Unexpected Association of Desacyl-Ghrelin with Physical Activity and Chronic Food Restriction: A Translational Study on Anorexia Nervosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1. Clinical Investigation
2.2. Experiment 2. Preclinical Investigation
3. Results
3.1. Experiment 1. Clinical Investigation
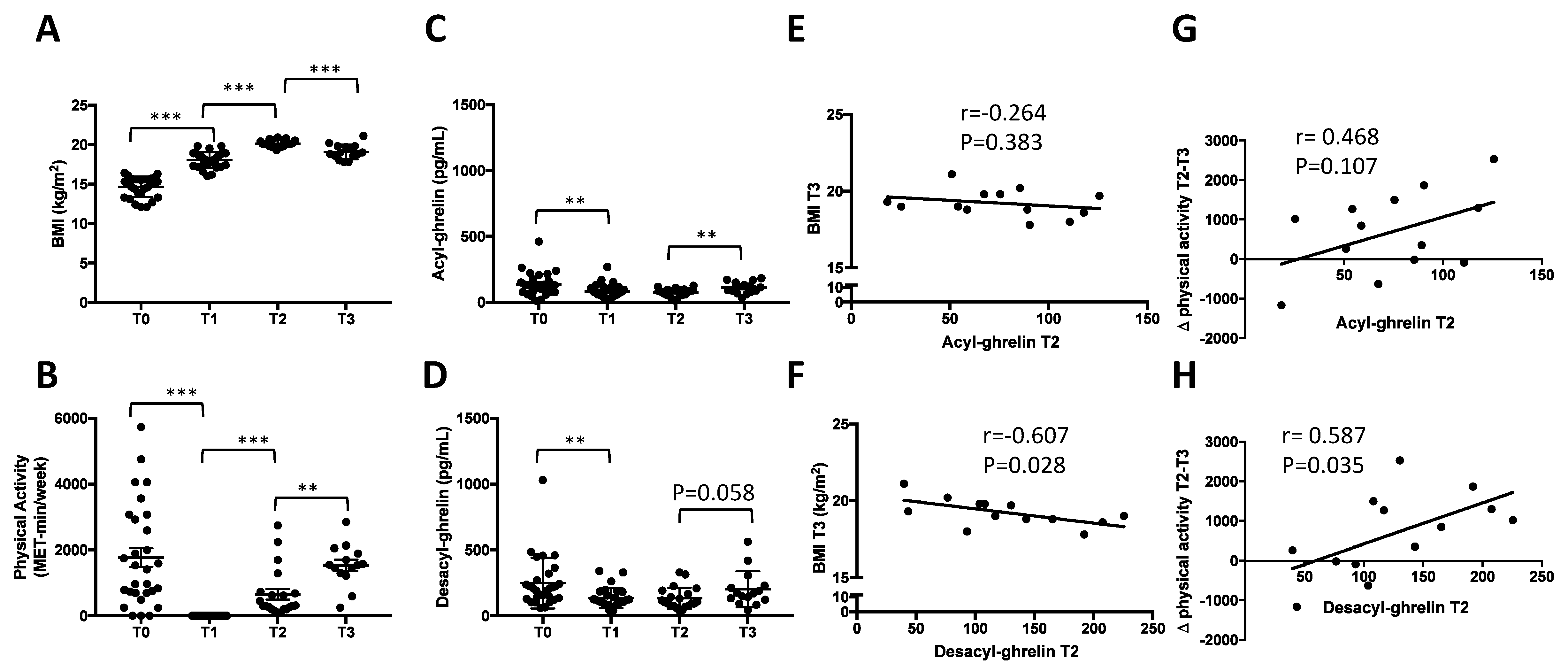
3.1.1. Longitudinal Evolution of BMI and Physical Activity during Inpatient Weight Recovery and One-Month Post Discharge
3.1.2. Rapid Decrease of Circulating AG and DAG during Refeeding Period
3.1.3. Early Increase of Circulating AG but Not DAG One Month after Discharge
3.1.4. Correlations between Circulating DAG after Weight Recovery (T2) and BMI at T3
3.2. Experiment 2. Preclinical Investigation
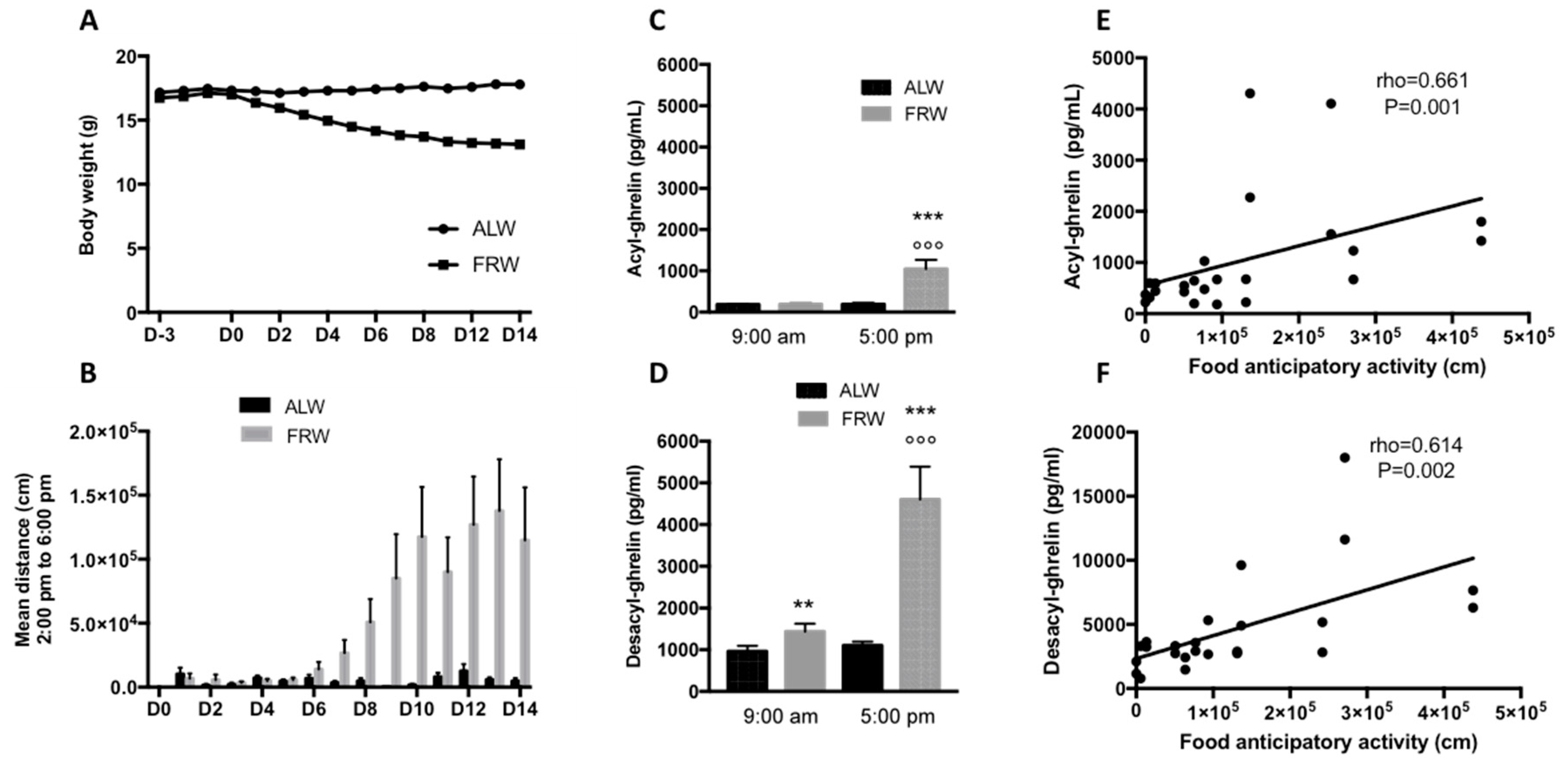
3.2.1. Short Term Food Restriction Protocol: Link between AG, DAG and Physical Activity
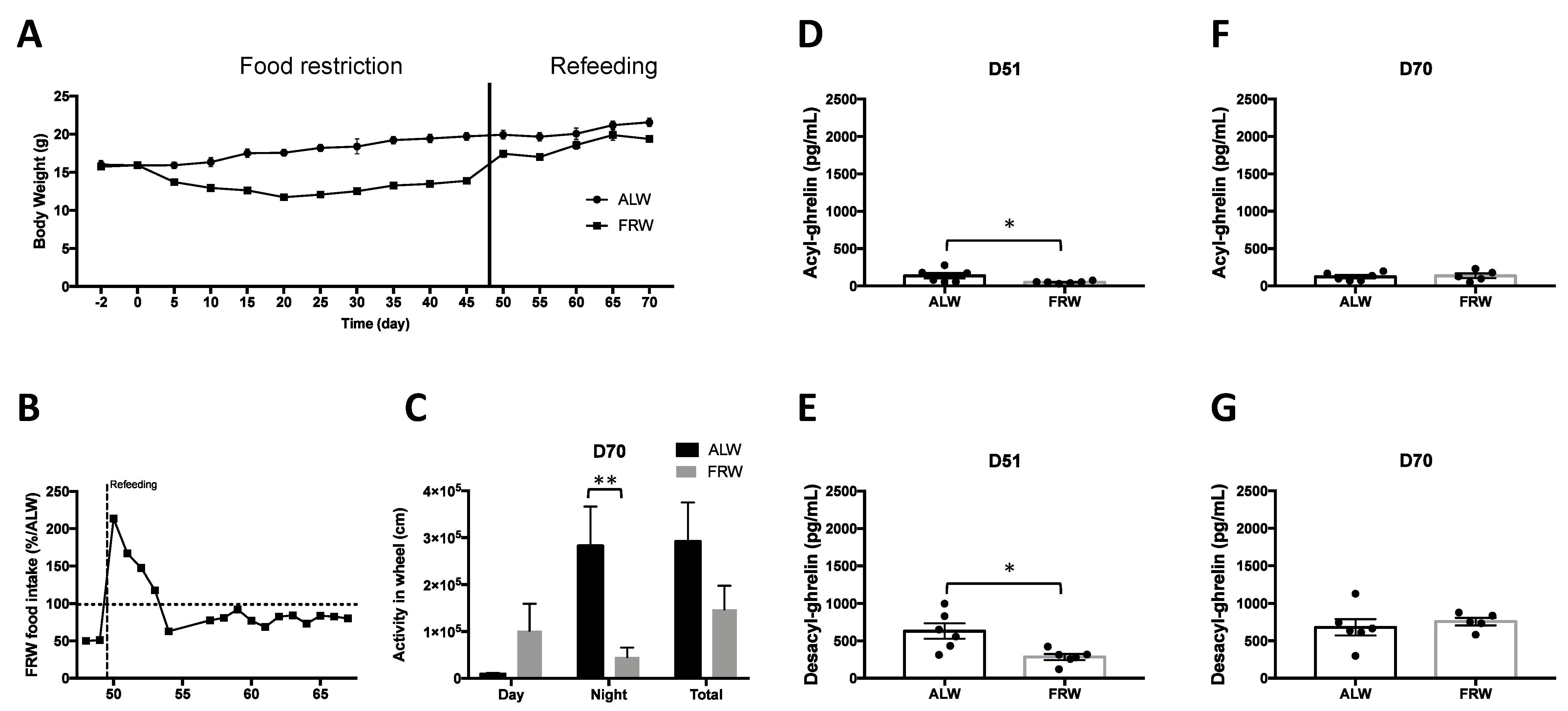
3.2.2. Long Term Food Restriction Protocol and Refeeding
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hebebrand, J.; Exner, C.; Hebebrand, K.; Holtkamp, C.; Casper, R.C.; Remschmidt, H.; Herpertz-Dahlmann, B.; Klingenspor, M. Hyperactivity in patients with anorexia nervosa and in semistarved rats: Evidence for a pivotal role of hypoleptinemia. Physiol. Behav. 2003, 79, 25–37. [Google Scholar] [CrossRef]
- Rizk, M.; Mattar, L.; Kern, L.; Berthoz, S.; Duclos, J.; Viltart, O.; Godart, N. Physical Activity in Eating Disorders: A Systematic Review. Nutrients 2020, 12, 183. [Google Scholar] [CrossRef]
- Solenberger, S.E. Exercise and eating disorders: A 3-year inpatient hospital record analysis. Eat. Behav. 2001, 2, 151–168. [Google Scholar] [CrossRef]
- Taranis, L.; Meyer, C. Associations between specific components of compulsive exercise and eating-disordered cognitions and behaviors among young women. Int. J. Eat. Disord. 2011, 44, 452–458. [Google Scholar] [CrossRef]
- Steinhausen, H.-C. The outcome of anorexia nervosa in the 20th century. Am. J. Psychiatry 2002, 159, 1284–1293. [Google Scholar] [CrossRef]
- Strober, M.; Freeman, R.; Morrell, W. The long-term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int. J. Eat. Disord. 1997, 22, 339–360. [Google Scholar] [CrossRef]
- Treasure, J.; Zipfel, S.; Micali, N.; Wade, T.; Stice, E.; Claudino, A.; Schmidt, U.; Frank, G.K.; Bulik, C.M.; Wentz, E. Anorexia nervosa. Nat. Rev. Dis. Primers 2015, 1, 15074. [Google Scholar] [CrossRef]
- Duncan, L.; Yilmaz, Z.; Gaspar, H.; Walters, R.; Goldstein, J.; Anttila, V.; Bulik-Sullivan, B.; Ripke, S.; Eating Disorders Working Group of the Psychiatric Genomics Consortium; Thornton, L.; et al. Significant Locus and Metabolic Genetic Correlations Revealed in Genome-Wide Association Study of Anorexia Nervosa. Am. J. Psychiatry 2017, 174, 850–858. [Google Scholar] [CrossRef]
- Schorr, M.; Miller, K.K. The endocrine manifestations of anorexia nervosa: Mechanisms and management. Nat. Rev. Endocrinol. 2017, 13, 174–186. [Google Scholar] [CrossRef]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef]
