Physiological and Proteomic Approaches to Address the Active Role of Botrytis cinerea Inoculation in Tomato Postharvest Ripening
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Culture
2.2. Plant Material and Experimental Design
2.3. Quality and Ripening-Related Attributes
2.4. Metadata Analysis of Proteomic Study in B. Cinerea Inoculated Tomatoes
2.5. Statistical Analysis
3. Results
3.1. Impact of B. cinerea on Qualitative and Antioxidant Characteristics
3.2. Impact of B. cinerea on Protein Composition of Tomato Fruit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, S.; Elad, Y. Botrytis—The Fungus, the Pathogen and its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2016; p. 488. [Google Scholar]
- Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N. Botrytis spp. and diseases they cause in agricultural systems—an introduction. In Botrytis: Biology, Pathology and Control; Springer: Dordrecht, The Netherlands, 2007; pp. 1–8. [Google Scholar]
- Tournas, V.H. Spoilage of vegetable crops by bacteria and fungi and related health hazards. Crit. Rev. Microbiol. 2005, 31, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; p. 952. [Google Scholar]
- Rama, M.V.; Narasimham, P. Potatoes and related crops. Fruits of the Solanaceae. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4658–4666. [Google Scholar]
- Tzortzakis, N.; Borland, A.; Singleton, I.; Barnes, J. Impact of atmospheric ozone-enrichment on quality-related attributes of tomato fruit. Postharvest Biol. Technol. 2007, 45, 317–325. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E. Botrytis cinerea (gray mold). In Postharvest Decay. Control Strategies; Bautista-Banos, S., Ed.; Elsevier: London, UK, 2014; p. 394. [Google Scholar]
- Tuffi, R.; Lovino, R.; Canese, S.; Cafiero, L.M.; Vitali, F. Effects of exposure to gaseous ozone and negative air ions on control of epiphytic flora and the development of Botrytis cinerea and Penicillium expansum during cold storage of strawberries and tomatoes. Ital. J. Food Sci. 2012, 24, 102–114. [Google Scholar]
- Tzortzakis, N.; Singleton, I.; Barnes, J. Deployment of low-level ozone-enrichment for the preservation of chilled fresh produce. Postharvest Biol. Technol. 2007, 43, 261–270. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Taybi, T.; Roberts, R.; Singleton, I.; Borland, A.; Barnes, J. Low-level atmospheric ozone exposure induces protection against Botrytis cinerea with down-regulation of ethylene-, jasmonate- and pathogenesis-related genes in tomato fruit. Postharvest Biol. Technol. 2011, 61, 152–159. [Google Scholar] [CrossRef]
