verA Gene is Involved in the Step to Make the Xanthone Structure of Demethylsterigmatocystin in Aflatoxin Biosynthesis
Abstract
1. Introduction
2. Results
2.1. Preparation of verA Disruptant
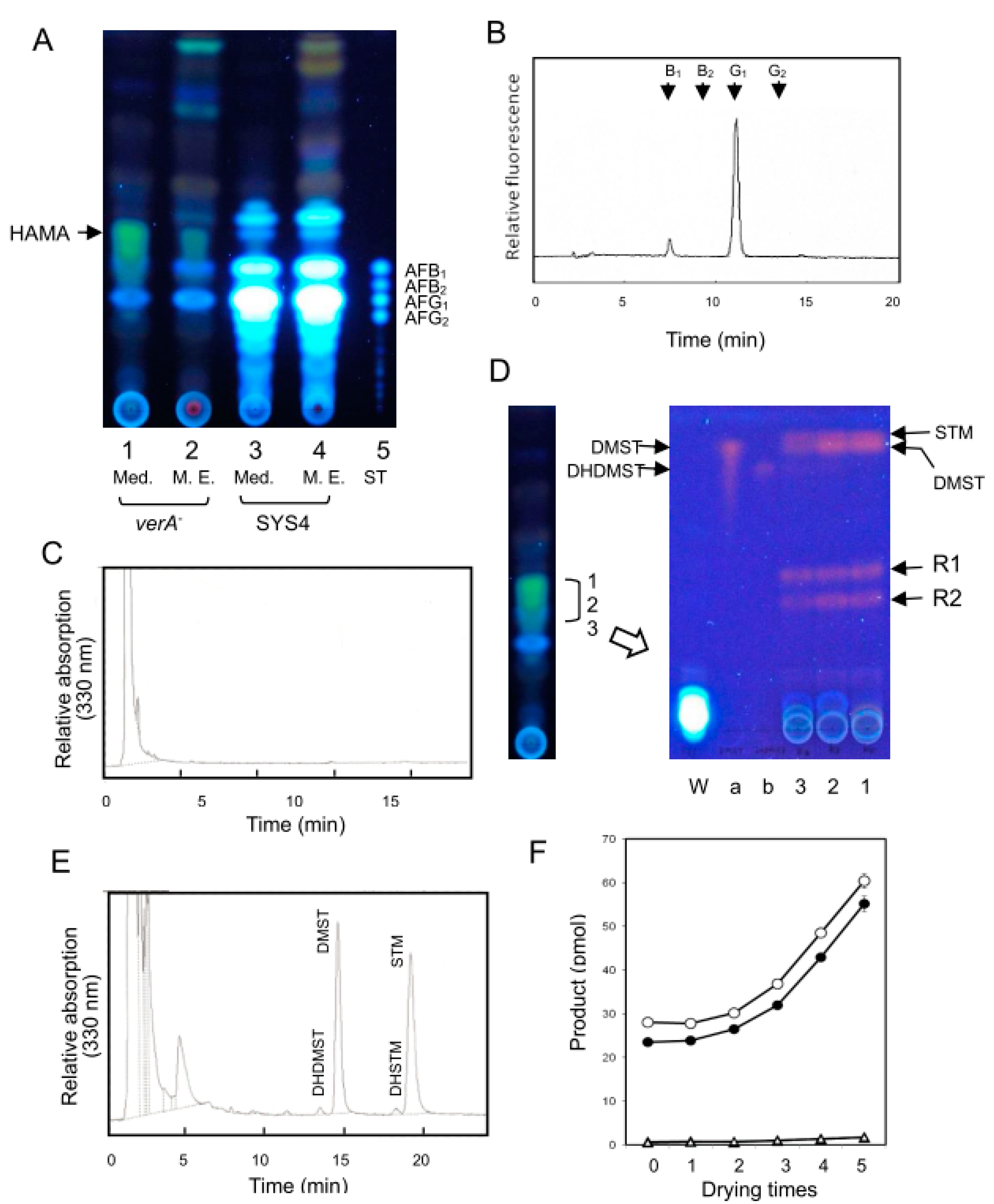
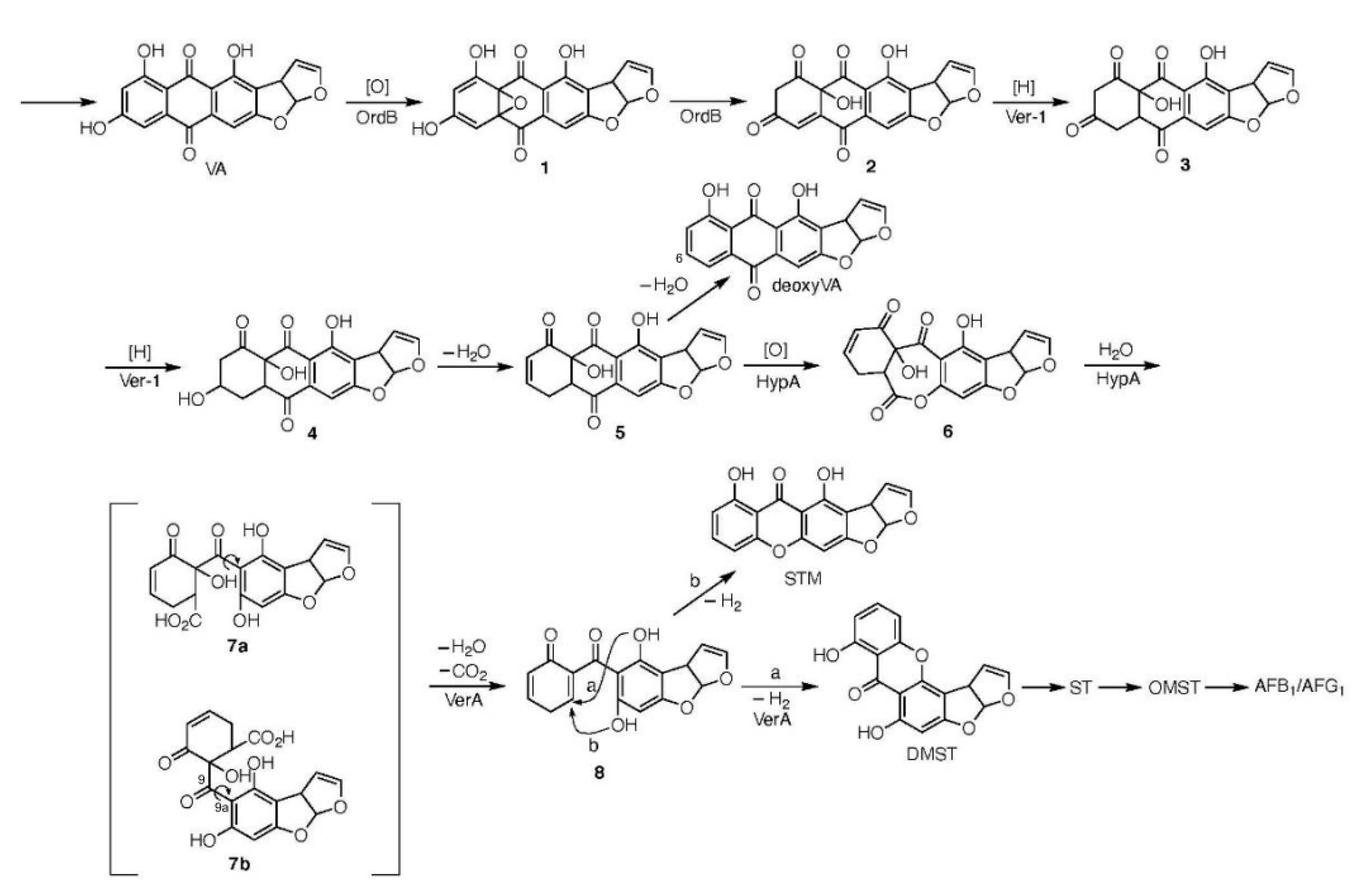
2.2. Characterizations of the Substances Accumulating in the verA Disruptant
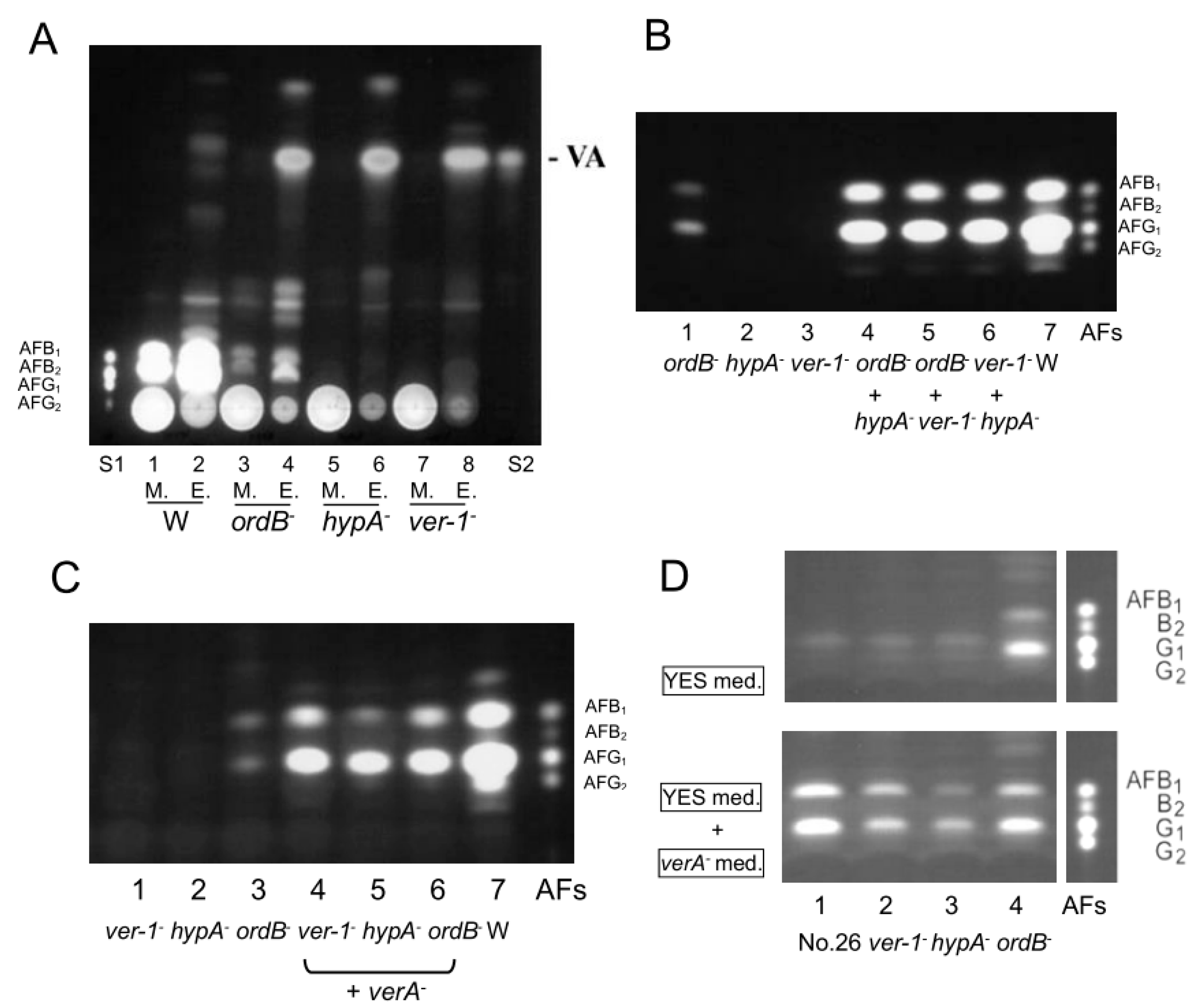
2.3. Relationships among the verA, ver-1, hypA and ordB Disruptants
2.4. The LC-MS Analysis of HAMA Pigment
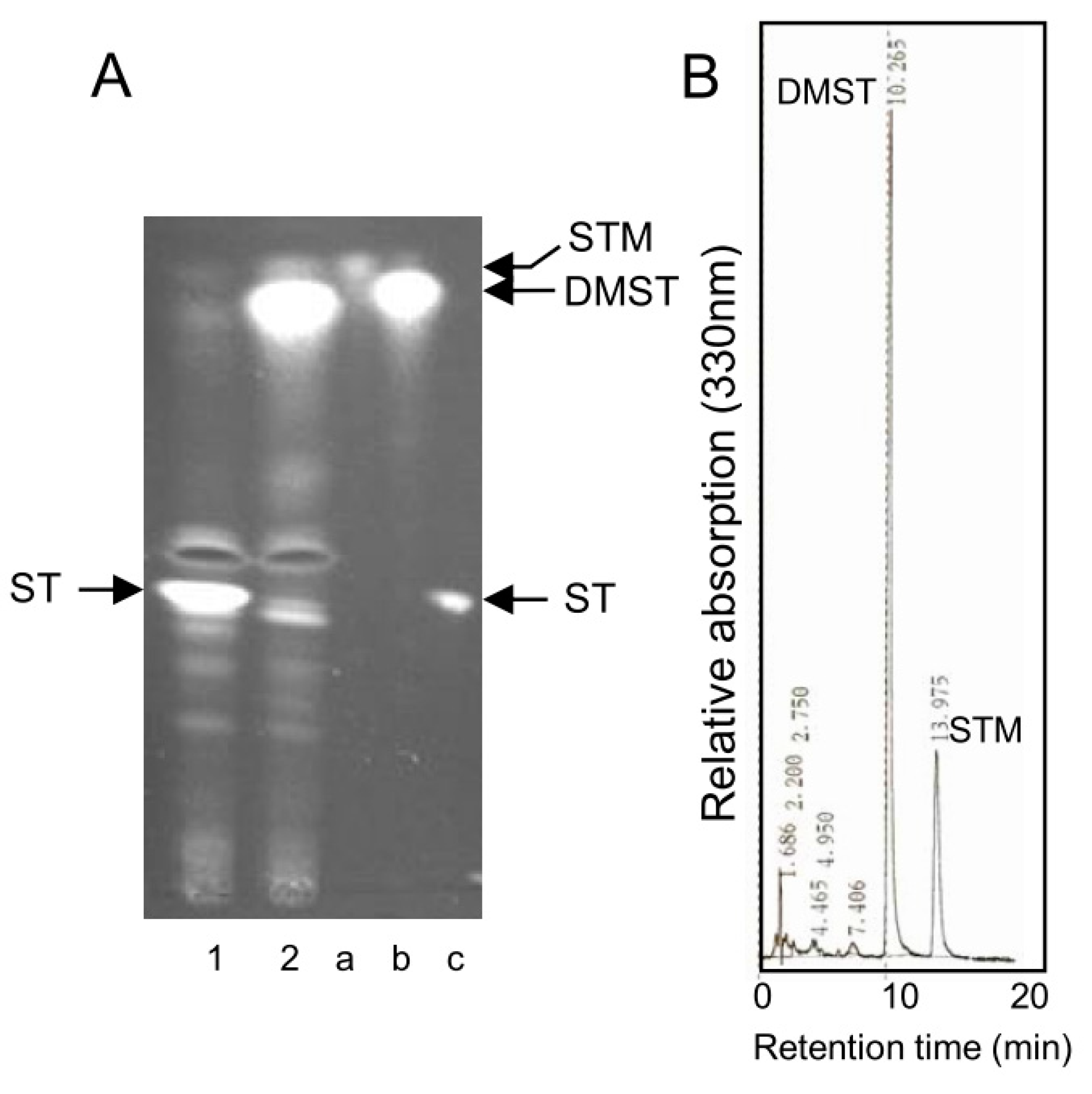
2.5. Accumulation of Small Amounts of STM Together with DMST in A. nidulans stcP-Disruptant
3. Discussion
3.1. verA Gene and HAMA Intermediate
3.2. The Postulated Pathway from VA to HAMA
3.3. The Postulated Pathway from HAMA to DMST
4. Materials and Methods
4.1. Fungal Strains
4.2. Metabolites
4.3. Media and Culture Conditions
4.4. Preparation of verA Disruptant
4.5. Preparation of ver-1, ordB, and hypA Deletion Mutants
4.6. TLC and HPLC Analyses of AFs
4.7. Feeding Experiments for Aflatoxin Production
4.8. Co-Incubation of Two Disruptants among verA, ver-1, ordB, and hypA Disruptants
4.9. Preparation of HAMA Pigment
4.10. Characterization of HAMA Pigment
4.11. LC-MS Measurements
4.12. Substances Accumulated by the stcP Disruptant of A. nidulans
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | aflatoxin |
| HAMA | a novel precursor of AF, which accumulates in the mycelia as well as the culture medium of the verA disruptant |
| VA | versicolorin A |
| DMST | demethylsterigmatocystin |
| STM | sterigmatin |
| PCR | polymerase chain reaction |
| TLC | thin-layer chromatography |
| HPLC | high-performance liquid chromatography |
| LC-MS | liquid chromatography-mass spectrometry |
| DNA | deoxyribonucleic acid |
References
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Peterson, S.W.; Wicklow, D.T.; Goto, T. Aspergillus pseudotamarii, a new aflatoxin producing species in Aspergillus section Flavi. Mycol. Res. 2001, 105, 233–239. [Google Scholar] [CrossRef]
