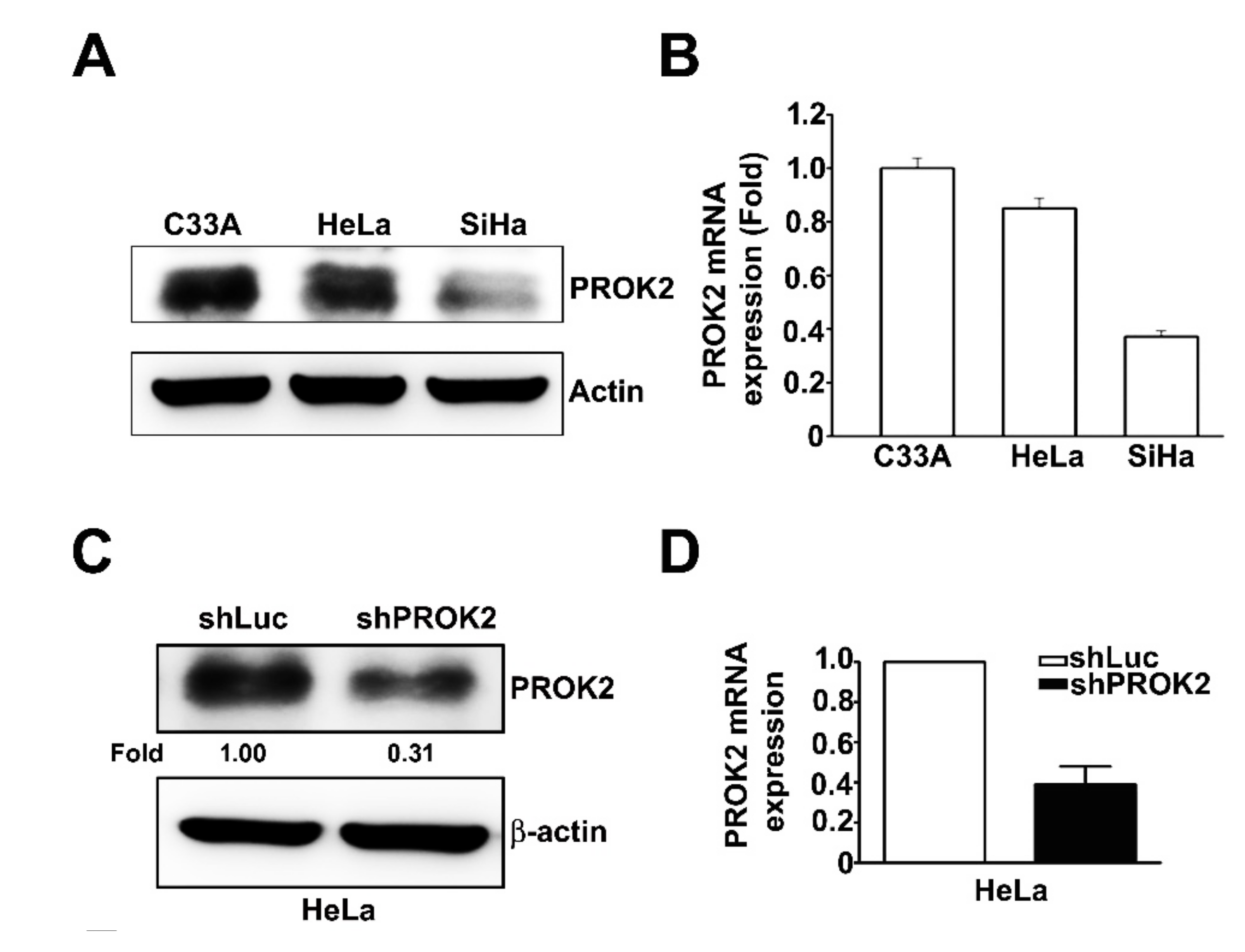
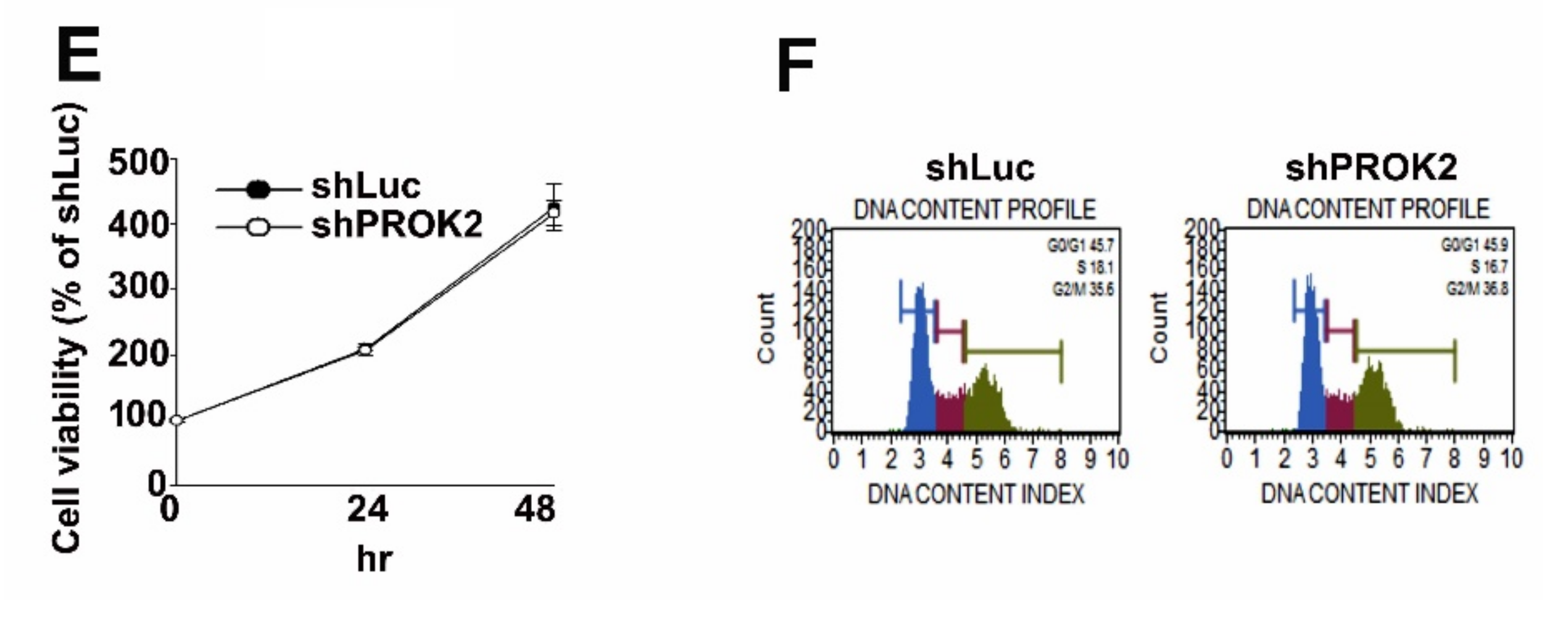
2.2. Effect of PROK2 on Cell Viability and Cell Cycle Regulation in Human Cervical Cancer Cells
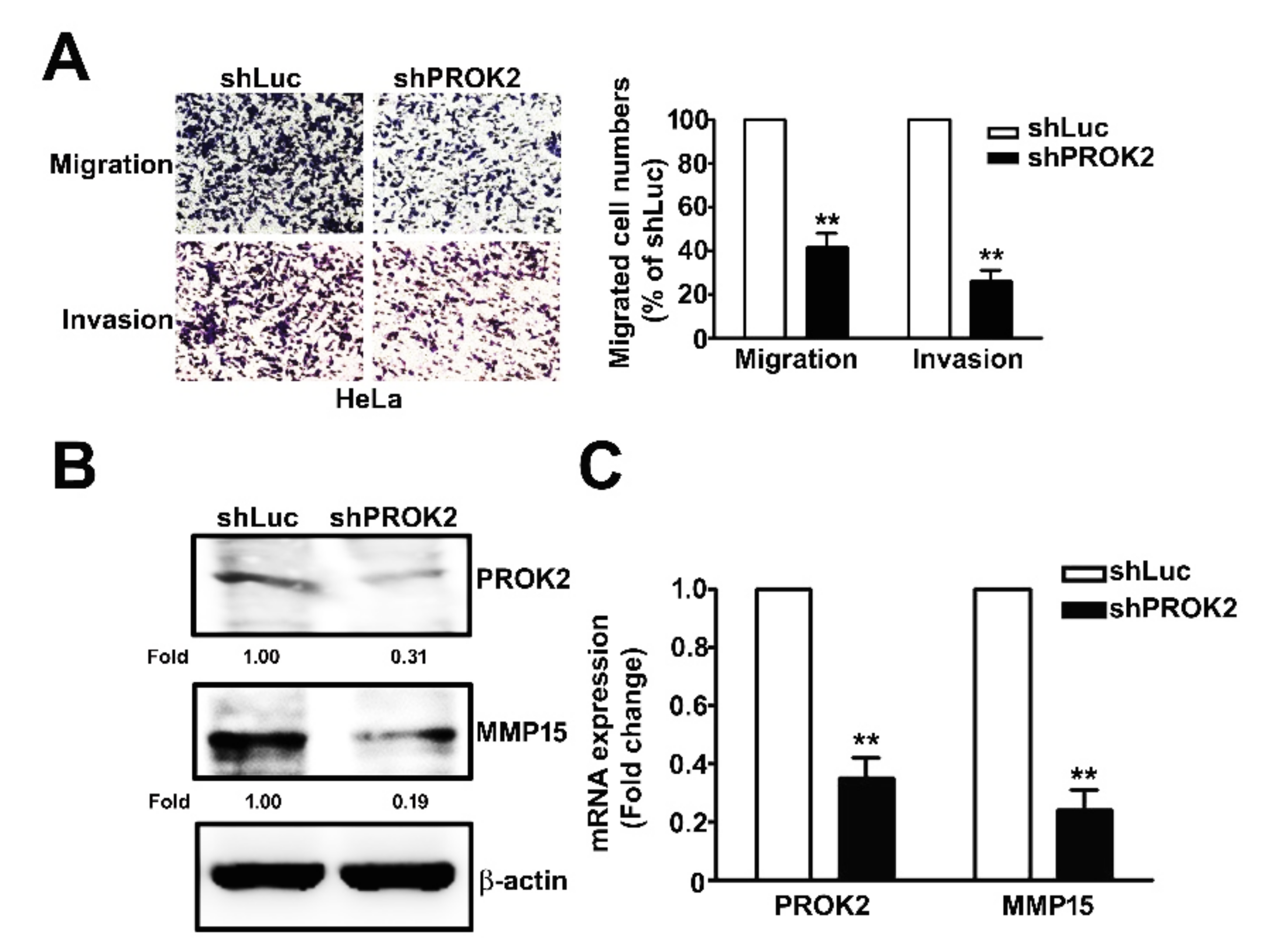
2.3. Knockdown of PROK2 Inhibits the Cell Migration and Invasion
2.4. Knockdown of PROK2 Reduced the Expression of MMP15 and is Associated with Poor Survival of Human Cervical Cancer Patients
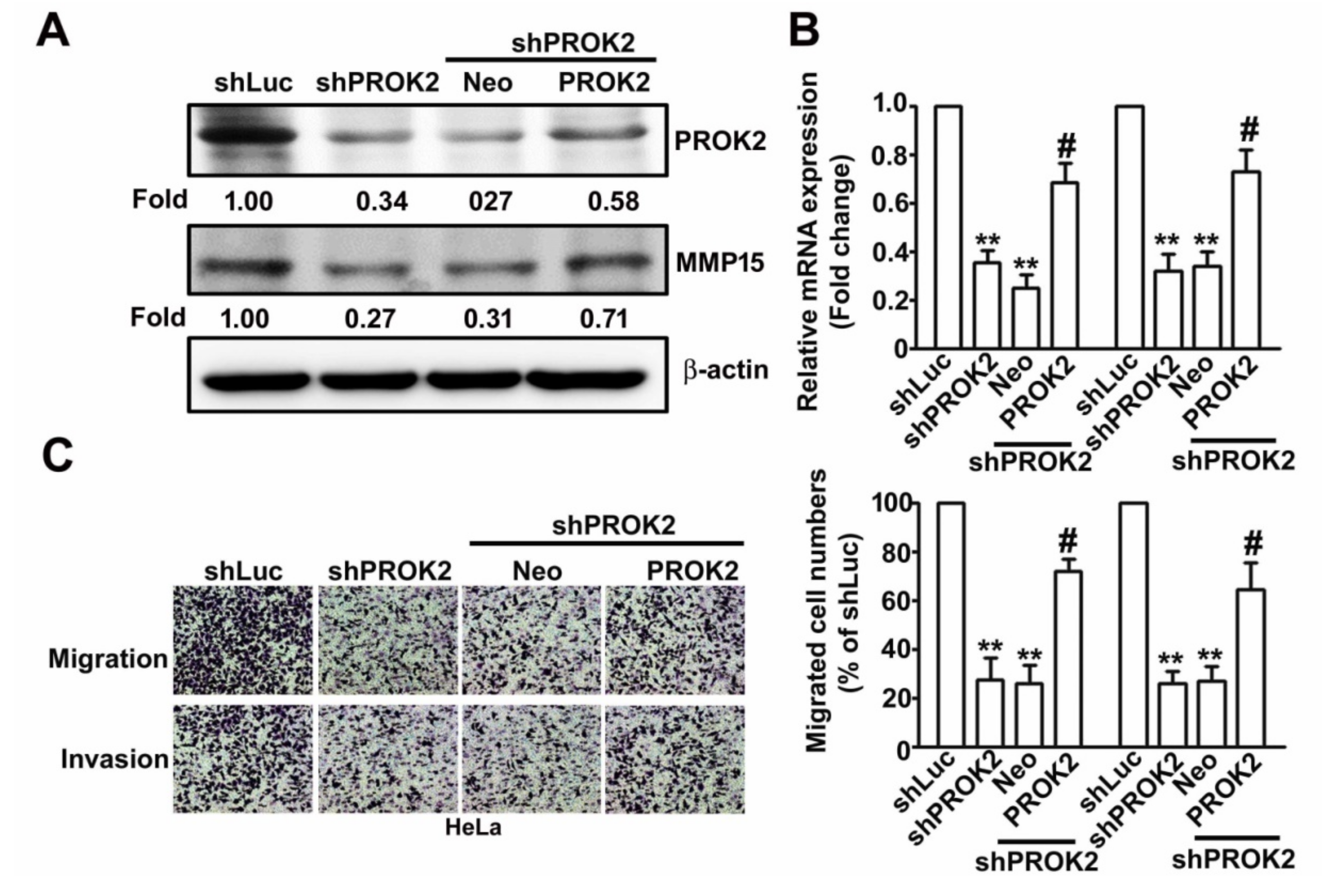
2.5. Knockdown PROK2 Inhibits Cell Migration and Invasion of Human Cervical Cancer HeLa Cells Through Targeting MMP15
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagent
4.2. Human Cervical Tissue Array and TCGA Database Analysis
4.3. Cell Lines
4.4. shRNA for Knockdown Endogenous PROK2 and Transfection Assay with PROK2 Plasmid
4.5. Cell Proliferation Assay
4.6. Cell Cycle Distribution by Flow Cytometry
4.7. Migration and Invasion Assay
4.8. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
4.9. Western Blotting Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Insinga, R.P.; Perez, G.; Wheeler, C.M.; Koutsky, L.A.; Garland, S.M.; Leodolter, S.; Joura, E.A.; Ferris, D.G.; Steben, M.; Hernandez-Avila, M.; et al. Incident Cervical HPV Infections in Young Women: Transition Probabilities for CIN and Infection Clearance. Cancer Epidemiol. Biomark. Prev. 2011, 20, 287–296. [Google Scholar] [CrossRef]
