Abstract
This study aims to investigate the effects of exogenous catalase (CAT), an antioxidative enzyme from microbial cultures, on intestinal development and microbiota in weaned piglets. Seventy-two weaned piglets were allotted to two groups and fed a basal diet or a basal diet containing 2.0 g/kg exogenous CAT. Results showed that exogenous CAT increased (p < 0.05) jejunal villus height/crypt depth ratio and intestinal factors (diamine oxidase and transforming growth factor-α) concentration. Moreover, dietary CAT supplementation enhanced the antioxidative capacity, and decreased the concentration of pro-inflammatory cytokine in the jejunum mucosa. Exogenous CAT did not affect the concentration of short-chain fatty acids, but decreased the pH value in colonic digesta (p < 0.05). Interestingly, the relative abundance of Bifidobacterium and Dialister were increased (p < 0.05), while Streptococcus and Escherichia-Shigella were decreased (p < 0.05) in colonic digesta by exogenous CAT. Accordingly, decreased (p < 0.05) predicted functions related to aerobic respiration were observed in the piglets fed the CAT diet. Our study suggests a synergic response of intestinal development and microbiota to the exogenous CAT, and provides support for the application of CAT purified from microbial cultures in the feed industry.
1. Introduction
Healthy intestines are not only beneficial to the digestion and absorption of nutrients, but also a crucial safeguard against the invasion of pathogens [1]. Gut microbiota is a category of microorganisms inhabiting the mammalian gastrointestinal tract, and are involved in maintaining intestinal health in human and monogastric animals, including providing nutrients, optimizing the intestinal immune system, and regulating the growth of the intestinal epithelial mucosa [2,3]. Weaning a pig from its sow is one of the most stressful events in a pig’s life [4]. Weaned piglets suffer from a huge stress response (especially oxidative stress) and changes in gut microbiota because of multiple factors such as environmental factors, weaning, and infection [5,6]. A previous study demonstrated that oxidative stress is strongly linked to the alteration of gut microbiota [7]. Moreover, oxidative stress suppresses intestinal development [8], weakens intestinal digestion and absorption [9], and induces mucosa inflammation and cell apoptosis [10], which can result in growth restriction, disease, and even death in piglets [11,12]. Therefore, decreasing postweaning oxidative stress is crucial for weaned piglets.
Hydrogen peroxide (H2O2), belonging to reactive oxygen species (ROS), is constantly generated from oxygen in all aerobic metabolism and pathogenic processes [13]. Excessive H2O2 is harmful to almost all cell components, so its rapid and efficient removal is essentially important for aerobically living organisms [14]. Catalase (CAT), an important antioxidative enzyme in the body, can break H2O2 down into O2 and H2O and reduce its damage to the body [15]. In turn, excess ROS also inhibits catalase activity [16]. Currently, exogenous CAT, which is purified from animal tissues or microorganisms [17,18], has been used in pharmaceuticals [19] and the food industry [20], as well as by biocatalyst [21] and other industries [22]. Wang [23] suggested that exogenous CAT supplementation in high-fat diets could increase rat antioxidative capacity and improve the intestinal flora structure. Our recent study showed that piglets fed diets supplemented with exogenous CAT had higher gain-to-feed ratios [24]. However, there was little information available in the scientific literature on the evaluation of the effects of dietary supplementation with exogenous CAT on intestinal development and gut microbial composition of weaned piglets.
In the present study, we investigated the effects of dietary exogenous CAT from microbial culture supplementation on the intestinal development and gut microbiota of weaned piglets, which could be helpful to understanding the microbial mechanisms behind exogenous CAT-modulated intestinal development, and could provide support for the application of CAT purified from microbial fermentation in the feed industry.
2. Materials and Methods
2.1. Ethical Approval
The present experiment was conducted at the Research Farm of Animal Nutrition Institute, Sichuan Agricultural University, Ya’an, China. The research protocol was approved by the Care and Use committee of Sichuan Agricultural University under ethic approval number SCAUAC201408-3.
2.2. Exogenous Catalase Production
The exogenous CAT production was provided by Liaoning Vetland Bio-Technology Co., Ltd., Liaoning, China. It was produced from Penicillium notatum (collection number: ACCC 30443), followed by spray drying and sieving to obtain powder production.
2.3. Experimental Design and Animal Management
A total of 72 Duroc × (Landrace × Yorkshire) crossbred weaned piglets (21 d of age, 6.90 ± 0.01 kg) were selected as experimental animals and housed in 12 pens with 6 piglets per pen. Pens were randomly assigned to one of the following two dietary treatments (n = 6) for a 35-d feed trial: (1) CON group (piglets were fed with a basal diet) and (2) CAT group (piglets were fed with the basal diet supplemented with 2.0 g/kg exogenous CAT). Diets were offered to piglets according to two-phase feeding programs (1–21 d and 22–35 d of trial). The basal diets were formulated to respect or exceed the nutrient requirements recommended by the National Research Council (NRC, 2012) for weaned piglets at different growth stages and the ingredient composition and nutrient levels are shown in Supplementary Table S1. The exogenous CAT was added to the basal diets at the expense of corn. Piglets were housed in a totally enclosed and temperature-controlled room with 12 pens (1.5 × 2.5 m) and had free access to feed and water [25]. The room temperature was set at 28 ± 1 °C for the first week and gradually decreased to 25 °C by the end of the trial. No vaccines or antibiotics were administered to these piglets throughout the experiment.
2.4. Sampling Procedure
At the end of the feeding trial, one healthy pig per pen with a similar body weight (BW) to the average pen weight (totally 12 piglets, 14.62 ± 0.20 kg) was selected and slaughtered immediately after deep anesthesia with Zoletile 50 (Virbac, France) administered by intramuscular injection at a dose of 0.1 mg/kg of BW. After evisceration, the small intestine was dissected and rapidly measured for weight. The small intestine in piglets was defined as described in Li et al. [26]. Briefly, intestinal segments (duodenum, jejunum, and ileum) were obtained by using the anatomical landmarks, with the pyloric-duodenal junction to the duodenal-jejunal junction being the duodenum, the duodenal-jejunal junction to the jejunal-ileal junction being the jejunum, and the jejunal-ileal junction to the ileocecal junction being the ileum. The relative weight of the intestine was calculated as the intestinal weight divided by the BW of piglet. The intestinal segments (about 2 cm) of tissues (duodenum, jejunum, and ileum), were immediately rinsed by physiological saline and fixed in 4% paraformaldehyde for 24 h for morphological examination. The rest jejunum was opened longitudinally and washed in ice-cold physiological saline solution. The mucosa tissue from jejunum was subsequently collected by scraping using a sterile glass slide, followed by being snap-frozen in liquid nitrogen and being stored at −80 °C until further analysis. The colonic digesta samples were collected on a clean bench, immediately placed in sterile bags, and stored at −80 °C for further analysis.
2.5. Mucosal Morphology Measurement
Fixed intestinal segments were embedded according to routine paraffin-embedding protocol. Serial sections with a 5-μm thickness were made using a microtome, and this was followed by hematoxylin and eosin staining. Villi height and crypt depth were determined as previously described in Li et al. [27]. Briefly, two transverse sections of each intestinal sample were prepared on one slide for morphometric analysis. A total of 12–20 intact, well-oriented crypt-villus units per sample were selected randomly and measured. Villus height measurements were taken from the tip to the base of the villus between individual villi, and crypt depth was measured from the valley between individual villi to the basal membrane. The crypt depth (μm) and villus height (μm) of the small intestine were measured using an Olympus BX51 microscope equipped with a DP70 digital camera (Olympus, Tokyo, Japan) and JD801 morphologic image analysis software (JEDA, Nanjing, Jiangsu, China). The ratio of villus height to crypt depth (VCR) was calculated as the villus height divided by the crypt depth.
2.6. Determination of H2O2 in Intestinal Tissue
The H2O2 level in jejunal mucosa tissues was determined according to the manufacturer’s instruction (Beyotime Biotech, Shanghai, China) as previously described in Luo et al. [5]. Briefly, the mucosa tissues were weighted and homogenized in an H2O2 lysis buffer (1:20, w/v) followed by centrifugation at 12,000× g for 10 min for supernatants. The absorbance was read at 560 nm after the sample solution (50 µL) was incubated with reaction solution (100 µL) at room temperature for 30 min. The H2O2 concentration was calculated by the standard curve made from the standard solutions.
