Antiobesity Effects of Gentiana lutea Extract on 3T3-L1 Preadipocytes and a High-Fat Diet-Induced Mouse Model
Abstract
1. Introduction
2. Results and Discussion
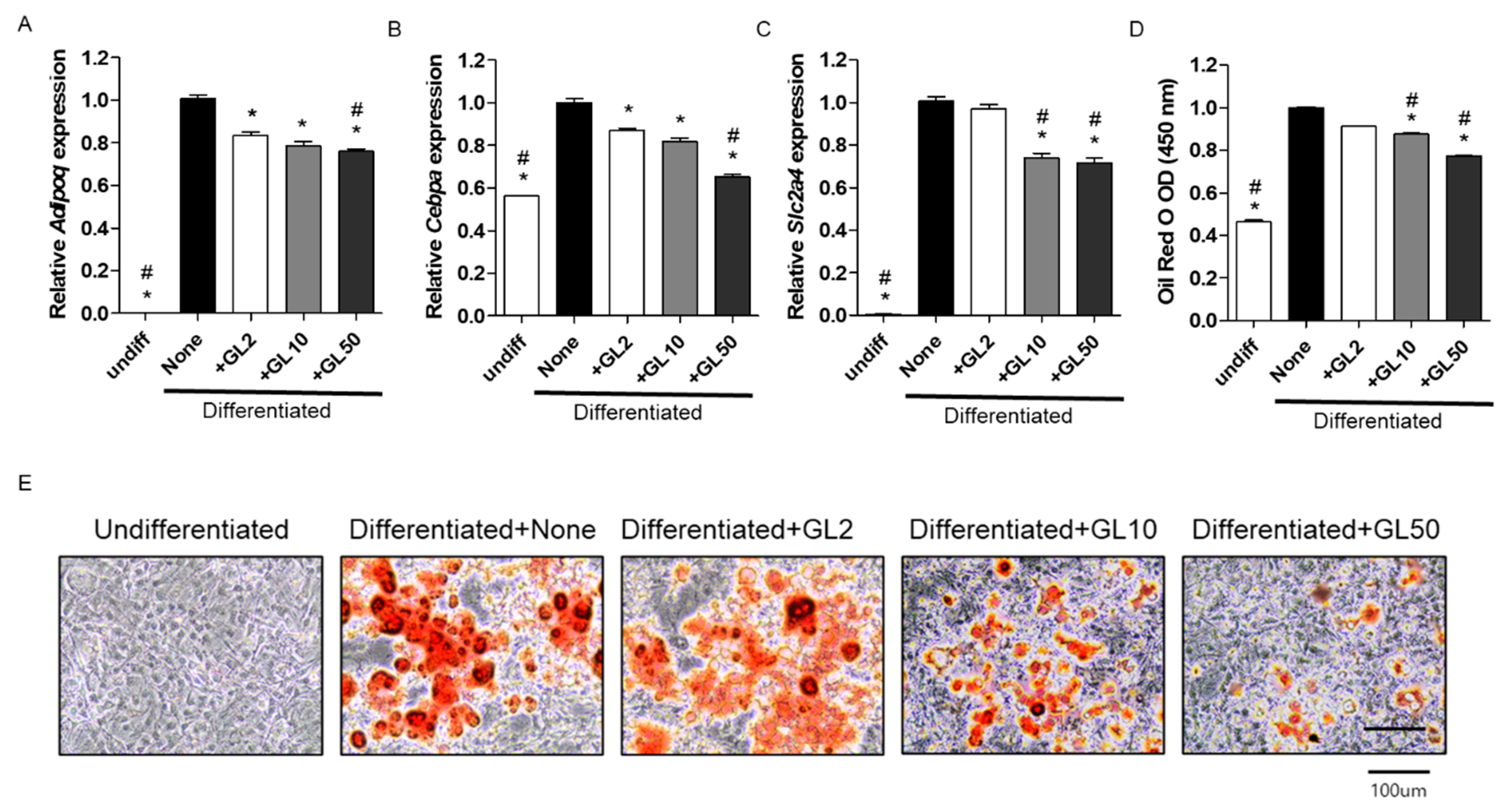
2.1. Gentiana lutea (GL) Decreased Adipocyte Differentiation in 3T3-L1 Cells
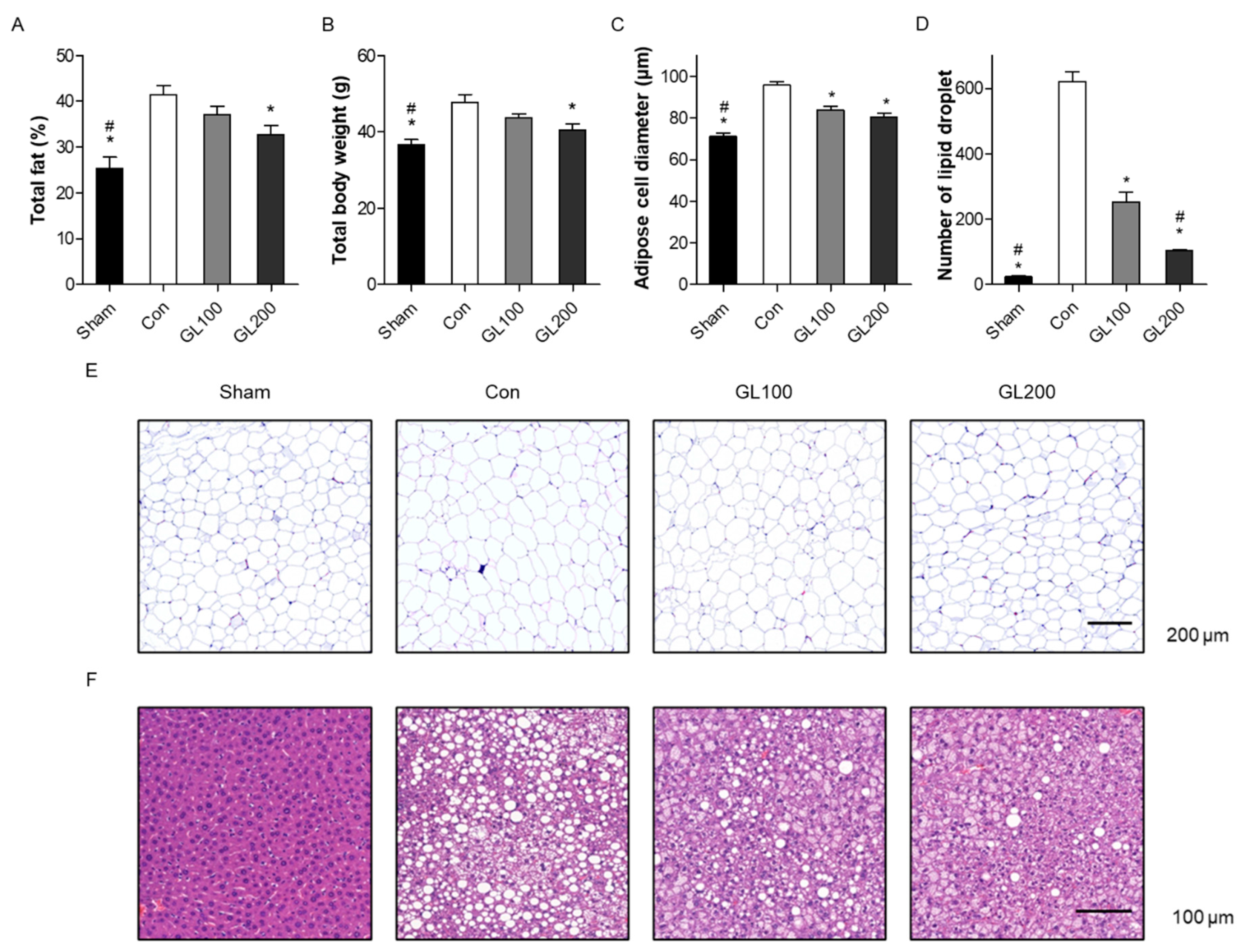
2.2. GL Reduced Obesity-Related Phenotypes in High-Fat Diet-Induced Mice
2.3. GL Inhibited High-Fat Diet-Induced Serum Levels of Obesity-Related Hormones
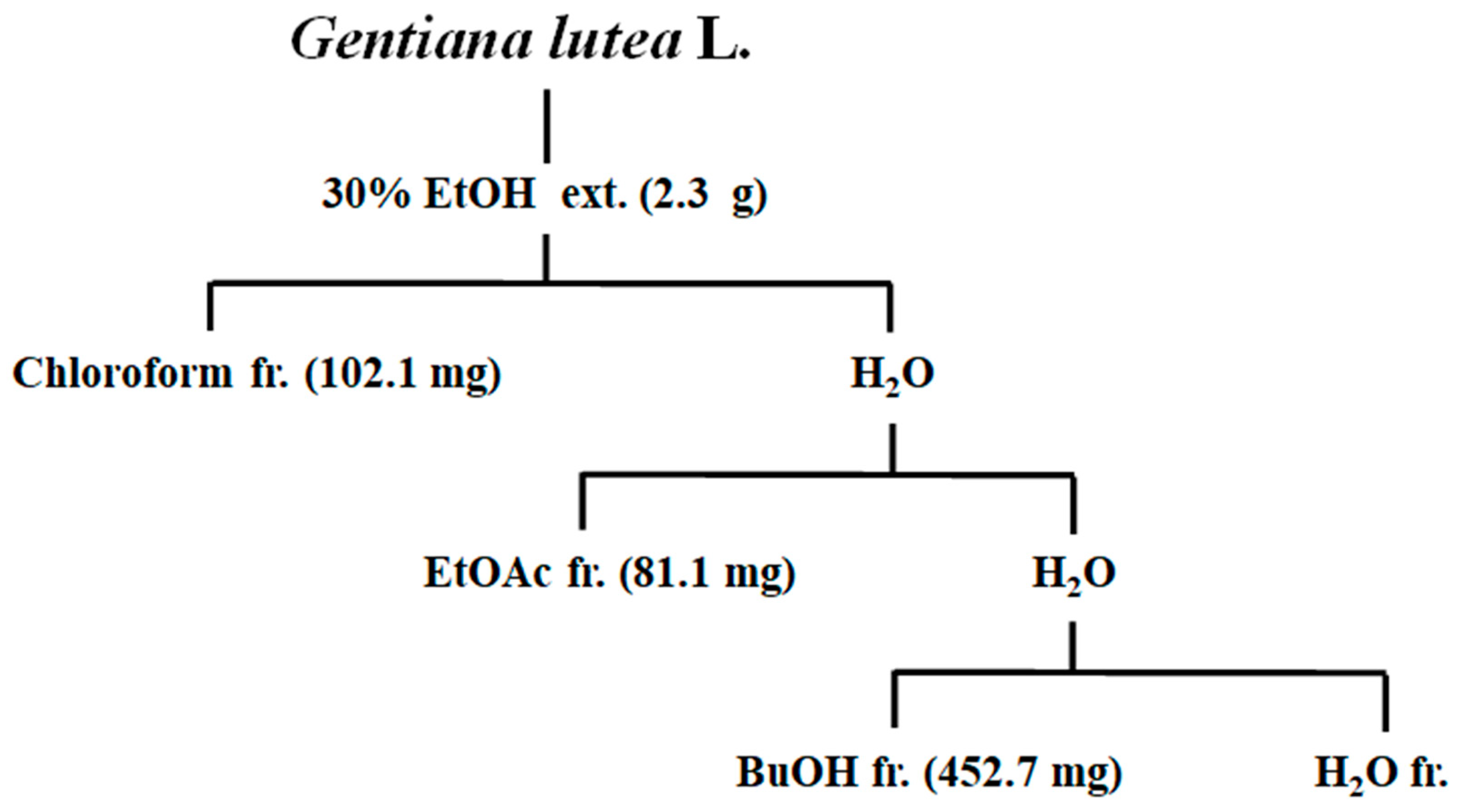
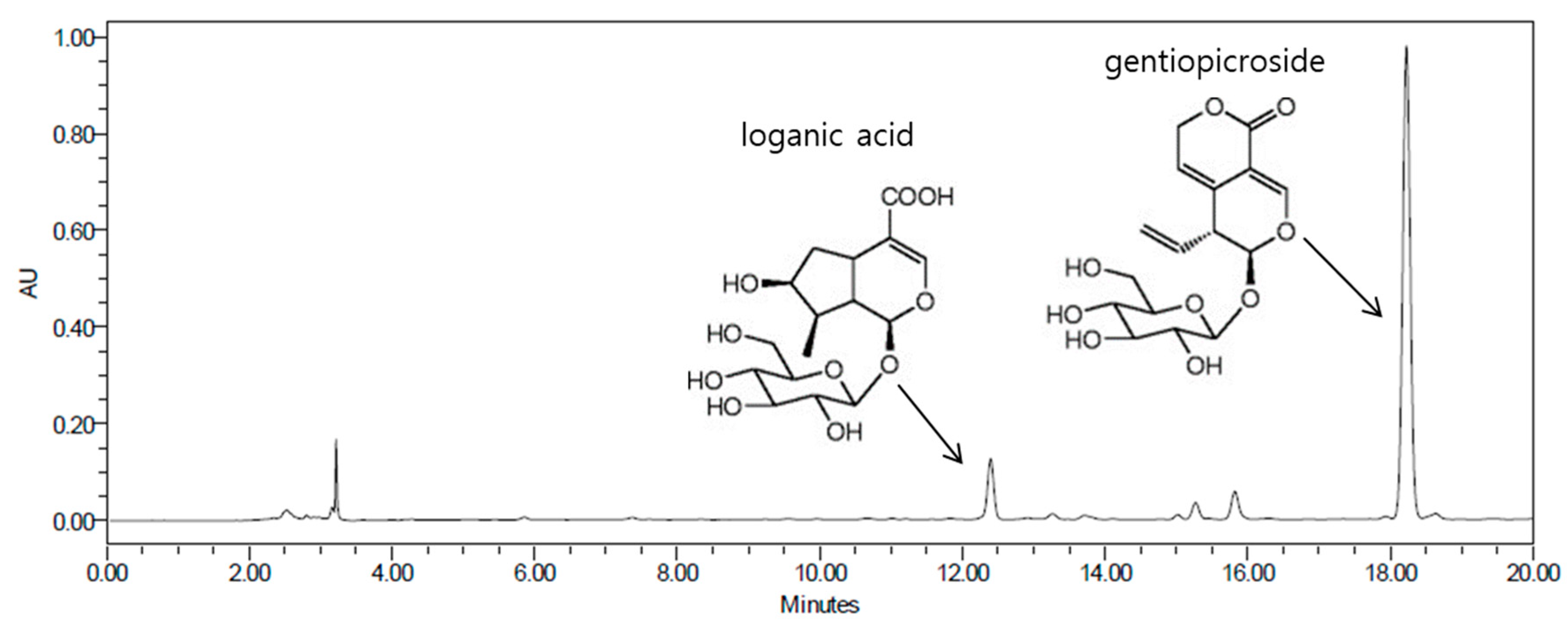
2.4. Extraction and Fractionation of Gentiana lutea Linne Roots
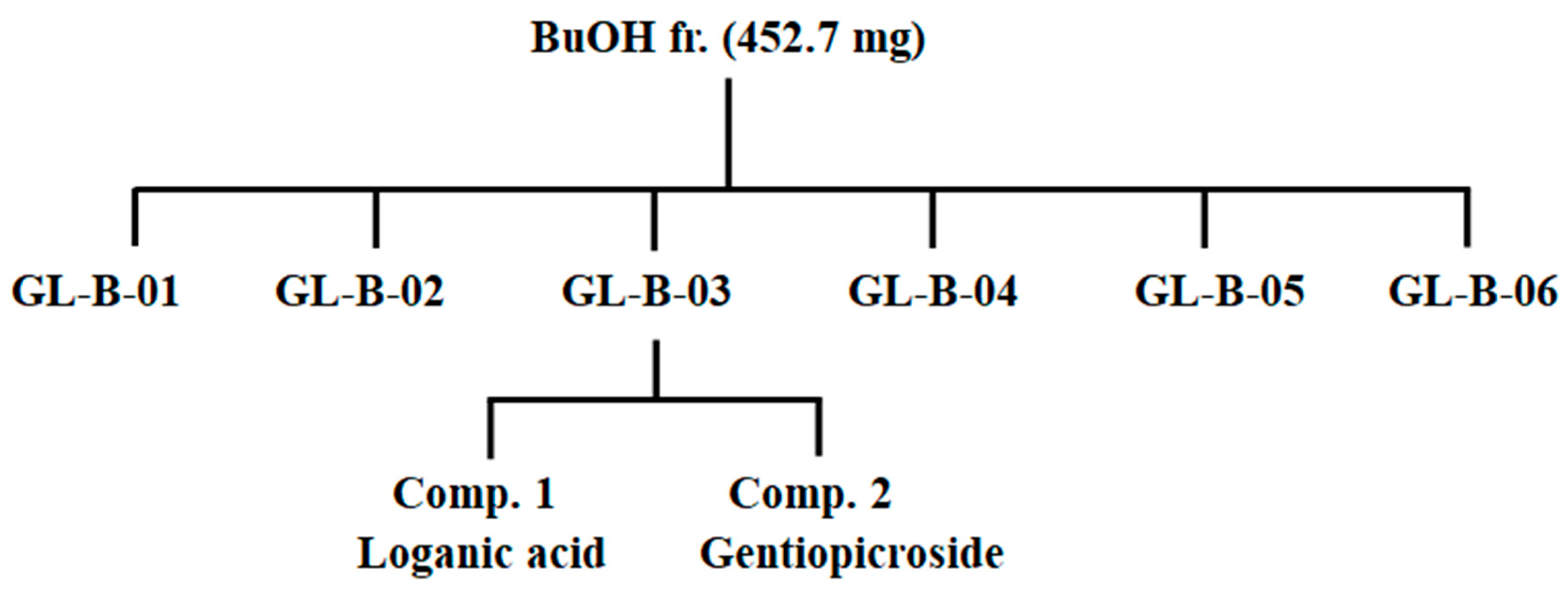
2.5. Isolation and Determination of Compounds 1 and 2 from the BuOH Fraction
2.6. Physicochemical and Spectroscopic Properties of Isolated Compounds
3. Materials and Methods Section
3.1. GL Extract and Cell Culture
3.2. Water-Soluble Tetrazolium Salt Assay
3.3. Oil Red O Staining of Adipocytes
3.4. Quantitative Reverse Transcription Polymerase Chain Reaction
3.5. In Vivo Experiments in Obesity Model Mice
3.6. Preparation of Tissue Samples and Histology
3.7. Blood Sampling and Serum Leptin and Insulin
