The Importance of Redox Status in the Frame of Lifestyle Approaches and the Genetics of the Lung Innate Immune Molecules, SP-A1 and SP-A2, on Differential Outcomes of COVID-19 Infection
Abstract
1. Introduction
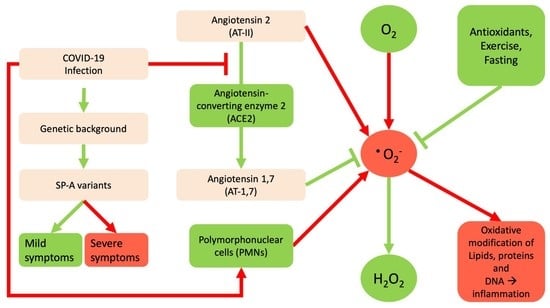
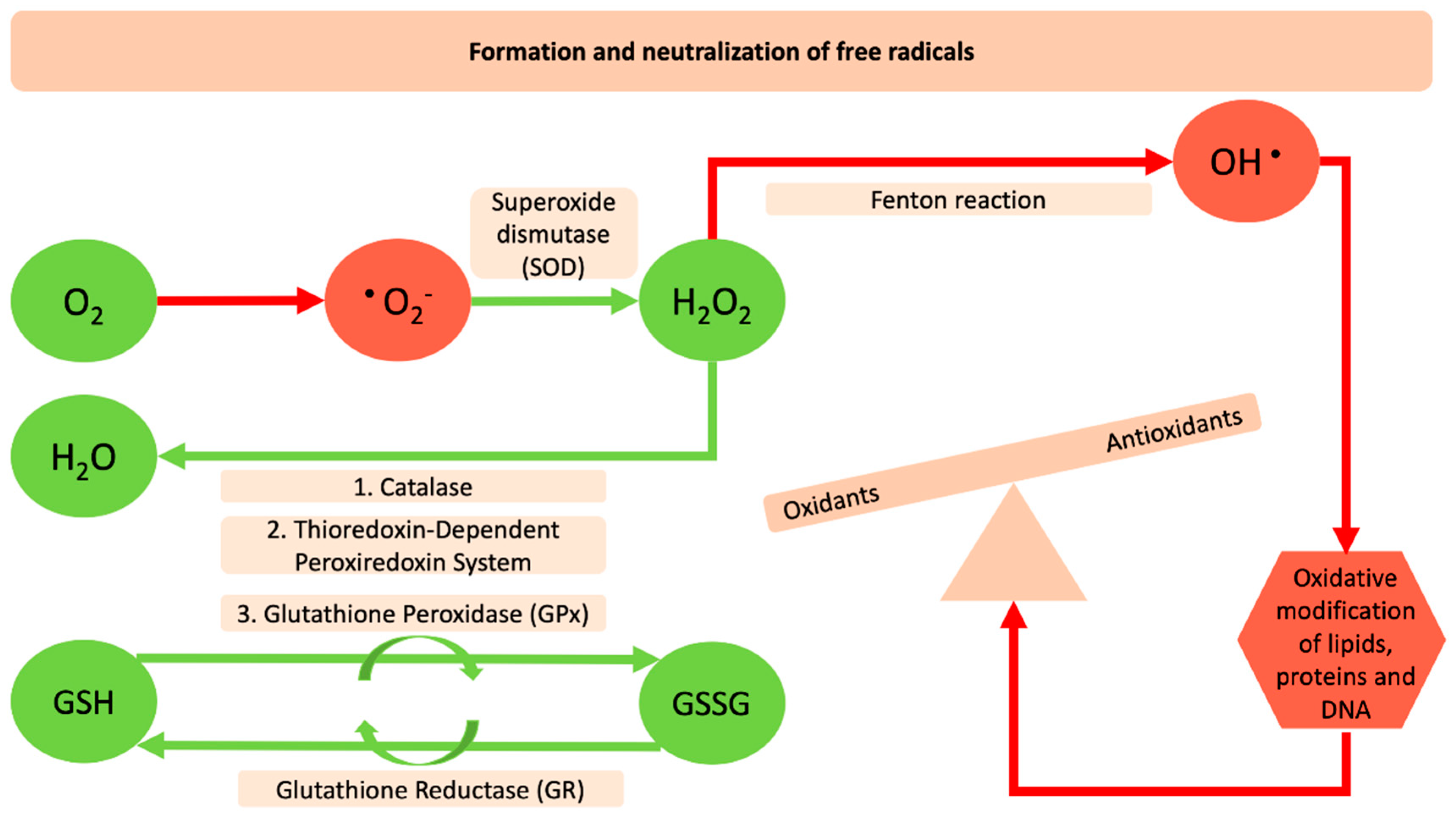
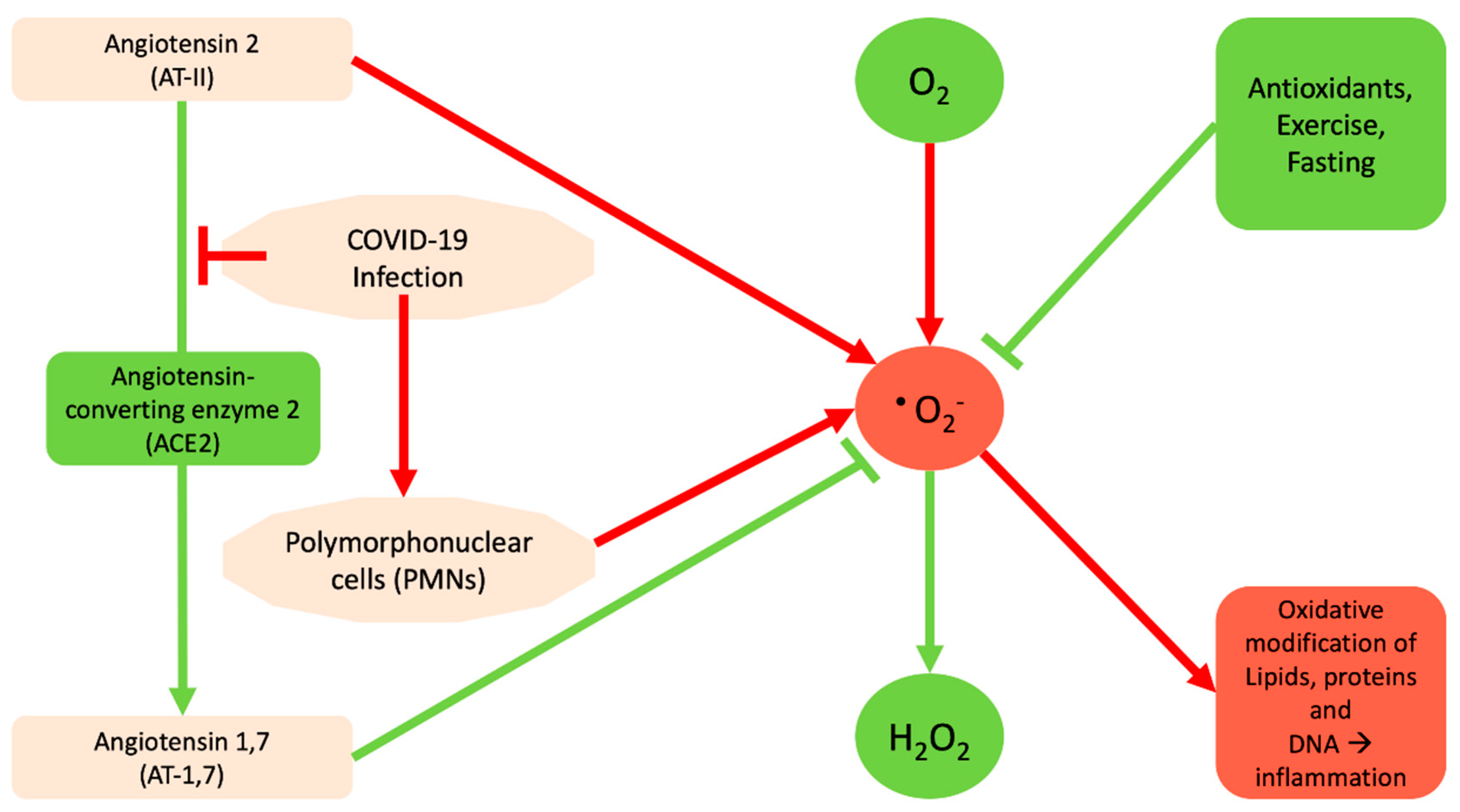
2. The Interrelation between Reactive Oxygen Species and SARS-Cov-2
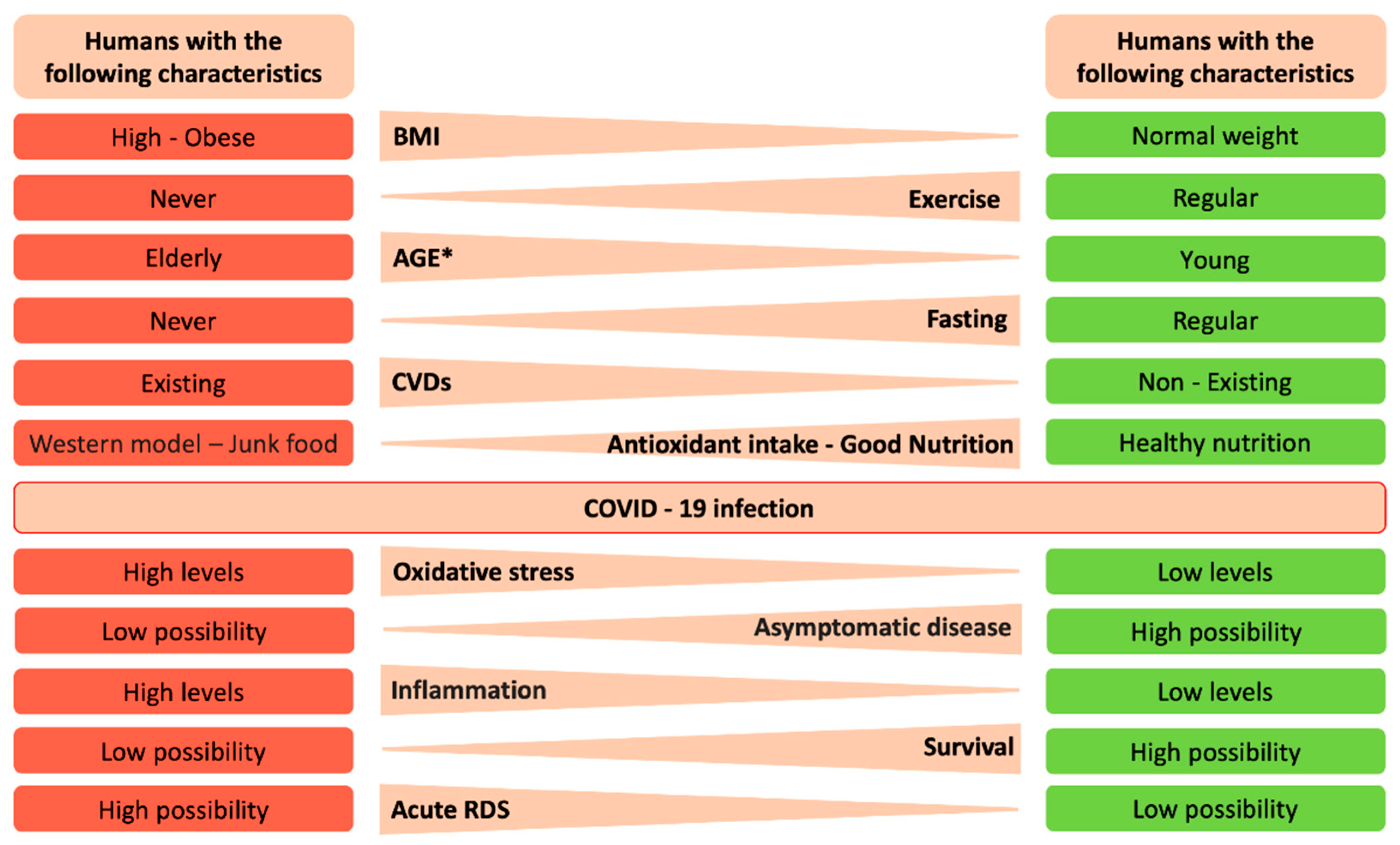
3. Lifestyle Changes
3.1. Antioxidants and Natural Dietary Regimens that Promote a Strong Host Antioxidant Environment
3.2. Exercise
3.3. Fasting
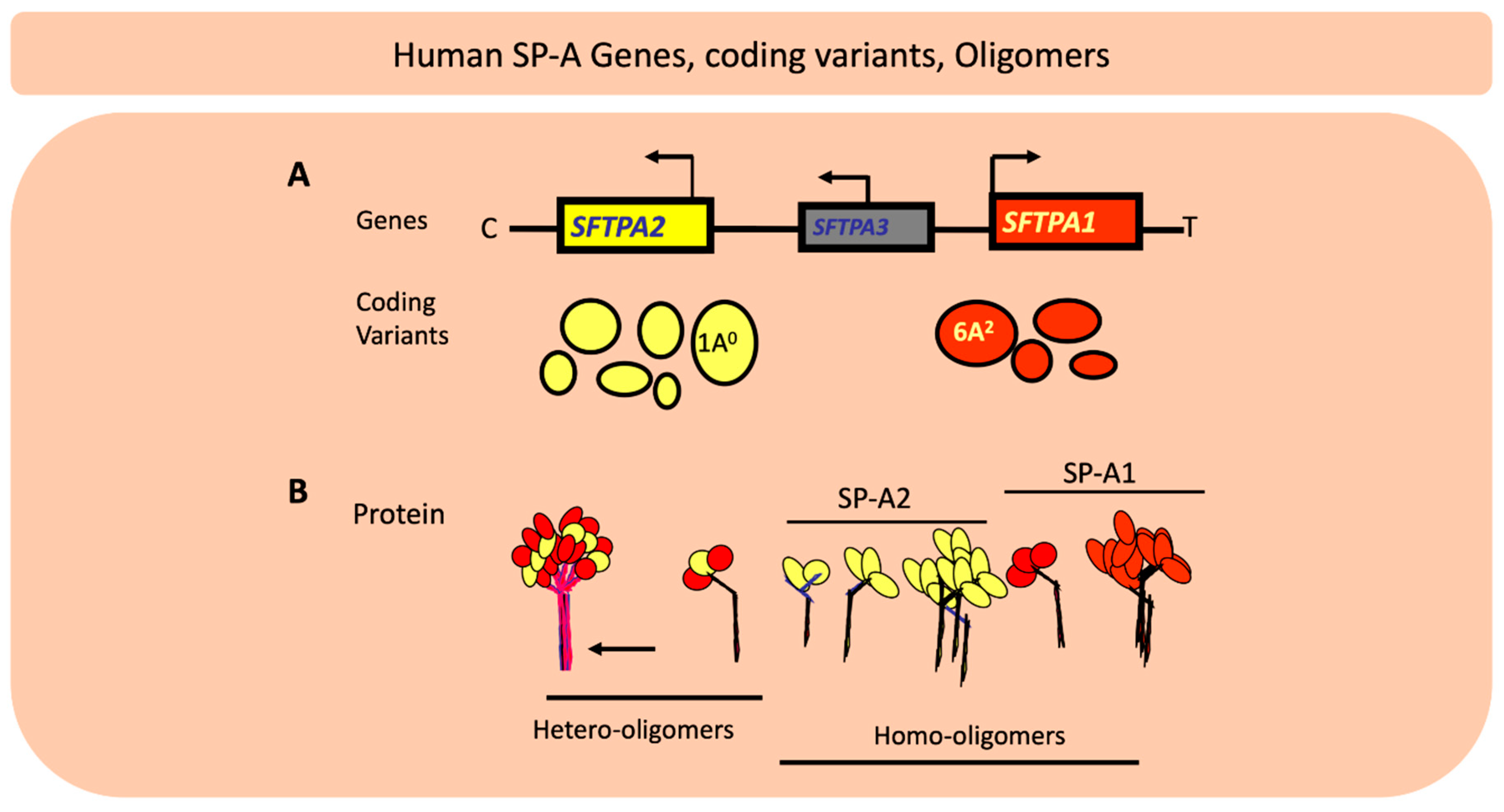
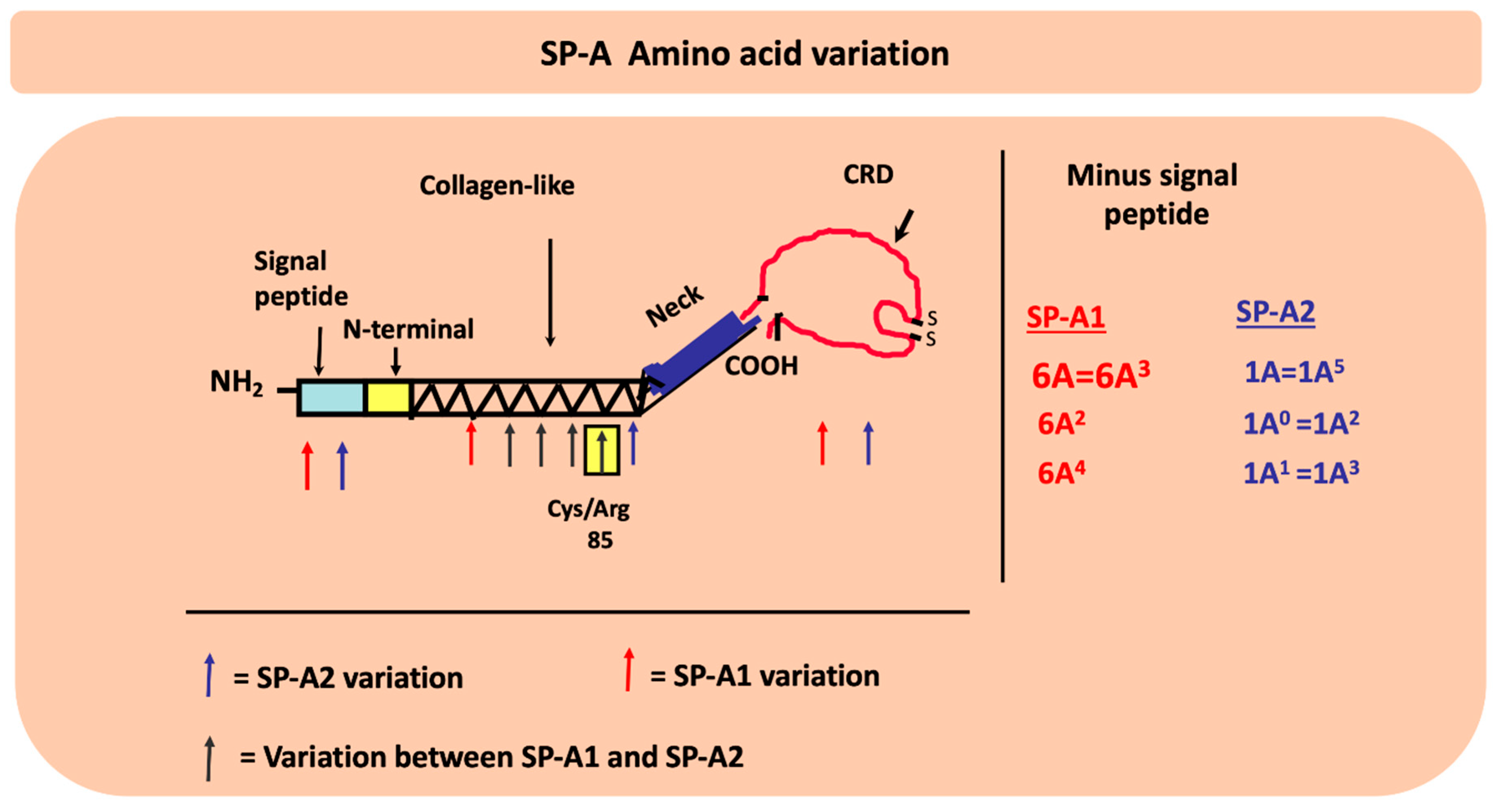
4. Genetic Complexity of the Lung Innate Immune Molecules, SP-A1 and SP-A2, and Its Role in Lung Health
4.1. What have We Learned from Preclinical and/or Human Studies with Regards to the Role of SP-A Genetics on Lung Health?