2.7. Intestinal Antioxidant Parameters Determination
The determination of intestinal antioxidant parameters was proceeded as previously described in Chen et al. [8]. The jejunal mucosa samples were homogenized in ice-cold saline solution (1:9, w/v), followed by centrifugation at 2500× g for 10 min at 4 °C. The supernatant was prepared for further determination. Intestinal mucosa antioxidant parameters included CAT, total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA), and were measured with the commercial kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, Jiangsu, China) according to the manufacturer’s instructions.
2.8. Intestinal Barrier Integrity Determination
The determination of jejunal mucosal barrier integrity was proceeded as described in a previous study [28]. Diamine oxidase (DAO) activities, transforming growth factor-α (TGF-α), trefoil factor family (TFF), and major histocompatibility complex class II (MHC-II) were analyzed by commercial porcine-specific ELISA kits (Beijing winter song Boye Biotechnology Co. Ltd., Beijing, China) according to the manufacturer’s instructions.
2.9. Intestine Mucosal Proinflammatory Cytokine and SIgA Determination
Jejunal mucosa was homogenized in ice-cold physiological saline (1:9, w/v) and the supernatant was collected. The levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in the jejunal mucosa were measured using a commercially available ELISA kit (R&D Systems Inc., Minneapolis, MN). The concentration of secretory immunoglobulin A (SIgA) in jejunum was determined using the sandwich ELISA kits (Beijing winter song Boye Biotechnology Co. Ltd., Beijing, China) according to the manufacturer’s instructions.
2.10. Digesta pH Values Determination
The method of digesta pH value determination was according to Chen et al. [28]. Briefly, about 5 g colonic digesta of each pig was immediately transferred into an ice-bathed sterile 10-mL centrifugal tube after the colon segments were removed, and the pH value of each sample was measured by pH meter (PHS-3C PH, Shanghai, China).
2.11. Short-Chain Fatty Acids Concentration Analysis
The short-chain fatty acids (SCFAs) in piglet colonic digesta were measured according to the protocol from a previous study [29]. Briefly, 0.7 g of colonic digesta samples were suspended in 1.5 mL of distilled water and allowed to stand for 30 min, followed by being centrifuged for 15 min at 15,000× g at 4 °C. Then 1 mL supernatant was transferred and mixed with metaphosphoric acid (0.2 mL, 25%, w/v) and crotonic acid (23.3 µL, 210 mmol/L). After standing at 4 °C for 30 min, the samples were centrifuged for 10 min at 15,000× g again. Then the supernatant was transferred and mixed with chromatographic methanol (1:1, v/v). After centrifugation at 10,000× g, an amount of 1 µL supernatant was analyzed using gas chromatography (Varian CP-3800 GC, USA).
2.12. Microbial Analysis
Bacterial genomic DNA was extracted from frozen colonic samples with the EZNA TM Stool DNA kit (Omega Bio-Tek, Norcross, Georgia, USA) according to the manufacturer’s protocol. DNA concentration and purity were monitored on 1% agarose gels. According to the concentration, DNA was diluted to 1 ng/μL using sterile water. The barcoded V4: 515F-806R primer (5′-GTGCCAGCMGCCGCGGTAA-3′ and 5′-GGACTACHVGGGTWTCTAAT-3′, respectively) were used to perform amplicon pyrosequencing on the Illumina HiSeq PE2500 platform (Novogene, Beijing, China). Generated 250-bp paired-end reads from the original DNA fragments were merged using Fast Length Adjustment of Short reads (FLASH) [30]. The sequences containing ambiguous bases or mismatches in the primer regions were filtered to obtain the high-quality clean sequence tags according to the Quantitative Insights Into Microbial Ecology (QIIME, V1.7.0) quality control process [31,32]. All sequencing data are available in the NCBI Sequence Read Archive (SRA) under accession PRJNA560659 (Illumina sequences). Then the tags were compared with the Gold database using the UCHIME algorithm to detect chimera sequences [33,34]. The effective tags were finally obtained after the chimera sequences were removed. The effective tags were performed by Uparse software (V7.0.1001) and sequences were clustered into the same operational taxonomic units (OTUs) at 97% sequence similarity [35], and were classified to different levels (phylum, class, order, family, genus, and species) by comparing sequences with the GreenGene database [36] using the Ribosomal Database Project (RDP) classifier (V2.2) [37]. Alpha and beta diversity were both calculated to compare the taxonomic data. The observed species (calculated unique OTUs), Shannon index (measured community diversity), and Chao 1 index (estimated community richness) were used to ascertain differences in alpha diversity based on different diets. A Wilcoxon rank-sum test was used to detect the statistical differences between two groups. Bray–Curtis distances were calculated and visualized by Principal Coordinate Analysis (PCoA) [38]. An analysis of similarity (ANOSIM) test was used to access significant differences among the microbial communities. All the analyses from clustering to alpha and beta diversity were performed with QIIME (V1.7.0) and displayed with R software (V2.15.3). Functional profiles of the prokaryotic community were annotated using the Functional Annotation of Prokaryotic Taxa (FAPROTAX) [39].
2.13. Statistical Analysis
The individual piglet was considered to be the experimental unit for all other variables. All indexes except microbial analysis were used to evaluate significance using the t-test procedure of SAS 9.0 (Institute Inc., Cary, NC, USA). Normality of the data was assessed using a Shapiro–Wilk’s statistic (W > 0.05). If the data did not follow a normal distribution, transformation was used to achieve normality. Values are expressed as mean ± standard error in tables and figures. Statistical significance was set at p < 0.05, and 0.05 < p < 0.10 was considered a trend toward significance.
3. Results
3.1. Effects of Exogenous CAT on Intestinal Relative Weight and Morphology
Effect of dietary exogenous catalase supplementation on the intestinal relative weight of weaned piglets is presented in Table 1. Piglets fed diets supplemented with exogenous CAT had heavier (p < 0.05) ileums and small intestines, and had a trend of increased jejunum weight compared with those fed the CON diet (p < 0.10).

Table 1.
Effect of dietary exogenous catalase supplementation on the intestinal relative weight of weaned piglets.
As shown in Table 2, the jejunal villus height and VCR of piglets fed the CAT diet were significantly higher than of those fed the CON diet (p < 0.05), and the duodenal villus height of piglets in the CAT group tended to be higher than of those in the CON group (p = 0.051). No significant difference was observed in ileum indexes (p > 0.05).

Table 2.
Effects of dietary exogenous catalase supplementation on intestinal morphology in weaned piglets.
3.2. Effects of Exogenous CAT on Intestinal Antioxidative Capacity
Effects of dietary exogenous catalase supplementation on the jejunum mucosa antioxidant parameters of weaned piglets are shown in Table 3. The jejunal mucosa CAT and SOD activity of piglets in the CAT group were significantly higher than in the CON group (p = 0.040), and piglets fed the CAT diet had significantly lower concentrations of MDA and H2O2 (p < 0.05). No significances were observed in GSH-Px and T-AOC activities (p > 0.05).

Table 3.
Effects of dietary exogenous catalase supplementation on jejunum mucosa antioxidant parameters in weaned piglets.
3.3. Effects of Exogenous CAT on Intestinal Mucosa Barrier Function
The concentrations of intestinal factors, DAO, and TGF-α in the jejunal mucosa of piglets fed the CAT diet were both significantly higher than piglets fed the CON diet (p < 0.05, Table 4). The concentrations of TFF and MHC-II were not significantly affected by dietary treatment (p > 0.05).

Table 4.
Effects of dietary exogenous catalase supplementation on jejunum mucosal factors in weaned piglets.
3.4. Effects of Exogenous CAT on Intestinal Mucosa Proinflammatory Cytokines and SIgA Concentration
Effects of dietary exogenous catalase supplementation on the jejunum mucosal proinflammatory cytokine and SIgA of weaned piglets are shown in Table 5. Dietary CAT significantly decreased (p < 0.05) the level of TNF-α and IL-6, and significantly increased (p = 0.025) the concentration of SIgA in the jejunum mucosa.

Table 5.
Effects of dietary exogenous catalase supplementation on the jejunum mucosal proinflammatory cytokines and SIgA of weaned piglets.
3.5. Effects of Exogenous CAT on Concentration of SCFAs and pH Values in Colonic Digesta
As shown in Table 6, significantly lower pH values were observed in the colonic digesta of piglets fed the CAT diet compared with those fed the CON diet (p = 0.006). Piglets fed the CAT diet tended to have a higher butyrate concentration in colonic digesta (p = 0.074).

Table 6.
Effects of dietary exogenous catalase supplementation on short-chain fatty acids concentration and pH values in the colonic digesta of weaned piglets.