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, T.I.A. From fat cells through an obesity theory. Eur. J. Clin. Nutr. 2018, 72, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Solas, M.; Milagro, F.I.; Martinez-Urbistondo, D.; Ramirez, M.J.; Martinez, J.A. Precision Obesity Treatments Including Pharmacogenetic and Nutrigenetic Approaches. Trends Pharmacol. Sci. 2016, 37, 575–593. [Google Scholar] [CrossRef] [PubMed]
- Yanovski, S.Z.; Yanovski, J.A. Long-term drug treatment for obesity: A systematic and clinical review. JAMA 2014, 311, 74–86. [Google Scholar] [CrossRef]
- Nicola, A.; Renato, B.; Carla, V. Use of morphological features and amarogentin content for characterization of wild yellow gentian (Gentiana lutea L.) populations in north-east Italy. Acta Bot. Gallica 2013, 160, 33–41. [Google Scholar]
- Balijagic, J.; Jankovic, T.; Zdunic, G.; Boskovic, J.; Savikin, K.; Godevac, D.; Stanojkovic, T.; Jovancevic, M.; Menkovic, N. Chemical profile, radical scavenging and cytotoxic activity of yellow gentian leaves (Genitaneae luteaefolium) grown in northern regions of Montenegro. Nat. Prod. Commun. 2012, 7, 1487–1490. [Google Scholar]