4.2. Do SP-A Genetics Play a Role in COVID-19?
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| ROS | Reactive Oxygen Species |
| O2•− | superoxide anion |
| H2O2 | hydrogen peroxide |
| HO• | hydroxyl radical |
| CAT | catalase |
| SOD | superoxide dismutase |
| Trx | thioredoxin |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| O2 | molecular oxygen |
| ACE2 | Angiotensin-converting enzyme 2 |
| AΤ-ΙΙ | Angiotensin 2 |
| AΤ-1,7 | Angiotensin 1,7 |
| NADPH oxidase | Nicotinamide adenine dinucleotide phosphate oxidase |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| GSH | reduced Glutathione |
| GSSG | Oxidized Glutathione |
| H2O2 | hydrogen peroxide |
| NO | Nitric Oxide |
| PMNs | Polymorphonuclear cells |
| IL-6 | Interleukin 6 |
| ICU | intensive care unit |
| COVID-19 | Coronavirus disease 2019 |
| COPD | Chronic obstructive pulmonary disease |
| BMI | Body mass index |
| CVD | cardiovascular disease |
| G6PD | Glucose-6-phosphate dehydrogenase deficiency |
| ECD | endothelial cell dysfunction |
| NAC | N-acetylcysteine |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| ARE | Antioxidant response element |
| ecSOD | Extracellular superoxide dismutase |
| TBARS | Thiobarbituric acid reactive substances |
| MDA | Malondialdehyde |
| mTOR | mammalian target of rapamycin |
| NF-κB | Nuclear factor-κB |
| AMPK | AMP-activated protein kinase |
| SIRT | Sirtuins |
| FoxO | Forkhead box O |
| BDNF | Brain-derived neurotrophic factor |
| TNFα | Tumor necrosis factor alpha |
| C-RP | c-reactive protein |
| SP-A | surfactant protein-A |
| SP-A1 | surfactant protein-A1 |
| SP-A2 | surfactant protein-A2 |
| OxS | oxidative stress |
| RSV | respiratory syncytial virus |
References
- World Health Organization. Available online: www.who.int (accessed on 11 March 2020).
