Effect of Supplementation with Hydroethanolic Extract of Campomanesia xanthocarpa (Berg.) Leaves and Two Isolated Substances from the Extract on Metabolic Parameters of Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Results
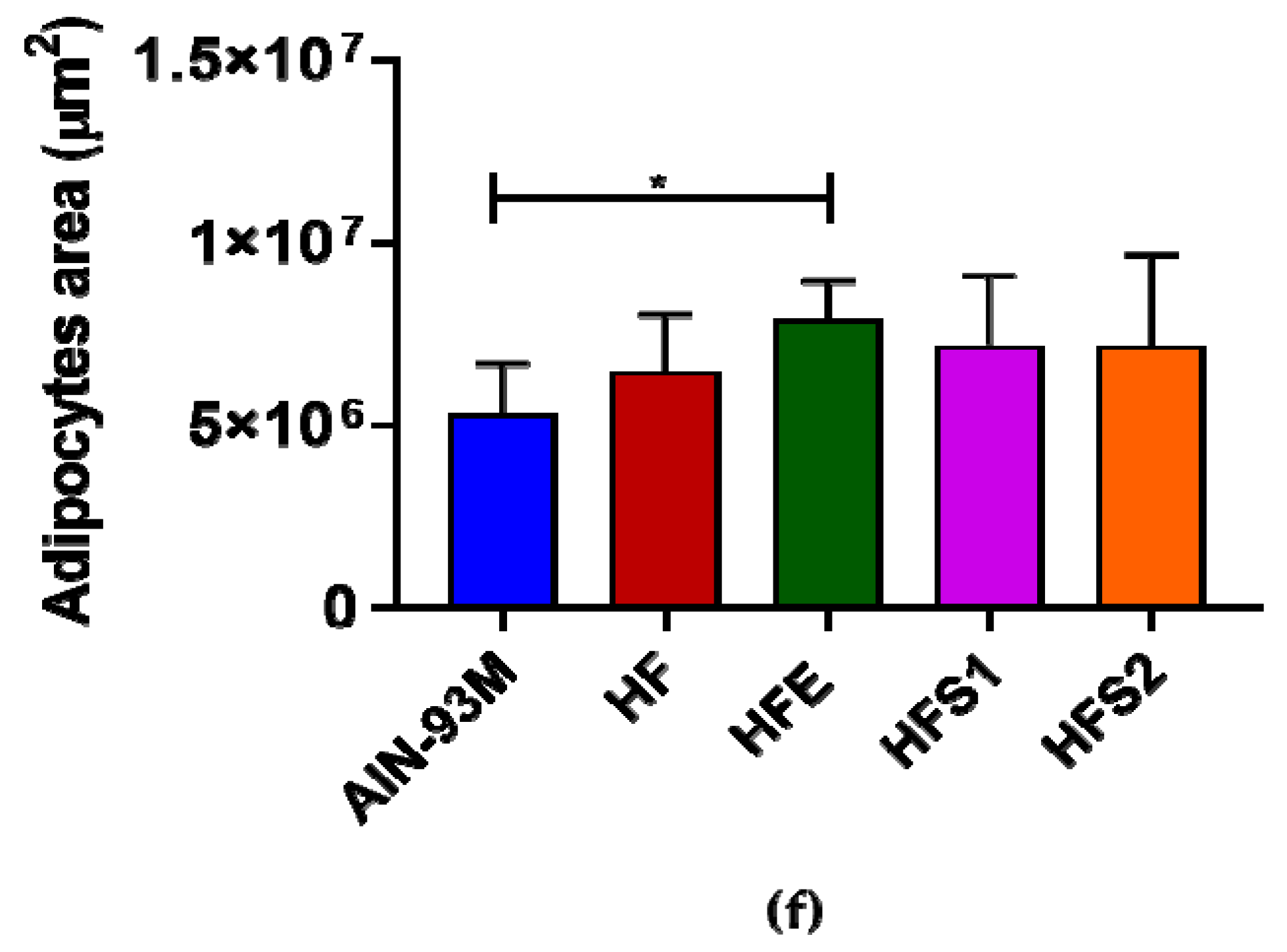
2.1. S1, S2 and Ext Have Not Influenced on Decreasing Body Weight and Visceral Adiposity
2.2. S1 Attenuated Food Intake; However, S2 and Ext Did Not Decrease Food and Calorie Intake