- Eskander, R.N.; Tewari, K.S. Chemotherapy in the treatment of metastatic, persistent, and recurrent cervical cancer. Curr. Opin. Obstet. Gynecol. 2014, 26, 314–321. [Google Scholar] [CrossRef]
- Feng, H.; Hu, Y.; Jin, P.; Meng, X.; Chen, Y.; Zhang, H. Intensity-modulated radiotherapy combined with iodine-125 seed implantation in non-central recurrence of cervical cancer: A case report and literature review. Oncol. Lett. 2017, 14, 4085–4091. [Google Scholar] [CrossRef]
- Liotta, L.A.; Stetler-Stevenson, W.G. Tumor invasion and metastasis: An imbalance of positive and negative regulation. Cancer Res. 1991, 51, 5054–5059. [Google Scholar]
- Yoon, W.-H.; Jung, Y.-J.; Kim, T.-D.; Li, G.; Park, B.-J.; Kim, J.-Y.; Lee, Y.-C.; Kim, J.-M.; Park, J.-I.; Park, H.-D.; et al. Gabexate Mesilate Inhibits Colon Cancer Growth, Invasion, and Metastasis by Reducing Matrix Metalloproteinases and Angiogenesis. Clin. Cancer Res. 2004, 10, 4517–4526. [Google Scholar] [CrossRef]
- Moro, N.; Mauch, C.; Zigrino, P. Metalloproteinases in melanoma. Eur. J. Cell Biol. 2014, 93, 23–29. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, H.; Wang, F.; Lv, J.; Lu, J.; Fang, Q.; Wang, F.; Lu, Y.; Zhang, S.; Xu, Y.; et al. The oncoprotein HBXIP facilitates metastasis of hepatocellular carcinoma cells by activation of MMP15 expression. Cancer Manag. Res. 2019, 11, 4529–4540. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.-Y.; Pan, C.-H.; Zhang, X.-Y.; Su, X.-Y. LncRNA MAFG-AS1 promotes the aggressiveness of breast carcinoma through regulating miR-339-5p/MMP15. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2838–2846. [Google Scholar]