- Plotto, A.; Roberts, D.D.; Roberts, R.G. Evaluation of plant essential oils as natural postharvest disease control of tomato (Lycopersicon esculentum). Acta Hort. 2003, 628, 737–745. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Essential oil: innovative tool to improve the preservation of fresh produce—A review. Fresh Prod. 2009, 3, 87–97. [Google Scholar]
- Guerra, I.C.D.; de Oliveira, P.D.L.; de Souza Pontes, A.L.; Lúcio, A.S.S.C.; Tavares, J.F.; Barbosa-Filho, J.M.; Madruga, M.S.; de Souza, E.L. Coatings comprising chitosan and Mentha piperita L. or Mentha×villosa Huds essential oils to prevent common postharvest mold infections and maintain the quality of cherry tomato fruit. Int. J. Food Microbiol. 2015, 214, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Sholberg, P.L.; Gaunce, A.P. Fumigation of fruit with acetic acid to prevent postharvest decay. HortScience 1995, 30, 1271–1275. [Google Scholar] [CrossRef]
- Charles, M.T.; Mercier, J.; Makhlouf, J.; Arul, J. Physiological basis of UV-C-induced resistance to Botrytis cinerea in tomato fruit. I. Role of pre- and post-challenge accumulation of the phytoalexin-rishitin. Postharvest Biol. Technol. 2008, 47, 10–20. [Google Scholar] [CrossRef]
- Fallik, E.; Ilic, Z.; Alkalai-Tuvia, S.; Copel, A.; Polevaya, Y. A short hot water rinsing and brushing reduces chilling injury and enhances resistance against Botrytis cinerea in fresh harvested tomato. Adv. Hortic. Sci. 2002, 16, 3–6. [Google Scholar]
- Seymour, G.B.; Granell, A. Fruit development and ripening. J. Exp. Bot. 2014, 65, 4489–4490. [Google Scholar] [CrossRef]
- Hückelhoven, R. Cell wall–associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 2007, 45, 101–127. [Google Scholar] [CrossRef]
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and necrotrophic lifestyle choice during postharvest disease development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef]
- Bargel, H.; Neinhuis, C. Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J. Exp. Bot. 2005, 56, 1049–1060. [Google Scholar] [CrossRef]
- Giovannoni, J. Molecular biology of fruit maturation and ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Vincenti, E.; Powell, A.L.T.; Cantu, D. Tomato transcriptome and mutant analyses suggest a role for plant stress hormones in the interaction between fruit and Botrytis cinerea. Front. Plant Sci. 2013, 4, 1–16. [Google Scholar] [CrossRef]