- Duriez, P.; Ramoz, N.; Gorwood, P.; Viltart, O.; Tolle, V. A Metabolic Perspective on Reward Abnormalities in Anorexia Nervosa. Trends Endocrinol. Metab. 2019, 30, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Viltart, O.; Duriez, P.; Tolle, V. Metabolic and neuroendocrine adaptations to undernutrition in anorexia nervosa: From a clinical to a basic research point of view. Horm. Mol. Biol. Clin. Investig. 2018, 36. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T.; Matsukura, S.; Kangawa, K.; Nakazato, M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- De Vriese, C.; Perret, J.; Delporte, C. Focus on the short- and long-term effects of ghrelin on energy homeostasis. Nutrition 2010, 26, 579–584. [Google Scholar] [CrossRef]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Tajiri, Y. Ghrelin and exercise: A possible virtuous circle. Diabetol. Int. 2017, 8, 347–349. [Google Scholar] [CrossRef]
- Tokudome, T.; Otani, K.; Miyazato, M.; Kangawa, K. Ghrelin and the heart. Peptides 2019, 111, 42–46. [Google Scholar] [CrossRef]
- Camargo-Silva, G.; Turones, L.C.; da Cruz, K.R.; Gomes, K.P.; Mendonça, M.M.; Nunes, A.; de Jesus, I.G.; Colugnati, D.B.; Pansani, A.P.; Pobbe, R.L.H.; et al. Ghrelin potentiates cardiac reactivity to stress by modulating sympathetic control and beta-adrenergic response. Life Sci. 2018, 196, 84–92. [Google Scholar] [CrossRef]
- Perello, M.; Dickson, S.L. Ghrelin signalling on food reward: A salient link between the gut and the mesolimbic system. J. Neuroendocrinol. 2015, 27, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Bake, T.; Edvardsson, C.E.; Cummings, C.J.; Dickson, S.L. Ghrelin’s effects on food motivation in rats are not limited to palatable foods. J. Neuroendocrinol. 2018, e12665. [Google Scholar] [CrossRef]
- Tolle, V.; Kadem, M.; Bluet-Pajot, M.-T.; Frere, D.; Foulon, C.; Bossu, C.; Dardennes, R.; Mounier, C.; Zizzari, P.; Lang, F.; et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J. Clin. Endocrinol. Metab. 2003, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Roca-Rivada, A.; Al-Massadi, O.; Seoane, L.M.; Camiña, J.P.; Casanueva, F.F. Peripheral leptin and ghrelin receptors are regulated in a tissue-specific manner in activity-based anorexia. Peptides 2010, 31, 1912–1919. [Google Scholar] [CrossRef]
- Méquinion, M.; Chauveau, C.; Viltart, O. The use of animal models to decipher physiological and neurobiological alterations of anorexia nervosa patients. Front. Endocrinol. (Lausanne) 2015, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Zigman, J.M.; Jones, J.E.; Lee, C.E.; Saper, C.B.; Elmquist, J.K. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 2006, 494, 528–548. [Google Scholar] [CrossRef]
- Delhanty, P.J.; Neggers, S.J.; van der Lely, A.J. Des-acyl ghrelin: A metabolically active peptide. Endocr. Dev. 2013, 25, 112–121. [Google Scholar] [CrossRef]
- Hosoda, H.; Kojima, M.; Matsuo, H.; Kangawa, K. Ghrelin and des-acyl ghrelin: Two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000, 279, 909–913. [Google Scholar] [CrossRef]
- Hassouna, R.; Grouselle, D.; Chiappetta, G.; Lipecka, J.; Fiquet, O.; Tomasetto, C.; Vinh, J.; Epelbaum, J.; Tolle, V. Combination of Selective Immunoassays and Mass Spectrometry to Characterize Preproghrelin-Derived Peptides in Mouse Tissues. Front. Neurosci. 2017, 11, 211. [Google Scholar] [CrossRef]