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.J.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Phelps, D.S.; Floros, J. Localization of pulmonary surfactant proteins using immunohistochemistry and tissue in situ hybridization. Exp. Lung Res. 1991, 17, 985–995. [Google Scholar] [CrossRef]
- Wang, G.; Umstead, T.M.; Phelps, D.S.; Al-Mondhiry, H.; Floros, J. The effect of ozone exposure on the ability of human surfactant protein a variants to stimulate cytokine production. Environ. Health Perspect. 2002, 110, 79–84. [Google Scholar] [CrossRef]
- Mikerov, A.N.; Wang, G.; Umstead, T.M.; Zacharatos, M.; Thomas, N.J.; Phelps, D.S.; Floros, J. Surfactant protein A2 (SP-A2) variants expressed in CHO cells stimulate phagocytosis of Pseudomonas aeruginosa more than do SP-A1 variants. Infect. Immun. 2007, 75, 1403–1412. [Google Scholar] [CrossRef]
- Mikerov, A.N.; Umstead, T.M.; Gan, X.; Huang, W.; Guo, X.; Wang, G.; Phelps, D.S.; Floros, J. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L121–L130. [Google Scholar] [CrossRef]
- Thorenoor, N.; Umstead, T.M.; Zhang, X.; Phelps, D.S.; Floros, J. Survival of Surfactant Protein-A1 and SP-A2 Transgenic Mice After Klebsiella pneumoniae Infection, Exhibits Sex-, Gene-, and Variant Specific Differences; Treatment With Surfactant Protein Improves Survival. Front. Immunol. 2018, 9, 2404. [Google Scholar] [CrossRef]
- Thorenoor, N.; Zhang, X.; Umstead, T.M.; Scott Halstead, E.; Phelps, D.S.; Floros, J. Differential effects of innate immune variants of surfactant protein-A1 (SFTPA1) and SP-A2 (SFTPA2) in airway function after Klebsiella pneumoniae infection and sex differences. Respir. Res. 2018, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef]
- Keidar, S.; Kaplan, M.; Gamliel-Lazarovich, A. ACE2 of the heart: From angiotensin I to angiotensin (1-7). Cardiovasc. Res. 2007, 73, 463–469. [Google Scholar] [CrossRef]
- Wang, W.; McKinnie, S.M.K.; Farhan, M.; Paul, M.; McDonald, T.; McLean, B.; Llorens-Cortes, C.; Hazra, S.; Murray, A.G.; Vederas, J.C.; et al. Angiotensin-Converting Enzyme 2 Metabolizes and Partially Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects in the Cardiovascular System. Hypertension 2016, 68, 365–377. [Google Scholar] [CrossRef]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000, 87, e1–e9. [Google Scholar] [CrossRef]
- Polizio, A.H.; Gironacci, M.M.; Tomaro, M.L.; Peña, C. Angiotensin-(1-7) blocks the angiotensin II-stimulated superoxide production. Pharmacol. Res. 2007, 56, 86–90. [Google Scholar] [CrossRef]
- Lovren, F.; Pan, Y.; Quan, A.; Teoh, H.; Wang, G.; Shukla, P.C.; Levitt, K.S.; Oudit, G.Y.; Al-Omran, M.; Stewart, D.J.; et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1377–H1384. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Zhang, Y.; Dong, X.-F.; Hao, Q.-Q.; Zhou, X.-M.; Yu, Q.-T.; Li, S.-Y.; Chen, X.; Tengbeh, A.F.; Dong, B.; et al. ACE2 and Ang-(1-7) protect endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response. Inflamm. Res. 2015, 64, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Stanhewicz, A.E.; Alexander, L.M. Local angiotensin-(1-7) administration improves microvascular endothelial function in women who have had preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R148–R155. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xiang, P.; Pu, L.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Meitzler, J.L.; Antony, S.; Wu, Y.; Juhasz, A.; Liu, H.; Jiang, G.; Lu, J.; Roy, K.; Doroshow, J.H. NADPH oxidases: A perspective on reactive oxygen species production in tumor biology. Antioxid. Redox Signal. 2014, 20, 2873–2889. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Hakim, Z.S.; Runge, M.S. Oxidative stress in atherogenesis and arterial thrombosis: The disconnect between cellular studies and clinical outcomes. J. Thromb. Haemost. 2005, 3, 254–267. [Google Scholar] [CrossRef]
- Wen, H.; Gwathmey, J.K.; Xie, L.-H. Oxidative stress-mediated effects of angiotensin II in the cardiovascular system. World J. Hypertens. 2012, 2, 34–44. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Bayraktutan, U. Free radicals, diabetes and endothelial dysfunction. Diabetes Obes. Metab. 2002, 4, 224–238. [Google Scholar] [CrossRef]
- SHI, Y.; Vanhoutte, P.M. Reactive oxygen-derived free radicals are key to the endothelial dysfunction of diabetes. J. Diabetes 2009, 1, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Gao, F.; Nelson, A.H.; Lopez, B.L.; Christopher, T.A.; Yue, T.L.; Barone, F.C. Oxidative inactivation of nitric oxide and endothelial dysfunction in stroke-prone spontaneous hypertensive rats. J. Pharmacol. Exp. Ther. 2001, 298, 879–885. [Google Scholar]
- Loscalzo, J. Oxidative stress in endothelial cell dysfunction and thrombosis. Pathophysiol. Haemost. Thromb. 2002, 32, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Becker, K.; Fuchs, D.; Gostner, J.M. Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. 2014, 6, 462–477. [Google Scholar] [CrossRef]
- Nasi, A.; McArdle, S.; Gaudernack, G.; Westman, G.; Melief, C.; Rockberg, J.; Arens, R.; Kouretas, D.; Sjölin, J.; Mangsbo, S. Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS-CoV-2 in an ageing population, consider N-acetylcysteine as early therapeutic intervention. Toxicol. Rep. 2020, 7, 768–771. [Google Scholar] [CrossRef]
- Veskoukis, A.S. Chapter 8—Redox Signaling and Antioxidant Defense in Pathogenic Microorganisms: A Link to Disease and Putative Therapy; Preedy, V.R.B.T.-P., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 87–95. ISBN 978-0-12-815972-9. [Google Scholar]
- Halasi, M.; Wang, M.; Chavan, T.S.; Gaponenko, V.; Hay, N.; Gartel, A.L. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem. J. 2013, 454, 201–208. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Tsatsakis, A.M.; Kouretas, D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperones 2012, 17, 11–21. [Google Scholar] [CrossRef]
- Marshall, K. Therapeutic applications of whey protein. Altern. Med. Rev. 2004, 9, 136–156. [Google Scholar] [PubMed]
- Veskoukis, A.; Kerasioti, E.; Priftis, A.; Kouka, P.; Spanidis, Y.; Makri, S.; Kouretas, D. A battery of translational biomarkers for the assessment of the in vitro and in vivo antioxidant action of plant polyphenolic compounds: The biomarker issue. Curr. Opin. Toxicol. 2019, 13, 99–109. [Google Scholar] [CrossRef]