- Wolfle, U.; Haarhaus, B.; Seiwerth, J.; Cawelius, A.; Schwabe, K.; Quirin, K.W.; Schempp, C.M. The Herbal Bitter Drug Gentiana lutea Modulates Lipid Synthesis in Human Keratinocytes In Vitro and In Vivo. Int. J. Mol. Sci. 2017, 18, 1814. [Google Scholar] [CrossRef]
- Aberham, A.; Pieri, V.; Croom, E.M., Jr.; Ellmerer, E.; Stuppner, H. Analysis of iridoids, secoiridoids and xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC-MS and RP-HPLC. J. Pharm. Biomed. Anal. 2011, 54, 517–525. [Google Scholar] [CrossRef]
- Nastasijevic, B.; Lazarevic-Pasti, T.; Dimitrijevic-Brankovic, S.; Pasti, I.; Vujacic, A.; Joksic, G.; Vasic, V. Inhibition of myeloperoxidase and antioxidative activity of Gentiana lutea extracts. J. Pharm. Biomed. Anal. 2012, 66, 191–196. [Google Scholar] [CrossRef]
- Menkovic, N.; Juranic, Z.; Stanojkovic, T.; Raonic-Stevanovic, T.; Savikin, K.; Zdunic, G.; Borojevic, N. Radioprotective activity of Gentiana lutea extract and mangiferin. Phytother. Res. 2010, 24, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Savikin, K.; Menkovic, N.; Zdunic, G.; Stevic, T.; Radanovic, D.; Jankovic, T. Antimicrobial activity of Gentiana lutea L. extracts. Z. Naturforsch. C J. Biosci. 