- Stevanovic, D.M.; Grefhorst, A.; Themmen, A.P.N.; Popovic, V.; Holstege, J.; Haasdijk, E.; Trajkovic, V.; van der Lely, A.-J.; Delhanty, P.J.D. Unacylated ghrelin suppresses ghrelin-induced neuronal activity in the hypothalamus and brainstem of male rats [corrected]. PLoS ONE 2014, 9, e98180. [Google Scholar] [CrossRef]
- Allas, S.; Caixàs, A.; Poitou, C.; Coupaye, M.; Thuilleaux, D.; Lorenzini, F.; Diene, G.; Crinò, A.; Illouz, F.; Grugni, G.; et al. AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: A randomized placebo-controlled trial. PLoS ONE 2018, 13, e0190849. [Google Scholar] [CrossRef] [PubMed]
- Beauloye, V.; Diene, G.; Kuppens, R.; Zech, F.; Winandy, C.; Molinas, C.; Faye, S.; Kieffer, I.; Beckers, D.; Nergårdh, R.; et al. High unacylated ghrelin levels support the concept of anorexia in infants with prader-willi syndrome. Orphanet J. Rare Dis. 2016, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Deighton, K.; Broom, D.R.; Wasse, L.K.; Douglas, J.A.; Burns, S.F.; Cordery, P.A.; Petherick, E.S.; Batterham, R.L.; Goltz, F.R.; et al. Individual Variation in Hunger, Energy Intake, and Ghrelin Responses to Acute Exercise. Med. Sci. Sports Exerc. 2017, 49, 1219–1228. [Google Scholar] [CrossRef]
- Dundar, A.; Kocahan, S.; Sahin, L. Associations of apelin, leptin, irisin, ghrelin, insulin, glucose levels, and lipid parameters with physical activity during eight weeks of regular exercise training. Arch. Physiol. Biochem. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, A.; Morpurgo, P.; Cappiello, V.; Agosti, F.; Marazzi, N.; Giordani, C.; Rigamonti, A.E.; Muller, E.E.; Spada, A. Exercise-induced effects on growth hormone levels are associated with ghrelin changes only in presence of prolonged exercise bouts in male athletes. J. Sports Med. Phys. Fit. 2008, 48, 97–101. [Google Scholar]
- Fathi, R.; Ghanbari-Niaki, A.; Kraemer, R.R.; Talebi-Garakani, E.; Saghebjoo, M. The effect of exercise intensity on plasma and tissue acyl ghrelin concentrations in fasted rats. Regul. Pept. 2010, 165, 133–137. [Google Scholar] [CrossRef]
- Hofmann, T.; Elbelt, U.; Haas, V.; Ahnis, A.; Klapp, B.F.; Rose, M.; Stengel, A. Plasma kisspeptin and ghrelin levels are independently correlated with physical activity in patients with anorexia nervosa. Appetite 2017, 108, 141–150. [Google Scholar] [CrossRef]
- Verhagen, L.A.W.; Egecioglu, E.; Luijendijk, M.C.M.; Hillebrand, J.J.G.; Adan, R.A.H.; Dickson, S.L. Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. Eur. Neuropsychopharmacol. 2011, 21, 384–392. [Google Scholar] [CrossRef]
- Wu, H.; van Kuyck, K.; Tambuyzer, T.; Luyten, L.; Aerts, J.-M.; Nuttin, B. Rethinking food anticipatory activity in the activity-based anorexia rat model. Sci. Rep. 2014, 4, 3929. [Google Scholar] [CrossRef]
- Mani, B.K.; Castorena, C.M.; Osborne-Lawrence, S.; Vijayaraghavan, P.; Metzger, N.P.; Elmquist, J.K.; Zigman, J.M. Ghrelin mediates exercise endurance and the feeding response post-exercise. Mol. Metab. 2018, 9, 114–130. [Google Scholar] [CrossRef]