- Alkan, N.; Friedlander, G.; Ment, D.; Prusky, D.; Fluhr, R. Simultaneous transcriptome analysis of Colletotrichum gloeosporioides and tomato fruit pathosystem reveals novel fungal pathogenicity and fruit defense strategies. New Phytol. 2015, 205, 801–815. [Google Scholar] [CrossRef]
- Cantu, D.; Blanco-Ulate, B.; Yang, L.; Labavitch, J.M.; Bennett, A.B.; Powell, A.L.T. Ripening-regulated susceptibility of tomato fruit to Botrytis cinerea requires NOR but not RIN or Ethylene. Plant Physiol. 2009, 150, 1434–1449. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Vincenti, E.; Cantu, D.; Powell, A.L.T. Ripening of tomato fruit and susceptibility to Botrytis cinerea. In Botrytis—The Fungus, the Pathogen and its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2016; pp. 387–412. [Google Scholar]
- Shah, P.; Powell, A.L.T.; Orlando, R.; Bergmann, C.; Gutierrez-Sanchez, G. Proteomic analysis of ripening tomato fruit infected by Botrytis cinerea. J. Proteome Res. 2012, 11, 2178–2192. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Taybi, T.; Antony, E.; Singleton, I.; Borland, A.; Barnes, J. Profiling shifts in protein complement in tomato fruit induced by atmospheric ozone-enrichment and/or wound-inoculation with Botrytis cinerea. Postharvest Biol. Technol. 2013, 78, 67–75. [Google Scholar] [CrossRef]
- Curto, M.; Camafeita, E.; Lopez, J.A.; Maldonado, A.M.; Rubiales, D.; Jorrín, J.V. A proteomic approach to study pea (Pisum sativum) responses to powdery mildew (Erysiphe pisi). Proteomics 2006, 6, 163–174. [Google Scholar] [CrossRef]
- Kaffamik, F.A.R.; Jones, A.M.E.; Rathjen, J.P.; Peck, S.C. Effector proteins of the bacterial pathogen Pseudomonas syringae alter the extracellular proteome of the host plant, Arabidopsis thaliana. Mol. Cell. Proteom. 2009, 8, 145–156. [Google Scholar] [CrossRef]
- Andrade, A.E.; Silva, L.P.; Pereira, J.L.; Noronha, E.F.; Reis, F.B.; Bloch, C.; Dos Santos, M.F.; Domont, G.B.; Franco, O.L.; Mehta, A. In vivo proteome analysis of Xanthomonas campestris pv. campestris in the interaction with the host plant Brassica oleracea. Fems Microbiol. Lett. 2008, 281, 167–174. [Google Scholar]
- Fernández-Acero, F.J.; Jorge, I.; Calvo, E.; Vallejo, I.; Carbú, M.; Camafeita, E.; López, J.A.; Cantoral, J.M.; Jorrín, J. Two-dimensional electrophoresis protein profile of the phytopathogenic fungus Botrytis cinerea. Proteomics 2006, 6, 88–96. [Google Scholar] [CrossRef]
