New Rare Ent-Clerodane Diterpene Peroxides from Egyptian Mountain Tea (Qourtom) and Its Chemosystem as Herbal Remedies and Phytonutrients Agents
Abstract
1. Introduction
2. Results and Discussion
3. Proposed Biosynthetic Pathway of the Isolated Compounds
4. Chemosystematic Significance
5. Materials and Methods
5.1. General Procedures
5.2. Plant Material
5.3. Extraction and Isolation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bilušić Vundać, V. Taxonomical and Phytochemical Characterisation of 10 Stachys Taxa Recorded in the Balkan Peninsula Flora: A Review. Plants 2019, 8, 32. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C.; Ceylan, O. Metabolite profiling and health benefits of Stachys cretica subsp. mersinaea as a medicinal food. Ind. Crop. Prod. 2019, 131, 85–89. [Google Scholar] [CrossRef]
- Goren, A.C. Use of Stachys species (Mountain Tea) as herbal tea and food. Rec. Nat. Prod. 2014, 8, 71. [Google Scholar]
- Goren, A.C.; Piozzi, F.; Akcicek, E.; Kılıç, T.; Çarıkçı, S.; Mozioğlu, E.; Setzer, W.N. Essential oil composition of twenty-two Stachys species (mountain tea) and their biological activities. Phytochem. Lett. 2011, 4, 448–453. [Google Scholar] [CrossRef]
- Gras, A.; Garnatje, T.; Ibáñez, N.; López-Pujol, J.; Nualart, N.; Vallès, J. Medicinal plant uses and names from the herbarium of Francesc Bolòs (1773–1844). J. Ethnopharmacol. 2017, 204, 142–168. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Zhuo, J.; Liu, B.; Long, C. Eating from the wild: Diversity of wild edible plants used by Tibetans in Shangri-la region, Yunnan, China. J. Ethnobiol. Ethnomed. 2013, 9, 28. [Google Scholar] [CrossRef]
- Kaya, A.; Demirci, B.; Doğu, S.; Dinç, M. Composition of the essential oil of Stachys sericantha, S. gaziantepensis, and S. mardinensis (Lamiaceae) from Turkey. Int. J. Food Prop. 2017, 20, 2639–2644. [Google Scholar] [CrossRef][Green Version]
- Polat, R.; Cakilcioglu, U.; Kaltalioglu, K.; Ulusan, M.D.; Turkmen, Z. An ethnobotanical study on medicinal plants in Espiye and its surrounding (Giresun-Turkey). J. Ethnopharmacol. 2015, 163, 1–11. [Google Scholar] [CrossRef]
- Serbetci, T.; Demirci, B.; Guzel, C.B.; Kultur, S.; Erguven, M.; Baser, K.H. Essential oil composition, antimicrobial and cytotoxic activities of two endemic Stachys cretica subspecies (Lamiaceae) from Turkey. Nat. Prod. Commun. 2010, 5, 1369–1374. [Google Scholar]
- Tundis, R.; Peruzzi, L.; Menichini, F. Phytochemical and biological studies of Stachys species in relation to chemotaxonomy: A review. Phytochemistry 2014, 102, 7–39. [Google Scholar] [CrossRef]