- Peterson, S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 2008, 100, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, N.; Kocsube, S.; Szekeres, A.; Raghavan, A.; Narendran, V.; Vagvolgyi, C.; Selvam, K.P.; Babu Singh, Y.R.; Kredics, L.; Varga, J.; et al. Keratitis caused by Aspergillus pseudotamarii. Med. Mycol. Case Rep. 2013, 2, 91–94. [Google Scholar] [CrossRef]
- Moore, G.G.; Mack, B.M.; Beltz, S.B. Genomic sequence of the aflatoxigenic filamentous fungus Aspergillus nomius. BMC Genom. 2015, 16. [Google Scholar] [CrossRef]
- Payne, G.A.; Brown, M.P. Genetics and physiology of aflatoxin biosynthesis. Annu. Rev. Phytopathol. 1998, 36, 329–362. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Horn, B.W. Ecology and Population Biology of Aflatoxigenic Fungi in Soil. Toxin Rev. 2003, 22, 351–379. [Google Scholar] [CrossRef]
- Lizarrage-Paulin, E.G.; Moreno-Martines, E.; Miranda-Castro, D.P. Aflatoxin and Their Impact on Human and Animal Health: An Emerging Problem. Aflatoxin-Biochemistry and Molecular Biology; IntechOpen: London, UK, 2015; pp. 1–28. [Google Scholar]
- Yabe, K.; Chihaya, N.; Hatabayashi, H.; Kito, M.; Hoshino, S.; Zeng, H.; Cai, J.; Nakajima, H. Production of M-/GM-group aflatoxins catalyzed by the OrdA enzyme in aflatoxin biosynthesis. Fungal Genet. Biol. 2012, 49. [Google Scholar] [CrossRef]
- Yu, J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins 2012, 4, 1024–1057. [Google Scholar] [CrossRef]
- Higginson, J.; DeVita, V.T. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. Am. Ind. Hyg. Assoc. J. 1980, 41. [Google Scholar] [CrossRef]
- Minto, R.E.; Townsend, C.A. Enzymology and Molecular Biology of Aflatoxin Biosynthesis. Chem. Rev. 1997, 97, 2537–2556. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, D.; Ehrlich, K.C.; Cleveland, T.E. Molecular genetic analysis and regulation of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2003, 61, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Yabe, K.; Nakajima, H. Enzyme reactions and genes in aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2004, 64, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered Pathway Genes in Aflatoxin Biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef]
