Study on the Infection Mechanism of Penicillium Digitatum on Postharvest Citrus (Citrus Reticulata Blanco) Based on Transcriptomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogen
2.2. Fruit
2.3. Sample Preparation
2.4. RNA Extraction
2.5. High Throughput Sequencing of Transcriptome
2.6. Bioinformatic Analysis of RNA-Seq Data
2.7. Validation of RNA-Seq Data by qRT-PCR
3. Results
3.1. Overview of RNA-Seq Data
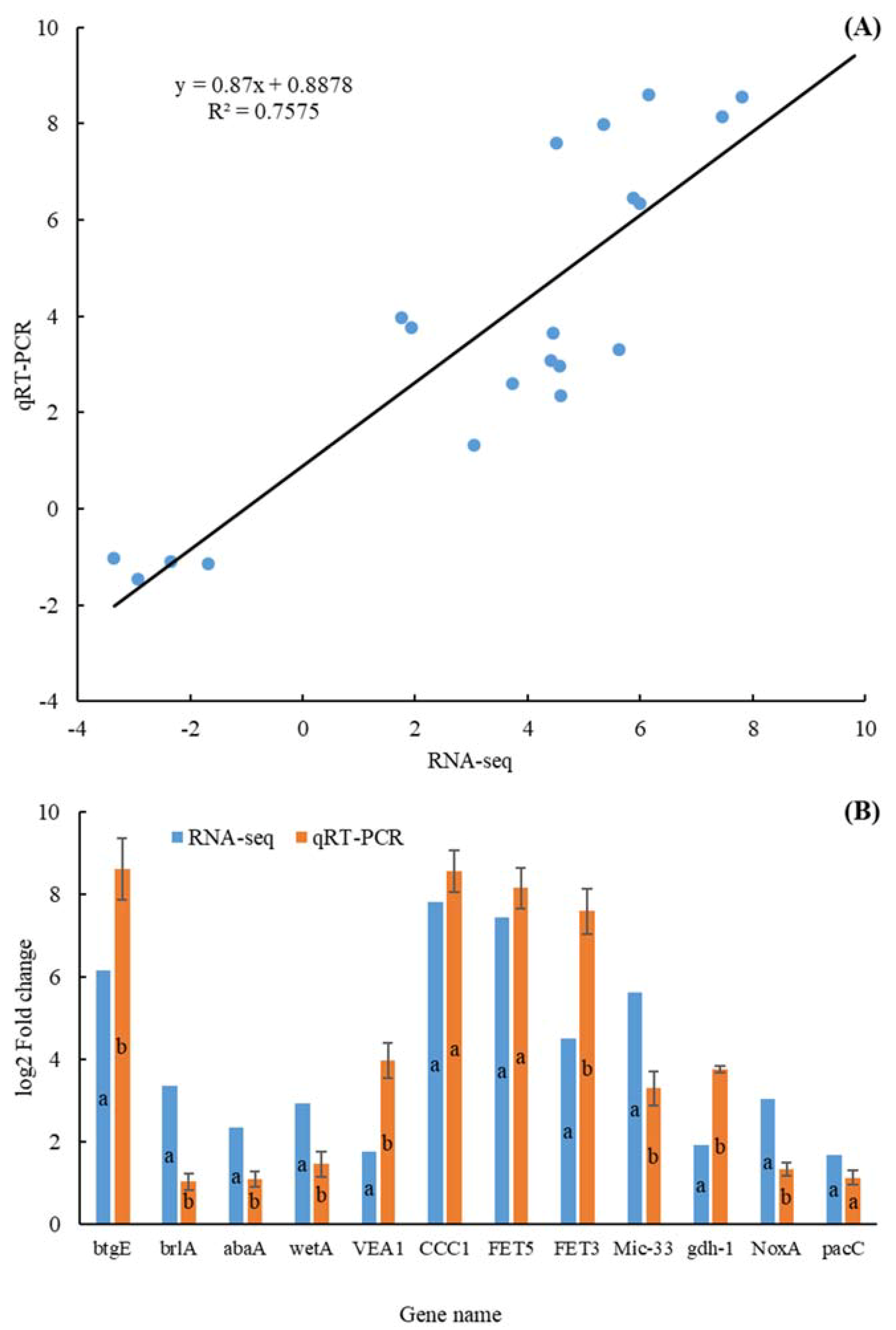
3.2. Validation of RNA-Seq Data by qRT-PCR
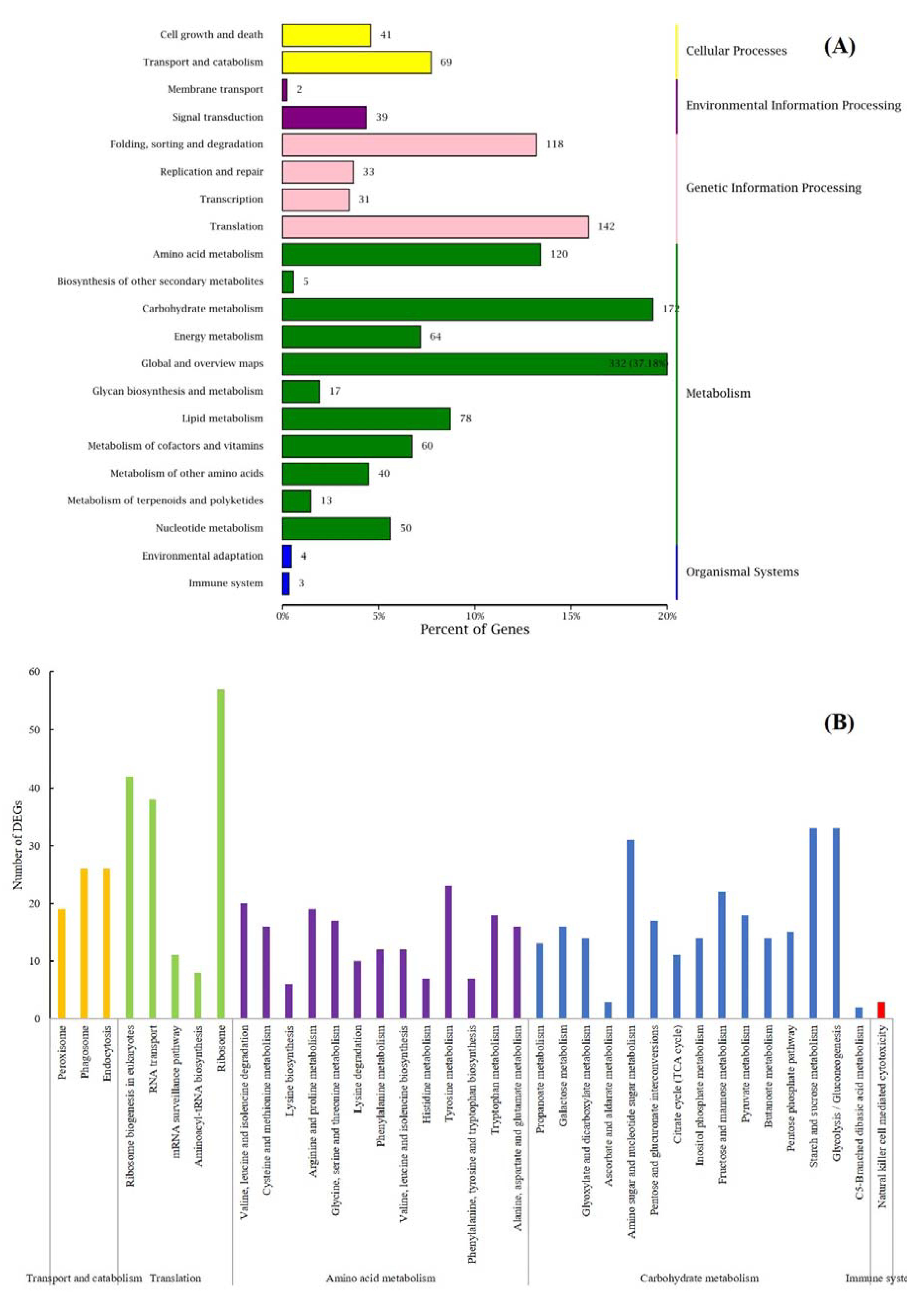
3.3. Clustering and Functional Enrichment of DEGs
3.4. Analysis of Key DEGs of P. Digitatum in Infection Process
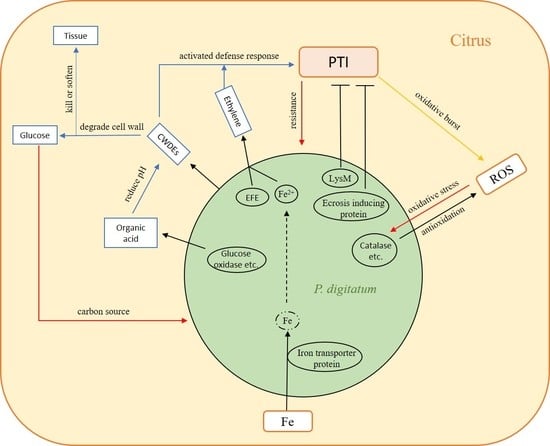
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Z.; Ma, Z.; Li, H. A molecular mechanism of azoxystrobin resistance in Penicillium digitatum UV mutants and a PCR-based assay for detection of azoxystrobin-resistant strains in packing- or store-house isolates. Int. J. Food Microbiol. 2009, 131, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Hibino, T. Tumorigenicity of citrinin in male F344 rats. Cancer Lett. 1983, 17, 281–287. [Google Scholar] [CrossRef]
- Zhu, J.; Xie, Q.; Li, H. Occurrence of imazalil-resistant biotype of Penicillium digitatum in China and the resistant molecular mechanism. J. Zhejiang Univ. Sci. A 2006, 7, 362–365. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Konstantinos, P.; Matthaios, M.M.; Joaquín, H.H.; Vasileios, Z. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar]
- Wang, M.; Sun, X.; Zhu, C.; Xu, Q.; Ruan, R.; Yu, D.; Li, H. PdbrlA, PdabaA and PdwetA control distinct stages of conidiogenesis in Penicillium digitatum. Res. Microbiol. 2015, 166, 56–65. [Google Scholar] [CrossRef]
- Ballester, A.R.; Marcet-Houben, M.; Levin, E.; Sela, N.; Selma-Lázaro, C.; Carmona, L.; Wisniewski, M.; Droby, S.; González-Candelas, L.; Gabaldón, T. Genome, transcriptome, and functional Analysis of Penicillium expansum provide new insights into secondary metabolism and pathogenicity. Mol. Plant Microbe Interact. 2015, 28, 232–248. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Luo, J.; Ye, B.; Zhou, Y.; Zhou, L.; Lai, T. Identification of differentially expressed genes involved in spore germination of Penicillium expansum by comparative transcriptome and proteome approaches. Microbiologyopen 2017, 7, e00562. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, X.; Zhang, X.; Zhao, L.; Yang, Q.; Boateng, N.A.S.; Ahima, J.; Zhang, H.; Liu, J. Comparative transcriptomic analysis of the interaction between Penicillium expansum and apple fruit (Malus pumila Mill.) during early stages of infection. Microorganisms 2019, 7, 495. [Google Scholar] [CrossRef]
- Prusky, D.; Lichter, A. Mechanisms modulating fungal attack in post-harvest pathogen interactions and their control. Eur. J. Plant Pathol. 2008, 121, 281–289. [Google Scholar] [CrossRef]
- Qian, X.; Yang, Q.; Zhang, Q.; Abdelhai, M.H.; Dhanasekaran, S.; Boateng, N.A.S.; Gu, N.; Zhang, H. Elucidation of the initial growth process and the infection mechanism of Penicillium digitatum on postharvest citrus (Citrus reticulata Blanco). Microorganisms 2019, 7, 485. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Darren, A.N.; Uma, T.S.; Michael, Y.G.; Yuri, I.W.; Aravind, L.; Eugene, V.K. Genome annotation using clusters of orthologous groups of proteins (COGs)—Towards understanding the first genome of a Crenarchaeon. Genome Biol. 2000, 1, research0009.1–research0009.19. [Google Scholar]
- Altermann, E.; Klaenhammer, T.R. PathwayVoyager: Pathway mapping using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. BMC Genom. 2005, 6, 60. [Google Scholar] [CrossRef]
- Pasquier, C.; Promponas, V.J.; Palaios, G.A.; Hamodrakas, J.S.; Hamodrakas, S.J. A novel method for predicting transmembrane segments in proteins based on a statistical analysis of the SwissProt database: The PRED-TMR algorithm. Protein Eng. Des. Sel. 1999, 12, 381–385. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Rodney, B.J.; Stacy, C.; Diana, H.; Rich, M.V.; Bhanu, R.; Barbara, R.; Brian, S.W.; Danso, A.A. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Zhu, P.; Xu, Z.; Cui, Z.; Zhang, Z.; Xu, L. Ethylene production by Alternaria alternata and its association with virulence on inoculated grape berries. Phytoparasitica 2017, 45, 273–279. [Google Scholar] [CrossRef]