- Kostyuchenko, O. Chemical composition and pharmacological properties of Stachys species. Rastit. Resur. 1983, 19, 407–413. [Google Scholar]
- Saeedi, M.; Morteza-Semnani, K.; Mahdavi, M.R.; Rahimi, F. Antimicrobial studies on extracts of four species of stachys. Indian J. Pharm. Sci. 2008, 70, 403–406. [Google Scholar] [PubMed]
- Skaltsa, H.D.; Lazari, D.M.; Chinou, I.B.; Loukis, A.E. Composition and antibacterial activity of the essential oils of Stachys candida and S. chrysantha from southern Greece. Planta Med. 1999, 65, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C. Phenolic ingredients and therapeutic potential of Stachys cretica subsp. smyrnaea for the management of oxidative stress, Alzheimer’s disease, hyperglycemia, and melasma. Ind. Crop. Prod. 2019, 127, 82–87. [Google Scholar] [CrossRef]
- Elfalleh, W.; Kirkan, B.; Sarikurkcu, C. Antioxidant potential and phenolic composition of extracts from Stachys tmolea: An endemic plant from Turkey. Ind. Crop. Prod. 2019, 127, 212–216. [Google Scholar] [CrossRef]
- Háznagy-Radnai, E.; Balogh, Á.; Czigle, S.; Máthé, I.; Hohmann, J.; Blazsó, G. Antiinflammatory activities of Hungarian Stachys species and their iridoids. Phytother. Res. 2012, 26, 505–509. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Kocak, M.S.; Uren, M.C.; Calapoglu, M.; Tepe, A.S. Potential sources for the management global health problems and oxidative stress: Stachys byzantina and S. iberica subsp. iberica var. densipilosa. Eur. J. Integr. Med. 2016, 8, 631–637. [Google Scholar] [CrossRef]
- Šliumpaitė, I.; Venskutonis, P.R.; Murkovic, M.; Ragažinskienė, O. Antioxidant properties and phenolic composition of wood betony (Betonica officinalis L., syn. Stachys officinalis L.). Ind. Crop. Prod. 2013, 50, 715–722. [Google Scholar]
- Maleki, N.; Garjani, A.; Nazemiyeh, H.; Nilfouroushan, N.; Eftekhar Sadat, A.T.; Allameh, Z.; Hasannia, N. Potent anti-inflammatory activities of hydroalcoholic extract from aerial parts of Stachys inflata on rats. J. Ethnopharmacol. 2001, 75, 213–218. [Google Scholar] [CrossRef]
- Iannuzzi, A.M.; Camero, C.M.; D’Ambola, M.; D’Angelo, V.; Amira, S.; Bader, A.; Braca, A.; De Tommasi, N.; Germano, M.P. Antiangiogenic Iridoids from Stachys ocymastrum and Premna resinosa. Planta Med. 2019, 85, 1034–1039. [Google Scholar] [CrossRef]