- Yabe, K.; Nakajima, H. Aflatoxin biosynthesis. Shokuhin Eiseigaku Zashi 2011, 52, 135–147. [Google Scholar] [CrossRef]
- Yabe, K.; Ando, Y.; Hamasaki, T. Desaturase Activity in the Branching Step between Aflatoxins B1 and G1 and Aflatoxins B2 and G2. Agric. Biol. Chem. 1991, 55, 1907–1911. [Google Scholar] [CrossRef]
- Yabe, K.; Nakamura, M.; Hamasaki, T. Enzymatic formation of G-group aflatoxins and biosynthetic relationship between G- and B-group aflatoxins. Appl. Environ. Microbiol. 1999, 65, 3867–3872. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; Chang, P.; Yu, J.; Cotty, P.J. Aflatoxin Biosynthesis Cluster Gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 2004, 70, 6518–6524. [Google Scholar] [CrossRef]
- Zeng, H.; Hatabayashi, H.; Nakagawa, H.; Cai, J.; Suzuki, R.; Sakuno, E.; Tanaka, T.; Ito, Y.; Ehrlich, K.C.; Nakajima, H.; et al. Conversion of 11-hydroxy-O-methylsterigmatocystin to aflatoxin G1 in Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2011, 90, 635–650. [Google Scholar] [CrossRef]
- Cole, R.J.; Cox, R.H. Sterigmatocystin. In Handbook of Toxic Fungal Metabolites; Cole, R.J., Cox, R.H., Eds.; Academic Press, Inc.: Cambridge, MA, USA, 1981; pp. 67–93. [Google Scholar]
- Hajjar, J.D.; Bennett, J.W.; Bhatnagar, D.; Bahu, R. Sterigmatocystin production by laboratory strains of Aspergillus nidulans. Mycol. Res. 1989, 93, 548–551. [Google Scholar] [CrossRef]