- Li, M.; Bullock, C.M.; Knauer, D.J.; Ehlert, F.J.; Zhou, Q.Y. Identification of Two Prokineticin cDNAs: Recombinant Proteins Potently Contract Gastrointestinal Smooth Muscle. Mol. Pharmacol. 2001, 59, 692–698. [Google Scholar] [CrossRef]
- Curtis, V.F.; Wang, H.; Yang, P.; McLendon, R.E.; Li, X.; Zhou, Q.-Y.; Wang, X.-F. A PK2/Bv8/PROK2 Antagonist Suppresses Tumorigenic Processes by Inhibiting Angiogenesis in Glioma and Blocking Myeloid Cell Infiltration in Pancreatic Cancer. PLoS ONE 2013, 8, e54916. [Google Scholar] [CrossRef]
- Kurebayashi, H.; Goi, T.; Shimada, M.; Tagai, N.; Naruse, T.; Nakazawa, T.; Kimura, Y.; Hirono, Y.; Yamaguchi, A. Prokineticin 2 (PROK2) is an important factor for angiogenesis in colorectal cancer. Oncotarget 2015, 6, 26242–26251. [Google Scholar] [CrossRef][Green Version]
- Hasnis, E.; Alishekevitz, D.; Gingis-Veltski, S.; Bril, R.; Fremder, E.; Voloshin, T.; Raviv, Z.; Karban, A.; Shaked, Y. Anti-Bv8 antibody and metronomic gemcitabine improve pancreatic adenocarcinoma treatment outcome following weekly gemcitabine therapy. Neoplasia 2014, 16, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Monnier, C.P.-P.J.; Piquet-Pellorce, C.; Feige, J.-J.; Musso, O.; Ment, B.T.; Turlin, B.; Ret, M.S.; Samson, M. Prokineticin 2/Bv8 is expressed in Kupffer cells in liver and is down regulated in human hepatocellular carcinoma. World J. Gastroenterol. 