- Barreto, R.S.; Quintans, J.S.; Amarante, R.K.; Nascimento, T.S.; Amarante, R.S.; Barreto, A.S.; Pereira, E.W.; Duarte, M.C.; Coutinho, H.D.; Menezes, I.R. Evidence for the involvement of TNF-α and IL-1β in the antinociceptive and anti-inflammatory activity of Stachys lavandulifolia Vahl.(Lamiaceae) essential oil and (-)-α-bisabolol, its main compound, in mice. J. Ethnopharmacol. 2016, 191, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Dehaghi, N.K.; Hajimehdipoor, H.; Sahebgharani, M.; Khanavi, M.; Mirshaki, Z. Antinociceptive effects of Stachys laxa. Planta Med. 2010, 76, 145. [Google Scholar]
- Öztürk, M.; Duru, M.E.; Aydoğrmuş-Öztürk, F.; Harmandar, M.; Mahlıçlı, M.; Kolak, U.; Ulubelen, A. GC-MS analysis and antimicrobial activity of essential oil of Stachys cretica subsp. smyrnaea. Nat. Prod. Commun. 2009, 4, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Skaltsa, H.D.; Demetzos, C.; Lazari, D.; Sokovic, M. Essential oil analysis and antimicrobial activity of eight Stachys species from Greece. Phytochemistry 2003, 64, 743–752. [Google Scholar] [CrossRef]
- Kokhdan, E.P.; Sadeghi, H.; Ghafoori, H.; Sadeghi, H.; Danaei, N.; Javadian, H.; Aghamaali, M.R. Cytotoxic effect of methanolic extract, alkaloid and terpenoid fractions of Stachys pilifera against HT-29 cell line. Res. Pharm. Sci. 2018, 13, 404–412. [Google Scholar]
- Ma, L.; Qin, C.; Wang, M.; Gan, D.; Cao, L.; Ye, H.; Zeng, X. Preparation, preliminary characterization and inhibitory effect on human colon cancer HT-29 cells of an acidic polysaccharide fraction from Stachys floridana Schuttl. ex Benth. Food Chem. Toxicol. 2013, 60, 269–276. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Elshamy, A.I.; Hamed, A.R.; Shams, K.A.; Hegazy, M.-E.F. Cytotoxic neo-clerodane diterpenes from Stachys aegyptiaca. Phytochem. Lett. 2018, 28, 32–36. [Google Scholar] [CrossRef]
- Nasrollahi, S.; Ghoreishi, S.M.; Ebrahimabadi, A.H.; Khoobi, A. Gas chromatography-mass spectrometry analysis and antimicrobial, antioxidant and anti-cancer activities of essential oils and extracts of Stachys schtschegleevii plant as biological macromolecules. Int. J. Biol. Macromol. 2019, 128, 718–723. [Google Scholar] [CrossRef]
- Ramak, P.; Talei, G.R. Chemical composition, cytotoxic effect and antimicrobial activity of Stachys koelzii Rech.f. essential oil against periodontal pathogen Prevotella intermedia. Microb. Pathog. 2018, 124, 272–278. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Nicoletti, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Vitali, L.A.; Petrelli, D.; Papa, F.; Vittori, S.; et al. Phytochemical analysis, biological evaluation and micromorphological study of Stachys alopecuros (L.) Benth. subsp. divulsa (Ten.) Grande endemic to central Apennines, Italy. Fitoterapia 2013, 90, 94–103. [Google Scholar] [CrossRef]