- Kerasioti, E.; Stagos, D.; Tzimi, A.; Kouretas, D. Increase in antioxidant activity by sheep/goat whey protein through nuclear factor-like 2 (Nrf2) is cell type dependent. Food Chem. Toxicol. 2016, 97, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kerasioti, E.; Stagos, D.; Georgatzi, V.; Bregou, E.; Priftis, A.; Kafantaris, I.; Kouretas, D. Antioxidant Effects of Sheep Whey Protein on Endothelial Cells. Oxid. Med. Cell. Longev. 2016, 2016, 6585737. [Google Scholar] [CrossRef] [PubMed]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef]
- Kerasioti, E.; Stagos, D.; Jamurtas, A.; Kiskini, A.; Koutedakis, Y.; Goutzourelas, N.; Pournaras, S.; Tsatsakis, A.M.; Kouretas, D. Anti-inflammatory effects of a special carbohydrate-whey protein cake after exhaustive cycling in humans. Food Chem. Toxicol. 2013, 61, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Kerasioti, E.; Stagos, D.; Tsatsakis, A.M.; Spandidos, D.A.; Taitzoglou, I.; Kouretas, D. Effects of sheep/goat whey protein dietary supplementation on the redox status of rats. Mol. Med. Rep. 2018, 17, 5774–5781. [Google Scholar] [CrossRef]
- Kerasioti, E.; Veskoukis, A.; Virgiliou, C.; Theodoridis, G.; Taitzoglou, I.; Kouretas, D. The Strong Antioxidant Sheep/Goat Whey Protein Protects Against mTOR Overactivation in Rats: A Mode of Action Mimicking Fasting. Antioxidants 2019, 8, 71. [Google Scholar] [CrossRef]
- Draganidis, D.; Karagounis, L.G.; Athanailidis, I.; Chatzinikolaou, A.; Jamurtas, A.Z.; Fatouros, I.G. Inflammaging and Skeletal Muscle: Can Protein Intake Make a Difference? J. Nutr. 2016, 146, 1940–1952. [Google Scholar] [CrossRef]
- Goutzourelas, N.; Stagos, D.; Spanidis, Y.; Liosi, M.; Apostolou, A.; Priftis, A.; Haroutounian, S.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D. Polyphenolic composition of grape stem extracts affects antioxidant activity in endothelial and muscle cells. Mol. Med. Rep. 2015, 12, 5846–5856. [Google Scholar] [CrossRef]
- Goutzourelas, N.; Stagos, D.; Demertzis, N.; Mavridou, P.; Karterolioti, H.; Georgadakis, S.; Kerasioti, E.; Aligiannis, N.; Skaltsounis, L.; Statiri, A.; et al. Effects of polyphenolic grape extract on the oxidative status of muscle and endothelial cells. Hum. Exp. Toxicol. 2014, 33, 1099–1112. [Google Scholar] [CrossRef]
- Kouka, P.; Priftis, A.; Stagos, D.; Angelis, A.; Stathopoulos, P.; Xinos, N.; Skaltsounis, A.-L.; Mamoulakis, C.; Tsatsakis, A.M.; Spandidos, D.A.; et al. Assessment of the antioxidant activity of an olive oil total polyphenolic fraction and hydroxytyrosol from a Greek Olea europea variety in endothelial cells and myoblasts. Int. J. Mol. Med. 2017, 40, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Makri, S.; Kafantaris, I.; Savva, S.; Ntanou, P.; Stagos, D.; Argyroulis, I.; Kotsampasi, B.; Christodoulou, V.; Gerasopoulos, K.; Petrotos, K.; et al. Novel Feed Including Olive Oil Mill Wastewater Bioactive Compounds Enhanced the Redox Status of Lambs. In Vivo 2018, 32, 291–302. [Google Scholar] [PubMed]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Kokka, E.; Kouka, P.; Terzopoulou, Z.; Gerasopoulos, K.; Stagos, D.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. Berl. 2017, 101, e108–e121. [Google Scholar] [CrossRef] [PubMed]
- Makri, S.; Kafantaris, I.; Stagos, D.; Chamokeridou, T.; Petrotos, K.; Gerasopoulos, K.; Mpesios, A.; Goutzourelas, N.; Kokkas, S.; Goulas, P.; et al. Novel feed including bioactive compounds from winery wastes improved broilers’ redox status in blood and tissues of vital organs. Food Chem. Toxicol. 2017, 102, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2018, 12, 246–255. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef]
- Matthaiou, C.M.; Goutzourelas, N.; Stagos, D.; Sarafoglou, E.; Jamurtas, A.; Koulocheri, S.D.; Haroutounian, S.A.; Tsatsakis, A.M.; Kouretas, D. Pomegranate juice consumption increases GSH levels and reduces lipid and protein oxidation in human blood. Food Chem. Toxicol. 2014, 73, 1–6. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.A.; Morrison, E.Y.; McGrowder, D.A.; Young, R.; Fraser, Y.T.P.; Zamora, E.M.; Alexander-Lindo, R.L.; Irving, R.R. Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complement. Altern. Med. 2008, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.N.; Bessa, A.; Jorge, M.L.M.P.; Oliveira, R.J.D.S.; de Mello, M.T.; De Agostini, G.G.; Jorge, P.T.; Espindola, F.S. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2012, 37, 334–344. [Google Scholar] [CrossRef]
- Azizbeigi, K.; Azarbayjani, M.A.; Peeri, M.; Agha-alinejad, H.; Stannard, S. The effect of progressive resistance training on oxidative stress and antioxidant enzyme activity in erythrocytes in untrained men. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, A.B.; Kafkas, M.E.; Kafkas, A.S.; Onal, Y.; Kiran, T.R. The effect of regular exercise and massage on oxidant and antioxidant parameters. Indian J. Physiol. Pharmacol. 2013, 57, 378–383. [Google Scholar]
- Johnson, M.L.; Irving, B.A.; Lanza, I.R.; Vendelbo, M.H.; Konopka, A.R.; Robinson, M.M.; Henderson, G.C.; Klaus, K.A.; Morse, D.M.; Heppelmann, C.; et al. Differential Effect of Endurance Training on Mitochondrial Protein Damage, Degradation, and Acetylation in the Context of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Mitranun, W.; Deerochanawong, C.; Tanaka, H.; Suksom, D. Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand. J. Med. Sci. Sports 2014, 24, e69–e76. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Adams, V.; Schulze, P.C.; Erbs, S.; Gielen, S.; Fiehn, E.; Möbius-Winkler, S.; Schubert, A.; Schuler, G.; Hambrecht, R. Antioxidative effects of exercise training in patients with chronic heart failure: Increase in radical scavenger enzyme activity in skeletal muscle. Circulation 2005, 111, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Onur, E.; Kabaroğlu, C.; Günay, O.; Var, A.; Yilmaz, O.; Dündar, P.; Tikiz, C.; Güvenç, Y.; Yüksel, H. The beneficial effects of physical exercise on antioxidant status in asthmatic children. Allergol. Immunopathol. Madr. 2011, 39, 90–95. [Google Scholar] [CrossRef]
- Beck, D.T.; Martin, J.S.; Casey, D.P.; Braith, R.W. Exercise training improves endothelial function in resistance arteries of young prehypertensives. J. Hum. Hypertens. 2014, 28, 303–309. [Google Scholar] [CrossRef]
- Soares, J.P.; Silva, A.M.; Oliveira, M.M.; Peixoto, F.; Gaivão, I.; Mota, M.P. Effects of combined physical exercise training on DNA damage and repair capacity: Role of oxidative stress changes. Age Dord. 2015, 37, 9799. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A time to fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M.; Whitehead, R.G.; Roberts, S.B.; Paul, A.A. Long-term energy balance in child-bearing Gambian women. Am. J. Clin. Nutr. 1981, 34, 2790–2799. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Borsellino, C.; Martindale, R.G. From Religion to Secularism: The Benefits of Fasting. Curr. Nutr. Rep. 2018, 7, 131–138. [Google Scholar] [CrossRef] [PubMed]