- Hitokoto, H.; Morozumi, S.; Wauke, T.; Sakai, S.; Yoshikawa, S. Chemically defined medium for high yields of sterigmatocystin. Mycopathologia 1982, 78, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Yu, J.H.; Kelkar, H.S.; Fernandes, M.; Nesbitt, T.C.; Keller, N.P.; Adams, T.H.; Leonard, T.J. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 1996, 93, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Jurjevic, Z.; Peterson, S.W.; Horn, B.W. Aspergillus section Versicolors: Nine new species and multilocus DNA sequence based phylogeny. IMA Fungus 2012, 3, 59–795. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C.; Montalbano, B.; Boué, S.M.; Bhatnagar, D. An aflatoxin biosynthesis cluster gene encodes a novel oxidase required for conversion of versicolorin A to sterigmatocystin. Appl. Environ. Microbiol. 2005, 71, 8963–8965. [Google Scholar] [CrossRef] [PubMed]
- Cary, J.W.; Ehrlich, K.C.; Bland, J.M.; Montalbano, B.G. The aflatoxin biosynthesis cluster gene, aflX, encodes an oxidoreductase involved in conversion of versicolorin a to demethylsterigmatocystin. Appl. Environ. Microbiol. 2006, 72, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Skory, C.D.; Chang, P.K.; Cary, J.; Linz, J.E. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 1992, 58, 3527–3537. [Google Scholar] [CrossRef]
- Keller, N.P.; Segner, S.; Bhatnagar, D.; Adams, T.H. stcS, a putative P-450 monooxygenase, is required for the conversion of versicolorin A to sterigmatocystin in Aspergillus nidulans. Appl. Environ. Microbiol. 1995, 61, 3628–3632. [Google Scholar] [CrossRef]
- Hamasaki, T.; Matsui, K.; Isono, K.; Hatsuda, Y. A new metabolite from Aspergillus versicolor. Agric. Biol. Chem. 1973, 37, 1769–1770. [Google Scholar] [CrossRef]
- Fukuyama, K.; Tsukuhara, T.; Katsube, Y.; Hamasaki, T.; Tanaka, N.; Ashida, T.; Kakudo, M. The crystal and molecular structure of sterigmatin, A metabolite of Aspergillus vesicolor. Bull. Chem. Soc. Jpn. 1975, 48, 1639–1640. [Google Scholar] [CrossRef]
- Fukuyama, K.; Tsukihara, T.; Hamasaki, T. Absolute Configuration of Sterigmatin, a Metabolite of Aspergillus versicolor. Agric. Biol. Chem. 1985, 49, 2465–2466. [Google Scholar] [CrossRef]