3.6. Effects of Exogenous CAT on Microbial Diversity in Colonic Digesta
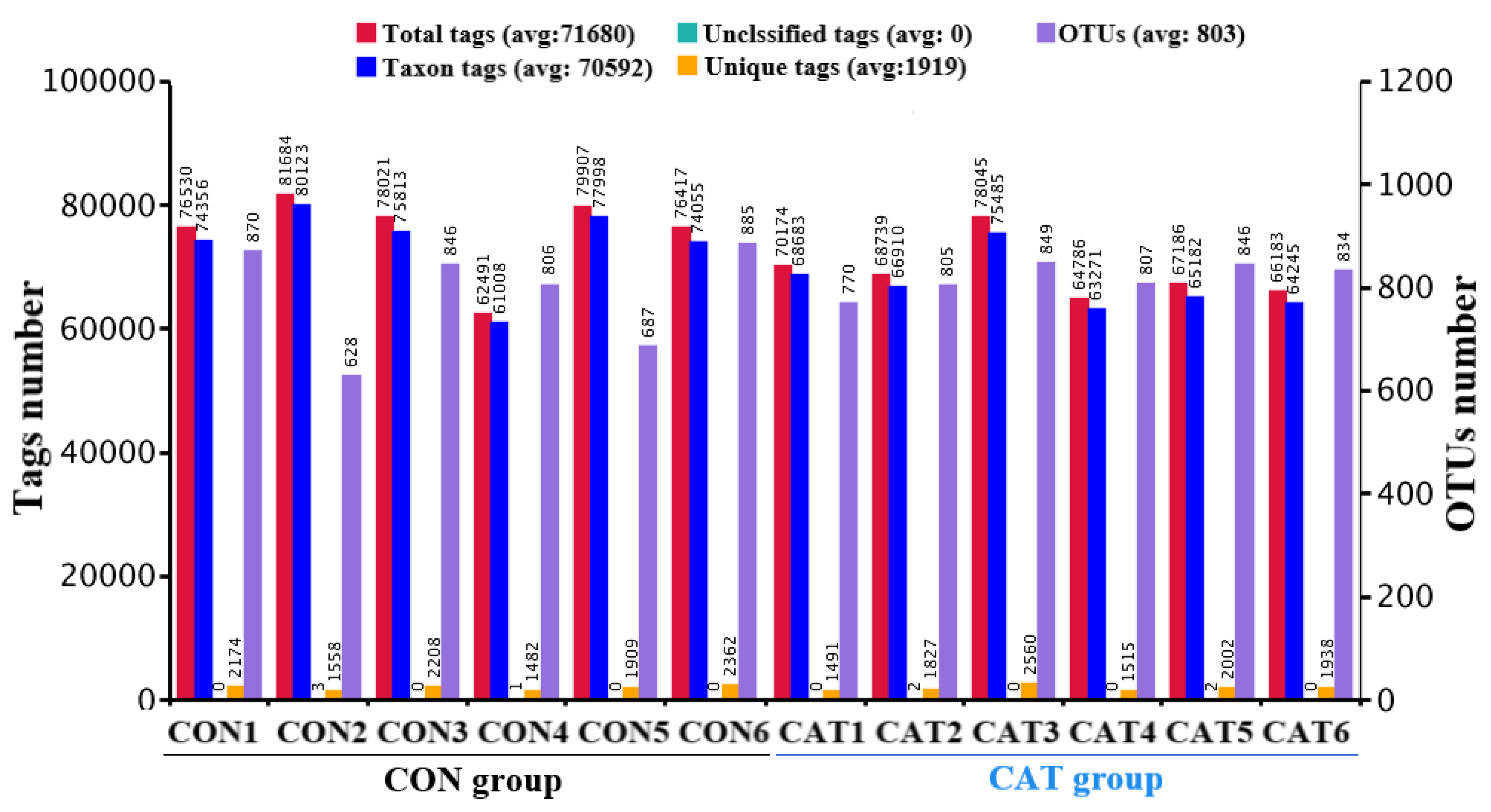
Total tags, taxon tags, unclassified tags, unique tags, and OTUs are shown in Figure 1. A total of 847,129 taxon tags were obtained from two treatments, with an average of 70,594.08 ± 1857.12 per sample. The species diversity of the samples was further studied and species were annotated on the representative sequence of OTUs. A total of 4722 OTUs were found in the CON group, with an average of 787.00 ± 43.06 per sample, while a total of 4911 OTUS were found in the CAT group, with an average of 818.50 ± 12.37 per sample.
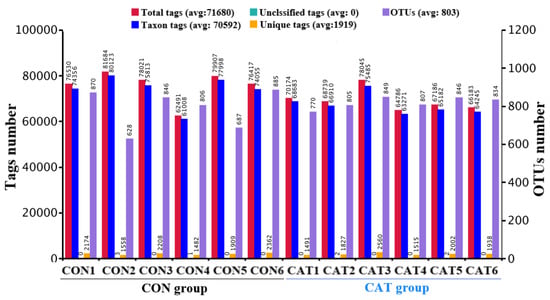
Figure 1.
Operational taxonomic unit (OTU) clustering and annotation per sample. CON 1, 2, 3, 4, 5, and 6 are colonic digesta samples from piglets fed with a basal diet; CAT 1, 2, 3, 4, 5, and 6 are colonic digesta samples from piglets fed with a basal diet supplemented with 2.0 g/kg exogenous catalase production.
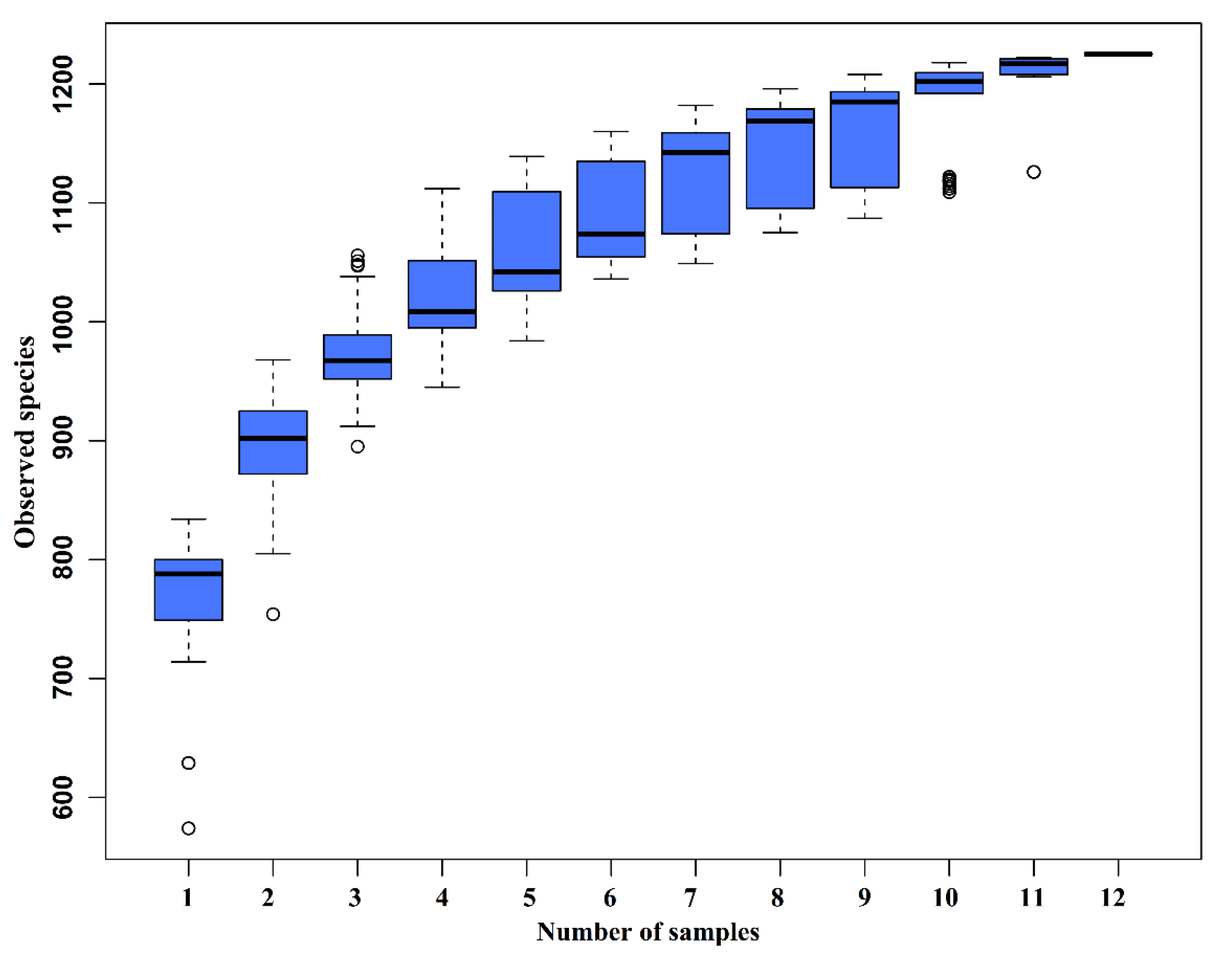
The species accumulation curves (SAC) (Figure 2) tended to flatten as the number of analyzed sequences increased up to 12, indicating that our samples were sufficient for OTU testing and could predict the species richness of samples.