2009, 64, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, R.; Chandel, S.; Upadhyay, S.; Bendre, R.; Ganugula, R.; Potunuru, U.R.; Giri, H.; Sahu, G.; Kumar, P.U.; Reddy, G.B.; et al. Gentiana lutea exerts anti-atherosclerotic effects by preventing endothelial inflammation and smooth muscle cell migration. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Lara-Castro, C.; Fu, Y.; Chung, B.H.; Garvey, W.T. Adiponectin and the metabolic syndrome: Mechanisms mediating risk for metabolic and cardiovascular disease. Curr. Opin. Lipidol. 2007, 18, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Seo, H.S.; Shin, K.O.; Park, K.; Park, B.C.; Kim, M.H.; Park, M.; Kim, C.D.; Seo, S.J. Adiponectin Enhances Human Keratinocyte Lipid Synthesis via SIRT1 and Nuclear Hormone Receptor Signaling. J. Investig. Dermatol. 2019, 139, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.L.; Robinson, C.E.; Gimble, J.M. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor gamma 2 promoter. Biochem. Biophys. Res. Commun. 1997, 240, 99–103. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J., Jr.; Liu, X.S.; et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef]
- Shepherd, P.R.; Gnudi, L.; Tozzo, E.; Yang, H.; Leach, F.; Kahn, B.B. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J. Biol. Chem. 1993, 268, 22243–22246. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Grinspoon, S.; Gulick, T.; Askari, H.; Landt, M.; Lee, K.; Anderson, E.; Ma, Z.; Vignati, L.; Bowsher, R.; Herzog, D.; et al. Serum leptin levels in women with anorexia nervosa. J. Clin. Endocrinol. Metab. 1996, 81, 3861–3863. [Google Scholar] [PubMed]
- Jiang, L.; Wang, Q.; Yu, Y.; Zhao, F.; Huang, P.; Zeng, R.; Qi, R.Z.; Li, W.; Liu, Y. Leptin contributes to the adaptive responses of mice to high-fat diet intake through suppressing the lipogenic pathway. PLoS ONE 2009, 4, e6884. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Sethi, J.K. Obesity: Adipogenesis and Insulin Resistance. IDrugs 2000, 3, 884–886. [Google Scholar]
- Benoit, S.C.; Clegg, D.J.; Seeley, R.J.; Woods, S.C. Insulin and leptin as adiposity signals. Recent Prog. Horm. Res. 2004, 59, 267–285. [Google Scholar] [CrossRef]