2008, 14, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, D.; Rossi, V.; Staibano, S.; De Rosa, G.; Chieffi, P.; Prezioso, D.; Mirone, V.; Mascolo, M.; Tramontano, D.; Bellastella, A.; et al. The Endocrine-Gland-Derived Vascular Endothelial Growth Factor (EG-VEGF)/Prokineticin 1 and 2 and Receptor Expression in Human Prostate: Up-Regulation of EG-VEGF/Prokineticin 1 with Malignancy. Endocrinology 2006, 147, 4245–4251. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Baba, T.; Muranaka, H.; Tanabe, Y.; Takahashi, C.; Matsugo, S.; Mukaida, N. Involvement of Prokineticin 2–expressing Neutrophil Infiltration in 5-Fluorouracil–induced Aggravation of Breast Cancer Metastasis to Lung. Mol. Cancer Ther. 2018, 17, 1515–1525. [Google Scholar] [CrossRef]
- Yoshida, Y.; Goi, T.; Kurebayashi, H.; Morikawa, M.; Hirono, Y.; Katayama, K. Prokineticin 2 expression as a novel prognostic biomarker for human colorectal cancer. Oncotarget 2018, 9, 30079–30091. [Google Scholar] [CrossRef][Green Version]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Ngan, E.S.-W.; Sit, F.Y.; Lee, K.L.; Miao, X.; Yuan, Z.; Wang, W.; Nicholls, J.M.; Wong, K.K.; Garcia-Barcelo, M.; Lui, V.C.; et al. Implications of Endocrine Gland-Derived Vascular Endothelial Growth Factor/Prokineticin-1 Signaling in Human Neuroblastoma Progression. Clin. Cancer Res. 2007, 13, 868–875. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Sui, R.; Chen, Y.; Liang, H.; Shi, J.; Piao, H. MicroRNA-374a Governs Aggressive Cell Behaviors of Glioma by Targeting Prokineticin 2. Technol. Cancer Res. Treat. 2019, 18, 1533033818821401. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, S.; Leng, J.; Xie, T.; Jamal, M.; Yin, Q.; Li, J.; He, C.; Dong, X.; Shao, L.; et al. The prognostic value of matrix metalloproteinase-7 and matrix metalloproteinase-15 in acute myeloid leukemia. J. Cell. Biochem. 2019, 120, 10613–10624. [Google Scholar] [CrossRef]
- Chen, L.; Di, D.; Luo, G.; Zheng, L.; Tan, Y.; Zhang, X.; Xu, N. Immunochemical staining of MT2-MMP correlates positively to angiogenesis of human esophageal cancer. Anticancer Res. 2010, 30, 4363–4368. [Google Scholar]
- Riddick, A.C.P.; Shukla, C.J.; Pennington, C.J.; Bass, R.; Nuttall, R.K.; Hogan, A.; Sethia, K.K.; Ellis, V.; Collins, A.T.; Maitland, N.J.; et al. Identification of degradome components associated with prostate cancer progression by expression analysis of human prostatic tissues. Br. J. Cancer 2005, 92, 2171–2180. [Google Scholar] [CrossRef]
- Aliakbari, M.; Mohammadian, E.; Esmaeili, A.; Pahlevanneshan, Z. Differential effect of polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles on BT-474 human breast cancer cell viability. Toxicol. Vitr. 2018, 54, 114–122. [Google Scholar] [CrossRef]