- Mackelvie, K.J.; Meneilly, G.S.; Elahi, D.; Wong, A.C.K.; Barr, S.I.; Chanoine, J.-P. Regulation of appetite in lean and obese adolescents after exercise: Role of acylated and desacyl ghrelin. J. Clin. Endocrinol. Metab. 2007, 92, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Shiiya, T.; Ueno, H.; Toshinai, K.; Kawagoe, T.; Naito, S.; Tobina, T.; Nishida, Y.; Shindo, M.; Kangawa, K.; Tanaka, H.; et al. Significant lowering of plasma ghrelin but not des-acyl ghrelin in response to acute exercise in men. Endocr. J. 2011, 58, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Tiryaki-Sonmez, G.; Ozen, S.; Bugdayci, G.; Karli, U.; Ozen, G.; Cogalgil, S.; Schoenfeld, B.; Sozbir, K.; Aydin, K. Effect of exercise on appetite-regulating hormones in overweight women. Biol. Sport 2013, 30, 75–80. [Google Scholar] [CrossRef]
- Khalsa, S.S.; Portnoff, L.C.; McCurdy-McKinnon, D.; Feusner, J.D. What happens after treatment? A systematic review of relapse, remission, and recovery in anorexia nervosa. J. Eat. Disord. 2017, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Méquinion, M.; Caron, E.; Zgheib, S.; Stievenard, A.; Zizzari, P.; Tolle, V.; Cortet, B.; Lucas, S.; Prévot, V.; Chauveau, C.; et al. Physical activity: Benefit or weakness in metabolic adaptations in a mouse model of chronic food restriction? Am. J. Physiol. Endocrinol. Metab. 2015, 308, E241–E255. [Google Scholar] [CrossRef]
- Duriez, P.; Eddarkaoui, S.; Blum, D.; Dickson, S.L.; Gorwood, P.; Tolle, V.; Viltart, O. Does physical activity associated with chronic food restriction alleviate anxiety like behaviour, in female mice? Horm. Behav. 2020, 124, 104807. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L.; Heiman, M.L.; Lehnert, P.; Fichter, M.; Tschöp, M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001, 145, 669–673. [Google Scholar] [CrossRef]
- Koyama, K.-I.; Yasuhara, D.; Nakahara, T.; Harada, T.; Uehara, M.; Ushikai, M.; Asakawa, A.; Inui, A. Changes in acyl ghrelin, des-acyl ghrelin, and ratio of acyl ghrelin to total ghrelin with short-term refeeding in female inpatients with restricting-type anorexia nervosa. Horm. Metab. Res. 2010, 42, 595–598. [Google Scholar] [CrossRef]
- Keel, P.K.; Dorer, D.J.; Franko, D.L.; Jackson, S.C.; Herzog, D.B. Postremission predictors of relapse in women with eating disorders. Am. J. Psychiatry 2005, 162, 2263–2268. [Google Scholar] [CrossRef]
- Van Furth, E.F.; van der Meer, A.; Cowan, K. Top 10 research priorities for eating disorders. Lancet Psychiatry 2016, 3, 706–707. [Google Scholar] [CrossRef]
- Merkestein, M.; Brans, M.A.D.; Luijendijk, M.C.M.; de Jong, J.W.; Egecioglu, E.; Dickson, S.L.; Adan, R.A.H. Ghrelin mediates anticipation to a palatable meal in rats. Obesity (Silver Spring) 2012, 20, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Mifune, H.; Tajiri, Y.; Sakai, Y.; Kawahara, Y.; Hara, K.; Sato, T.; Nishi, Y.; Nishi, A.; Mitsuzono, R.; Kakuma, T.; et al. Voluntary exercise is motivated by ghrelin, possibly related to the central reward circuit. J. Endocrinol. 2020, 244, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, S.; Sato, T.; Kangawa, K.; Nakazato, M. The Homeostatic Force of Ghrelin. Cell Metab. 2018, 27, 786–804. [Google Scholar] [CrossRef]