- Steyn, P.S.; Vleggaar, R. Austocystins. Six novel dihydrofuro [3′,2′:4,5]furo[3 ,2-b]xanthenones from Aspergillus ustus. J. Chem. Soc. Perkin Trans. 1974, 1, 2250–2256. [Google Scholar] [CrossRef]
- Cole, R.J.; Cox, R.H. Aflatoxins. In Handbook of Toxic Fungal Metabolites; Cole, R.J., Cox, R.H., Eds.; Academic Press: London, UK, 1981; pp. 1–66. [Google Scholar]
- Cole, R.J.; Cox, R.H. Versicolorin group. In Handbook of Toxic Fungal Metbolites; Cole, R.J., Cox, R.H., Eds.; Academic Press, Inc.: London, UK, 1981; pp. 94–127. [Google Scholar]
- Kelkar, H.S.; Keller, N.P.; Adams, T.H. Aspergillus nidulans stcP encodes an O-methyltransferase that is required for sterigmatocystin biosynthesis. Appl. Environ. Microbiol. 1996, 62, 4296–4298. [Google Scholar] [CrossRef] [PubMed]
- Motomura, M.; Chihaya, N.; Shinozawa, T.; Hamasaki, T.; Yabe, K. Cloning and characterization of the O-methyltransferase I gene (dmtA) from Aspergillus parasiticus associated with the conversions of demethylsterigmatocystin to sterigmatocystin and dihydrodemethylsterigmatocystin to dihydrosterigmatocystin in aflatoxin. Appl. Environ. Microbiol. 1999, 65, 4987–4994. [Google Scholar] [CrossRef] [PubMed]
- Kubodera, T.; Watanabe, M.; Yoshiuchi, K.; Yamashita, N.; Nishimura, A.; Nakai, S.; Gomi, K.; Hanamoto, H. Thiamine-regulated gene expression of Aspergillus oryzae thiA requires splicing of the intron containing a riboswitch-like domain in the 5′-UTR. FEBS Lett. 2003, 555, 516–520. [Google Scholar] [CrossRef]
- Henry, K.M.; Townsend, C.A. Ordering the reductive and cytochrome P450 oxidative steps in demethylsterigmatocystin formation yields general insights into the biosynthesis of aflatoxin and related fungal metabolites. J. Am. Chem. Soc. 2005, 127, 3724–3733. [Google Scholar] [CrossRef]
- Yabe, K.; Nakamura, H.; Ando, Y.; Terakado, N.; Nakajima, H.; Hamasaki, T. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by a novel tip culture method. Appl. Environ. Microbiol. 1988, 54, 2096–2100. [Google Scholar] [CrossRef]