- Fernández-Acero, F.J.; Colby, T.; Harzen, A.; Cantoral, J.M.; Schmidt, J. Proteomic analysis of the phytopathogenic fungus Botrytis cinerea during cellulose degradation. Proteomics 2009, 9, 2892–2902. [Google Scholar] [CrossRef]
- Shah, P.; Atwood, J.A.; Orlando, R.; Mubarek, H.E.; Podila, G.K.; Davis, M.R. Comparative proteomic analysis of Botrytis cinerea secretome. J. Proteome Res. 2009, 8, 1123–1130. [Google Scholar] [CrossRef]
- Rep, M.; Dekker, H.L.; Vossen, J.H.; De Boer, A.D.; Houterman, P.M.; Speijer, D.; Back, J.W.; De Koster, C.G.; Cornelissen, B.J.C. Mass spectrometric identification of isoforms of PR proteins in xylem sap of fungus-infected tomato. Plant Physiol. 2002, 130, 904–917. [Google Scholar] [CrossRef]
- Dahal, D.; Heintz, D.; Van Dorsselaer, A.; Braun, H.P.; Wydra, K. Pathogenesis and stress related, as well as metabolic proteins are regulated in tomato stems infected with Ralstonia solanacearum. Plant Physiol. Biochem. 2009, 47, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Corpillo, D.; Gardini, G.; Vaira, A.M.; Basso, M.; Aime, S.; Accotto, G.P.; Fasano, M. Proteomics as a tool to improve investigation of substantial equivalence in genetically modified organisms: The case of a virus-resistant tomato. Proteomics 2004, 4, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Casado-Vela, J.; Sellés, S.; Martínez, R.B. Proteomic analysis of tobacco mosaic virus-infected tomato (Lycopersicon esculentum M.) fruits and detection of viral coat protein. Proteomics 2006, 6 (Suppl 1), S196–S206. [Google Scholar] [CrossRef]
- Chen, S.; Gollop, N.; Heuer, B. Proteomic analysis of salt-stressed tomato (Solanum lycopersicum) seedlings: Effect of genotype and exogenous application of glycinebetaine. J. Exp. Bot. 2009, 60, 2005–2019. [Google Scholar] [CrossRef]
- Hahn, A.; Bublak, D.; Schleiff, E.; Scharf, K.D. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell 2011, 23, 741–755. [Google Scholar] [CrossRef]
- Barsan, C.; Sanchez-Bel, P.; Rombaldi, C.; Egea, I.; Rossignol, M.; Kuntz, M.; Zouine, M.; Latché, A.; Bouzayen, M.; Pech, J.C. Characteristics of the tomato chromoplast revealed by proteomic analysis. J. Exp. Bot. 2010, 61, 2413–2431. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Miner, D.P.; Larson, M.; McDowell, E.; Gang, D.R.; Wilkerson, C.; Last, R.L. Studies of a biochemical factory: Tomato trichome deep expressed sequence tag sequencing and proteomics. Plant Physiol. 2010, 153, 1212–1223. [Google Scholar] [CrossRef]