- Mistlberger, R.E. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 1994, 18, 171–195. [Google Scholar] [CrossRef]
- Mistlberger, R.E. Neurobiology of food anticipatory circadian rhythms. Physiol. Behav. 2011, 104, 535–545. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.-C.; Zimmerman, C.A.; Essner, R.A.; Knight, Z.A. Hunger neurons drive feeding through a sustained, positive reinforcement signal. Elife 2016, 5. [Google Scholar] [CrossRef]
- Challet, E. Circadian clocks, food intake, and metabolism. Prog. Mol. Biol. Transl. Sci. 2013, 119, 105–135. [Google Scholar] [CrossRef]
- LeSauter, J.; Hoque, N.; Weintraub, M.; Pfaff, D.W.; Silver, R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc. Natl. Acad. Sci. USA 2009, 106, 13582–13587. [Google Scholar] [CrossRef]
- Blum, I.D.; Patterson, Z.; Khazall, R.; Lamont, E.W.; Sleeman, M.W.; Horvath, T.L.; Abizaid, A. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience 2009, 164, 351–359. [Google Scholar] [CrossRef]
- Gunapala, K.M.; Gallardo, C.M.; Hsu, C.T.; Steele, A.D. Single gene deletions of orexin, leptin, neuropeptide Y, and ghrelin do not appreciably alter food anticipatory activity in mice. PLoS ONE 2011, 6, e18377. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.F.; Katsuyama, A.M.; Pavlovski, I.; Michalik, M.; Patterson, Z.; Parfyonov, M.; Smit, A.N.; Marchant, E.G.; Chung, S.H.; Chung, J.; et al. Circadian mechanisms of food anticipatory rhythms in rats fed once or twice daily: Clock gene and endocrine correlates. PLoS ONE 2014, 9, e112451. [Google Scholar] [CrossRef] [PubMed]
- Dailey, M.J.; Stingl, K.C.; Moran, T.H. Disassociation between preprandial gut peptide release and food-anticipatory activity. Endocrinology 2012, 153, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Toshinai, K.; Yamaguchi, H.; Sun, Y.; Smith, R.G.; Yamanaka, A.; Sakurai, T.; Date, Y.; Mondal, M.S.; Shimbara, T.; Kawagoe, T.; et al. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology 2006, 147, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Delhanty, P.J.D.; Neggers, S.J.; van der Lely, A.J. Should we consider des-acyl ghrelin as a separate hormone and if so, what does it do? Front. Horm. Res. 2014, 42, 163–174. [Google Scholar] [CrossRef]
- Goymann, W.; Lupi, S.; Kaiya, H.; Cardinale, M.; Fusani, L. Ghrelin affects stopover decisions and food intake in a long-distance migrant. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef]
- Guisinger, S. Adapted to flee famine: Adding an evolutionary perspective on anorexia nervosa. Psychol. Rev. 2003, 110, 745–761. [Google Scholar] [CrossRef]
- Willner, P. Validation criteria for animal models of human mental disorders: Learned helplessness as a paradigm case. Prog. Neuropsychopharmacol. Biol. Psychiatry 1986, 10, 677–690. [Google Scholar] [CrossRef]
- Holliday, A.; Blannin, A. Appetite, food intake and gut hormone responses to intense aerobic exercise of different duration. J. Endocrinol. 2017, 235, 193–205. [Google Scholar] [CrossRef]
