Whey Protein Hydrolysate and Pumpkin Pectin as Nutraceutical and Prebiotic Components in a Functional Mousse with Antihypertensive and Bifidogenic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrolysis of Cheese Whey
2.2. Preparation of Mousse Samples
2.3. In Vitro Bioassays
2.3.1. Antioxidant Activity Assay
2.3.2. Angiotensin Converting Enzyme Inhibition Assay
2.4. Animal Studies
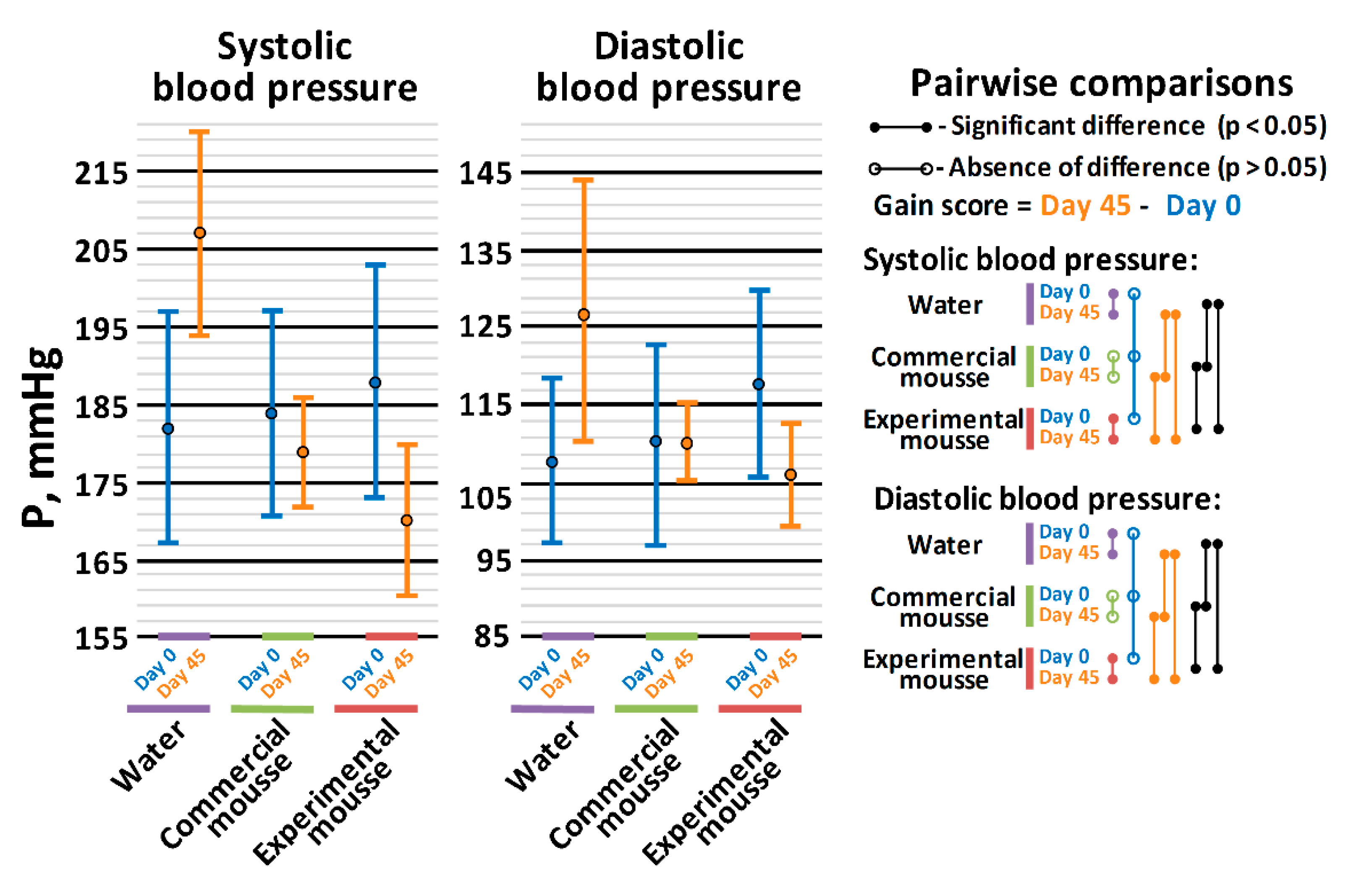
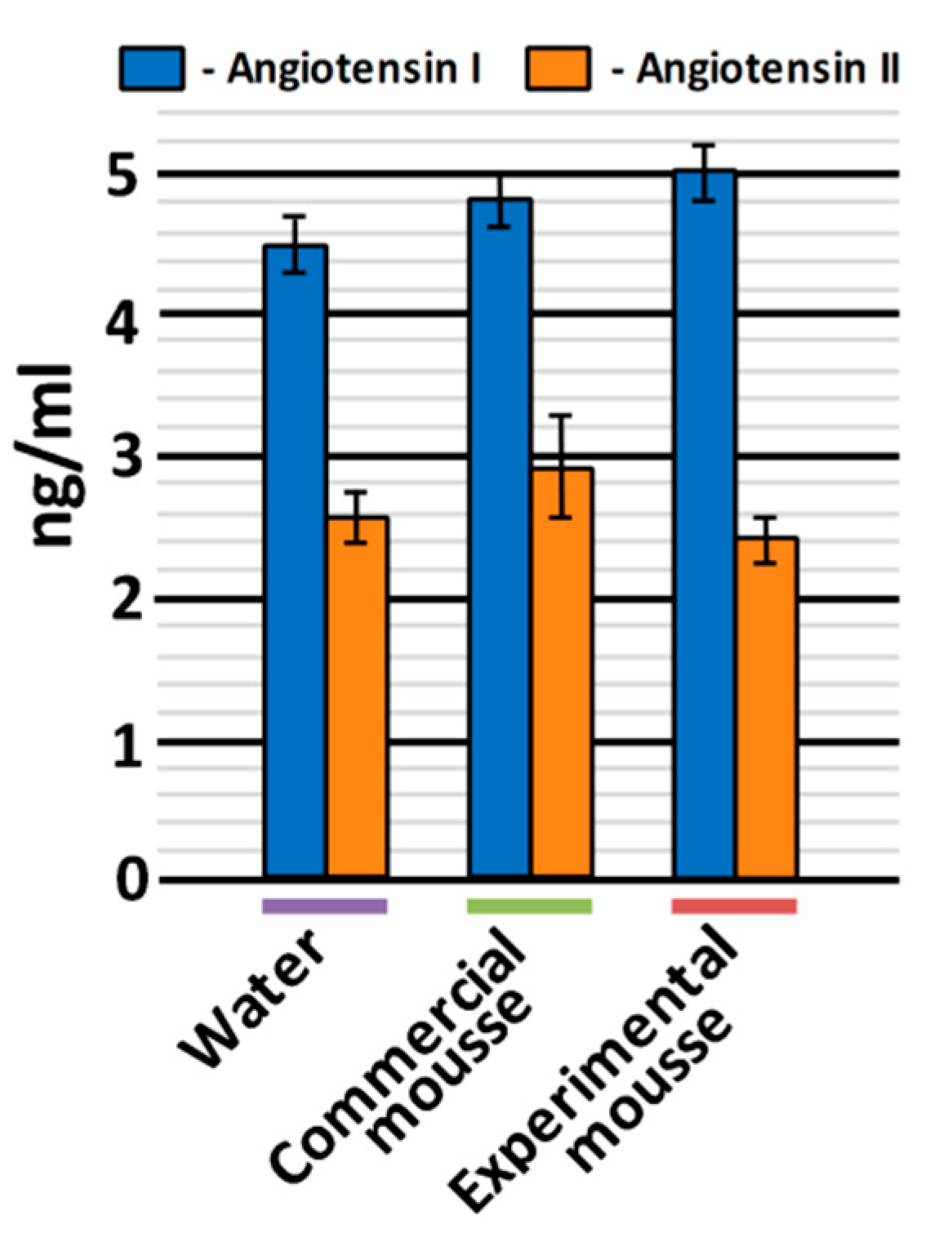
2.4.1. Assay of Antihypertensive Effect in Spontaneously Hypertensive Rats (SHRs)
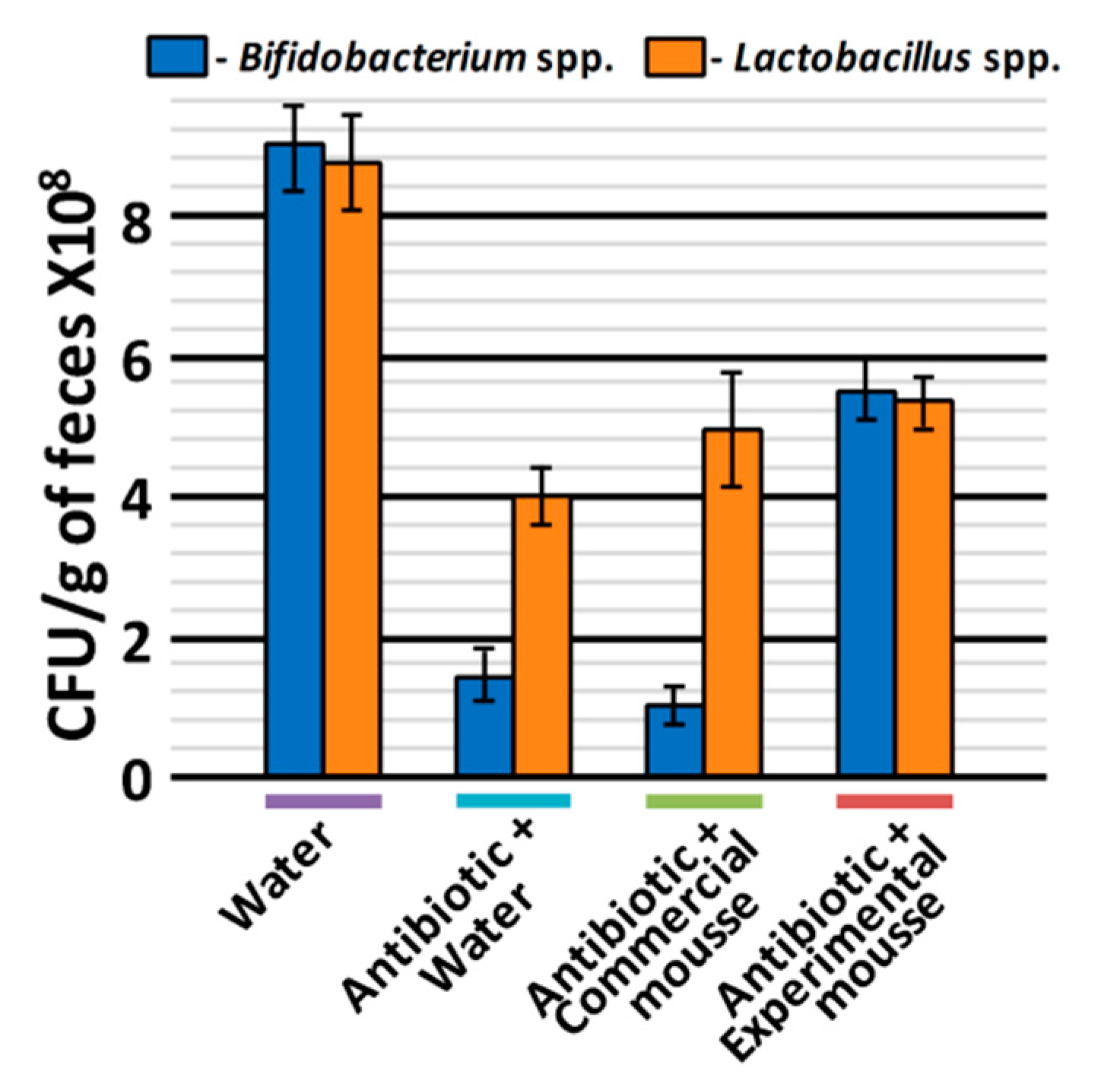