- Catalá, C.; Howe, K.J.; Hucko, S.; Rose, J.K.C.; Thannhauser, T.W. Towards characterization of the glycoproteome of tomato (Solanum lycopersicum) fruit using Concanavalin A lectin affinity chromatography and LC-MALDI-MS/MS analysis. Proteomics 2011, 11, 1530–1544. [Google Scholar] [CrossRef]
- Saravanan, R.S.; Rose, J.K.C. A critical evaluation of sample extraction techniques for enhanced proteomic analysis of recalcitrant plant tissues. Proteomics 2004, 4, 2522–2532. [Google Scholar] [CrossRef]
- Casado-Vela, J.; Sellés, S.; Martínez, R.B. Proteomic approach to blossom-end rot in tomato fruits (Lycopersicon esculentum M.): Antioxidant enzymes and the pentose phosphate pathway. Proteomics 2005, 5, 2488–2496. [Google Scholar] [CrossRef]
- Iwahashi, Y.; Hosoda, H. Effect of heat stress on tomato fruit protein expression. Electrophoresis 2000, 21, 1766–1771. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikou, A.; Tzortzakis, N. Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. New Zeal. J. Crop Hortic. Sci. 2016, 44, 203–217. [Google Scholar] [CrossRef]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaish 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Tzanakaki, K.; Conomakis, C.D. Effect of origanum oil and vinegar on the maintenance of postharvest quality of tomato. Food Nutr. Sci. 2011, 2, 974–982. [Google Scholar] [CrossRef][Green Version]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crop. Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Cantu, D.; Vicente, A.R.; Greve, L.C.; Dewey, F.M.; Bennett, A.B.; Labavitch, J.M.; Powell, A.L.T. The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proc. Natl. Acad. Sci. USA 2008, 105, 859–864. [Google Scholar] [CrossRef]
- Bui, T.T.A.; Wright, S.A.I.; Falk, A.B.; Vanwalleghem, T.; Van Hemelrijck, W.; Hertog, M.L.A.T.M.; Keulemans, J.; Davey, M.W. Botrytis cinerea differentially induces postharvest antioxidant responses in ‘Braeburn’ and ‘Golden Delicious’ apple fruit. J. Sci. Food Agric. 2019, 99, 5662–5670. [Google Scholar] [CrossRef]