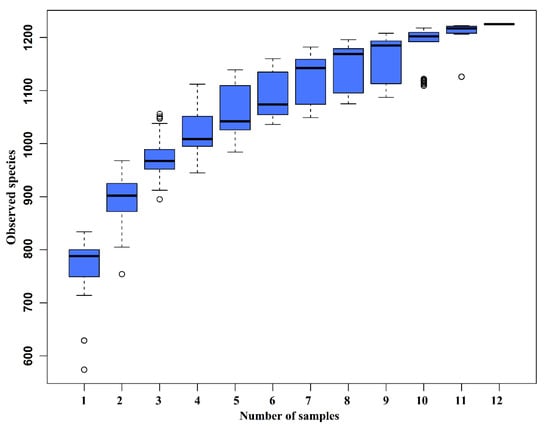
Figure 2.
Species accumulation curves (SAC). The SAC tends to flatten as the number of analyzed sequences increases up to 12, indicating that our samples were sufficient for OTU testing and could predict the species richness of samples.
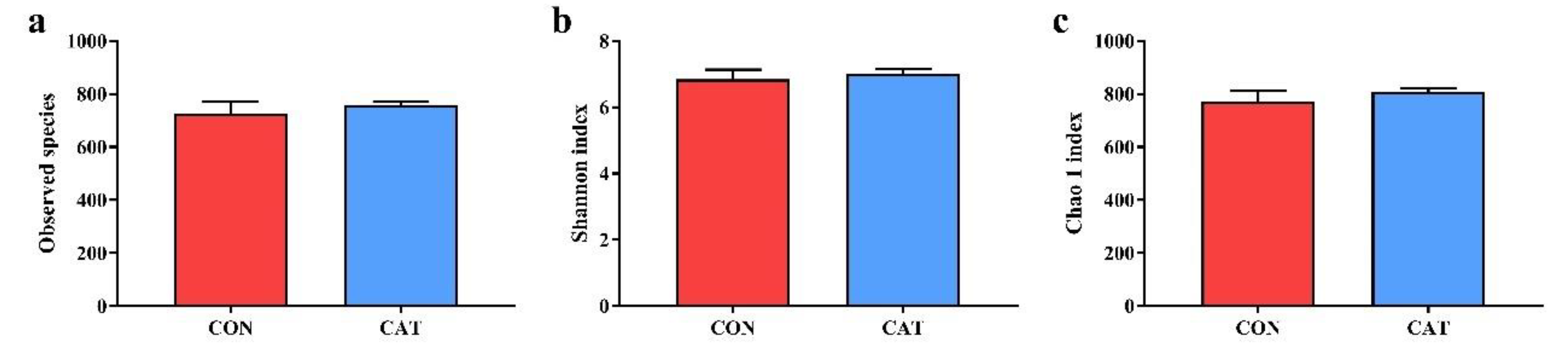
To assess colonic microbial community structures, observed species (Figure 3a), Shannon index (Figure 3b), and Chao 1 index (Figure 3c) were calculated, but no significant differences were observed in the three indexes (p > 0.05).
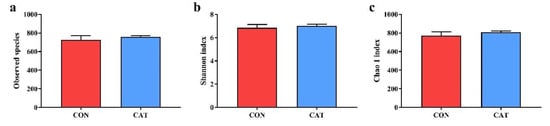
Figure 3.
Difference on bacteria community diversity and richness between the two treatments. (a) Observed species; (b) Shannon index; (c) Chao 1 index. CON, piglets fed with a basal diet; CAT, piglets fed with a basal diet supplemented with 2.0 g/kg exogenous catalase production. Values are mean ± standard error (n = 6).
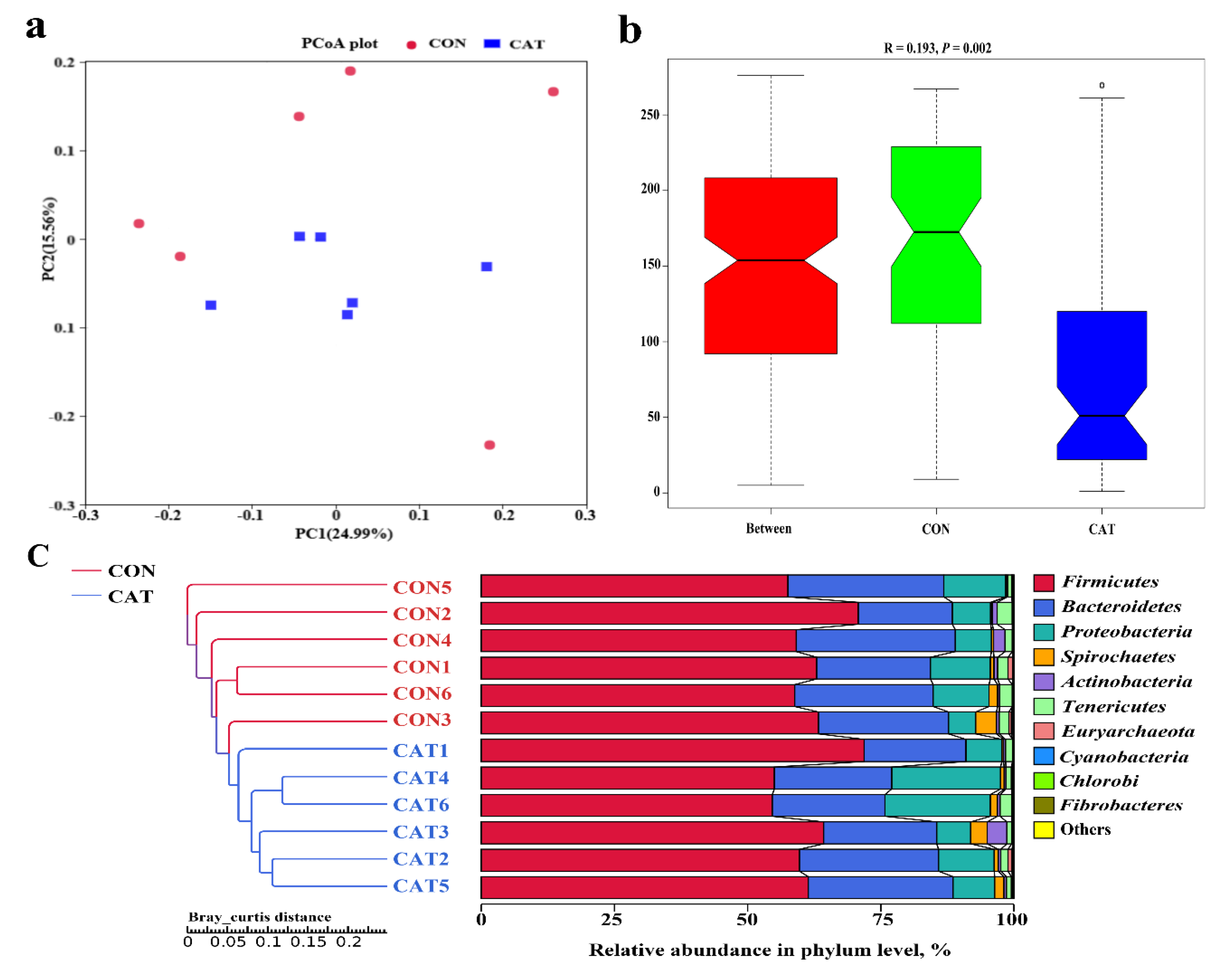
The PCoA profile for piglet colonic samples based on the Bray–Curtis distance (Figure 4a) showed a clear separation between the CON group and CAT group. The ANOSIM (Figure 4b) showed that two groups had significantly different microbiota community structures (p < 0.05). An unweighted pair-group method with an arithmetic mean (UPGMA) phylogenetic tree (Figure 4c) constructed based on the Bray–Curtis distance showed that samples CON1, CON2, CON3, CON4, CON5, and CON6 formed the first group, while CAT1, CAT2, CAT3, CAT4, CAT5, and CAT6 formed the second group, suggesting the two groups had obvious differences in their microbial communities.