- Afouxenidi, A.; Milošević-Ifantis, T.; Skaltsa, H. Secondary metabolites from Stachys tetragona Boiss. & Heldr. ex Boiss. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2018, 81, 83–85. [Google Scholar]
- Cincinelli, R.; Scaglioni, L.; Arnold, N.A.; Dallavalle, S. Structure and absolute configuration of new acidic metabolites from Stachys ehrenbergii. Tetrahedron Lett. 2011, 52, 5972–5975. [Google Scholar] [CrossRef]
- Demirtas, I.; Gecibesler, I.H.; Yaglioglu, A.S. Antiproliferative activities of isolated flavone glycosides and fatty acids from Stachys byzantina. Phytochem. Lett. 2013, 6, 209–214. [Google Scholar] [CrossRef]
- Guo, H.; Saravanakumar, K.; Wang, M.-H. Total phenolic, flavonoid contents and free radical scavenging capacity of extracts from tubers of Stachys affinis. Biocatal. Agric. Biotechnol. 2018, 15, 235–239. [Google Scholar] [CrossRef]
- Kumar, D.; Bhat, Z.A. Apigenin 7-glucoside from Stachys tibetica Vatke and its anxiolytic effect in rats. Phytomedicine 2014, 21, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Serrilli, A.M.; Ramunno, A.; Piccioni, F.; Serafini, M.; Ballero, M. Flavonoids and iridoids from Stachys corsica. Nat. Prod. Res. 2005, 19, 561–565. [Google Scholar] [CrossRef]
- Adinolfi, M.; Barone, G.; Lanzetta, R.; Laonigro, G.; Mangoni, L.; Parrilli, M. Diterpenes from Stachys recta. J. Nat. Prod. 1984, 47, 541–543. [Google Scholar] [CrossRef]
- Farooq, U.; Naz, S.; Sarwar, R.; Khan, A.; Khan, A.; Rauf, A.; Khan, H.; Ahmad, M.; Hameed, S. Isolation and characterization of two new diterpenoids from Stachys parviflora: Antidiarrheal potential in mice. Phytochem. Lett. 2015, 14, 198–202. [Google Scholar] [CrossRef]
- Fazio, C.; Passannanti, S.; Paternostro, M.P.; Arnold, N.A. Diterpenoids from Stachys mucronata. Planta Med. 1994, 60, 499. [Google Scholar] [CrossRef]
- Hegazy, M.F.; Hamed, A.R.; El-Kashoury, E.-S.A.; Shaheen, A.M.; Tawfik, W.A.; Paré, P.W.; Abdel-Sattar, E.; Stachaegyptin, A.C. Neo-clerodane diterpenes from Stachys aegyptiaca. Phytochem. Lett. 2017, 21, 151–156. [Google Scholar] [CrossRef]
- Mohamed, A.E.-H.H.; Mohamed, N. A new trans-neo clerodane diterpene from Stachys aegyptiaca. Nat. Prod. Res. 2014, 28, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, M.P.; Maggio, A.M.; Piozzi, F.; Servettaz, O. Labdane diterpenes from Stachys plumosa. J. Nat. Prod. 2000, 63, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Endo, Y.; Miyase, T.; Yoshizaki, F. Iridoid glycoside constituents of Stachys lanata. J. Nat. Prod. 2008, 71, 1768–1770. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, S.; Wang, P.; Luo, Q.; Huang, X.; Xu, Q.; Qin, J.; Liang, C.; Chen, X. A syringic acid derivative and two iridoid glycosides from the roots of Stachys geobombycis and their antioxidant properties. Nat. Prod. Res. 2019, 33, 681–686. [Google Scholar] [CrossRef]