- Reid, I.R.; Evans, M.C.; Cooper, G.J.; Ames, R.W.; Stapleton, J. Circulating insulin levels are related to bone density in normal postmenopausal women. Am. J. Physiol. 1993, 265, E655–E659. [Google Scholar] [CrossRef]
- Osegbe, I.; Okpara, H.; Azinge, E. Relationship between serum leptin and insulin resistance among obese Nigerian women. Ann. Afr. Med. 2016, 15, 14–19. [Google Scholar] [CrossRef]
- Di, L.; Li, N.; Zu, L.; Wang, K.; Zhao, Y.; Wang, Z. Three New Iridoid Glucosides from the Roots of Patrinia scabra. Bull. Korean Chem. Soc. 2011, 32, 3251–3254. [Google Scholar] [CrossRef]
- Zeng, W.; Han, H.; Tao, Y.; Yang, L.; Wang, Z.; Chen, K. Identification of bio-active metabolites of gentiopicroside by UPLC/Q-TOF MS and NMR. Biomed. Chromatogr. 2013, 27, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, J.; Yeo, S.; Kim, G.; Ko, E.H.; Lee, S.W.; Li, W.Y.; Choi, C.W.; Jeong, S.Y. Antiadipogenic Effects of Loganic Acid in 3T3-L1 Preadipocytes and Ovariectomized Mice. Molecules 2018, 23, 1663. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Nam, S.J.; Lee, H.I.; Lee, J.; Leutou, A.S.; Ri Ham, J.; Lee, M.K. Gentiopicroside isolated from Gentiana scabra Bge. inhibits adipogenesis in 3T3-L1 cells and reduces body weight in diet-induced obese mice. Bioorg. Med. Chem. Lett. 2019, 29, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.; Lee, C.G.; Kim, J.; Yeo, S.; Kim, J.A.; Choi, C.W.; Jeong, S.