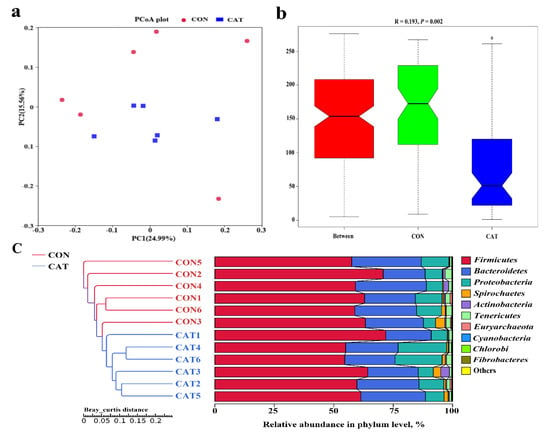
Figure 4.
The beta diversity of microbial communities in two treatments. (a) The principal coordinate analysis (PCoA) profile of the two groups displayed with the Bray–Curtis distance. Each dot represents one sample from each group. The percent variation explained by each principal coordinate is indicated on the X and Y axis. (b) Analysis of similarity (ANOSIM). R value is scaled to lie between −1 and +l. Generally, 0 < R < 1 and p < 0.05 represents that there were significant differences between the groups. (c) Unweighted pair-group method with arithmetic mean (UPGMA) phylogenetic tree constructed based on the Bray–Curtis distance. The left panel shows the phylogenic tree, and the right panel shows the relative abundance of each sample at the phylum level. CON 1, 2, 3, 4, 5, and 6 are colonic digesta samples from piglets fed with a basal diet; CAT 1, 2, 3, 4, 5, and 6 are colonic digesta samples from piglets fed with a basal diet supplemented with 2.0 g/kg exogenous catalase production.
3.7. Effects of Exogenous CAT on Microbial Relative Abundances in Colonic Digesta
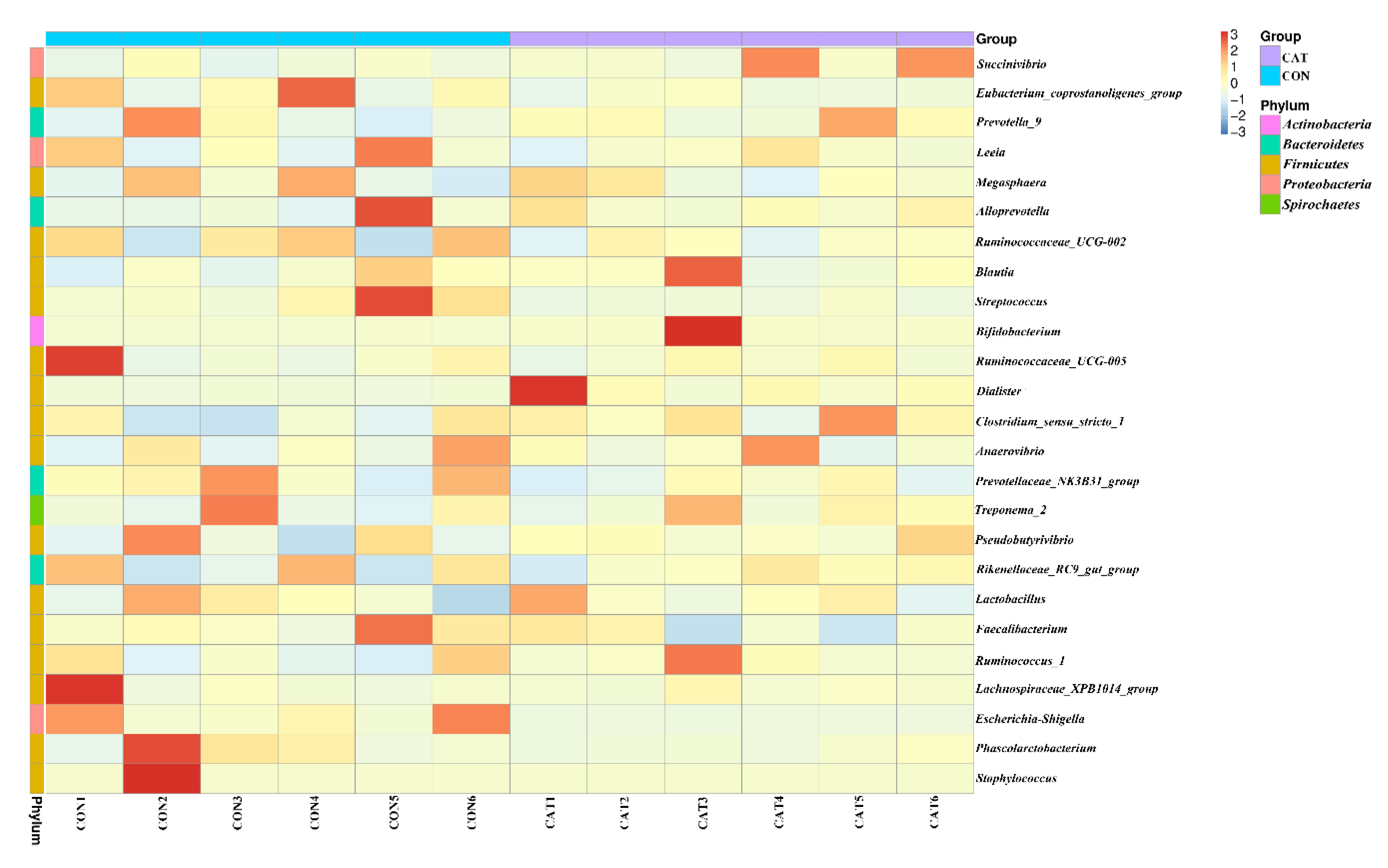
The relative abundance at the phylum level in piglet colonic microbiota (top 10 phyla) is shown in Figure 4c. Firmicutes and Bacteroidetes were the most predominant phyla. There was no significant difference observed in the top 10 phyla between the CON group and the CAT group (p > 0.05).
The relative abundance at the genus level in piglet colonic microbiota (top 25 genera) is shown in Supplemental Table S2. The colonic samples were dominated by three genera: Lactobacillus, Succinivibrio, and Alloprevotella. The heatmap (Figure 5) exhibits the abundance of the selected genera across all the samples, clearly showing that there were significant differences in the genus distribution between the CON and CAT treatments. Dietary exogenous CAT supplementation significantly decreased the relative abundance of Streptococcus (p = 0.021) and Escherichia-Shigella (p = 0.006), and significantly increased the relative abundance of Dialister (p = 0.008) and Bifidobacterium (p = 0.023).
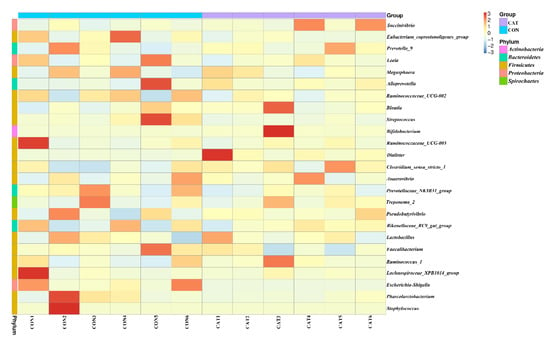
Figure 5.
Bacterial community heatmap analysis at the genus level. CON 1, 2, 3, 4, 5, and 6 are colonic digesta samples from piglets fed with a basal diet; CAT 1, 2, 3, 4, 5, and 6 are colonic digesta samples from piglets fed with a basal diet supplemented with 2.0 g/kg exogenous catalase production.