- Hosoda, H.; Doi, K.; Nagaya, N.; Okumura, H.; Nakagawa, E.; Enomoto, M.; Ono, F.; Kangawa, K. Optimum collection and storage conditions for ghrelin measurements: Octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin. Chem. 2004, 50, 1077–1080. [Google Scholar] [CrossRef]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.F.; Perello, M.; Choi, D.L.; Magrisso, I.J.; Kirchner, H.; Pfluger, P.T.; Tschoep, M.; Zigman, J.M.; Benoit, S.C. GOAT induced ghrelin acylation regulates hedonic feeding. Horm. Behav. 2012, 62, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, H.; Gutierrez, J.A.; Solenberg, P.J.; Pfluger, P.T.; Czyzyk, T.A.; Willency, J.A.; Schurmann, A.; Joost, H.G.; Jandacek, R.; Hale, J.E.; et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat. Med. 2009, 15, 741–745. [Google Scholar] [CrossRef]
- Heppner, K.M.; Piechowski, C.L.; Müller, A.; Ottaway, N.; Sisley, S.; Smiley, D.L.; Habegger, K.M.; Pfluger, P.T.; Dimarchi, R.; Biebermann, H.; et al. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes 2014, 63, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Delhanty, P.J.D.; Neggers, S.J.; van der Lely, A.J. Mechanisms in endocrinology: Ghrelin: The differences between acyl- and des-acyl ghrelin. Eur. J. Endocrinol. 2012, 167, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, A.; Inui, A.; Fujimiya, M.; Sakamaki, R.; Shinfuku, N.; Ueta, Y.; Meguid, M.M.; Kasuga, M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 2005, 54, 18–24. [Google Scholar] [CrossRef]
- Asakawa, A.; Ataka, K.; Fujino, K.; Chen, C.-Y.; Kato, I.; Fujimiya, M.; Inui, A. Ghrelin family of peptides and gut motility. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. 3), 73–74. [Google Scholar] [CrossRef]
- Robichon, L.; Adda, S.; Tolle, V.; Viltart, O. Deacyl-ghrelin: A potential role on the food anticipatory activity in a mouse model of chronic food restriction? Eur. Neuropsychopharmacol. 2019, 29, S189–S190. [Google Scholar] [CrossRef]
- Gahete, M.D.; Córdoba-Chacón, J.; Salvatori, R.; Castaño, J.P.; Kineman, R.D.; Luque, R.M. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol. Cell. Endocrinol. 2010, 317, 154–160. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Nelson, T.A.S.; Guschina, I.A.; Parsons, L.C.; Lewis, C.L.; Brown, R.C.; Christian, H.C.; Davies, J.S.; Wells, T. Unacylated ghrelin promotes adipogenesis in rodent bone marrow via ghrelin O-acyl transferase and GHS-R1a activity: Evidence for target cell-induced acylation. Sci. Rep. 2017, 7, 45541. [Google Scholar] [CrossRef]
- Gortan Cappellari, G.; Zanetti, M.; Semolic, A.; Vinci, P.; Ruozi, G.; Falcione, A.; Filigheddu, N.; Guarnieri, G.; Graziani, A.; Giacca, M.; et al. Unacylated Ghrelin Reduces Skeletal Muscle Reactive Oxygen Species Generation and Inflammation and Prevents High-Fat Diet-Induced Hyperglycemia and Whole-Body Insulin Resistance in Rodents. Diabetes 2016, 65, 874–886. [Google Scholar] [CrossRef]
- Delhanty, P.J.D.; van der Eerden, B.C.J.; van der Velde, M.; Gauna, C.; Pols, H.A.P.; Jahr, H.; Chiba, H.; van der Lely, A.J.; van Leeuwen, J.P.T.M. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J. Endocrinol. 2006, 188, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Jerlhag, E.; Egecioglu, E.; Dickson, S.L.; Douhan, A.; Svensson, L.; Engel, J.A. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict. Biol. 2007, 12, 6–16. [Google Scholar] [CrossRef]