-Y. Antiobesity Effects of Gentiana lutea Extract on 3T3-L1 Preadipocytes and a High-Fat Diet-Induced Mouse Model. Molecules 2020, 25, 2453. https://doi.org/10.3390/molecules25102453
Park E, Lee CG, Kim J, Yeo S, Kim JA, Choi CW, Jeong S-Y. Antiobesity Effects of Gentiana lutea Extract on 3T3-L1 Preadipocytes and a High-Fat Diet-Induced Mouse Model. Molecules. 2020; 25(10):2453. https://doi.org/10.3390/molecules25102453
Chicago/Turabian StylePark, Eunkuk, Chang Gun Lee, Junho Kim, Subin Yeo, Ji Ae Kim, Chun Whan Choi, and Seon-Yong Jeong. 2020. "Antiobesity Effects of Gentiana lutea Extract on 3T3-L1 Preadipocytes and a High-Fat Diet-Induced Mouse Model" Molecules 25, no. 10: 2453. https://doi.org/10.3390/molecules25102453
APA StylePark, E., Lee, C. G., Kim, J., Yeo, S., Kim, J. A., Choi, C. W., & Jeong, S.-Y. (2020). Antiobesity Effects of Gentiana lutea Extract on 3T3-L1 Preadipocytes and a High-Fat Diet-Induced Mouse Model. Molecules, 25(10), 2453. https://doi.org/10.3390/molecules25102453