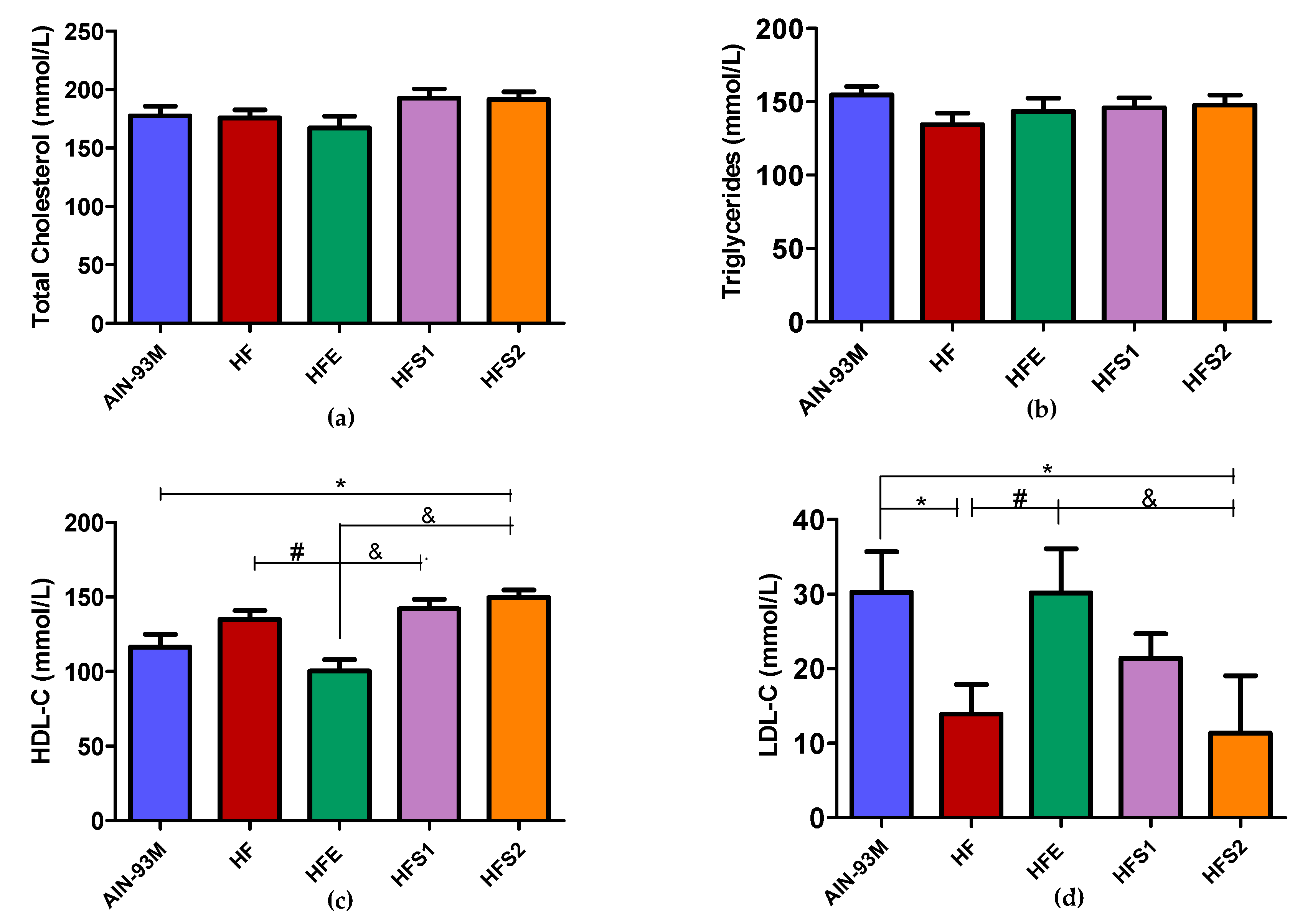
2.3. Ext Diminished HDL-C and Increased LDL-C Levels, Whereas S1 and S2 Diminished LDL-C and Increased HDL-C Levels Compared to HFE Group
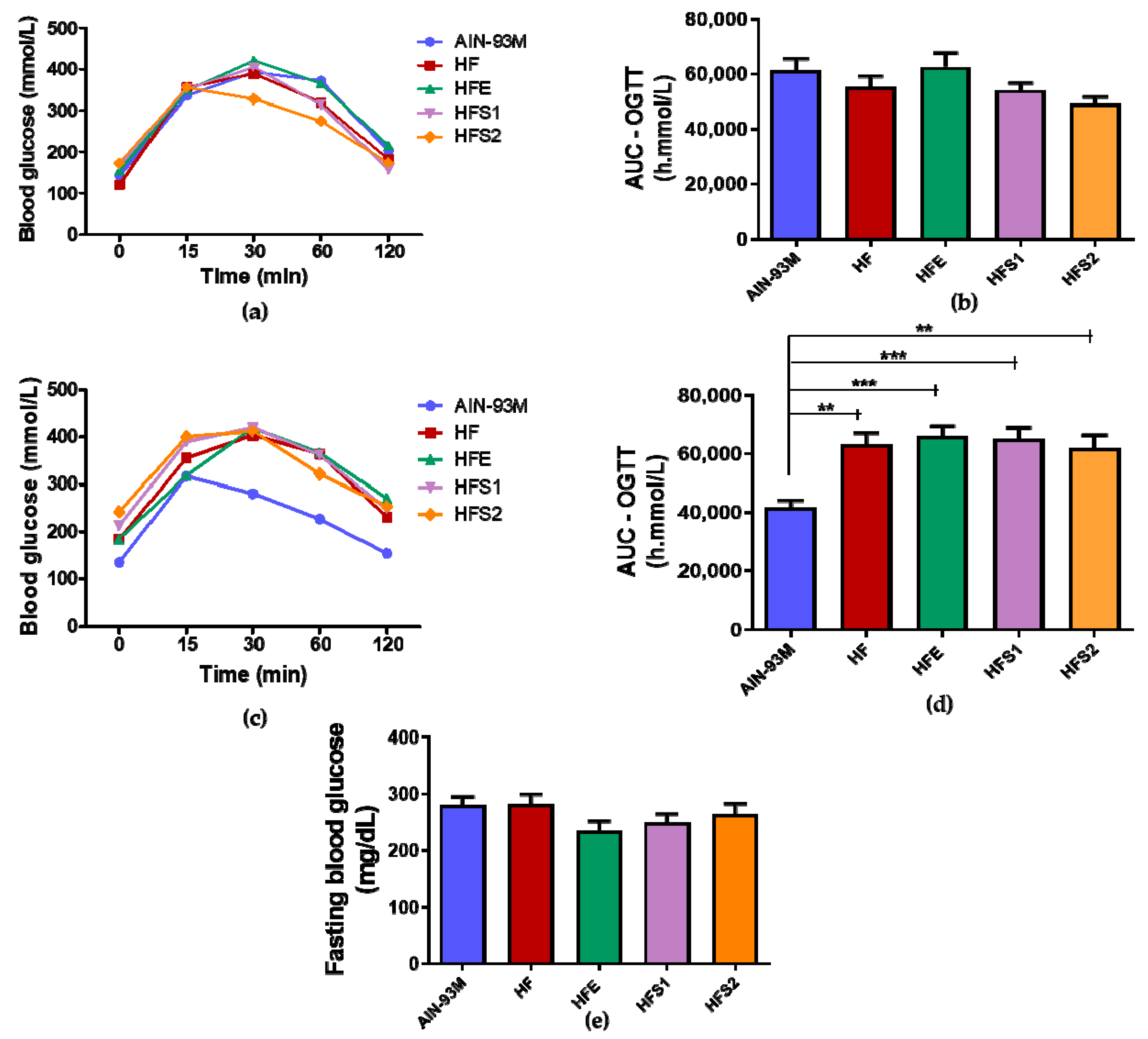
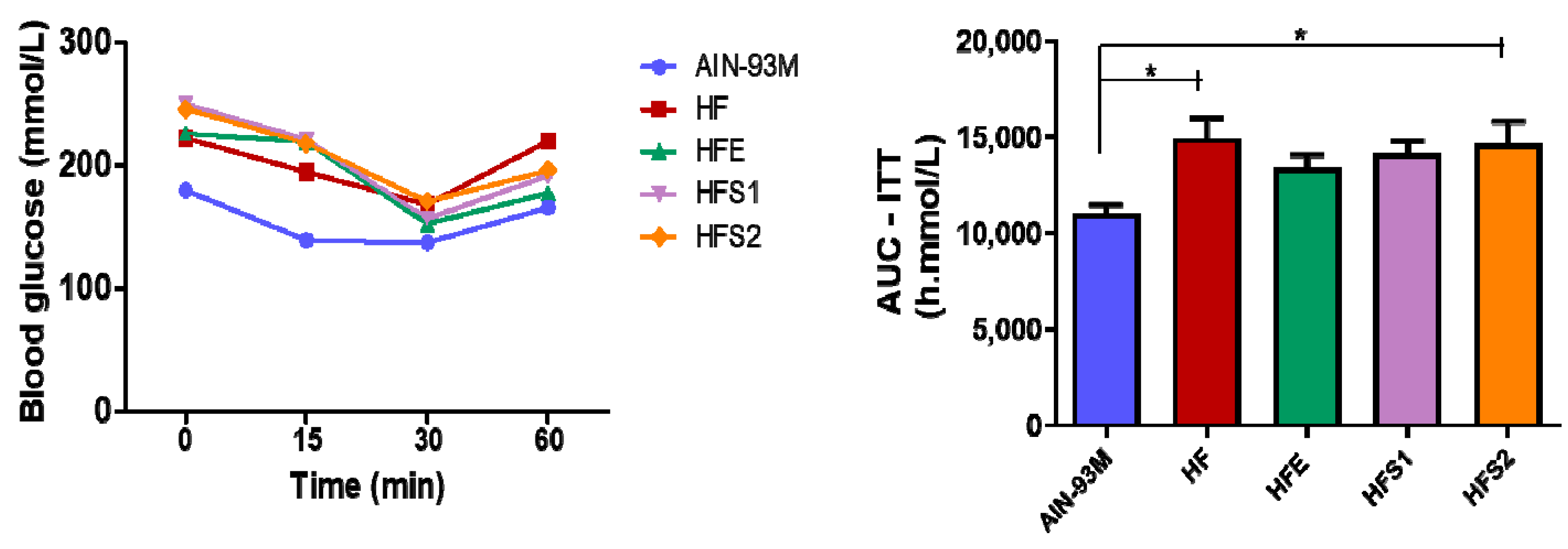
2.4. S1, S2, and Ext Have Not Influenced on Glycemic Metabolism
2.5. S1, S2 and Ext Have Not Influenced on Insulin Parameters
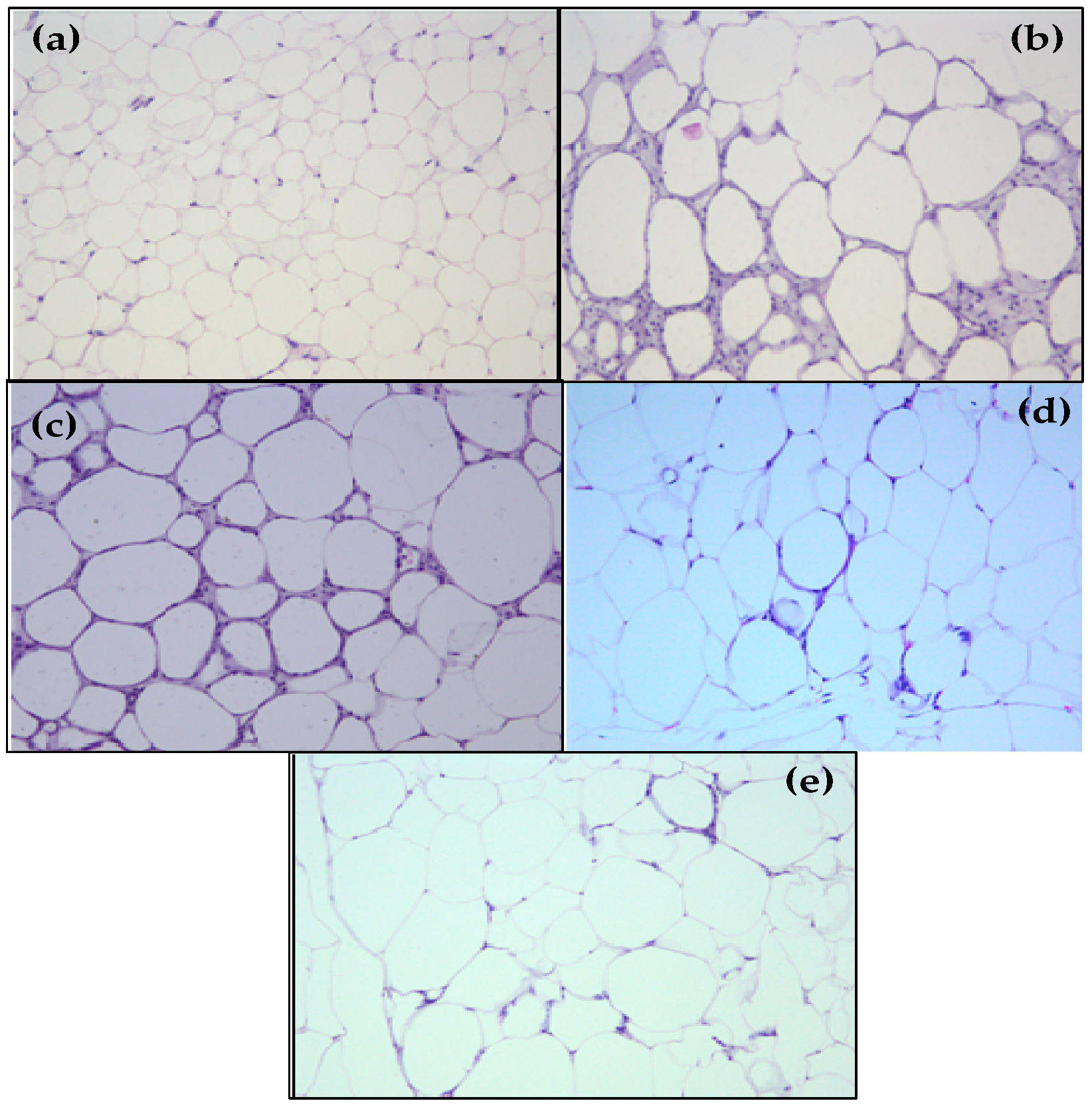