- Yabe, K.; Ando, Y.; Hamasaki, T. Biosynthetic Relationship among Aflatoxins B1, B2, G1, and G2. Microbiology 1988, 54, 2101–2106. [Google Scholar] [CrossRef]
- Hamasaki, T.; Renbutsu, M.; Hatsuda, Y. A Red Pigment from Aspergillus versicolor (Vuillemin) Tiraboschi. Agric. Biol. Chem. 1967, 31, 1513–1514. [Google Scholar] [CrossRef]
- Yabe, K.; Ando, Y.; Hamasaki, T. A metabolic grid among versiconal hemiacetal acetate, versiconol acetate, versiconol and versiconal during aflatoxin biosynthesis. J. Gen. Microbiol. 1991, 137. [Google Scholar] [CrossRef]
- Hamasaki, T.; Hatsuda, Y.; Terashima, N.; Renbutsu, M. The Structure of a New Metabolite of Aspergillus versicolor. Agric. Biol. Chem. 1965, 29, 696–697. [Google Scholar] [CrossRef]
- Hatsuda, Y.; Kuyama, S. Studies on the metabolic products of Aspergillus versicolor. I. Cultivation of Aspergillus versicolor, isolation and purification of metabolic products. J. Agric. Chem. Soc. Jpn. 1954, 28, 989–992. [Google Scholar]
- Hatsuda, Y.; Hamasaki, T.; Ishida, M.; Matsui, K.; Hara, S. Dihydrosterigmatocystin and dihydrodemethylsterigmatocystin, new metabolites from Aspergillus versicolor. Agric. Biol. Chem. 1972, 36, 521–522. [Google Scholar]
- Hatsuda, Y.; Kuyama, S.; Terashima, N. Studies on the metabolic products of Aspergillus versicolor. II. The physical and chemical properties and the chemical structure of sterigmatocystin. J. Agric. Chem. Soc. Jpn. 1954, 28, 992–997. [Google Scholar]
- Wen, Y.; Hatabayashi, H.; Arai, H.; Kitamoto, H.K.; Yabe, K. Function of the cypX and moxY genes in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 2005, 71. [Google Scholar] [CrossRef]
- Kubodera, T.; Yamashita, N.; Nishimura, A. Pyrithiamine Resistance Gene (ptrA) of Aspergillus oryzae: Cloning, characterization and application as a dominant selectable marker for transformation. Biosci. Biotechnol. Biochem. 2000, 1416–1421. [Google Scholar] [CrossRef]
- Yabe, K.; Ando, Y.; Ito, M.; Terakado, N. Simple method for screening aflatoxin-producing molds by UV photography. Appl. Environ. Microbiol. 1987, 53. [Google Scholar] [CrossRef]