- de Toledo, F.W.; Grundler, F.; Bergouignan, A.; Drinda, S.; Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 2019, 14, e0209353. [Google Scholar]
- de Toledo, F.W.; Buchinger, A.; Burggrabe, H.; Hölz, G.; Kuhn, C.; Lischka, E.; Lischka, N.; Lützner, H.; May, W.; Ritzmann-Widderich, M.; et al. Fasting therapy—An expert panel update of the 2002 consensus guidelines. Complement. Med. Res. 2013, 20, 434–443. [Google Scholar] [CrossRef]
- Mesnage, R.; Grundler, F.; Schwiertz, A.; Le Maho, Y.; de Toledo, F.W. Changes in human gut microbiota composition are linked to the energy metabolic switch during 10 d of Buchinger fasting. J. Nutr. Sci. 2019, 8, e36. [Google Scholar] [CrossRef]
- Cherif, A.; Roelands, B.; Meeusen, R.; Chamari, K. Effects of Intermittent Fasting, Caloric Restriction, and Ramadan Intermittent Fasting on Cognitive Performance at Rest and During Exercise in Adults. Sports Med. 2016, 46, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; La Bounty, P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Effects of intermittent fasting on glucose and lipid metabolism. Proc. Nutr. Soc. 2017, 76, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Medina, J.P.; Popovich, I.; Thorwald, M.A.; Viscarra, J.A.; Rodriguez, R.; Sonanez-Organis, J.G.; Lam, L.; Peti-Peterdi, J.; Nakano, D.; Nishiyama, A.; et al. Angiotensin receptor-mediated oxidative stress is associated with impaired cardiac redox signaling and mitochondrial function in insulin-resistant rats. Am. J. Physiol. Circ. Physiol. 2013, 305, H599–H607. [Google Scholar] [CrossRef]
- Vázquez-Medina, J.P.; Soñanez-Organis, J.G.; Rodriguez, R.; Viscarra, J.A.; Nishiyama, A.; Crocker, D.E.; Ortiz, R.M. Prolonged fasting activates Nrf2 in post-weaned elephant seals. J. Exp. Biol. 2013, 216, 2870–2878. [Google Scholar] [CrossRef]
- de Toledo, F.W.; Grundler, F.; Goutzourelas, N.; Tekos, F.; Vassi, E.; Mesnage, R.; Kouretas, D. Influence of Long-Term Fasting on Blood Redox Status in Humans. Antioxidants 2020, 9, 496. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., III; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obes. Silver Spring 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.I.; El-Serafi, A.T.; Jahrami, H.A.; Sherif, N.M.; Hassan, R.E.; Awadallah, S.; Faris, M.A.-I.E. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res. Clin. Pract. 2019, 155, 107801. [Google Scholar] [CrossRef]
- Halberg, N.; Henriksen, M.; Söderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.-H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Telljohann, R.; Maudsley, S.; et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Samimi, M.; Taghizadeh, M.; Esmaillzadeh, A. Effects of Ramadan Fasting on Glucose Homeostasis, Lipid Profiles, Inflammation and Oxidative Stress in Women with Polycystic Ovary Syndrome in Kashan, Iran. Arch. Iran. Med. 2015, 18, 806–810. [Google Scholar] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Faris, M.A.-I.E.; Kacimi, S.; Al-Kurd, R.A.; Fararjeh, M.A.; Bustanji, Y.K.; Mohammad, M.K.; Salem, M.L. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr. Res. 2012, 32, 947–955. [Google Scholar] [CrossRef]
- Aliasghari, F.; Izadi, A.; Gargari, B.P.; Ebrahimi, S. The Effects of Ramadan Fasting on Body Composition, Blood Pressure, Glucose Metabolism, and Markers of Inflammation in NAFLD Patients: An Observational Trial. J. Am. Coll. Nutr. 2017, 36, 640–645. [Google Scholar] [CrossRef]
- Kroeger, C.M.; Klempel, M.C.; Bhutani, S.; Trepanowski, J.F.; Tangney, C.C.; Varady, K.A. Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: Relationship to adipokine modulations. Nutr. Metab. Lond. 2012, 9, 98. [Google Scholar] [CrossRef]
- Karinch, A.M.; Floros, J. 5’ splicing and allelic variants of the human pulmonary surfactant protein A genes. Am. J. Respir. Cell Mol. Biol. 1995, 12, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.R.; Floros, J. Organization of the human SP-A and SP-D loci at 10q22-q23. Physical and radiation hybrid mapping reveal gene order and orientation. Am. J. Respir. Cell Mol. Biol. 1998, 18, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Bruns, G.; Stroh, H.; Veldman, G.M.; Latt, S.A.; Floros, J. The 35 kd pulmonary surfactant-associated protein is encoded on chromosome 10. Hum. Genet. 1987, 76, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Floros, J.; Hoover, R.R. Genetics of the hydrophilic surfactant proteins A and D. Biochim. Biophys. Acta 1998, 1408, 312–322. [Google Scholar] [CrossRef]
- Floros, J.; Wang, G.; Lin, Z. Genetic diversity of human SP-A, a molecule with innate host defense and surfactant-related functions; characteristics, primary function, and significance. Curr. Pharm. 2005, 3, 87–95. [Google Scholar] [CrossRef]
- Floros, J.; Wang, G.; Mikerov, A.N. Genetic complexity of the human innate host defense molecules, surfactant protein A1 (SP-A1) and SP-A2--impact on function. Crit. Rev. Eukaryot Gene Expr. 2009, 19, 125–137. [Google Scholar] [CrossRef]
- DiAngelo, S.; Lin, Z.; Wang, G.; Phillips, S.; Ramet, M.; Luo, J.; Floros, J. Novel, non-radioactive, simple and multiplex PCR-cRFLP methods for genotyping human SP-A and SP-D marker alleles. Dis. Markers 1999, 15, 269–281. [Google Scholar] [CrossRef]
- Voss, T.; Melchers, K.; Scheirle, G.; Schäfer, K.P. Structural comparison of recombinant pulmonary surfactant protein SP-A derived from two human coding sequences: Implications for the chain composition of natural human SP-A. Am. J. Respir. Cell Mol. Biol. 1991, 4, 88–94. [Google Scholar] [CrossRef]
- Wang, G.; Bates-Kenney, S.R.; Tao, J.-Q.; Phelps, D.S.; Floros, J. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry 2004, 43, 4227–4239. [Google Scholar] [CrossRef]
- Floros, J.; Wang, G. A point of view: Quantitative and qualitative imbalance in disease pathogenesis; pulmonary surfactant protein A genetic variants as a model. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 129, 295–303. [Google Scholar] [CrossRef]
- Wang, G.; Myers, C.; Mikerov, A.; Floros, J. Effect of cysteine 85 on biochemical properties and biological function of human surfactant protein A variants. Biochemistry 2007, 46, 8425–8435. [Google Scholar] [CrossRef] [PubMed]