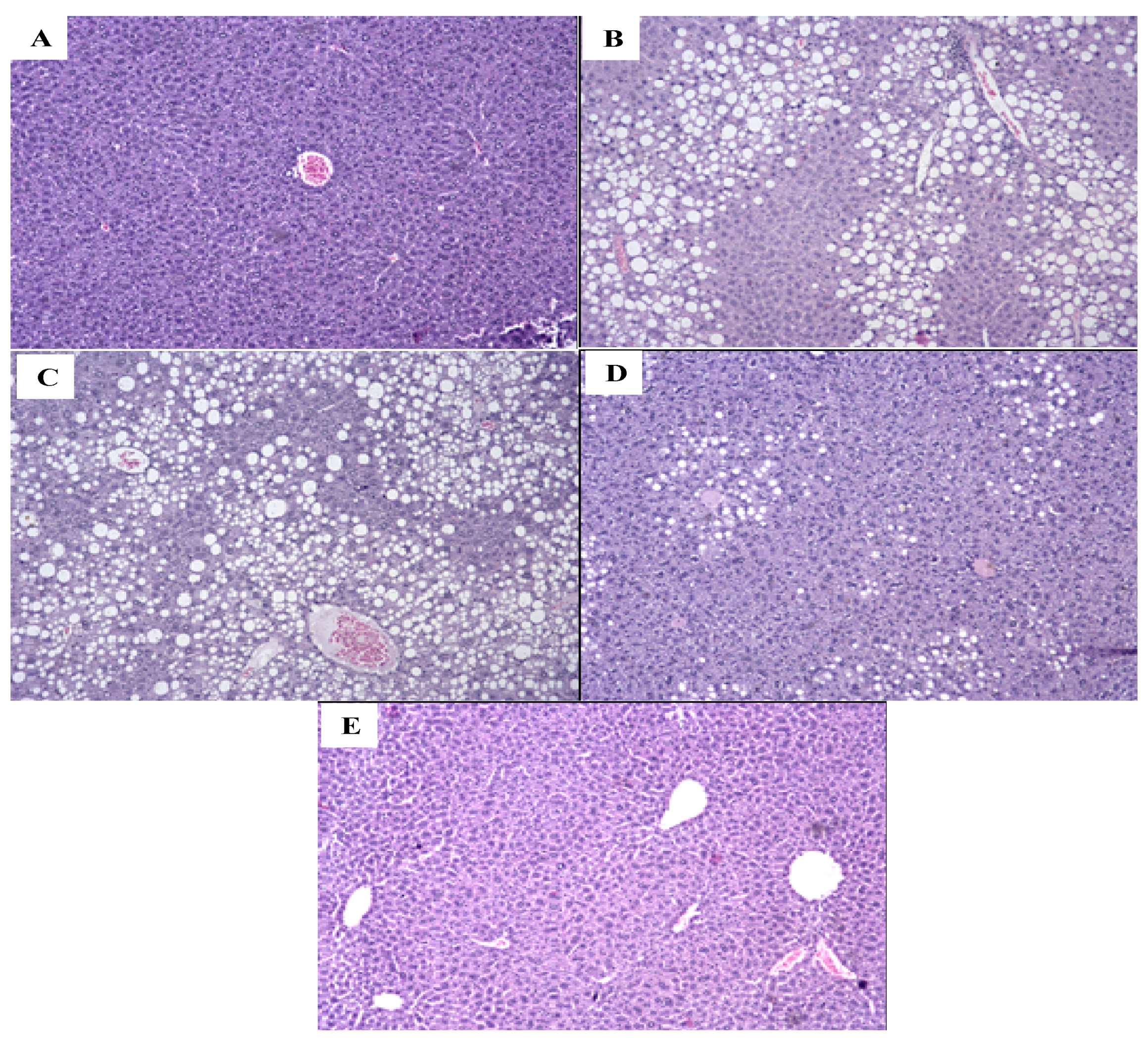
2.6. S1, S2 and Ext Have Not Attenuated Hepatic Steatosis Percentage
2.7. Ext, S1, and S2 Had No Influence on Pancreas Hypertrophy
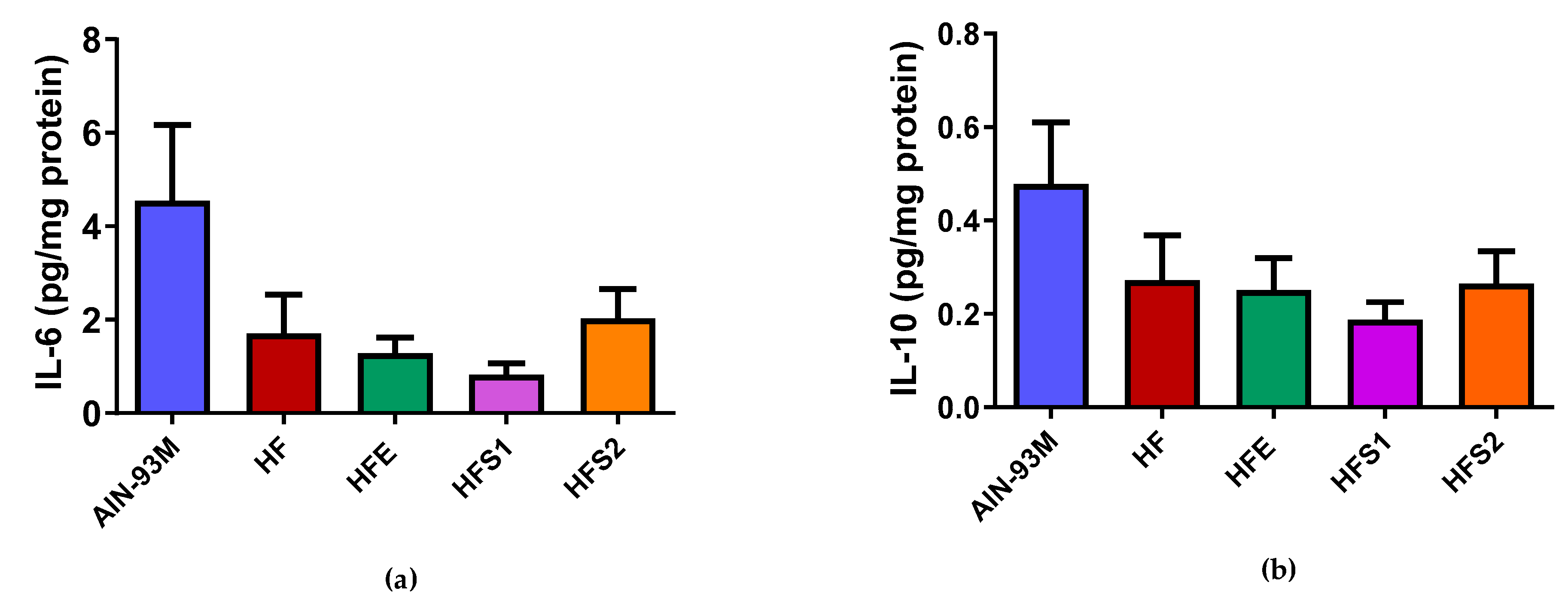
2.8. S1, S2, and Ext Groups Had No Influence on IL-6 and IL-10 Protein Expression in Epididymal Adipose Tissue
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of Extract
4.2. Isolation and Identification of Substances
4.3. Ethics Statement
4.4. Animal Care and Experimental Procedure
4.5. Body Weight and Diet Intake
4.6. Biochemical Analysis
4.7. Oral Glucose Tolerance Test
4.8. Insulin Tolerance Test
4.9. Quantification of Cytokine of Adipose Tissue
4.10. Assessment of Body Fat and Liver Weight
4.11. Histological Analysis: Epididimal Adipose Tissue, Liver, and Pancreas
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 17 March 2019).