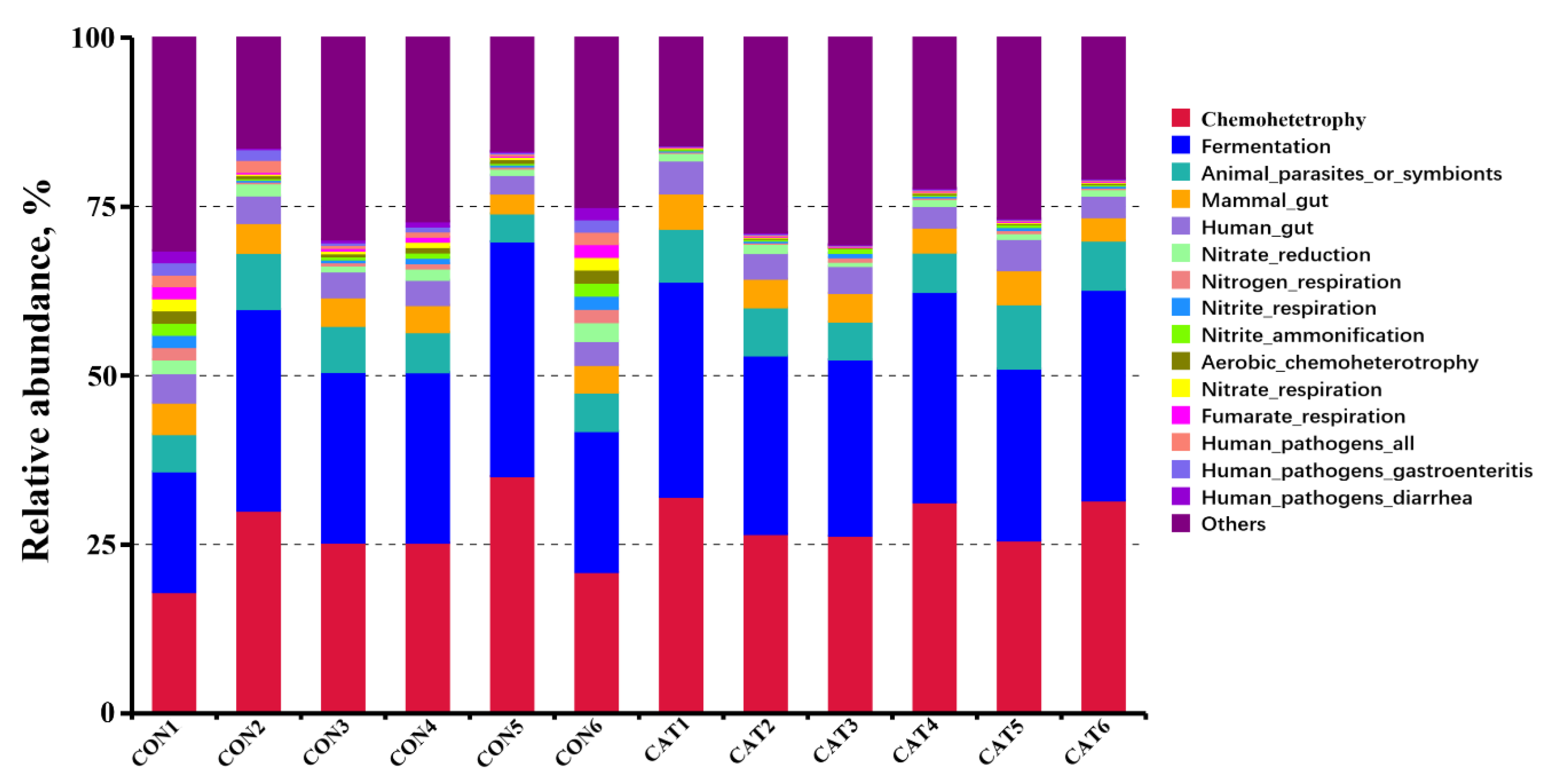
3.8. Effects of Exogenous CAT on Microbial Functional Characteristics
Relative abundance of the top 10 predicted functions of colonic microbiota in two experimental treatments are shown Supplemental Table S3 and displayed with a bar chart in Figure 6. Microbial functions were predicted using FAPROTAX based on the relative abundance of colonic microbes. Of the 10 functions, “Chemoheterotrophy” and “Fermentation” were the top two functional annotations in the two treatments. Dietary exogenous CAT supplementation significantly decreased (p < 0.05) the relative abundance of “Aerobic_chemoheterotrophay”, “Nitrate_respiration”, “Fumarate_respiration”, “Human_pathogens_all”, “Human_pathogens_gastroenteritis”, and “Human_pathogens_diarrhea”, and tended to decrease (p < 0.10) the relative abundance of “Nitrate_reduction”, “Nitrogen_respiration”, and “Nitrite_respiration”.
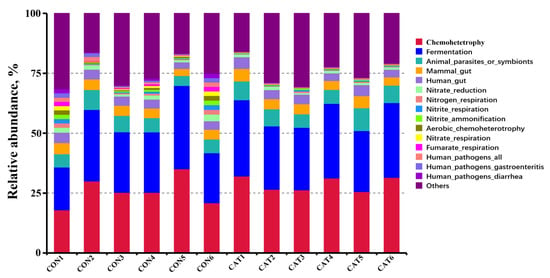
Figure 6.
Relative abundance of the predicted function of colonic microbiota in two experimental treatments. CON 1, 2, 3, 4, 5, and 6 are colonic digesta samples from piglets fed with a basal diet; CAT 1, 2, 3, 4, 5, and 6 are colonic digesta samples from piglets fed with a basal diet supplemented with 2.0 g/kg exogenous catalase production.
4. Discussion
In the current study, dietary CAT supplementation increased the weight of ileums and small intestines, and benefited jejunal villus height and VCR. The weight of intestines has been considered to be an important indicator of intestine development [26], and it has been thought that higher intestinal weight is related to a thicker intestinal wall and mucus layer [40,41]. Intestinal morphology is an important indicator of the health of intestines. The height of the villus determines the ability of the small intestine to absorb nutrients, and the VCR is regarded as a reliable measure to evaluate nutrient absorption capacity of the small intestine [11]. Decreased villus height and VCR are always accompanied by a decrease in digestibility [42]. It was illuminated in this study that exogenous CAT might be conducive to digestion and the absorption of nutrients in the small intestine. Consistently, a previous study showed that exogenous supplementation increased feed conversion efficiency [24]. Further, the maturity and integrity of the intestinal epithelium barrier, which plays a key role in the digestion and absorption of nutrients and provides a physical barrier to protect against infection in the intestinal tract, were evaluated through measuring the concentration of DAO, TGF-α, TFF, and MHC-II in the jejunal mucosa. In the current study, exogenous CAT supplementation increased the concentration of DAO and TGF-α. DAO was found in high concentrations in the small intestine. In the gut, DAO is synthesized by maternal enterocytes and stored in a plasma membrane-associated vesicular structure in the intestinal epithelial cells, and is usually considered to be an indicator by which to evaluate the integrity of the intestinal barrier [43,44]. TGF-α, which serves as a polypeptide ligand of the epidermal growth factor or TNF-α receptor, has been reported as being produced by the intestinal epithelial cell and able to maintain the integrity of intestinal epithelial cells [45]. A previous study showed that increased DAO and TGF-α concentrations were associated with enhanced antioxidative capacity in the jejunal tissue [11,28]. The results also suggested a beneficial effect of exogenous CAT on intestinal epithelial barrier functions.
Intestine mucosal damage was usually associated with inflammation [8]. Previous studies have reported that weaning stress could easily trigger intestinal inflammation in pigs [46]. Dietary exogenous CAT supplementation decreased the concentration of pro-inflammatory factors (TNF-α and IL-6) and increased the concentration of SIgA. TNF-α is a pleiotropic cytokine produced by activated macrophages, with some metabolic effects on lipid metabolism [47]. TNF-α signaling can induce activation of the NF-κB signaling pathway, which is considered to be a key inducer of inflammation and programmed cell death [48]. TNF-α can also activate IL-6 production [49], which is in accord with the increased IL-6 level in the present study. IL-6, considered to be an inflammatory biomarker, is a multifunctional cytokine that plays a central role in regulating the balance between the IL-17-producing Th17 cells and regulatory T cells [50]. Activated Th17 cells can produce inflammatory mediators leading to chronic inflammation [51]. SIgA is secreted by intestinal lamina propria plasmacytes, and serves as the first line of specific defense in the mucosal immune system of the intestine [52]. The concentration of intestinal SIgA is reduced along with the number of bacteria adhered to the mucosa as it increases [53].
Early weaned piglets are prone to oxidative stress, which often causes damage to the intestinal mucosa [8]. A previous study showed that decreasing oxidative stress was a main reason for improving intestinal development in weaned piglets [28]. In the current study, the redox status in the jejunal mucosa was evaluated through monitoring the oxidative and antioxidative items. We found that dietary exogenous CAT decreased H2O2 and MDA concentrations in the jejunal mucosa of piglets. H2O2 is one of the major ROSs in the body, and MDA, a secondary product of lipid oxidation, has been widely considered to be an index by which to monitor the degree of lipid peroxidation [29]. Both H2O2 and MDA are closely associated with cell damage. A previous study showed that piglets weaned earlier than normal suckling piglets always had higher H2O2 and MDA levels and lower antioxidative enzymes activities in the intestinal tissues [54]. It has been suggested that piglets fed a diet supplemented with exogenous CAT have lower oxidative stress, which might be related to the increased activities of intestinal CAT and SOD. CAT and SOD are endogenous to important antioxidative enzymes, playing important roles in preventing oxidative damage. SOD can convert ROSs into H2O2, then CAT can degrade the H2O2 to water and oxygen [55]. The results in the mice also showed that diets enriched with exogenous CAT could increase intestinal antioxidative enzyme activities such as CAT, SOD, and GSH-Px, while alleviating the intestinal oxidative stress induced by a high-fat diet [23]. Therefore, exogenous CAT could improve intestinal development by enhancing intestinal antioxidative capacity.