- Karinch, A.M.; Floros, J. Translation in vivo of 5’ untranslated-region splice variants of human surfactant protein-A. Biochem. J. 1995, 307, 327–330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silveyra, P.; Wang, G.; Floros, J. Human SP-A1 (SFTPA1) variant-specific 3’ UTRs and poly(A) tail differentially affect the in vitro translation of a reporter gene. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L523–L534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silveyra, P.; DiAngelo, S.L.; Floros, J. An 11-nt sequence polymorphism at the 3’UTR of human SFTPA1 and SFTPA2 gene variants differentially affect gene expression levels and miRNA regulation in cell culture. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L106–L119. [Google Scholar] [CrossRef]
- García-Verdugo, I.; Wang, G.; Floros, J.; Casals, C. Structural analysis and lipid-binding properties of recombinant human surfactant protein a derived from one or both genes. Biochemistry 2002, 41, 14041–14053. [Google Scholar] [CrossRef]
- Wang, G.; Taneva, S.; Keough, K.M.W.; Floros, J. Differential effects of human SP-A1 and SP-A2 variants on phospholipid monolayers containing surfactant protein B. Biochim. Biophys. Acta 2007, 1768, 2060–2069. [Google Scholar] [CrossRef][Green Version]
- Phelps, D.S.; Umstead, T.M.; Silveyra, P.; Hu, S.; Wang, G.; Floros, J. Differences in the alveolar macrophage proteome in transgenic mice expressing human SP-A1 and SP-A2. J. Proteom. Genom. Res. 2013, 1, 2–26. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, E.; Pascual, A.; Arroyo, R.; Floros, J.; Perez-Gil, J. Human Pulmonary Surfactant Protein SP-A1 Provides Maximal Efficiency of Lung Interfacial Films. Biophys. J. 2016, 111, 524–536. [Google Scholar] [CrossRef]
- Tsotakos, N.; Phelps, D.S.; Yengo, C.M.; Chinchilli, V.M.; Floros, J. Single-cell analysis reveals differential regulation of the alveolar macrophage actin cytoskeleton by surfactant proteins A1 and A2: Implications of sex and aging. Biol. Sex Differ. 2016, 7, 18. [Google Scholar] [CrossRef]
- Noutsios, G.T.; Thorenoor, N.; Zhang, X.; Phelps, D.S.; Umstead, T.M.; Durrani, F.; Floros, J. SP-A2 contributes to miRNA-mediated sex differences in response to oxidative stress: Pro-inflammatory, anti-apoptotic, and anti-oxidant pathways are involved. Biol. Sex Differ. 2017, 8, 37. [Google Scholar] [CrossRef]
- Wang, G.; Umstead, T.M.; Hu, S.; Mikerov, A.N.; Phelps, D.S.; Floros, J. Differential Effects of Human SP-A1 and SP-A2 on the BAL Proteome and Signaling Pathways in Response to Klebsiella pneumoniae and Ozone Exposure. Front. Immunol. 2019, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Thorenoor, N.; Kawasawa, Y.I.; Gandhi, C.K.; Zhang, X.; Floros, J. Differential Impact of Co-expressed SP-A1/SP-A2 Protein on AM miRNome; Sex Differences. Front. Immunol. 2019, 10, 1960. [Google Scholar] [CrossRef] [PubMed]
- Noutsios, G.T.; Thorenoor, N.; Zhang, X.; Phelps, D.S.; Umstead, T.M.; Durrani, F.; Floros, J. Major Effect of Oxidative Stress on the Male, but Not Female, SP-A1 Type II Cell miRNome. Front. Immunol. 2019, 10, 1514. [Google Scholar] [CrossRef] [PubMed]
- Phelps, D.S.; Umstead, T.M.; Floros, J. Sex differences in the acute in vivo effects of different human SP-A variants on the mouse alveolar macrophage proteome. J. Proteom. 2014, 108, 427–444. [Google Scholar] [CrossRef]
- Nalian, A.; Umstead, T.M.; Yang, C.-H.; Silveyra, P.; Thomas, N.J.; Floros, J.; McCormack, F.X.; Chroneos, Z.C. Structural and Functional Determinants of Rodent and Human Surfactant Protein A: A Synthesis of Binding and Computational Data. Front. Immunol. 2019, 10, 2613. [Google Scholar] [CrossRef]
- Floros, J.; Thomas, N. Genetic variations of surfactant proteins and lung injury. In Surfactant Pathogenesis and Treatment of Lung Disease; Nakos, G., Papathanasiou, A., Eds.; Research Signpost: Kerala, India, 2009; pp. 25–48. [Google Scholar]
- Silveyra, P.; Floros, J. Genetic variant associations of human SP-A and SP-D with acute and chronic lung injury. Front. Biosci. 2012, 17, 407–429. [Google Scholar] [CrossRef]
- Wang, G.; Phelps, D.S.; Umstead, T.M.; Floros, J. Human SP-A protein variants derived from one or both genes stimulate TNF-alpha production in the THP-1 cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L946–L954. [Google Scholar] [CrossRef]
- Mikerov, A.N.; Umstead, T.M.; Huang, W.; Liu, W.; Phelps, D.S.; Floros, J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L150–L158. [Google Scholar] [CrossRef][Green Version]
- Thorenoor, N.; Kawasawa, Y.I.; Ghandi, C.K.; Floros, J. Sex-specific regulation of gene expression networks by surfactant protein A (SP-A) variants in alveolar macrophages in response to Klebsiella pneumoniae. Front. Immunol. 2020, 11, 1290. [Google Scholar] [CrossRef]
- D’Ovidio, F.; Floros, J.; Aramini, B.; Lederer, D.; DiAngelo, S.L.; Arcasoy, S.; Sonett, J.R.; Robbins, H.; Shah, L.; Costa, J.; et al. Donor surfactant protein A2 polymorphism and lung transplant survival. Eur. Respir. J. 2020, 55, 1900618. [Google Scholar]
- D’Ovidio, F.; Kaneda, H.; Chaparro, C.; Mura, M.; Lederer, D.; Di Angelo, S.; Takahashi, H.; Gutierrez, C.; Hutcheon, M.; Singer, L.G.; et al. Pilot study exploring lung allograft surfactant protein A (SP-A) expression in association with lung transplant outcome. Am. J. Transpl. 2013, 13, 2722–2729. [Google Scholar] [CrossRef]
- Karinch, A.M.; Deiter, G.; Ballard, P.L.; Floros, J. Regulation of expression of human SP-A1 and SP-A2 genes in fetal lung explant culture. Biochim. Biophys. Acta 1998, 1398, 192–202. [Google Scholar] [CrossRef]
- Wang, G.; Guo, X.; Floros, J. Human SP-A 3’-UTR variants mediate differential gene expression in basal levels and in response to dexamethasone. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L738–L748. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Guo, X.; Floros, J. Differences in the translation efficiency and mRNA stability mediated by 5’-UTR splice variants of human SP-A1 and SP-A2 genes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L497–L508. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Guo, X.; Silveyra, P.; Kimball, S.R.; Floros, J. Cap-independent translation of human SP-A 5’-UTR variants: A double-loop structure and cis-element contribution. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L635–L647. [Google Scholar] [CrossRef]
- Noutsios, G.T.; Silveyra, P.; Bhatti, F.; Floros, J. Exon B of human surfactant protein A2 mRNA, alone or within its surrounding sequences, interacts with 14-3-3; role of cis-elements and secondary structure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L722–L735. [Google Scholar] [CrossRef]
- Noutsios, G.T.; Ghattas, P.; Bennett, S.; Floros, J. 14-3-3 isoforms bind directly exon B of the 5’-UTR of human surfactant protein A2 mRNA. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L147–L157. [Google Scholar] [CrossRef]