- World Health Organization (WHO). Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 17 March 2019).
- Kones, A.; Rumana, U. Cardiometabolic diseases of civilization: History and maturation of an evolving global threat. An update and call to action. Ann. Med. 2017, 49, 260–274. [Google Scholar] [CrossRef]
- Johnson, R.J.; Stenvinkel, P.; Martin, S.L.; Jani, A.; Sanchez-Lozada, L.G.; Hill, J.O.; Lanaspa, M.A. Redefining metabolic syndrome as a fat storage condition based on studies of comparative physiology. Obesity 2013, 21, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef] [Green Version]
- Zivkovic, A.M.; German, J.B.; Sanyal, A.J. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2007, 86, 285–300. [Google Scholar] [CrossRef]
- Naseri, R.; Farzaei, F.; Haratipour, P.; Nabavi, S.F.; Habtemariam, S.; Farzaei, M.H.; Khodarahmi, R.; Tewari, D.; Momtaz, S. Anthocyanins in the management of metabolic syndrome: A pharmacological and biopharmaceutical review. Front. Pharmacol. 2018, 9, 1–19. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan for the Prevention and Control of NCDs 2013–2020. Available online: https://www.who.int/nmh/events/ncd_action_plan/en/ (accessed on 17 March 2019).
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin. Drug Saf. 2012, 11, 459–471. [Google Scholar] [CrossRef]
- da Silva, E.; Salmazzo, G.; Arrigo, J.; Oliveira, R.; Kassuya, C.; Cardoso, C. Anti-inflammatory evaluation and toxicological analysis of Campomanesia xanthocarpa Berg. Inflammation 2016, 39, 1462–1468. [Google Scholar] [CrossRef]
- Sahib, N.; Saari, N.; Ismail, A.; Khatib, A.; Mahomoodally, F.; Hamid, A. Plants metabolites as potential antiobesity agents. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Markman, B.; Bacchi, E.; Kato, E. Antiulcerogenic effects of Campomanesia xanthocarpa. J. Ethnopharmacol. 2004, 94, 55–57. [Google Scholar] [CrossRef]
- Souza-Moreira, T.; Salvagnini, L.; Santos, E.; Silva, V.; Moreira, R.; Salgado, H.; Pietro, R. Antidiarrheal activity of Campomanesia xanthocarpa fruit. J. Med. Food 2011, 14, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.; Steffens, R.; Jablonski, A.; Hertz, P.; Rios, A.; Vizzotto, M.; Flôres, S. Characterization and antioxidant potential of Brazilian fruits from the Myrtaceae family. J. Agric. Food Chem. 2012, 60, 3061–3067. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, C.M.L.; Inada, A.C.; Marcelino, G.; Figueiredo, P.S.; Arakaki, D.G.; Hiane, P.A.; Cardoso, C.A.L.; Guimarães, R.C.A.; Freitas, K.C. Therapeutic potential of Brazilian Cerrado Campomanesia species on metabolic dysfunctions. Molecules 2018, 23, 2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biavatti, M.; Farias, C.; Curtius, F.; Brasil, L.; Hort, S.; Schuster, L.; Leite, S.; Prado, S. Preliminary studies on Campomanesia xanthocarpa (Berg.) and Cuphea carthagenensis (Jacq.) J.F. Macbr. aqueous extract: Weight control and biochemical parameters. J. Ethnopharmacol. 2004, 93, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Klafke, J.; Pereira, R.; Hirsch, G.; Parisi, M.; Porto, F.; de Almeida, A.; Rubin, F.; Schmidt, A.; Beutler, H.; Nascimento, S.; et al. Study of oxidative and inflammatory parameters in ldlr-ko mice treated with hypercholestrolemic diet: Comparison between the use of Campomanesia xanthocarpa and acetylsalicylic acid. Phytomedicine 2016, 23, 1227–1234. [Google Scholar] [CrossRef]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Iizuka, M.; Schneider, S.; Ogasawara, K.; Mukainaka, T.; Iwatsuki, K.; Suzuki, T.; Nishino, H. Chalcones, coumarins and flavonones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett. 2003, 201, 133–137. [Google Scholar] [CrossRef]
- Zhang, T.; Yamashita, Y.; Yasuda, M.; Yamamoto, N.; Ashida, H. Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat-diet-fed C57BL/6 mice. Food Funct. 2015, 6, 135–145. [Google Scholar] [CrossRef]
- Bak, E.-J.; Choi, K.C.; Jang, S.; Woo, G.H.; Yoon, H.G.; Na, Y.; Yoo, Y.J.; Lee, Y.; Jeong, Y.; Cha, J.H. Licochalcone F alleviates glucose tolerance and chronic inflammation in diet-induced obese mice through Akt and p38 MAPK. Clin. Nutr. 2016, 35, 414–421. [Google Scholar] [CrossRef]
- Ohnogi, H.; Hayami, S.; Kudo, Y.; Deguchi, S.; Mizutani, S.; Enoki, T.; Tanimura, Y.; Aoi, W.; Naito, Y.; Kato, I.; et al. Angelica keiskei extract improves insulin resistance and hypertriglyceridemia in rats fed a high-fructose drink. Biosci. Biotechnol. Biochem. 2012, 76, 928–932. [Google Scholar] [CrossRef]
- Goristein, S.; Leontowicz, H.; Leontowicz, M.; Krzeminski, R.; Gralak, M.; Delgado-Lincoln, E.; Ayala, A.L.M.; Katrich, E.; Trakhtenberg, S. Changes in plasma lipid and antioxidant activity in rats as a result of naringin and red grapefruit supplementation. J. Agric. Food Chem. 2005, 53, 3223–3228. [Google Scholar] [CrossRef]