- Petrasch, S.; Silva, C.J.; Mesquida-Pesci, S.D.; Gallegos, K.; van den Abeele, C.; Papin, V.; Fernandez-Acero, F.J.; Knapp, S.J.; Blanco-Ulate, B. Infection strategies deployed by Botrytis cinerea, Fusarium acuminatum, and Rhizopus stolonifer as a function of tomato fruit ripening stage. Front. Plant Sci. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, U.; Viefhues, A. Reactive oxygen species in the botrytis—Host interaction. In Botrytis: The fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 269–289. [Google Scholar]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.K.; Anderson, M.D.; Martin, B.A.; Stewart, C.R. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 1994, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yahraus, T.; Chandra, S.; Legendre, L.; Low, P.S. Evidence for a mechanically induced oxidative burst. Plant Physiol. 1995, 109, 1259–1266. [Google Scholar] [CrossRef]
- Mittler, R.; Zilinskas, B.A. Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J. Biol. Chem. 1992, 267, 21802–21807. [Google Scholar]
- McGonigle, B.; Keeler, S.J.; Lau, S.M.C.; Koeppe, M.K.; O’Keefe, D.P. A genomics approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol. 2000, 124, 1105–1120. [Google Scholar] [CrossRef]
- Ranford, J.C.; Coates, A.R.M.; Henderson, B. Chaperonins are cell-signalling proteins: The unfolding biology of molecular chaperones. Expert Rev. Mol. Med. 2000, 2, 1–17. [Google Scholar] [CrossRef]
- Hara, M.; Fujinaga, M.; Kuboi, T. Metal binding by citrus dehydrin with histidine-rich domains. J. Exp. Bot. 2005, 56, 2695–2703. [Google Scholar] [CrossRef]
- Moyano, E.; Portero-Robles, I.; Medina-Escobar, N.; Valpuesta, V.; Muñoz-Blanco, J.; Caballero, J.L. A fruit-specific putative dihydroflavonol 4-reductase gene is differentially expressed in strawberry during the ripening process. Plant Physiol. 1998, 117, 711–716. [Google Scholar] [CrossRef][Green Version]
- Hensel, M.; Holden, D.W. Molecular genetic approaches for the study of virulence in both pathogenic bacteria and fungi. Microbiology 1996, 142, 1049–1058. [Google Scholar] [CrossRef]
- Konishi, H.; Ishiguro, K.; Komatsu, S. A proteomics approach towards understanding blast fungus infection of rice grown under different levels of nitrogen fertilization. Proteomics 2001, 1, 1162–1171. [Google Scholar] [CrossRef]
- Chan, Z.; Qin, G.; Xu, X.; Li, B.; Tian, S. Proteome approach to characterize proteins induced by antagonist yeast and salicylic acid in peach fruit. J. Proteome Res. 2007, 6, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Chan, Z.; Wang, Q.; Xu, X.; Meng, X.; Qin, G.; Li, B.; Tian, S. Functions of defense-related proteins and dehydrogenases in resistance response induced by salicylic acid in sweet cherry fruits at different maturity stages. Proteomics 2008, 8, 4791–4807. [Google Scholar] [CrossRef]