Gut microbiota plays a vital role in host health by providing nutrients, modulating gastrointestinal development, shaping the immune system, and competitively inhibiting pathogens [56,57], while the gastrointestinal tract offers a physical environment for microorganisms [28]. The intestinal mucosa is a particularly dynamic environment where the host constantly interacts with trillions of commensal microorganisms, and periodically interacts with pathogens of diverse natures [58]. A mature mucosa barrier serves as a primary innate defense against pathogens, and an increased abundance of harmful bacteria will destroy the dynamic environment and result in intestinal barrier dysfunction [44]. Oxidative stress will modify the intestinal barrier function and finally contribute to the pathogenesis of gut inflammation and microbial flora disorders [7,10]. A previous study showed that dietary exogenous CAT supplementation could induce changes in the intestinal microflora of mice [23]. Similarly, exogenous CAT supplementation significantly changed the structures of gut microbiota in weaned piglets in the current study. Moreover, exogenous CAT increased the relative abundance of Bifidobacterium and Dialister, and decreased the relative abundance of Streptococcus and Escherichia-Shigella. Bifidobacterium, considered to be a probiotic bacteria, could decrease inflammation by inhibiting the growth of pathogens via the production of organic acids, and could release soluble factors that suppress the secretion of pro-inflammatory cytokines by immune cells [59,60]. A decreased pH value and harmful bacteria (Streptococcus and Escherichia-Shigella) were found in the colonic digesta of piglets fed the CAT diet in the present study. Wang et al. [61] have also reported that supplementation of Bifidobacteria improves gut barrier function by reducing the damage of the intestinal mucosa. Moreover, the abundance of Bifidobacterium was positive to antioxidative capacity [62]. Members of the Dialister genus are asaccharolytic obligately anaerobic gram-negative coccobacilli, and negatively associated with pro-inflammatory cytokine response [63,64]. Streptococcus and Escherichia-Shigella are both pathogenic bacteria. Higher abundance of Streptococcus is related to numerous inflammatory response [65], and Escherichia-Shigella is prevalent in patients with inflammatory bowel disease [66]. This indicates that dietary exogenous CAT supplementation increases the abundance of beneficial bacteria and decreases the abundance of harmful bacteria. The results of predicted functions are associated with changes in gut microbiota. The intestinal microflora is dominated by diverse anaerobes, providing both a health benefit to the host and a barrier to infection [67,68,69]. Escherichia-Shigella, a facultative anaerobe, can use nitrate as a terminal electron acceptor for anaerobic respiration [70], while Bifidobacterium and Dialister are anaerobic bacteria. It was reported that the inflammatory host response selectively enhances the growth of commensal Enterobacteriaceae by generating electron acceptors for anaerobic respiration [71]. In our study, decreased predicted functions related to aerobic respiration, such as “Aerobic_chemoheterotrophay”, “Nitrate_respiration”, and “Fumarate_respiration”, were observed in the piglets fed the CAT diet. The results suggest that dietary exogenous CAT could promote a proliferation of beneficial bacteria and inhibit the proliferation of harmful bacteria.
5. Conclusions
In conclusion, the present study indicated that a diet supplemented with exogenous CAT from Penicillium notatum shows beneficial effects in improving intestinal development and function, and in increasing the abundance of beneficial bacteria in the colons of weaned piglets. These findings will be helpful in enhancing our understanding of the mechanisms of dietary exogenous CAT in modulating gut health, and will provide support for the application of CAT purified from microbial cultures in the feed industry.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/3/391/s1, Table S1: Ingredients composition and nutrient levels of basal diets (as-fed basis); Table S2: Effects of dietary exogenous catalase supplementation on the relative abundance of colonic microbiota at the genus level; Table S3: Effects of dietary exogenous catalase supplementation on the colonic microbiota functions.
Author Contributions
Conceptualization, Y.L., X.Z. (Xilun Zhao), and D.W.; Data curation, Y.L. (Yang Li), X.Z. (Xilun Zhao), and L.Z.; Formal analysis, Y.L. (Yang Li), X.Z. (Xilun Zhao), and L.Z.; Funding acquisition, Z.L. and D.W.; Investigation, L.Z., X.Z. (Xiaoyan Zhan), and D.W.; Methodology, Y.L. (Yang Li), X.Z. (Xilun Zhao), L.Z., L.C., and D.W.; Project administration, Y.L. (Yang Li), X.Z. (Xilun Zhao), and L.Z.; Resources, Z.L. and D.W.; Software, Y.L. (Yang Li); Supervision, Y.Z., Y.L. (Yan Lin), Z.F., L.C., B.F., S.X., J.L., and D.W.; Validation, D.W.; Visualization, Y.L. (Yang Li), X.Z. (Xilun Zhao), L.Z., X.Z. (Xiaoyan Zhan), and B.F.; Writing—original draft, Y.L. (Yang Li); Writing—review & editing, Y.Z., Y.L., and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Sichuan Province “135” Breeding Tackle Project [NO. 2016NYZ0052].
Acknowledgments
We are grateful for the support and provision of exogenous catalase by Vetland Bio-Technology Co., Ltd., China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berkes, J.; Viswanathan, V.K.; Savkovic, S.D.; Hecht, G. Intestinal epithelial responses to enteric pathogens: Effects on the tight junction barrier, ion transport, and inflammation. Gut 2003, 52, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechn. 2013, 4, 19. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning induced hepatic oxidative stress; apoptosis; and aminotransferases through mapk signaling pathways in piglets. Oxid. Med. Cell Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nie, Y.; Chen, J.; Zhang, Y.; Wang, Z.; Fan, Q.; Yan, X. Gradual changes of gut microbiota in weaned miniature piglets. Front. Microbiol. 2016, 7, 1727. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Ding, Y.; Le, G.; Yonghui, S. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl. Microbiol. Biotechn. 2013, 97, 1689–1697. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581. [Google Scholar] [CrossRef]
- Chen, J.; Xie, H.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Luo, Y.; Luo, J.; He, J. Chlorogenic acid improves intestinal development via suppressing mucosa inflammation and cell apoptosis in weaned pigs. Acs Omega 2018, 3, 2211–2219. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Yu, B.; Chen, D.; Mao, X.; Zheng, P.; Luo, J.; He, J. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 2018, 96, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease, an epiphenomenon or the cause? Digest. Dis. Sci. 2007, 52, 2015. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.J.; Vass, E. Hydroxyl radical formation via iron-mediated Fenton chemistry is inhibited by methylated catechols. BBA-Gen. Subjects 1998, 1425, 159–167. [Google Scholar] [CrossRef]
- Zamocky, M.; Furtmüller, P.G.; Obinger, C. Evolution of catalases from bacteria to humans. Antioxid. Redox Sign. 2008, 10, 1527–1548. [Google Scholar] [CrossRef]
- Schrader, M.; Fahimi, H.D. Peroxisomes and oxidative stress. BBA-Mol. Cell Res. 2006, 1763, 1755–1766. [Google Scholar] [CrossRef]
- Kono, Y.; Fridovich, I. Superoxide radical inhibits catalase. J. Biol. Chem. 1982, 257, 5751–5754. [Google Scholar]
- Nakamura, K.; Watanabe, M.; Sasaki, Y.; Ikeda, T. Purification and characterization of liver catalase in acatalasemic beagle dog, comparison with normal dog liver catalase. Int. J. Biochem. Cell B. 2000, 32, 89–98. [Google Scholar] [CrossRef]
- Yumoto, I.; Ichihashi, D.; Iwata, H.; Istokovics, A.; Ichise, N.; Matsuyama, H.; Okuyama, H.; Kawasaki, K. Purification and characterization of a catalase from the facultatively psychrophilic bacterium Vibrio rumoiensis S-1T exhibiting high catalase activity. J. Bacteriol. 2000, 182, 1903. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.M.; Russell, W.J.; Veal, C.F. Endogenous and exogenous catalase in reoxygenation lung injury. J. Appl. Physiol. 1992, 72, 858–864. [Google Scholar] [CrossRef]