- Zhang, T.; Sawada, K.; Yamamoto, N.; Ashida, H. 4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica keiskei) suppress differentiation of preadipocytes to adipocytes via AMPK and MAPK pathways. Mol. Nutr. Food Res. 2013, 57, 1729–1740. [Google Scholar] [CrossRef]
- Guo, W.W.; Wang, X.; Chen, X.Q.; Ba, Y.Y.; Zhang, N.; Xu, R.R.; Zhao, W.W.; Wu, X. Flavonones from Penthorum chinense ameliorate hepatic steatosis by activating SIRT1/AMPK pathway in HepG2 cells. Int. J. Mol. Sci. 2018, 19, 2555. [Google Scholar] [CrossRef] [Green Version]
- Salmazzo, G.R.; Verdan, M.H.; Silva, F.; Cicarelli, R.M.; Mota, J.D.S.; Salvador, M.J.; de Carvalho, J.E.; Cardoso, C.A.L. Chemical composition and antiproliferative, antioxidant and trypanocidal activities of the fruits from Campomanesia xanthocarpa (Mart.) O. Berg (Myrtaceae). Nat. Prod. Res. 2019, 15, 1–5. [Google Scholar] [CrossRef]
- Klafke, J.; da Silva, M.; Panigas, T.; Belli, K.; de Oliveira, M.; Barichello, M.; Rigo, F.; Rossato, M.; dos Santos, A.; Pizzolatti, M.; et al. Effects of Campomanesia xanthocarpa on biochemical, hematological and oxidative stress parameters in hypercholesterolemic patients. J. Ethnopharmacol. 2010, 127, 299–305. [Google Scholar] [CrossRef]
- Viecili, P.; Borges, D.; Kirsten, K.; Malheiros, J.; Viecili, E.; Melo, R.; Trevisan, G.; da Silva, M.; Bochi, G.; Moresco, R.; et al. Effects of Campomanesia xanthocarpa on inflammatory processes, oxidative stress, endothelial dysfunction and lipid biomarkers in hypercholesterolemic individuals. Atherosclerosis 2014, 234, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Z. Resistin’s, Obesity and insulin resistance: The continuing disconnect between rodents and humans. J. Endocrinol. Investig. 2016, 6, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Wang, S.; Woods, L.C.H.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yaday, H. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Long-term green tea extract supplementation does not affect absorption, resting energy expenditure, and body composition in adults. J. Nutr. 2015, 145, 864–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angiletta, C.J.; Griffin, L.E.; Steele, C.N.; Baer, D.J.; Novotny, J.A.; Davy, K.P.; Neilson, A.P. Impact of short-term flavanol supplementation on fasting plasma trimethylamine N-oxide concentrations in obese adults. Food Funct. 2018, 9, 5350–5361. [Google Scholar] [CrossRef] [Green Version]
- Bitok, E.; Rajaram, S.; Jaceldo-Siegl, K.; Oda, K.; Sala-Vila, A.; Serra-Mir, M.; Ros, E.; Sabaté, J. Effects of long-term walnut supplementation on body weight in free-living elderly: Results of a randomized controlled trial. Nutrients 2018, 10, 1317. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, S.J.; Jonarkar, A.V.; Barnes, A.; Maranon, R.O. A new approach to study the sex differences in adipose tissue. J. Biomed. Sci. 2018, 25, 1–12. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Gui, J.; Su, Z. Natural products with anti-obesity effects and different mechanisms of action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Wang, Y.; Ho, C.T. Natural dietary products and their effects on appetite control. J. Agric. Food Chem. 2017, 66, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.J. Central and peripheral neuroendocrine peptides and signaling in appetite regulation: Considerations for obesity pharmacotherapy. Obes. Rev. 2008, 9, 108–120. [Google Scholar] [CrossRef]
- Murray, S.; Tulloch, A.; Gold, M.S.; Avena, N.M. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nature 2014, 10, 540–552. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.C.; do Espirito Santo, B.L.S.; Paniago, G.M.; Portugal, L.C.; Freitas, D.S.; Alcantara, G.B.; Filiú, W.F.O.; Freitas, K.C.; dos Santos, E.F.; Hiane, P.A. Metabolic evaluation of the effects of a hyperlipidic diet for obesity induction and standard normolipid diet (AIN93) consumption in Wistar rats. Int. J. Dev. Res. 2017, 7, 17461–17471. [Google Scholar]
- Ardisson, L.P.; Minicucci, M.F.; Azevedo, P.S.; Chiuso-Minicucci, F.; Matsubara, B.B.; Matsubara, L.M.; Santos, P.S.; Assalin, H.B.; Novo, R.; Novelli, E.L.; et al. Influence of AIN-93M diet on mortality and cardiac remodeling after myocardial infarction in rats. Int. J. Card 2012, 156, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.F.; Amaral, M.S.; Oliveira, S.L.; Barbosa, J.N.; Cabral, C.R., Jr.; Melo, I.S.; Bueno, N.B.; Freitas, J.D.; Sant’ana, A.G.; Ataíde, T.R. Dietary intake of AIN-93 standard diet induces fatty liver with altered hepatic fatty acid profile in Wistar rats. Nutr. Hosp. 2015, 31, 2140–2146. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [Green Version]