- Carrera, O.; Adan, R.A.H.; Gutierrez, E.; Danner, U.N.; Hoek, H.W.; van Elburg, A.A.; Kas, M.J.H. Hyperactivity in anorexia nervosa: Warming up not just burning-off calories. PLoS ONE 2012, 7, e41851. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Nakahara, K.; Maruyama, K.; Suzuki, Y.; Hayashi, Y.; Kangawa, K.; Murakami, N. Central and peripheral des-acyl ghrelin regulates body temperature in rats. Biochem. Biophys. Res. Commun. 2013, 430, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M.; Galvani, C.; El Ghoch, M.; Capelli, C.; Lanza, M.; Calugi, S.; Dalle Grave, R. Assessment of physical activity in anorexia nervosa and treatment outcome. Med. Sci. Sports Exerc. 2013, 45, 1643–1648. [Google Scholar] [CrossRef]
- Di Lodovico, L.; Gorwood, P. The relationship between moderate to vigorous physical activity and cognitive rigidity in anorexia nervosa. Psychiatry Res. 2020, 284, 112703. [Google Scholar] [CrossRef]
- Rizk, M.; Lalanne, C.; Berthoz, S.; Kern, L.; EVHAN Group; Godart, N. Problematic Exercise in Anorexia Nervosa: Testing Potential Risk Factors against Different Definitions. PLoS ONE 2015, 10, e0143352. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Taranis, L.; Touyz, S. Excessive exercise in the eating disorders: A need for less activity from patients and more from researchers. Eur. Eat. Disord. Rev. 2008, 16, 81–83. [Google Scholar] [CrossRef]
- Eddy, K.T.; Dorer, D.J.; Franko, D.L.; Tahilani, K.; Thompson-Brenner, H.; Herzog, D.B. Diagnostic crossover in anorexia nervosa and bulimia nervosa: Implications for DSM-V. Am. J. Psychiatry 2008, 165, 245–250. [Google Scholar] [CrossRef]
- Schaumberg, K.; Jangmo, A.; Thornton, L.M.; Birgegård, A.; Almqvist, C.; Norring, C.; Larsson, H.; Bulik, C.M. Patterns of diagnostic transition in eating disorders: A longitudinal population study in Sweden. Psychol. Med. 2019, 49, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Komaki, G.; Nishimura, H.; Naruo, T.; Okabe, K.; Kawai, K.; Takii, M.; Oka, T.; Kodama, N.; Nakamoto, C.; et al. A ghrelin gene variant may predict crossover rate from restricting-type anorexia nervosa to other phenotypes of eating disorders: A retrospective survival analysis. Psychiatr. Genet. 2010, 20, 153–159. [Google Scholar] [CrossRef] [PubMed]
| T0 (n = 29) | T1 (n = 28) | T2-T3 (n = 13) | ||||
|---|---|---|---|---|---|---|
| Mean +/−SD | % | Mean +/−SD | % | % | % | |
| Age (years) | 25.8+/−6.8 | 26.2 +/−6.44 | 26+/−7 | |||
| AN-Duration (years) | 7.4+/−5.4 | 7.4+/−5.5 | 8.8+/−6.5 | |||
| Age of onset | 18.4+/−3.8 | 18.4 +/−3.9 | 17.2+/−1.9 | |||
| AN Subtype | ||||||
| AN-R | 59% | 57% | 60% | |||
| AN-BP | 41% | 43% | 40% | |||
| BMI at inclusion (kg/m2) | 14.6+/−1.3 | 14.6+/−1.4 | 14.8+/−1.2 | |||
| Partner ship | ||||||
| Single | 79% | 78% | 69% | |||
| In a relationship | 21% | 22% | 31% | |||
| Daily psychiatric drugs | 62% | 62% | 62% | |||
| EDI-2 at inclusion | ||||||
| Total | 116+/−5 | 118+/−52 | 114+/−53.7 | |||
| Drive for thinness | 11.9+/−7.4 | 12.2+/−7.3 | 11.5+/−8.6 | |||
| Bulimia | 4.9+/−7 | 5.1+/−7.1 | 5+/−7.62 | |||
| Body dissatisfaction | 17+/−6.9 | 17.1+/−7 | 17+/−6.7 | |||