- Chu, H.D.; Leeder, J.G.; Gilbert, S.G. Immobilized catalase reactor for use in peroxide sterilization of dairy products. J. Food Sci. 1975, 40, 641–643. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Miyazaki, Y.; Kudo, Y.; Fukunaga, K.; Nakao, K. Glucose oxidation catalyzed by liposomal glucose oxidase in the presence of catalase-containing liposomes. Biotechnol. Progr. 2006, 22, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Paar, A.; Costa, S.; Tzanov, T.M.; Robra, K.H.; Cavaco-Paulo, A. Thermo-alkali-stable catalases from newly isolated Bacillus sp for the treatment and recycling of textile bleaching effluents. J. Biotechnol. 2001, 89, 147–153. [Google Scholar] [CrossRef]
- Wang, H. Effects of Exogenous Catalase on Tissue Antioxidant Capacity and Intestinal Microflora. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2016. [Google Scholar]
- Li, Y.; Zhao, X.; Jiang, X.; Chen, L.; Hong, L.; Zhuo, Y.; Lin, Y.; Fang, Z.; Che, L.; Feng, B.; et al. Effects of dietary supplementation with exogenous catalase on growth performance, oxidative stress and hepatic apoptosis in weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. (accepted). [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Lin, Y.; Fang, Z.F.; Che, L.Q.; Xu, S.Y.; Wu, D. Effects of replacing soybean meal with detoxified Jatropha curcas kernel meal in the diet on growth performance and histopathological parameters of growing pigs. Anim. Feed Sci. Tech. 2015, 204, 18–27. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Lin, Y.; Fang, Z.F.; Che, L.Q.; Xu, S.Y.; Wu, D. Substitution of soybean meal with detoxified Jatropha curcas kernel meal, Effects on performance; nutrient utilization; and meat edibility of growing pigs. Asian Austral. J. Anim. 2018, 31, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Liu, H.; Yang, Y.; He, J.; Cao, M.; Yang, M.; Zhong, W.; Lin, Y.; Zhuo, Y.; et al. Effects of the ratio of insoluble fiber to soluble fiber in gestation diets on sow performance and offspring intestinal development. Animals 2019, 9, 422. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Zheng, P.; Luo, Y.; Huang, Z.; Luo, J.; Mao, X.; Yu, J.; He, J. Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation. Appl. Microbiol. Biot. 2019, 1, 12. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zhang, L.; Yang, Y.; Lin, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Feng, B.; Xu, S.; et al. Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int. J. Mol. Sci. 2020, 21, 31. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH, fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE, highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes; a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac, a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Cao, M.; Che, L.; Wang, J.; Yang, M.; Su, G.; Fang, Z.; Lin, Y.; Xu, S.; Wu, D. Effects of maternal over-and undernutrition on intestinal morphology; enzyme activity, and gene expression of nutrient transporters in newborn and weaned pigs. Nutrition 2014, 30, 1442–1447. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. e21. [Google Scholar] [CrossRef]
- Heo, J.; Opapeju, F.; Pluske, J.; Kim, J.; Hampson, D.; Nyachoti, C. Gastrointestinal health and function in weaned pigs, a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. An. N. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Sakata, Y.; Tso, P. Nutrient-induced inflammation in the intestine. Curr. Opin. Clin. Nutr. 2011, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Nieto, N.; Torres, M.; Fernandez, M.; Giron, M.; Rios, A.; Suárez, M.D.; Gil, A. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Digest. Dis. Sci. 2000, 45, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Polk, W.H.; Awad, J.A.; Arteaga, C.L.; Nanney, L.B.; Wargovich, M.J.; Kraus, E.R.; Boland, C.R.; Coffey, R.J. Transforming growth factor alpha protection against drug-induced injury to the rat gastric mucosa in vivo. Eur. J. Clin. Invest. 1992, 90, 2409–2421. [Google Scholar] [CrossRef] [PubMed]
- McCracken, B.A.; Spurlock, M.E.; Roos, M.A.; Zuckermann, F.A.; Gaskins, H.R. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J. Nutr. 1999, 129, 613–619. [Google Scholar] [CrossRef]
- Gitto, E.; Romeo, C.; Reiter, R.J.; Impellizzeri, P.; Pesce, S.; Basile, M.; Antonuccio, P.; Trimarchi, G.; Gentile, C.; Barberi, I. Melatonin reduces oxidative stress in surgical neonates. J. Pediatr. Surg. 2004, 39, 184–189. [Google Scholar] [CrossRef]
- Van Antwerp, D.J.; Martin, S.J.; Kafri, T.; Green, D.R.; Verma, I.M. Suppression of TNF-α-induced apoptosis by NF-κB. Science 1996, 274, 787–789. [Google Scholar] [CrossRef]
- Raeburn, C.D.; Sheppard, F.; Barsness, K.A.; Arya, J.; Harken, A.H. Cytokines for surgeons. Am. J. Surg. 2002, 183, 268–273. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6, regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Beringer, A.; Noack, M.; Miossec, P. IL-17 in chronic inflammation: From discovery to targeting. Trends Mol. Med. 2016, 22, 230–241. [Google Scholar] [CrossRef]
- Ushida, K.; Kameue, C.; Tsukahara, T.; Fukuta, K.; Nakanishi, N. Decreasing traits of fecal immunoglobulin A in neonatal and weaning piglets. J. Vet. Med. Sci. 2008, 70, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Cara, D.; Fretez, S.; Cunha, F.; Vieira, E.; Nicoli, J.; Vieira, L. Saccharomyces boulardii stimulates sIgA production and the phagocytic system of gnotobiotic mice. J. Appl. Microbiol. 2000, 89, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, C.; Chen, X.; Cai, X.; Yang, S.; Sheng, Y.; Wang, T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 2014, 30, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds, evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008, 6, 776. [Google Scholar] [CrossRef] [PubMed]
- Camara-Lemarroy, C.R.; Metz, L.M.; Yong, V.W. Focus on the gut-brain axis, multiple sclerosis; the intestinal barrier and the microbiome. World J. Gastroentero. 2018, 24, 4217. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135. [Google Scholar] [CrossRef]
- Sinclair, N.; Stokes, J. Factors which control maximal growth of bacteria. J. Bacteriol. 1962, 83, 1147–1154. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Kamiya, S.; Hanawa, T.; Fukuda, M.; Kawakami, H.; Kamiya, S.; Hanawa, T. Effect of probiotic bacterial strains of Lactobacillus, Bifidobacterium, and Enterococcus on enteroaggregative Escherichia coli. J. Infect Chemother. 2010, 16, 10–18. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, G.; Yao, Y.; Guo, S.; Lu, K.; Sheng, Z. The role of Bifidobacteria in gut barrier function after thermal injury in rats. J. Trauma Acute Care 2006, 61, 650–657. [Google Scholar] [CrossRef]
- Awney, H.A. The effects of Bifidobacteria on the lipid profile and oxidative stress biomarkers of male rats fed thermally oxidized soybean oil. Biomarkers 2011, 16, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Tito, R.Y.; Cypers, H.; Joossens, M.; Varkas, G.; Van Praet, L.; Glorieus, E.; Van den Bosch, F.; De Vos, M.; Raes, J.; Elewaut, D. Brief report: Dialister as a microbial marker of disease activity in spondyloarthritis. Arthritis Rheumatol. 2017, 69, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Hantsoo, L.; Jašarević, E.; Criniti, S.; McGeehan, B.; Tanes, C.; Sammel, M.D.; Elovitz, M.A.; Compher, C.; Wu, G.; Epperson, C.N. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain Behav. Immu 2019, 75, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Tsatsaronis, J.A.; Walker, M.J.; Sanderson-Smith, M.L. Host responses to group a streptococcus, cell death and inflammation. PLoS Pathog. 2014, 10, e1004266. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, W.; Zhou, R.; Ng, S.C.; Li, J.; Huang, M.; Zhou, F.; Wang, X.; Shen., B.; Kamm, M.A.; et al. Characteristics of fecal and mucosa-associated microbiota in Chinese patients with inflammatory bowel disease. Medicine 2014, 93. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.V. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Freter, R. Mechanisms that control the microflora in the large intestine. In Human Intestinal Microflora in Health and Disease; Hentges, D.J., Ed.; Academic Press: Cambridge, MA, USA, 1983; pp. 33–54. [Google Scholar]
- Hentges, D.J. Role of the intestinal microflora in host defense against infection. In Human Intestinal Microflora in Health Disease; Hentges, D.J., Ed.; Academic Press: Cambridge, MA, USA, 1983; pp. 311–331. [Google Scholar]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother–neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Xavier, M.N.; Thiennimitr, P.; Poon, V.; Keestra, A.M.; Laughlin, R.C.; Gomez, G.; Wu, J.; Lawhon, S.D.; et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013, 339, 708–711. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).