- Jia, Y.C.; Wang, J.Y.; Liu, Y.Y.; Li, B.; Guo, H.; Zang, A.M. LncRNA MAFG-AS1 facilitates the migration and invasion of NSCLC cell via sponging miR-339-5p from MMP15. Cell Biol. Int. 2019, 43, 384–393. [Google Scholar] [CrossRef]
- Lin, A.; Xu, H.-H.; Xu, D.-P.; Zhang, X.; Wang, Q.; Yan, W.H. Multiple steps of HLA-G in ovarian carcinoma metastasis: Alter NK cytotoxicity and induce matrix metalloproteinase-15 (MMP-15) expression. Hum. Immunol. 2013, 74, 439–446. [Google Scholar] [CrossRef]
- Ito, H.; Noda, K.; Yoshida, K.; Otani, K.; Yoshiga, M.; Oto, Y.; Saito, S.; Kurosaka, D. Prokineticin 2 antagonist, PKRA7 suppresses arthritis in mice with collagen-induced arthritis. BMC Musculoskelet. Disord. 2016, 17, 387. [Google Scholar] [CrossRef]
- Chiang, K.-C.; Lai, C.-Y.; Chiou, H.-L.; Lin, C.-L.; Chen, Y.-S.; Kao, S.-H.; Hsieh, Y.-H. Timosaponin AIII inhibits metastasis of renal carcinoma cells through suppressing cathepsin C expression by AKT/miR-129-5p axis. J. Cell. Physiol. 2019, 234, 13332–13341. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Su, C.-W.; Hsieh, Y.-S.; Chen, P.-N.; Lin, C.-W.; Yang, S.-F. Carbonic Anhydrase III Promotes Cell Migration and Epithelial–Mesenchymal Transition in Oral Squamous Cell Carcinoma. Cells 2020, 9, 704. [Google Scholar] [CrossRef]
- Wu, M.-H.; Lin, C.-L.; Chiou, H.-L.; Yang, S.-F.; Lin, C.-Y.; Liu, C.-J.; Hsieh, Y.-H. Praeruptorin A Inhibits Human Cervical Cancer Cell Growth and Invasion by Suppressing MMP-2 Expression and ERK1/2 Signaling. Int. J. Mol. Sci. 2017, 19, 10. [Google Scholar] [CrossRef]