| Ineffectiveness | 16+/−8.9 | 16.5+/−8.7 | 15.5+/−9.3 | |||
| Perfectionism | 7.5+/−4.4 | 7.3+/−4.4 | 7.7+/−4.6 | |||
| Interpersonal distrust | 9.4+/−4.8 | 9.3+/−4.9 | 9.4+/−4.5 | |||
| Interoceptive awareness | 14.7+/−7.3 | 14.8+/−7.4 | 14.2+/−5.9 | |||
| Maturity fears | 8.3+/−6.5 | 8.6+/−6.4 | 8.2+/−7.5 | |||
| Asceticism | 9+/−5.74 | 9.3+/−5.8 | 9+/−6.32 | |||
| Impulse Regulation | 7+/−7.25 | 7+/−7.3 | 6+/−7.4 | |||
| Social Insecurity | 11+/−5.52 | 10.8+/−5.4 | 11+/−6.3 | |||
| BMI T2 | PA T2 1 | AG T2 | DAG T2 | BMI T3 | PA T3 | AG T3 | DAG T31 | Δ BMI | Δ PA | Δ AG | Δ DAG | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI T2 | — | |||||||||||
| PA T2 1 | 0.007 | — | ||||||||||
| AG T2 | 0.107 | −0.322 | — | |||||||||
| DAG T2 | −0.378 | −0.609 * | 0.205 | — | ||||||||
| BMI T3 | 0.533 | 0.111 | −0.264 | −0.607 * | — | |||||||
| PA T3 | −0.188 | 0.165 | 0.159 | 0.192 | −0.038 | — | ||||||
| AG T3 | −0.107 | 0.146 | 0.369 | 0.186 | −0.510 | 0.527 | — | |||||
| DAG T3 1 | −0.302 | 0.069 | 0.346 | 0.319 | −0.494 | 0.301 | 0.445 | — | ||||
| Δ BMI | 0.087 | 0.141 | −0.369 | −0.511 | 0.890 *** | 0.050 | −0.543 | −0.278 | — | |||
| Δ PA | −0.121 | −0.664 * | 0.468 | 0.587 * | −0.204 | 0.613 * | 0.412 | 0.159 | −0.175 | — | ||
| Δ AG | −0.190 | 0.085 | −0.492 | 0.002 | −0.257 | 0.344 | 0.628 * | 0.143 | −0.200 | −0.006 | — | |
| Δ DAG | −0.222 | 0.198 | 0.355 | −0.063 | −0.101 | −0.015 | −0.072 | 0.791 ** | 0.001 | −0.113 | −0.364 | — |
| ALW | FRW | ||||
|---|---|---|---|---|---|
| AG | DAG | AG | DAG | ||
| Activity in Wheel | Total | 0.887 * | 0.609 | 0.699 | 0.960 * |
| Day | −0.297 | −0.407 | 0.569 | 0.820 | |
| Night | 0.888 * | 0.614 | 0.117 | 0.049 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duriez, P.; Robichon, L.; Dardennes, R.; Lavoisy, G.; Grouselle, D.; Epelbaum, J.; Ramoz, N.; Gorwood, P.; Tolle, V.; Viltart, O. Unexpected Association of Desacyl-Ghrelin with Physical Activity and Chronic Food Restriction: A Translational Study on Anorexia Nervosa. J. Clin. Med. 2020, 9, 2782. https://doi.org/10.3390/jcm9092782
Duriez P, Robichon L, Dardennes R, Lavoisy G, Grouselle D, Epelbaum J, Ramoz N, Gorwood P, Tolle V, Viltart O. Unexpected Association of Desacyl-Ghrelin with Physical Activity and Chronic Food Restriction: A Translational Study on Anorexia Nervosa. Journal of Clinical Medicine. 2020; 9(9):2782. https://doi.org/10.3390/jcm9092782
Chicago/Turabian StyleDuriez, Philibert, Lauralee Robichon, Roland Dardennes, Guillaume Lavoisy, Dominique Grouselle, Jacques Epelbaum, Nicolas Ramoz, Philip Gorwood, Virginie Tolle, and Odile Viltart. 2020. "Unexpected Association of Desacyl-Ghrelin with Physical Activity and Chronic Food Restriction: A Translational Study on Anorexia Nervosa" Journal of Clinical Medicine 9, no. 9: 2782. https://doi.org/10.3390/jcm9092782
APA StyleDuriez, P., Robichon, L., Dardennes, R., Lavoisy, G., Grouselle, D., Epelbaum, J., Ramoz, N., Gorwood, P., Tolle, V., & Viltart, O. (2020). Unexpected Association of Desacyl-Ghrelin with Physical Activity and Chronic Food Restriction: A Translational Study on Anorexia Nervosa. Journal of Clinical Medicine, 9(9), 2782. https://doi.org/10.3390/jcm9092782