- Bayram, O.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.J.; Keller, N.P.; Yu, J.H.; et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Abouna, S.; Legendre, L.; Manteau, S.; Lambert, B. Differential regulation by ambient pH of putative virulence factor secretion by the phytopathogenic fungus Botrytis cinerea. FEMS Microbiol. Ecol. 2003, 43, 359–366. [Google Scholar]
- Vilanova, L.; Viñas, I.; Torres, R.; Usall, J.; Buron-Moles, G.; Teixidó, N. Acidification of apple and orange hosts by Penicillium digitatum and Penicillium expansum. Int. J. Food Microbiol. 2014, 178, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Arenas, Y.C.; Kalkman, E.R.I.C.; Schouten, A.; Dieho, M.; Vredenbregt, P.; Uwumukiza, B.; Ruiz, M.O.; Van Kan, J.A.L. Functional analysis and mode of action of phytotoxic Nep1-like proteins of Botrytis cinerea. Physiol. Mol. Plant Pathol. 2010, 74, 376–386. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Geraats, B.P.; Linthorst, H.J. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef]
- Pesis, E.; Marinansky, R. Volatile production induced by Penicillium digitatum in orange fruit and in culture. J. Phytopathol. 1990, 128, 306–314. [Google Scholar] [CrossRef]
- Zhu, P.; Xu, L.; Zhang, C.; Toyoda, H.; Gan, S. Ethylene produced by Botrytis cinerea can affect early fungal development and can be used as a marker for infection during storage of grapes. Postharvest Biol. Technol. 2012, 66, 23–29. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Van Esse, H.P.; Van’t Klooster, J.W.; Bolton, M.D.; Yadeta, K.A.; van Baarlen, P.; Boeren, S.; Vervoort, J.; de Wit, P.J.; Thomma, B.P. The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defense. Plant Cell 2008, 20, 1948–1963. [Google Scholar] [CrossRef]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; et al. Metabolic priming by a secreted fungal effector. Nature 2011, 478, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, N.M.; Raman, V. Roles and delivery mechanisms of fungal effectors during infection development: Common threads and new directions. Curr. Opin. Microbiol. 2012, 15, 692–698. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, R.; van Esse, H.P.; Kombrink, A.; Shinya, T.; Desaki, Y. Conserved fungal LysM effector Ecp6 prevents chitin-triggered immunity in plants. Science 2010, 329, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Faris, J.D.; Oliver, R.P.; Syme, R.; McDonald, M.C.; McDonald, B.A.; Solomon, P.S.; Lu, S.; Shelver, W.L.; et al. The cysteine rich necrotrophic effector SnTox1 produced by Stagonospora nodorum triggers susceptibility of wheat lines harboring Snn1. PLoS. Pathog. 2012, 8. [Google Scholar] [CrossRef]
- van Esse, H.P.; Bolton, M.D.; Stergiopoulos, I.; de Wit, P.J.; Thomma, B.P. The chitin-binding Cladosporium fulvum effector protein Avr4 is a virulence factor. Mol. Plant Microbe Interact. 2007, 20, 1092–2101. [Google Scholar] [CrossRef]
- Van den Burg, H.A.; Harrison, S.J.; Joosten, M.H.; Vervoort, J.; de Wit, P.J. Cladosporium fulvum Avr4 protects fungal cell walls against hydrolysis by plant chitinases accumulating during infection. Mol. Plant Microbe Interact. 2006, 19, 1420–1430. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Qian, X.; Dhanasekaran, S.; Boateng, N.A.S.; Yan, X.; Zhu, H.; He, F.; Zhang, H. Study on the Infection Mechanism of Penicillium Digitatum on Postharvest Citrus (Citrus Reticulata Blanco) Based on Transcriptomics. Microorganisms 2019, 7, 672. https://doi.org/10.3390/microorganisms7120672
Yang Q, Qian X, Dhanasekaran S, Boateng NAS, Yan X, Zhu H, He F, Zhang H. Study on the Infection Mechanism of Penicillium Digitatum on Postharvest Citrus (Citrus Reticulata Blanco) Based on Transcriptomics. Microorganisms. 2019; 7(12):672. https://doi.org/10.3390/microorganisms7120672
Chicago/Turabian StyleYang, Qiya, Xin Qian, Solairaj Dhanasekaran, Nana Adwoa Serwah Boateng, Xueli Yan, Huimin Zhu, Fangtao He, and Hongyin Zhang. 2019. "Study on the Infection Mechanism of Penicillium Digitatum on Postharvest Citrus (Citrus Reticulata Blanco) Based on Transcriptomics" Microorganisms 7, no. 12: 672. https://doi.org/10.3390/microorganisms7120672
APA StyleYang, Q., Qian, X., Dhanasekaran, S., Boateng, N. A. S., Yan, X., Zhu, H., He, F., & Zhang, H. (2019). Study on the Infection Mechanism of Penicillium Digitatum on Postharvest Citrus (Citrus Reticulata Blanco) Based on Transcriptomics. Microorganisms, 7(12), 672. https://doi.org/10.3390/microorganisms7120672