- Lin, C.-L.; Ying, T.-H.; Yang, S.-F.; Wang, S.-W.; Cheng, S.; Lee, J.-J.; Hsieh, Y.-H. Transcriptional Suppression of miR-7 by MTA2 Induces Sp1-Mediated KLK10 Expression and Metastasis of Cervical Cancer. Mol. Ther. Nucleic Acids 2020, 20, 699–710. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-H.; Wu, P.-R.; Hsieh, Y.-H.; Lin, C.-L.; Liu, C.-J.; Ying, T.-H. Silencing PROK2 Inhibits Invasion of Human Cervical Cancer Cells by Targeting MMP15 Expression. Int. J. Mol. Sci. 2020, 21, 6391. https://doi.org/10.3390/ijms21176391
Wu M-H, Wu P-R, Hsieh Y-H, Lin C-L, Liu C-J, Ying T-H. Silencing PROK2 Inhibits Invasion of Human Cervical Cancer Cells by Targeting MMP15 Expression. International Journal of Molecular Sciences. 2020; 21(17):6391. https://doi.org/10.3390/ijms21176391
Chicago/Turabian StyleWu, Min-Hua, Pei-Ru Wu, Yi-Hsien Hsieh, Chia-Liang Lin, Chung-Jung Liu, and Tsung-Ho Ying. 2020. "Silencing PROK2 Inhibits Invasion of Human Cervical Cancer Cells by Targeting MMP15 Expression" International Journal of Molecular Sciences 21, no. 17: 6391. https://doi.org/10.3390/ijms21176391
APA StyleWu, M.-H., Wu, P.-R., Hsieh, Y.-H., Lin, C.-L., Liu, C.-J., & Ying, T.-H. (2020). Silencing PROK2 Inhibits Invasion of Human Cervical Cancer Cells by Targeting MMP15 Expression. International Journal of Molecular Sciences, 21(17), 6391. https://doi.org/10.3390/ijms21176391