- Cai, J.; Zeng, H.; Shima, Y.; Hatabayashi, H.; Nakagawa, H.; Ito, Y.; Adachi, Y.; Nakajima, H.; Yabe, K. Involvement of the nadA gene in formation of G-group aflatoxins in Aspergillus parasiticus. Fungal Genet. Biol. 2008, 45, 1081–1093. [Google Scholar] [CrossRef]

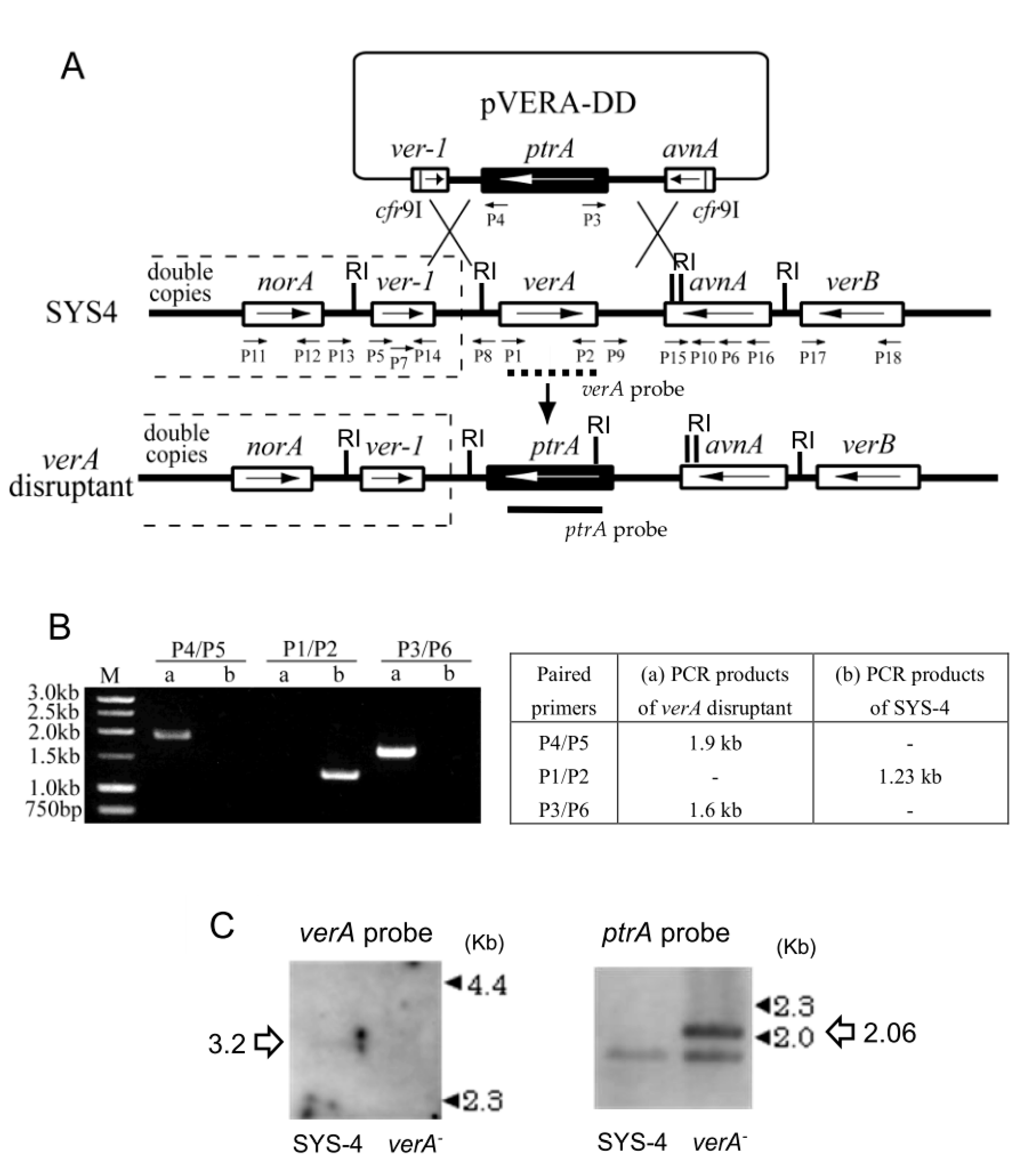

| Primer | Sequence | Position on AY371490 | DNA |
|---|---|---|---|
| P1 | 5′-GTTTCGACTCCCTCGGC | 44119–44135 | verA |
| P2 | 5′-CTCATCGTACGCTGGCG | 45349–45333 | verA |
| P3 | 5′-TGGGATCCCGTAATCAATTGCCC | 38–16 * | ptrA |
| P4 | 5′-GCAAGAGCGGCTCATCGTCA | 1998–2017 * | ptrA |
| P5 | 5′-ATGTCGGATAATCACCGTTTAG | 42020–42041 | ver-1 |
| P6 | 5′-AACCCGAGCCATCTGCACCA | 47140–47121 | avnA |
| P7 | 5′-CCCCGCTCGAGTTTCACCGATGAGCAGGTAG (XhoI) ** | 42696–42715 | ver-1 |
| P8 | 5′-AAAACTGCAGGGAGAGTACCAGGTTCGCTT (PstI) ** | 43894–43875 | verA-up |
| P9 | 5′-GGGGTACCCCTGCTGTTACGAGCTATTC (KpnI) ** | 45542–45561 | verA-down |
| P10 | 5′-GAAGATCTGCAAAGTAACCGCATCGTGC (BglII) ** | 46994–46975 | avnA |
| P11 | 5′-ATGGTTCTCCCTACTGCTCC | 39983–40002 | norA |
| P12 | 5′-CATTTTGAGGCAGAACCAAAG | 41208–41187 | norA |
| P13 | 5′-AGGCTCAGTCACTTGTTCC | 41368–41386 | norA-down |
| P14 | 5′-TTATCGAAAAGCGCCACCAT | 42919–42900 | ver-1 |
| P15 | 5′-GGTCCGATGCTGAACGG | 46718–46734 | avnA |
| P16 | 5′-CATAGTCCCTGAGGCGG | 47828–47812 | avnA |
| P17 | 5′-GCGTAGGCCAGATTGCG | 48662–48678 | verB |
| P18 | 5′-GGTCCACTGCTATGGCG | 49947–49931 | verB |
| P19 | 5′-GGGGTACCGGGCAATTGATTACGGGATCCCA (KpnI) ** | 16–38 * | ptrA |
| P20 | 5′- AAAACTGCAGTGACGATGAGCCGCTCTTGC (PstI) ** | 2017–1998 * | ptrA |
| Strain | AFs (ng/250 µL Culture Medium) 1 | |||
|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | |
| verA disruptant | n.d. | n.d. | 0.1 ± 0.0 | n.d. |
| SYS4 | 363 ± 12 | 17 ± 1 | 1056 ± 71 | 30 ± 2 |
| Strain | Total Concentrations of AFs Formed (ng/250 µL Culture Medium) 2 | ||||
|---|---|---|---|---|---|