- Delazar, A.; Delnavazi, M.R.; Nahar, L.; Moghadam, S.B.; Mojarab, M.; Gupta, A.; Williams, A.S.; Mukhlesur Rahman, M.; Sarker, S.D. Lavandulifolioside B: A new phenylethanoid glycoside from the aerial parts of Stachys lavandulifolia Vahl. Nat. Prod. Res. 2011, 25, 8–16. [Google Scholar] [CrossRef]
- Nishimura, H.; Sasaki, H.; Inagaki, N.; Chin, M.; Mitsuhashi, H. Nine phenethyl alcohol glycosides from Stachys sieboldii. Phytochemistry 1991, 30, 965–969. [Google Scholar] [CrossRef]
- Melek, F.; Radwan, A.; El-Ansari, M.; El-Gindi, O.; Hilal, S.; Genenah, A. Diterpenes from Stachys aegyptiaca. Fitoterapia 1992, 63, 269–276. [Google Scholar]
- El-Ansari, M.; Abdalla, M.; Saleh, N.; Barron, D.; Le Quere, J. Flavonoid constituents of Stachys aegyptiaca. Phytochemistry 1991, 30, 1169–1173. [Google Scholar] [CrossRef]
- El-Ansari, M.A.; Nawwar, M.A.; Saleh, N.A. Stachysetin, a diapigenin-7-glucoside-p, p′-dihydroxy-truxinate from Stachys aegyptiaca. Phytochemistry 1995, 40, 1543–1548. [Google Scholar] [CrossRef]
- El-Desoky, S.; Hawas, U.W.; Sharaf, M. A new flavone glucoside from Stachys aegyptiaca. Chem. Nat. Compd. 2007, 43, 542–543. [Google Scholar] [CrossRef]
- Sharaf, M. Isoscutellarein 8-o-(6-trans-p-coumaroyl)-β-D-glucoside from Stachys aegyptiaca. Fitoter. (Milano) 1998, 69, 355–357. [Google Scholar]
- Halim, A.; Mashaly, M.; Zaghloul, A.; El-Fattah, H.A.; De Pooter, H. Chemical constituents of the essential oils of Origanum syriacum and Stachys aegyptiaca. Int. J. Pharm. 1991, 29, 183–187. [Google Scholar] [CrossRef]
- Shaheen, A.M.; Saleh, I.A.; El-Kashoury, E.-S.A.; Tawfik, W.A.; Omar, E.A.; Hegazy, M.-E.F.; Abdel-Sattar, E. Microwave-assisted extraction as an alternative tool for extraction of Stachys aegyptiaca essential oil. Egypt. Pharmaceut. J. 2017, 16, 98–102. [Google Scholar]
- Fazio, C.; Passannanti, S.; Paternostro, M.P.; Piozzi, F. Neo-clerodane diterpenoids from Stachys rosea. Phytochemistry 1992, 31, 3147–3149. [Google Scholar] [CrossRef]
- Whitson, E.L.; Thomas, C.L.; Henrich, C.J.; Sayers, T.J.; McMahon, J.B.; McKee, T.C. Clerodane diterpenes from Casearia arguta that act as synergistic TRAIL sensitizers. J. Nat. Prod. 2010, 73, 2013–2018. [Google Scholar] [CrossRef]