- Nie, T.; Matsuzaka, T.; Suzuki, M.; Nakano, Y.; Hui, Z.; Yokoo, T.; Suzuki-Kemuriyama, N.; Kuba, M.; Okajima, Y.; Takeuchi, Y.; et al. Ablation of Elovl6 protects pancreatic islets from high-fat diet-induced impairment of insulin secretion. Biochem. Piophys. Res. Commun. 2014, 450, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Vinagre, A.P.S.; Rönnau, A.D.R.O.; Pereira, S.F.; Silveira, L.U.; Wiilland, E.F.; Suyenaga, E.S. Anti-diabetic effects of Campomanesia xanthocarpa (Berg) leaf decoction. Braz. J. Pharm. Sci. 2010, 46, 169–177. [Google Scholar] [CrossRef]
- Zhang, T.; Yamamoto, N.; Ashida, H. Chalcones suppress fatty-acid induced lipid accumulation through a LKB1/AMPK signaling pathway in HepG2 cells. Food Funct. 2014, 5, 1131–1141. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, M.S.; Park, Y.B.; Kim, S.R.; Lee, M.K.; Jung, U.J. Garcinia cambogia attenuated diet-induced adiposity but exacerbates hepatic collagen accumulation and inflammation. World J. Gastroenterol. 2013, 19, 4689–4701. [Google Scholar] [CrossRef]
- Galindo, L.A.; Pultrini, A.M.; Costa, M. Biological effects of Ocimum gratissimum L. are due to synergic action among multiple compounds present in essential oil. J. Nat. Med. 2010, 63, 436–441. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Lenquiste, S.A.; Marineli, R.S.; Moraes, E.A.; Dionisio, A.P.; Brito, E.S.; Junior, M.R.M. Jaboticaba peel and jaboticaba peel aqueous extract shows in vitro and in vivo antioxidant properties in obesity model. Food Res. Int. 2015, 77, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Nery, C.S.; Pinheiro, I.L.; Muniz, G.S.; Vasconcelos, D.A.A.; França, S.P.; Nascimento, E. Murinometric evaluations and feed efficiency in rats from reduced litter during lactation and submitted or not to swimming exercise. Rev. Bras. Med. Esporte 2011, 17, 49–55. [Google Scholar] [CrossRef]
- Salerno, P.S.V. Efeito da Dieta de Cafeteria em Ratos Submetidos ao Estresse Crônico Variado: Análise de Parâmetros Bioquímicos e Comportamentais. Master’s Thesis, Universidade Federal de Pelotas, Pelotas, Brazil, 2014. [Google Scholar]
- Santos, S.H.S.; Fernandes, L.R.; Mario, E.G.; Ferreira, A.V.M.; Pôrto, L.C.J.; Alvarez-Leite, J.I.; Botion, L.M.; Bader, M.; Alenina, N.; Santos, R.A.S. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes 2008, 57, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Sasso, S.; Souza, P.C.S.; Santana, L.F.; Cardoso, C.A.L.; Alves, F.M.; Portugal, L.C.; Faria, B.B.; da Silva, A.F.; Motta-Castro, A.R.C.; Soares, L.S.; et al. Use of an extract of Annona muricata Linn to prevent high-fat diet induced metabolic disorders in C57BL/6 mice. Nutrients 2019, 11, 1509. [Google Scholar] [CrossRef] [Green Version]
- White, P.A.; Cercato, L.M.; Batista, V.S.; Camargo, E.A.; De Lucca, W., Jr.; Oliveira, A.S.; Silva, F.T.; Goes, T.C.; Oliveira, E.R.; Moraes, V.R.; et al. Aqueous extract of Chrysobalanus icaco leaves, in lower doses, prevent fat gain in obese high-fat fed mice. J. Ethnopharmacol. 2016, 179, 92–100. [Google Scholar] [CrossRef]
- Teixeira, H.M.; Ribas-Filho, J.M.; Nassif, P.A.N.; Dietz, U.A.; Henriques, G.S.; Aoki, S.; Pizzol, F.D. Avaliação morfométrica da mucosa do intestino grosso após derivação jejunoileal em ratos. Arq. Bras. Cir. Dig. 2006, 19, 140–145. [Google Scholar]
- Moraes, C.M. Avaliação das Alterações Inflamatórias e Funcionais do Pulmão no Curso da Pancreatite Aguda Experimental Induzida por Ceruleína. Master’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2013. [Google Scholar]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Chandran, R.; Parimelazhagan, T.; Shanmugam, S.; Thankarajan, S. Antidiabetic activity of Syzygium calophyllifolium in Streptozotocin-Nicotinamide induced Type-2 diabetic rats. Biomed. Pharmacother. 2016, 82, 547–554. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Wang, B.; Zhao, J.; Yan, X.; Huang, Y.; Du, M.; Zhu, M.J. Maternal obesity exacerbates insulitis and type 1 diabetes in non-obese diabetic mice. Reproduction 2014, 148, 73–79. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |
| Parameter | Groups | ||||
|---|---|---|---|---|---|
| AIN-93M | HF | HFE (100 mg/kg) | HFS1 (1 mg/kg) | HFS2 (1 mg/kg) | |
| Initial body weight (g) | 36.08 ± 1.11 | 37.45 ± 1.13 | 38.09 ± 1.17 | 35.83 ± 0.89 | 36.16 ± 1.33 |
| Final body weight (g) # | 37.33 ± 1.18 | 45.09 ± 1.80 | 49.09 ± 1.86 *** | 42.41 ± 1.78 | 43.75 ± 2.81 |
| Body weight gain (g) # | 1.25 ± 0.55 | 7.63 ± 0.83 ** | 11.00 ± 1.10 *** | 6.58 ± 1.20 | 7.58 ± 1.67 |
| Total visceral fat (g) | 2.326 ± 0.239 | 4.000 ± 0.351 * | 4.796 ± 0.229 *** | 3.708 ± 0.419 | 4.186 ± 0.534 ** |
| Adiposity index (%) | 6.091 ± 0.537 | 8.721 ± 0.560 * | 9.440 ± 0.292 ** | 8.504 ± 0.739 | 9.169 ± 0.851 * |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| AIN-93M | HF | HFE (100 mg/kg) | HFS1 (1 mg/kg) | HFS2 (1 mg/kg) | |
| Food intake (4th week) (g) | 112.33 ± 2.59 | 95.90 ± 2.64 * | 98.09 ± 3.55 | 93.41 ± 3.24 ** | 96.16 ± 5.58 * |