- Mehta, A.; Rosato, Y.B. Differentially expressed proteins in the interaction of Xanthomonas axonopodis pv. citri with leaf extract of the host plant. Proteomics 2001, 1, 1111–1118. [Google Scholar] [CrossRef]
- Tahara, S.T.; Mehta, A.; Rosato, Y.B. Proteins induced by Xanthomonas axonopodis pv. passiflorae with leaf extract of the host plant (Passifiorae edulis). Proteomics 2003, 3, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Smolka, M.B.; Martins, D.; Winck, F.V.; Santoro, C.E.; Castellari, R.R.; Ferrari, F.; Brum, I.J.; Galembeck, E.; Della Coletta Filho, H.; Machado, M.A.; et al. Proteome analysis of the plant pathogen Xylella fastidiosa reveals major cellular and extracellular proteins and a peculiar codon bias distribution. Proteomics 2003, 3, 224–237. [Google Scholar] [CrossRef]

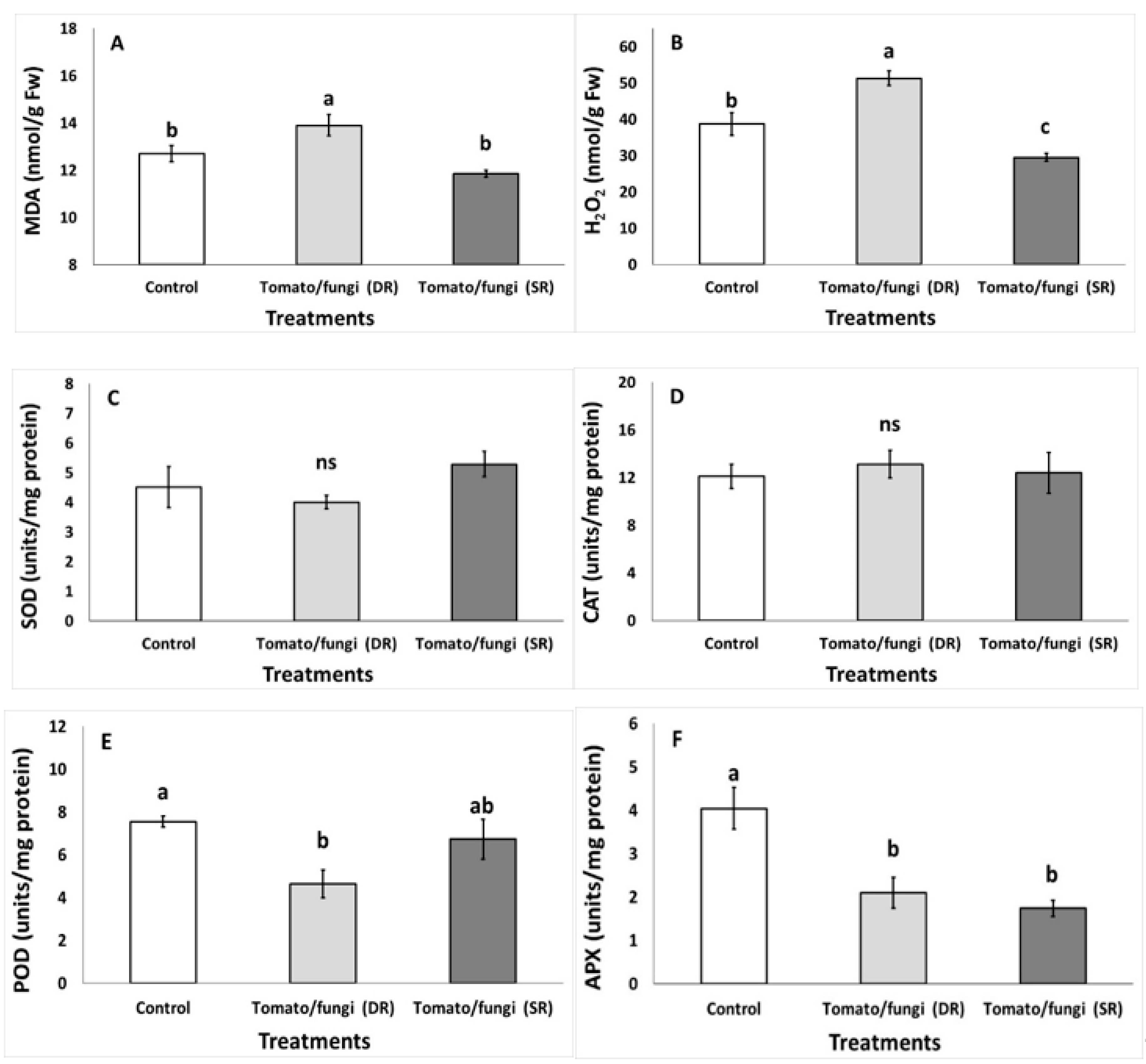
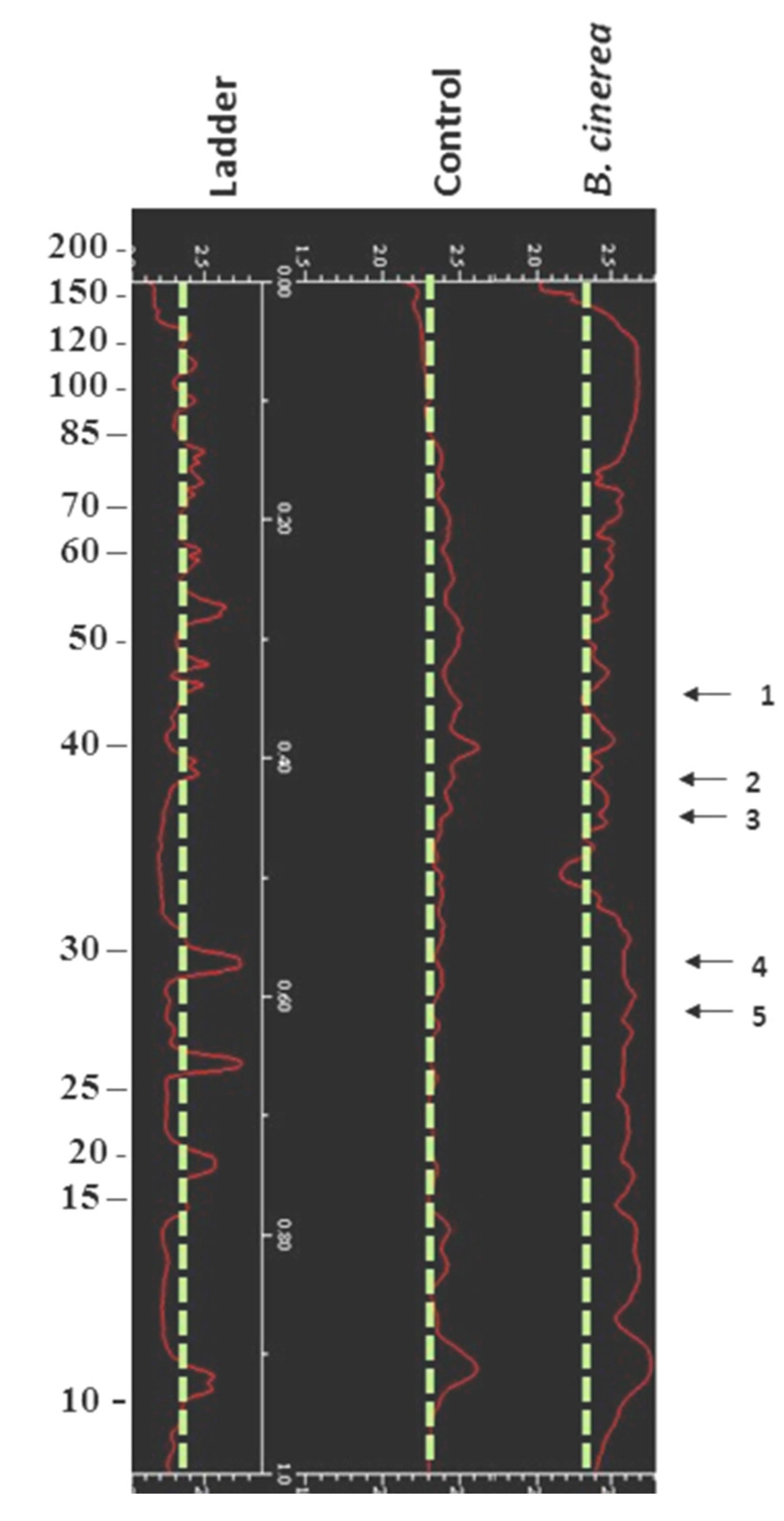

| Quality Parameters | Control | Tomato Tissue with Fungal Lesion (DR) | Tomato Tissue Without Fungal Lesion (SR) |
|---|---|---|---|
| Weight loss (%) | 0.335 ± 0.030 a | 0.259 ± 0.031 a | |
| Fruit firmness (N) | 11.08 ± 0.43 a | 9.37 ± 0.46 b | 10.99 ± 0.55 a |
| Total soluble solids (%) | 3.80 ± 0.20 a | 3.72 ± 0.18 a | 4.01 ± 0.02 a |
| Titratable acidity (citric acid %) | 1.76 ± 0.06 b | 1.60 ± 0.08 b | 2.01 ± 0.06 a |
| Ripening index (TSS/TA) | 2.15 ± 0.07 a | 2.34 ± 0.19 a | 1.98 ± 0.06 a |
| Colour L * | 40.98 ± 0.37 a | 40.48 ± 0.61 a | 40.56 ± 0.59 a |
| Colour a * | 23.44 ± 0.61 a | 24.63 ± 0.82 a | 24.15 ± 1.08 a |
| Colour b * | 25.60 ± 0.71 a | 26.73 ± 0.68 a | 25.32 ± 0.44 a |
| Chroma | 34.74 ± 0.73 a | 36.37 ± 0.95 a | 35.03 ± 0.99 a |
| Ascorbic acid (mg/g Fw) | 17.66 ± 1.16 b | 21.93 ± 1.77 a | 14.15 ± 1.00 b |
| Lycopene (nmol/g Fw) | 17.14 ± 2.69 a | 27.34 ± 3.80 a | 19.93 ± 2.95 a |
| β-carotene (nmol/g Fw) | 6.74 ± 1.02 b | 10.66 ± 0.49 a | 7.75 ± 0.93 ab |
| Phenols (mg GAE/g Fw) | 0.39 ± 0.031 a | 0.36 ± 0.043 a | 0.34 ± 0.019 a |
| FRAP (mg Trolox/g Fw) | 3.44 ± 0.12 b | 4.38 ± 0.31 a | 3.31 ± 0.20 b |
| DPPH (mg Trolox/g Fw) | 0.22 ± 0.00 a | 0.30 ± 0.04 a | 0.24 ± 0.02 a |
| ABTS (mg Trolox/g Fw) | 0.07 ± 0.01 a | 0.11 ± 0.01 a | 0.11 ± 0.00 a |
| Proteins Level | Control | B. cinerea infected Tomatoes |
|---|---|---|
| Protein yield (μg/g Fw) | 140.8 ± 5.31 b | 150.1 ± 4.98 a |
| Increased proteins by fungi | 29 ± 5 (9) | |