- Zhao, S.; Ling, J.; Li, Z.; Wang, S.; Hu, J.; Wang, N. Nine new diterpenes from the leaves of plantation-grown Cunninghamia lanceolata. Bioorg. Med. Chem. Lett. 2015, 25, 1483–1489. [Google Scholar] [CrossRef]
- Lourenço, A.; dela Torre, M.C.; Rodríguez, B.; Harada, N.; Ono, H.; Uda, H.; Bruno, M.; Piozzi, F.; Savona, G. The absolute stereoschemistry at C-12 in 12-hydroxylated neo-clerodane diterpenoids. Tetrahedron 1992, 48, 3925–3934. [Google Scholar]
- Puebla, P.; Lopez, J.L.; Guerrero, M.; Carron, R.; Martin, M.L.; San Roman, L.; San Feliciano, A. Neo-clerodane diterpenoids from Croton schiedeanus. Phytochemistry 2003, 62, 551–555. [Google Scholar] [CrossRef]
- Sattar, E.A.; Mossa, J.S.; Muhammad, I.; El-Feraly, F.S. Neo-clerodane diterpenoids from Teucrium yemense. Phytochemistry 1995, 40, 1737–1741. [Google Scholar] [CrossRef]
- Jiménez-Barbero, J. 1H-NMR spectroscopy as a tool for establishing the C-12 stereochemistry and the conformation of the side chain in 12-hydroxylated Neo-clerodanes isolated from Teucrium species. Tetrahedron 1993, 49, 6921–6930. [Google Scholar] [CrossRef]
- Savona, G.; Piozzi, F.; Bruno, M.; Domínguez, G.; Rodríguez, B.; Servettaz, O. Teucretol, a neo-clerodane diterpenoid from Teucrium creticum. Phytochemistry 1987, 26, 3285–3288. [Google Scholar] [CrossRef]
- Sato, A.; Kurabayashi, M.; Nagahori, H.; Ogiso, A.; Mishima, H. Chettaphanin-I, a novel furanoditerpenoid. Tetrahedron Lett. 1970, 1095–1098. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschke, S.L.; Lee, K.-H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef]
- Ovenden, S.P.; Capon, R.J. Nuapapuin A and sigmosceptrellins D and E: New norterpene cyclic peroxides from a southern Australian marine sponge, Sigmosceptrella sp. J. Nat. Prod. 1999, 62, 214–218. [Google Scholar] [CrossRef]
- Piozzi, F.; Bruno, M. Diterpenoids from roots and aerial parts of the genus Stachys. Rec. Nat. Prod. 2011, 5, 1–11. [Google Scholar]
- Tundis, R.; Bonesi, M.; Pugliese, A.; Nadjafi, F.; Menichini, F.; Loizzo, M.R. Tyrosinase, acetyl-and butyryl-cholinesterase inhibitory activity of Stachys lavandulifolia Vahl (Lamiaceae) and its major constituents. Rec. Nat. Prod. 2015, 9, 81–93. [Google Scholar]
- Fazio, C.; Paternostro, M.P.; Passannanti, S.; Piozzi, F. Further neo-clerodane diterpenoids from Stachys rosea. Phytochemistry 1994, 37, 501–503. [Google Scholar] [CrossRef]
- Farooq, U.; Ayub, K.; Hashmi, M.A.; Sarwar, R.; Khan, A.; Khan, S.S.; Khan, A.; Mumtaz, A. Spectroscopic and Density Functional Theory Studies of a New Rosane Type Diterpenoid from Stachys parviflora. Rec. Nat. Prod. 2015, 9, 329–335. [Google Scholar]