- Aramini, B.; Geraghty, P.; Lederer, D.J.; Costa, J.; DiAngelo, S.L.; Floros, J.; D’Ovidio, F. Surfactant protein A and D polymorphisms and methylprednisolone pharmacogenetics in donor lungs. J. Thorac. Cardiovasc. Surg. 2019, 157, 2109–2117. [Google Scholar] [CrossRef]
- Tagaram, H.R.S.; Wang, G.; Umstead, T.M.; Mikerov, A.N.; Thomas, N.J.; Graff, G.R.; Hess, J.C.; Thomassen, M.J.; Kavuru, M.S.; Phelps, D.S.; et al. Characterization of a human surfactant protein A1 (SP-A1) gene-specific antibody; SP-A1 content variation among individuals of varying age and pulmonary health. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L1052–L1063. [Google Scholar] [CrossRef]
- Wang, Y.; Voelker, D.R.; Lugogo, N.L.; Wang, G.; Floros, J.; Ingram, J.L.; Chu, H.W.; Church, T.D.; Kandasamy, P.; Fertel, D.; et al. Surfactant protein A is defective in abrogating inflammation in asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L598–L606. [Google Scholar] [CrossRef]
- Kim, D.; Quinn, J.; Pinsky, B.; Shah, N.H.; Brown, I. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA 2020, 323, 2085–2086. [Google Scholar] [CrossRef] [PubMed]
- LeVine, A.M.; Gwozdz, J.; Stark, J.; Bruno, M.; Whitsett, J.; Korfhagen, T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J. Clin. Investig. 1999, 103, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Kronqvist, N.; Spalluto, C.M.; Griffiths, M.; Staples, K.J.; Wilkinson, T.; Holmskov, U.; Sorensen, G.L.; Rising, A.; Johansson, J.; et al. Novel expression of a functional trimeric fragment of human SP-A with efficacy in neutralisation of RSV. Immunobiology 2017, 222, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.J.; Fan, R.; Diangelo, S.; Hess, J.C.; Floros, J. Haplotypes of the surfactant protein genes A and D as susceptibility factors for the development of respiratory distress syndrome. Acta Paediatr. 2007, 96, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Roschewski, M.; Lionakis, M.S.; Sharman, J.P.; Roswarski, J.; Goy, A.; Monticelli, M.A.; Roshon, M.; Wrzesinski, S.H.; Desai, J.V.; Zarakas, M.A.; et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020, 5, eabd0110. [Google Scholar] [CrossRef]
- Floros, J.; Phelps, D.S. Is the role of lung innate immune molecules, SP-A1 and SP-A2, and of the alveolar macrophage being overlooked in COVID-19 diverse outcomes? Pneumon 2020, 33. Available online: http://www.pneumon.org/online-first/newsid789/777 (accessed on 19 June 2020).
- Phelps, D.S.; Floros, J.; Taeusch, H.W.J. Post-translational modification of the major human surfactant-associated proteins. Biochem. J. 1986, 237, 373–377. [Google Scholar] [CrossRef]
- Floros, J.; Steinbrink, R.; Jacobs, K.; Phelps, D.; Kriz, R.; Recny, M.; Sultzman, L.; Jones, S.; Taeusch, H.W.; Frank, H.A. Isolation and characterization of cDNA clones for the 35-kDa pulmonary surfactant-associated protein. J. Biol. Chem. 1986, 261, 9029–9033. [Google Scholar]
- Phelps, D.S.; Floros, J. Proline hydroxylation alters the electrophoretic mobility of pulmonary surfactant-associated protein A. Electrophoresis 1988, 9, 231–233. [Google Scholar] [CrossRef]
- Floros, J.; Phelps, D.S.; Taeusch, H.W. Biosynthesis and in vitro translation of the major surfactant-associated protein from human lung. J. Biol. Chem. 1985, 260, 495–500. [Google Scholar]
- Floros, J.; Phelps, D.S.; Kourembanas, S.; Taeusch, H.W. Primary translation products, biosynthesis, and tissue specificity of the major surfactant protein in rat. J. Biol. Chem. 1986, 261, 828–831. [Google Scholar] [PubMed]
- Watson, A.; Sørensen, G.L.; Holmskov, U.; Whitwell, H.J.; Madsen, J.; Clark, H. Generation of novel trimeric fragments of human SP-A and SP-D after recombinant soluble expression in E. coli. Immunobiology 2020, 225, 151953. [Google Scholar] [CrossRef] [PubMed]
- Oosting, R.S.; Van Iwaarden, J.F.; Van Bree, L.; Verhoef, J.; Van Golde, L.M.; Haagsman, H.P. Exposure of surfactant protein A to ozone in vitro and in vivo impairs its interactions with alveolar cells. Am. J. Physiol. 1992, 262, L63–L68. [Google Scholar] [CrossRef] [PubMed]
- Mikerov, A.N.; Haque, R.; Gan, X.; Guo, X.; Phelps, D.S.; Floros, J. Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: Sex differences. Respir. Res. 2008, 9, 77. [Google Scholar] [CrossRef]
- Janic, B.; Umstead, T.M.; Phelps, D.S.; Floros, J. Modulatory effects of ozone on THP-1 cells in response to SP-A stimulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L317–L325. [Google Scholar] [CrossRef]
- Stagos, D.; Umstead, T.M.; Phelps, D.S.; Skaltsounis, L.; Haroutounian, S.; Floros, J.; Kouretas, D. Inhibition of ozone-induced SP-A oxidation by plant polyphenols. Free Radic. Res. 2007, 41, 357–366. [Google Scholar] [CrossRef]
- Samet, J.M.; Hatch, G.E.; Horstman, D.; Steck-Scott, S.; Arab, L.; Bromberg, P.A.; Levine, M.; McDonnell, W.F.; Devlin, R.B. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am. J. Respir. Crit. Care Med. 2001, 164, 819–825. [Google Scholar] [CrossRef]
- Steck-Scott, S.; Arab, L.; Craft, N.E.; Samet, J.M. Plasma and lung macrophage responsiveness to carotenoid supplementation and ozone exposure in humans. Eur. J. Clin. Nutr. 2004, 58, 1571–1579. [Google Scholar] [CrossRef][Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekos, F.; Skaperda, Z.; Goutzourelas, N.; Phelps, D.S.; Floros, J.; Kouretas, D. The Importance of Redox Status in the Frame of Lifestyle Approaches and the Genetics of the Lung Innate Immune Molecules, SP-A1 and SP-A2, on Differential Outcomes of COVID-19 Infection. Antioxidants 2020, 9, 784. https://doi.org/10.3390/antiox9090784
Tekos F, Skaperda Z, Goutzourelas N, Phelps DS, Floros J, Kouretas D. The Importance of Redox Status in the Frame of Lifestyle Approaches and the Genetics of the Lung Innate Immune Molecules, SP-A1 and SP-A2, on Differential Outcomes of COVID-19 Infection. Antioxidants. 2020; 9(9):784. https://doi.org/10.3390/antiox9090784
Chicago/Turabian StyleTekos, Fotios, Zoi Skaperda, Nikolaos Goutzourelas, David S. Phelps, Joanna Floros, and Demetrios Kouretas. 2020. "The Importance of Redox Status in the Frame of Lifestyle Approaches and the Genetics of the Lung Innate Immune Molecules, SP-A1 and SP-A2, on Differential Outcomes of COVID-19 Infection" Antioxidants 9, no. 9: 784. https://doi.org/10.3390/antiox9090784
APA StyleTekos, F., Skaperda, Z., Goutzourelas, N., Phelps, D. S., Floros, J., & Kouretas, D. (2020). The Importance of Redox Status in the Frame of Lifestyle Approaches and the Genetics of the Lung Innate Immune Molecules, SP-A1 and SP-A2, on Differential Outcomes of COVID-19 Infection. Antioxidants, 9(9), 784. https://doi.org/10.3390/antiox9090784