| Decreased proteins by fungi | 60 ± 4 (39) | |
| Novel by fungi | 0 ± 0 (0) | |
| Total | 89 ± 2 (48) |
| Spot No | Identification, Putative Function, Species/EC b) | Protein Expression |
|---|---|---|
| 1 | Superoxide dismutase (Cu-Zn), chloroplast precussor, Lycopersicon esculentum/1.15.1.1 | ↑ |
| 2 | Farnesyl pyrophosphate synthase, synthesis of farnesyl pyrophosphate, L. esculentum/2.5.1.1 | - |
| 3 | Ulp1 protease-like, Oryza sativa (japonica cultivar-group)/ | - |
| 4 | Dehydrin 2, Pisum sativum | - |
| 5 | Thioredoxin peroxidase, L. esculentum/1.11.1.- | - |
| 11 | Chaperonin 21 precursor, L. esculentum | - |
| 13 | Chaperonin 21 precursor, L. esculentum | ↑ |
| 15 | IN2-1 glutathione transferase, Arabidopsis thaliana/2.5.1.18 | - |
| 17 | Inorganic pyrophosphatase, A. thaliana/3.6.1.1 | ↑ |
| 19 | Putative NAD-dependent malate dehydrogenase, Solanum tuberosum/1.1.1.37 | ↑ |
| 20 | Putative NAD-dependent malate dehydrogenase, S. tuberosum/1.1.1.37 | ↑ |
| 21 | Putative NAD-dependent malate dehydrogenase, S. tuberosum/1.1.1.37 | - |
| 25 | Glyceraldehyde 3-phosphate dehydrogenase, L. esculentum/1.2.1.12 | - |
| 28 | Hypothetical protein, O. sativa japonica cultivar-group)/ | - |
| 29 | 1-Aminocyclopropane-1-carboxylate oxidase homolog (protein E8), L. esculentum/1.14.17.4 | ↑ |
| 31 | L-ascorbate peroxidase 1 cytosolic, A. thaliana | ↑ |
| 32 | dihydroflavonol-4-reductase, L. esculentum/1.1.1.219 | ↓ |
| 35 | Invertase, L. esculentum/3.2.1.26 | ↓ |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzortzakis, N. Physiological and Proteomic Approaches to Address the Active Role of Botrytis cinerea Inoculation in Tomato Postharvest Ripening. Microorganisms 2019, 7, 681. https://doi.org/10.3390/microorganisms7120681
Tzortzakis N. Physiological and Proteomic Approaches to Address the Active Role of Botrytis cinerea Inoculation in Tomato Postharvest Ripening. Microorganisms. 2019; 7(12):681. https://doi.org/10.3390/microorganisms7120681
Chicago/Turabian StyleTzortzakis, Nikolaos. 2019. "Physiological and Proteomic Approaches to Address the Active Role of Botrytis cinerea Inoculation in Tomato Postharvest Ripening" Microorganisms 7, no. 12: 681. https://doi.org/10.3390/microorganisms7120681
APA StyleTzortzakis, N. (2019). Physiological and Proteomic Approaches to Address the Active Role of Botrytis cinerea Inoculation in Tomato Postharvest Ripening. Microorganisms, 7(12), 681. https://doi.org/10.3390/microorganisms7120681