| VHA | VA | DMST | ST | OMST | |
| verA− | n.d. | n.d. | 13.1 ± 0.0 | 64.7 ± 3.2 | 165.4 ± 3.2 |
| NIAH-26 | 18.4 ± 2.5 | 11.0 ± 0.2 | 14.0 ± 0.7 | 47.0 ± 0.2 | 169.4 ± 2.9 |
| Strain | AFs (ng/250 µL Culture Medium) 1 | |||
|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | |
| Exp.1: [pks-fas-1]− strain | ||||
| Intact | 136 ± 3 (100%) | 5 ± 0 (100%) | 230 ± 2 (100%) | 5 ± 0 (100%) |
| Heat treatment | 10 ± 1 (7%) | 1 ± 0 (11%) | 13 ± 1 (6%) | 1 ± 0 (10%) |
| Exp.2: NIAH-26 mutant | ||||
| Intact (DW) | 210 ± 8 (100%) | 4 ± 0 (100%) | 129 ± 8 (100%) | 2 ± 0 (100%) |
| Acid (pH 1.5) | 185 ± 19 (89%) | 4 ± 1 (96%) | 82 ± 36 (64%) | 2 ± 1 (74%) |
| Alkaline (pH 11) | 102 ± 0 (49%) | 2 ± 0 (50%) | 55 ± 8 (43%) | 1 ± 0 (46%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Cai, J.; Hatabayashi, H.; Nakagawa, H.; Nakajima, H.; Yabe, K. verA Gene is Involved in the Step to Make the Xanthone Structure of Demethylsterigmatocystin in Aflatoxin Biosynthesis. Int. J. Mol. Sci. 2020, 21, 6389. https://doi.org/10.3390/ijms21176389
Zeng H, Cai J, Hatabayashi H, Nakagawa H, Nakajima H, Yabe K. verA Gene is Involved in the Step to Make the Xanthone Structure of Demethylsterigmatocystin in Aflatoxin Biosynthesis. International Journal of Molecular Sciences. 2020; 21(17):6389. https://doi.org/10.3390/ijms21176389
Chicago/Turabian StyleZeng, Hongmei, Jingjing Cai, Hidemi Hatabayashi, Hiroyuki Nakagawa, Hiromitsu Nakajima, and Kimiko Yabe. 2020. "verA Gene is Involved in the Step to Make the Xanthone Structure of Demethylsterigmatocystin in Aflatoxin Biosynthesis" International Journal of Molecular Sciences 21, no. 17: 6389. https://doi.org/10.3390/ijms21176389
APA StyleZeng, H., Cai, J., Hatabayashi, H., Nakagawa, H., Nakajima, H., & Yabe, K. (2020). verA Gene is Involved in the Step to Make the Xanthone Structure of Demethylsterigmatocystin in Aflatoxin Biosynthesis. International Journal of Molecular Sciences, 21(17), 6389. https://doi.org/10.3390/ijms21176389