| Food intake (8th week) (g) # | 102.91 ± 3.47 | 86.60 ± 2.02 | 85.63 ± 1.75 * | 84.16 ± 1.44 * | 82.91 ± 4.03 * |
| Food intake (13th week) (g) # | 135.50 ± 5.46 | 107.16 ± 4.67 * | 107.72 ± 2.83 | 103.75 ± 2.01 *,& | 100.75 ± 5.41 * |
| Total food intake (total g) # | 350.75 ± 10.89 | 292.54 ± 10.11 * | 291.45 ± 7.65 | 281.33 ± 5.84 * | 279.83 ± 14.66 * |
| Daily caloric intake (kcal/day) | 14.80 ± 0.46 | 17.22 ± 0.59 * | 17.16 ± 0.45 * | 16.57 ± 0.34 | 16.47 ± 0.85 |
| FEI | 37.22 ± 1.18 | 44.96 ± 1.79 | 48.96 ± 1.86 *** | 42.29 ± 1.78 | 43.62 ± 2.81 |
| WGPCI | 36.72 ± 1.29 | 45.06 ± 1.80 * | 49.06 ± 1.86 *** | 42.39 ± 1.78 | 43.72 ± 2.81 |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| AIN-93M (n = 12) | HF (n = 11) | HFE (100 mg/kg) (n = 11) | HFS1 (1 mg/kg) (n = 10) | HFS2 (1 mg/kg) (n = 12) | |
| Hepatic steatosis (p = 0.0006 *) | |||||
| <5% | 100.0 (12) | 18.2 (2) | 0.0 (0) | 30.0 (3) | 58.3 (7) |
| 5 to 33% | 0.0 (0) | 27.2 (3) | 36.4 (4) | 30.0 (3) | 16.7 (2) |
| 34 to 66% | 0.0 (0) | 36.4 (4) | 27.3 (3) | 40.0 (4) | 8.3 (1) |
| >66% | 0.0 (0) | 18.2 (2) | 36.4 (4) | 0.0 (0) | 16.7 (2) |
| Microvesicular steatosis (p = 0.003) | |||||
| Absence | 100.0 (12) | 81.8 (9) | 36.4 (4) | 60,0 (6) | 91.7 (11) |
| Presence | 0.0 (0) | 18.2 (2) | 63.6 (7) | 40.0 (4) | 8.3 (1) |
| Lobular inflammation (p = 0.003) | |||||
| Absence | 100.0 (12) | 63.6 (7) | 9.1 (1) | 30.0 (3) | 50.0 (6) |
| <1 focus/field | 0.0 (0) | 36.4 (4) | 72.7 (8) | 50.0 (5) | 33.3 (4) |
| 2–4 foci/field | 0.0 (0) | 0.0 (0) | 18.2 (2) | 20.0 (2) | 16.7 (2) |
| Ballooning a | |||||
| Absence | 100.0 (12) | 100.0 (11) | 100.0 (11) | 100.0 (10) | 100.0 (12) |
| Mallory’s hyaline a | |||||
| Absence | 100.0 (12) | 100.0 (11) | 100.0 (11) | 100.0 (10) | 100.0 (12) |
| Apoptosis (p = 0.48) | |||||
| Absence | 100.0 (12) | 100.0 (11) | 90.9 (10) | 90.0 (9) | 100.0 (12) |
| Presence | 0.0 (0) | 0.0 (0) | 9.1 (1) | 10.0 (1) | 0.0 (0) |
| Glycogenated nucleus | |||||
| None/rare | 100.0 (12) | 100.0 (11) | 81.8 (9) | 100.0 (10) | 100.0 (12) |
| Some | 0.0 (0) | 0.0 (0) | 18.2 (1) | 0.0 (0) | 0.0 (0) |
| Parameters | Experimental Groups % (n) | ||||
|---|---|---|---|---|---|
| AIN-93M (n = 12) | HF (n = 11) | HFE (100 mg/kg) (n = 11) | HFS1 (1 mg/kg) (n = 10) | HFS2 (1 mg/kg) (n = 11) | |
| Islets of Langerhans (p = 0.04 *) | |||||
| No alteration | 100.0 (12) | 45.5 (11) * | 63.6 (7) | 80.0 (8) | 81.8 (9) |
| Hypertrophy | 0.0 (0) | 54.6 (6) | 36.4 (4) | 20.0 (2) | 18.2 (2) |
| Pancreatic acini (**) | |||||
| No alteration | 100.0 (12) | 100.0 (11) | 100.0 (11) | 100.0 (10) | 100.0 (11) |
| Inflammatory cells (p = 0.17) | |||||
| No alteration Peri-insulitis | 100.0 (12)0.0 (0) | 81.8 (9)18.2 (2) | 81.8 (9)18.2 (2) | 100.0 (10)0.0 (0) | 100.0 (11)0.0 (0) |
| Experimental Groups (g/kg) | AIN-93M Diet | High-Fat Diet (HF) |
|---|---|---|
| Composition (g/kg) | ||
| Lard | – | 320.00 |
| Corn starch | 630.692 | 320.692 |
| Casein (>85% of protein) | 140.00 | 140.00 |
| Cellulose | 50.00 | 50.00 |
| Vitamin mix | 10.00 | 10.00 |
| Mineral mix | 35.00 | 35.00 |
| Soybean oil | 40.00 | 20.00 |
| L-cistin | 1.80 | 1.80 |
| Choline bitartrate | 2.50 | 2.50 |
| Sucrose | 100.00 | 100.00 |
| Tert-butylhydroquinone | 0.008 | 0.008 |
| Energy (kcal/g) | 3802.8 | 5302.8 |
| Carbohydrates (%) | 75.81 | 31.73 |
| Protein (%) | 14.73 | 10.56 |
| Lipids (%) | 9.47 | 57.71 |
| Calories/g of diet | 3.80 | 5.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardozo, C.M.L.; Inada, A.C.; Cardoso, C.A.L.; Filiú, W.F.d.O.; Farias, B.B.d.; Alves, F.M.; Tatara, M.B.; Croda, J.H.R.; Guimarães, R.d.C.A.; Hiane, P.A.; et al. Effect of Supplementation with Hydroethanolic Extract of Campomanesia xanthocarpa (Berg.) Leaves and Two Isolated Substances from the Extract on Metabolic Parameters of Mice Fed a High-Fat Diet. Molecules 2020, 25, 2693. https://doi.org/10.3390/molecules25112693
Cardozo CML, Inada AC, Cardoso CAL, Filiú WFdO, Farias BBd, Alves FM, Tatara MB, Croda JHR, Guimarães RdCA, Hiane PA, et al. Effect of Supplementation with Hydroethanolic Extract of Campomanesia xanthocarpa (Berg.) Leaves and Two Isolated Substances from the Extract on Metabolic Parameters of Mice Fed a High-Fat Diet. Molecules. 2020; 25(11):2693. https://doi.org/10.3390/molecules25112693
Chicago/Turabian StyleCardozo, Carla Maiara Lopes, Aline Carla Inada, Claudia Andrea Lima Cardoso, Wander Fernando de Oliveira Filiú, Bernardo Barcelar de Farias, Flávio Macedo Alves, Mariana Bento Tatara, Júlio Henrique Rosa Croda, Rita de Cássia Avellaneda Guimarães, Priscila Aiko Hiane, and et al. 2020. "Effect of Supplementation with Hydroethanolic Extract of Campomanesia xanthocarpa (Berg.) Leaves and Two Isolated Substances from the Extract on Metabolic Parameters of Mice Fed a High-Fat Diet" Molecules 25, no. 11: 2693. https://doi.org/10.3390/molecules25112693