- Santos, E.A.; Quintela, A.L.; Ferreira, E.G.; Sousa, T.S.; Pinto, F.; Hajdu, E.; Carvalho, M.S.; Salani, S.; Rocha, D.D.; Wilke, D.V.; et al. Cytotoxic Plakortides from the Brazilian Marine Sponge Plakortis angulospiculatus. J. Nat. Prod. 2015, 78, 996–1004. [Google Scholar] [CrossRef]
- Xu, T.; Feng, Q.; Jacob, M.R.; Avula, B.; Mask, M.M.; Baerson, S.R.; Tripathi, S.K.; Mohammed, R.; Hamann, M.T.; Khan, I.A.; et al. The marine sponge-derived polyketide endoperoxide plakortide F acid mediates its antifungal activity by interfering with calcium homeostasis. Antimicrob. Agents. Chemother. 2011, 55, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Ruider, S.A.; Carreira, E.M. A unified strategy to Plakortin pentalenes: Total syntheses of (±)-gracilioethers E and F. Org. Lett. 2016, 18, 220–223. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4 and 5 are available from the authors. |
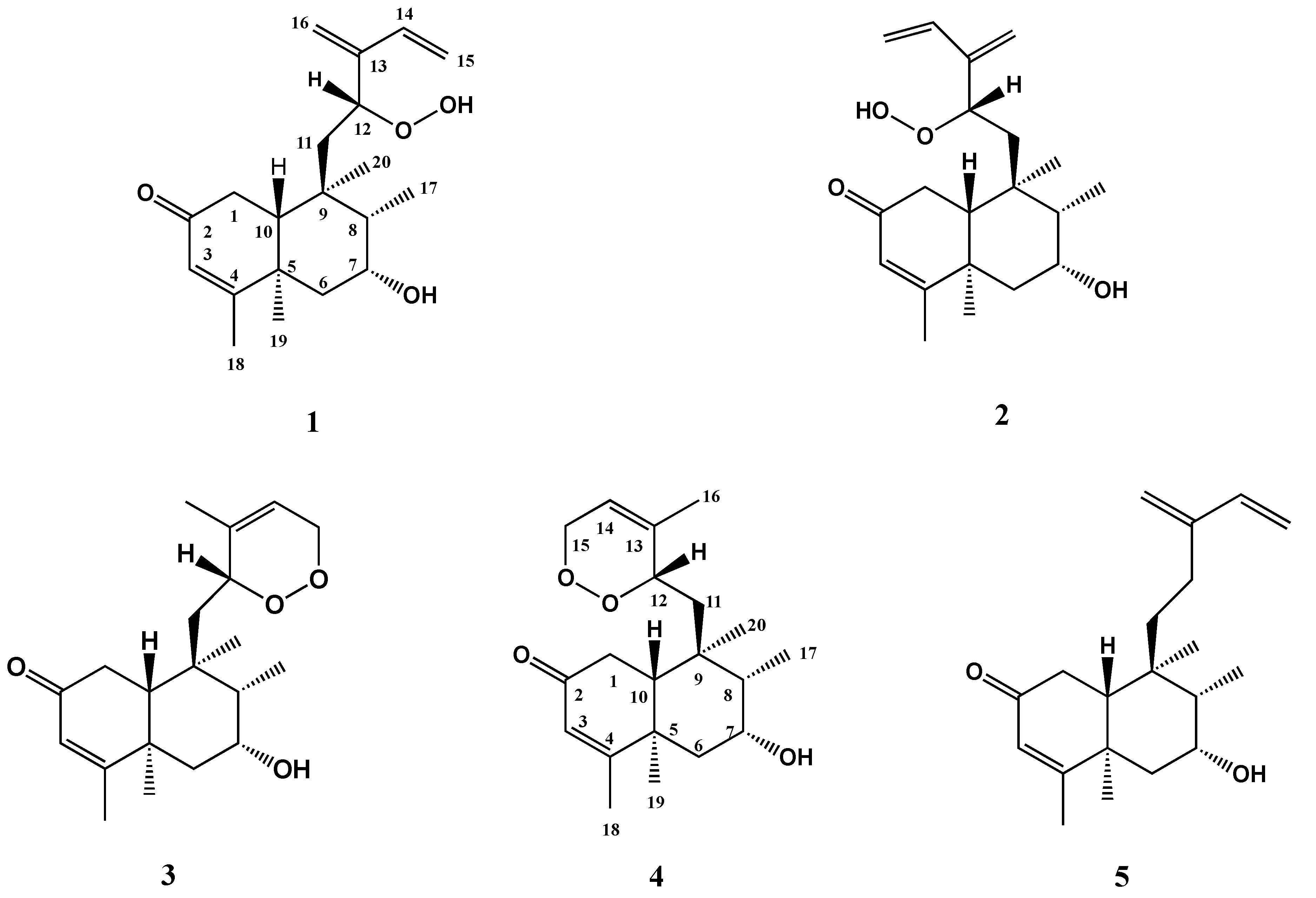
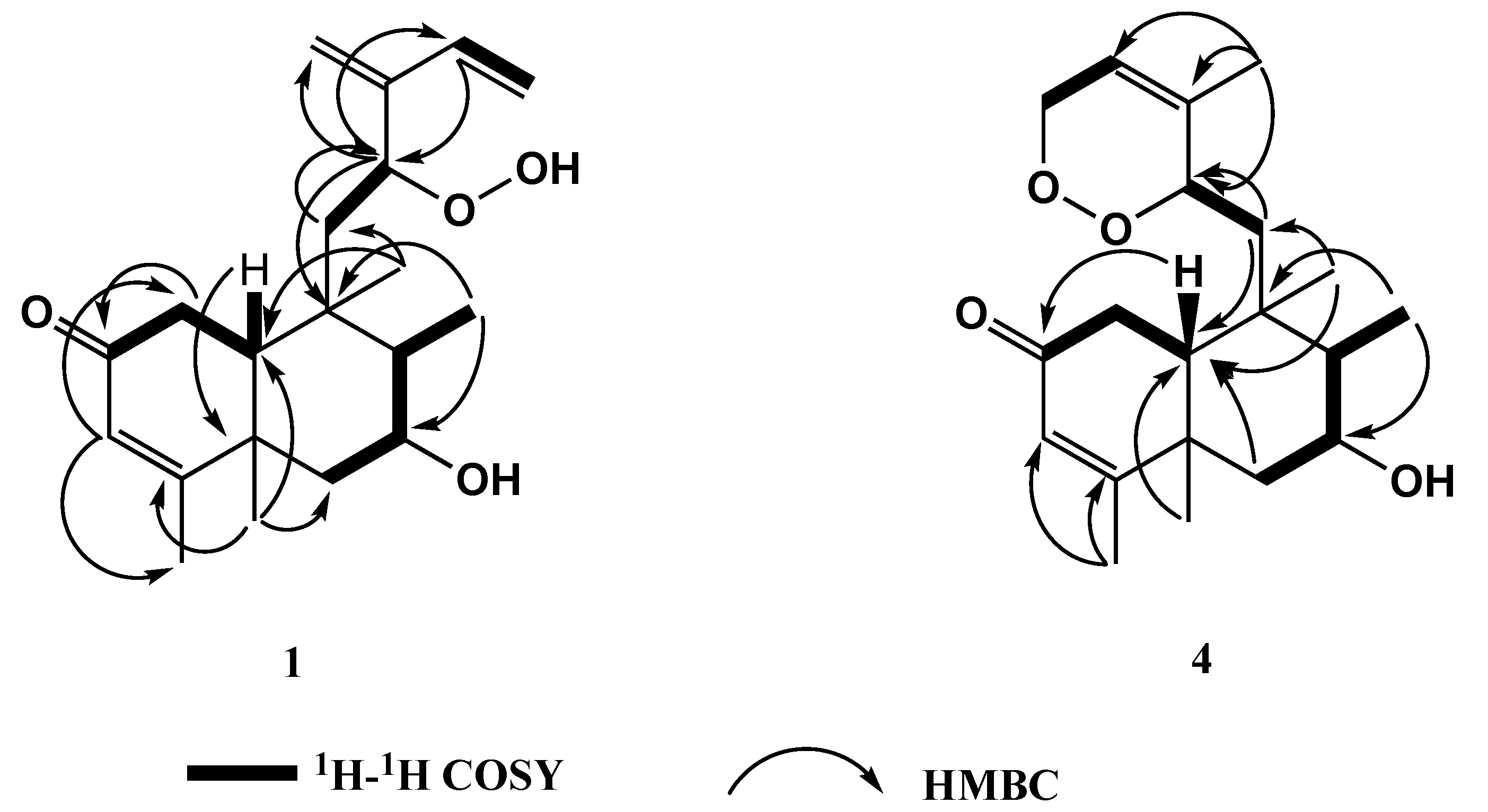
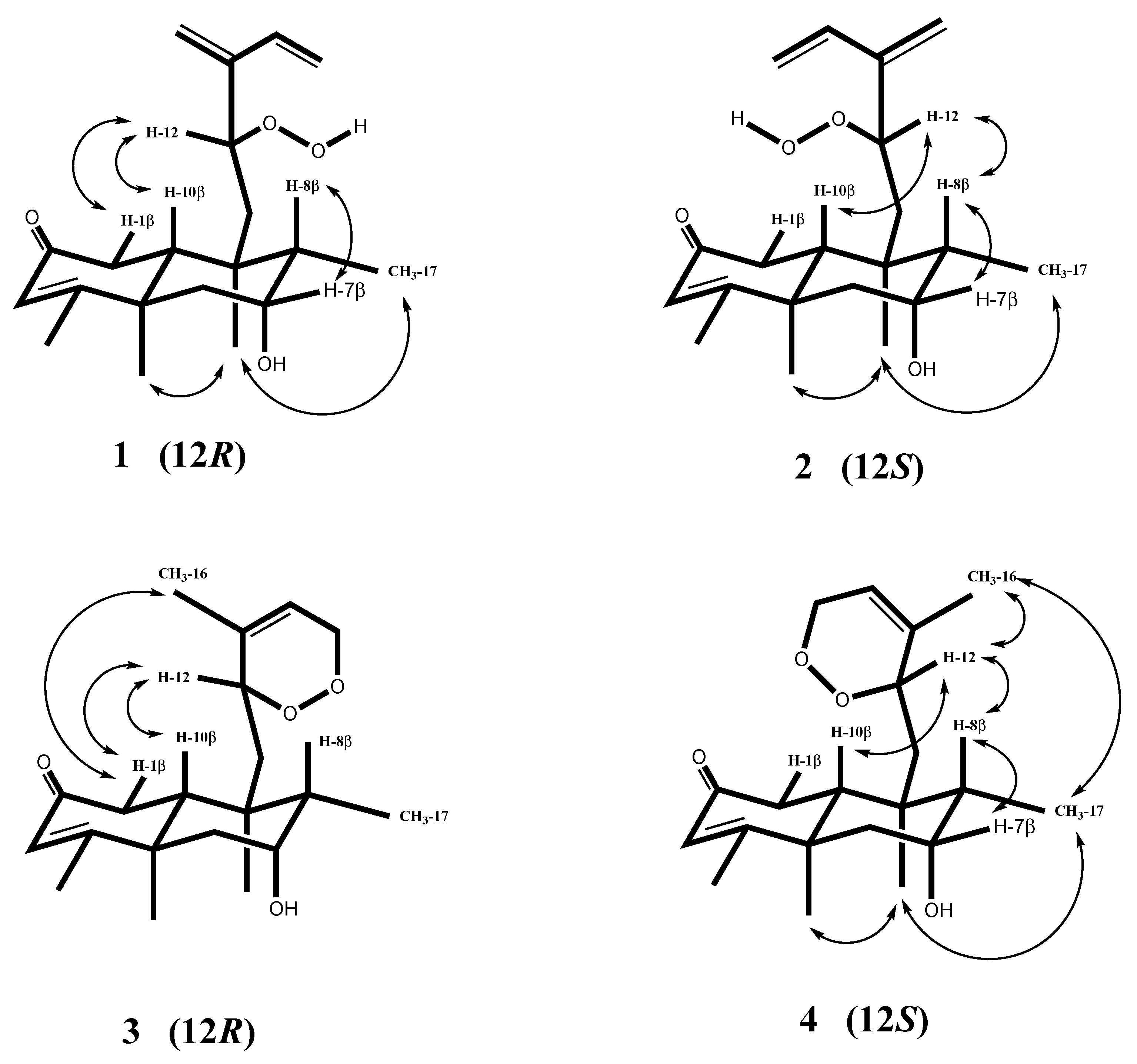
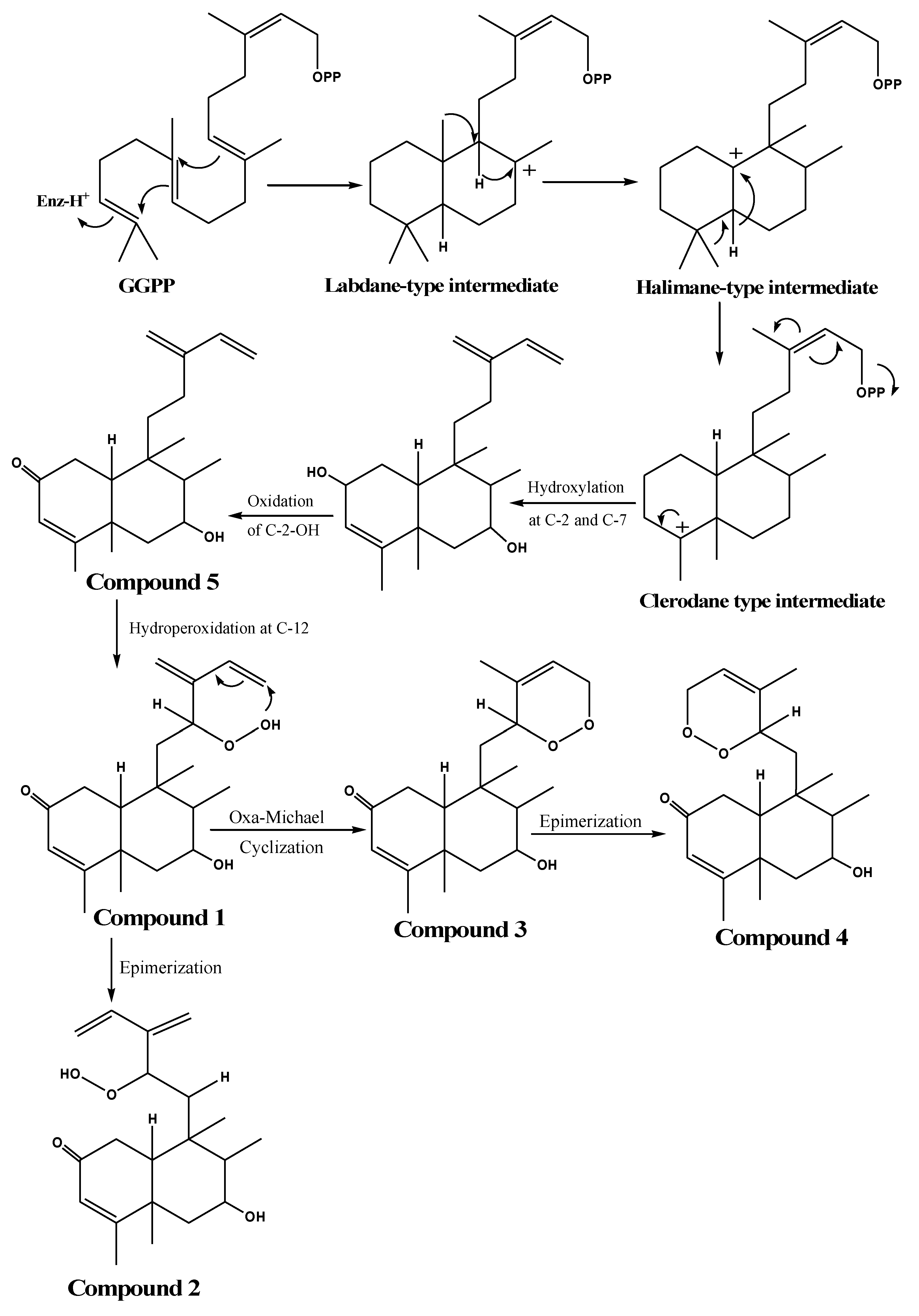
| Position | 1 | 2 | 3 a | 4 |
|---|---|---|---|---|
| 1α | 2.41 dd, (17.0, 14.0) | 2.41 dd (17.0, 14.4) | 2.52 dd (17.0, 14.0) | 2.41 m * |
| 1β | 2.29 dd (17.0, 3.4) | 2.60 dd (17.0, 2.8) | 2.32 dd (17.0, 3.4) | 2.80 dd (17.0, 3.4) |
| 2 | --- | --- | --- | --- |
| 3 | 5.68 br s | 5.68 br s | 5.69 br s | 5.69 br s |
| 4 | --- | --- | --- | --- |
| 5 | --- | --- | --- | --- |
| 6α | 2.20 dd (14.0, 2.7) | 2.22 dd (14.0, 2.7) | 2.19 dd (14.0, 2.7) | 2.17 dd (14.0, 2.7) |
| 6β | 1.60 dd (14.0, 3.4) | 1.63 dd (14.0, 3.4) | 1.57 dd (14.0, 3.4) | 1.57 dd (14.0, 3.4) |
| 7 | 4.09 br d (3.4) | 4.11 br d (2.4) | 4.07 m | 4.11 br d (2.7) |
| 8 | 1.90 m * | 1.71 m | 2.06 m | 1.69 m * |
| 9 | --- | --- | --- | --- |
| 10 | 2.14 dd (14.0, 3.4) | 2.25 dd (14.0, 2.8) | 2.11 dd (14.0, 3.4) | 2.41 m * |
| 11a | 1.64 dd (16.5, 7.5) | 1.62 dd (16.5, 7.5) | 1.91 dd (14.0, 10.5) | 1.96 dd (16.5, 10.3) |
| 11b | 1.50 dd (16.5, 2.0) | 1.52 dd (16.5, 2.0) | 1.44 m * | 1.42 m * |
| 12 | 4.66 dd (7.5, 2.7) | 4.66 d (8.2) | 4.18 br d (10.5) | 4.18 d (10.3) |
| 13 | --- | --- | --- | --- |
| 14 | 6.29 dd (17.0, 11.0) | 6.31 dd (17.0, 11.0) | 5.58 br s | 5.57 br d (2.5) |
| 15a | 5.49 d (17.0) | 5.45 d (17.0) | 4.61 br d (14.0) | 4.61 br dd (14.4, 2.5) |
| 15b | 5.17 d (11.0) | 5.15 d (11.0) | 4.28 br d (14.0) | 4.29 br d (14.4) |
| 16a | 5.23 s | 5.23 s | 1.73 s | 1.71 s |
| 16b | 5.13 s | 5.18 s | --- | --- |
| 17 | 1.09 d (7.0) | 0.99 d (7.5) | 1.13 d (7.0) | 1.06 d (7.0) |
| 18 | 1.92 s | 1.91 s | 1.91 s | 1.88 s |
| 19 | 1.39 s | 1.39 s | 1.42 s | 1.39 s |
| 20 | 1.02 s | 1.01 s | 1.07 s | 1.07 s |
| C | 1 | 2 | 3 a | 4 | ||
|---|---|---|---|---|---|---|
| δC | δC | DEPT | δC | δC | DEPT | |
| 1 | 35.3 | 35.5 | CH2 | 35.4 | 35.3 | CH2 |
| 2 | 199.8 | 200.9 | C=O | 199.8 | 200.7 | C=O |
| 3 | 125.1 | 125.2 | CH | 125.0 | 125.5 | CH |
| 4 | 172.9 | 172.7 | C | 173.1 | 172.2 | C |
| 5 | 39.6 | 39.0 | C | 38.8 | 38.8 | C |
| 6 | 41.9 | 42.0 | CH2 | 41.2 | 41.4 | CH2 |
| 7 | 73.2 | 73.2 | CH | 73.3 | 73.3 | CH |
| 8 | 39.8 | 39.6 | CH | 39.7 | 38.8 | CH |
| 9 | 39.6 | 39.5 | C | 39.6 | 39.2 | C |
| 10 | 46.4 | 46.6 | CH | 45.9 | 46.4 | CH |
| 11 | 41.2 | 41.3 | CH2 | 38.0 | 38.0 | CH2 |
| 12 | 83.7 | 82.6 | CH | 79.2 | 79.0 | CH |
| 13 | 146.3 | 146.9 | C | 134.7 | 134.2 | C |
| 14 | 134.8 | 135.3 | CH | 118.7 | 119.1 | CH |
| 15 | 116.4 * | 115.5 | CH2 | 69.9 | 69.8 | CH2 |
| 16 | 116.5 * | 115.6 | CH2 | 19.1 | 19.0 | CH3 |
| 17 | 12.8 | 12.7 | CH3 | 12.5 | 12.6 | CH3 |
| 18 | 19.4 | 19.2 | CH3 | 19.7 | 19.4 | CH3 |
| 19 | 20.2 | 20.4 | CH3 | 20.3 | 20.6 | CH3 |
| 20 | 19.1 | 19.1 | CH3 | 19.4 | 19.3 | CH3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussien, T.A.; Mahmoud, A.A.; Mohamed, N.S.; Shahat, A.A.; El-Seedi, H.R.; Hegazy, M.-E.F. New Rare Ent-Clerodane Diterpene Peroxides from Egyptian Mountain Tea (Qourtom) and Its Chemosystem as Herbal Remedies and Phytonutrients Agents. Molecules 2020, 25, 2172. https://doi.org/10.3390/molecules25092172
Hussien TA, Mahmoud AA, Mohamed NS, Shahat AA, El-Seedi HR, Hegazy M-EF. New Rare Ent-Clerodane Diterpene Peroxides from Egyptian Mountain Tea (Qourtom) and Its Chemosystem as Herbal Remedies and Phytonutrients Agents. Molecules. 2020; 25(9):2172. https://doi.org/10.3390/molecules25092172
Chicago/Turabian StyleHussien, Taha A., Ahmed A. Mahmoud, Naglaa S. Mohamed, Abdelaaty A. Shahat, Hesham R. El-Seedi, and Mohamed-Elamir F. Hegazy. 2020. "New Rare Ent-Clerodane Diterpene Peroxides from Egyptian Mountain Tea (Qourtom) and Its Chemosystem as Herbal Remedies and Phytonutrients Agents" Molecules 25, no. 9: 2172. https://doi.org/10.3390/molecules25092172
APA StyleHussien, T. A., Mahmoud, A. A., Mohamed, N. S., Shahat, A. A., El-Seedi, H. R., & Hegazy, M.-E. F. (2020). New Rare Ent-Clerodane Diterpene Peroxides from Egyptian Mountain Tea (Qourtom) and Its Chemosystem as Herbal Remedies and Phytonutrients Agents. Molecules, 25(9), 2172. https://doi.org/10.3390/molecules25092172