2.4.2. Assay of Bifidogenic Effect in Wistar Rats
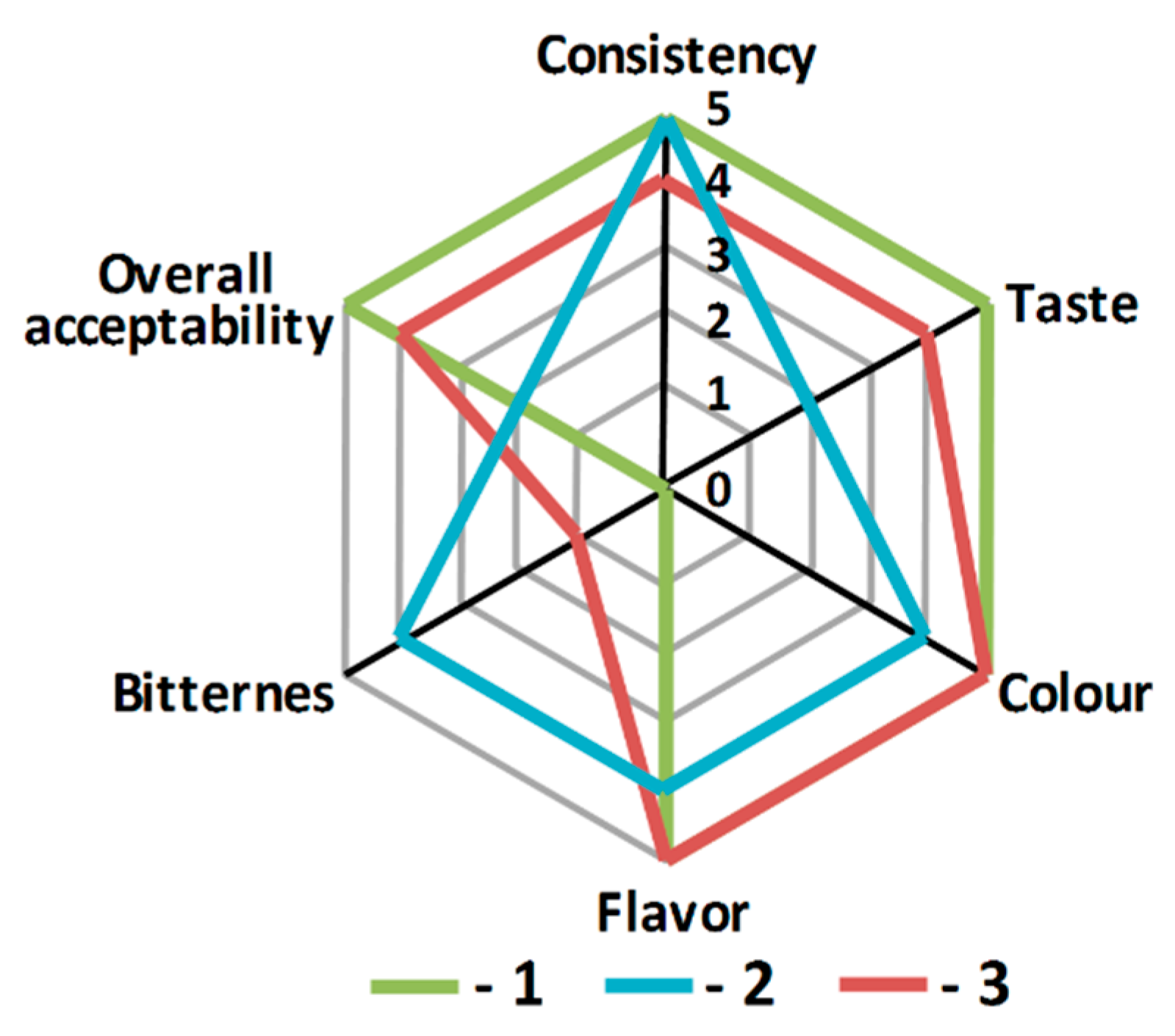
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Antioxidant and ACEI Activities of Mousse Samples
3.2. In Vivo Hypotensive Properties of Mousse Samples
3.3. Prebiotic Effect In Vivo
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keservani, R.K.; Kesharwani, R.K.; Sharma, A.K.; Gautam, S.P.; Verma, S.K. Nutraceutical formulations and challenges. Dev. New Funct. Food Nutraceutical Prod. 2017, 161–177. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Hardy, G. Nutraceuticals and functional foods: Introduction and meaning. Nutrition 2000, 16, 688–689. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Kopaei, M.R. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Rivellese, A.A.; Ciciola, P.; Costabile, G.; Vetrani, C.; Vitale, M. The possible role of nutraceuticals in the prevention of cardiovascular disease. High Blood Press. Cardiovasc. Prev. 2019, 26, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Girija, A.R. Peptide nutraceuticals. Pept. Appl. Biomed. Biotechnol. Bioeng. 2018, 157–181. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Del Mar Contreras, M.; Recio, I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive protein hydrolysates in the functional food ingredient industry: Overcoming current challenges. Food Rev. Int. 2017, 33, 217–246. [Google Scholar] [CrossRef]
- Ahhmed, A.M.; Muguruma, M. A review of meat protein hydrolysates and hypertension. Meat Sci. 2010, 86, 110–118. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Grassi, D.; Tocci, G.; Galletti, F.; Borghi, C.; Ferri, C. Nutrients and Nutraceuticals for the Management of High Normal Blood Pressure: An Evidence-Based Consensus Document. High Blood Press. Cardiovasc. Prev. 2019, 26, 9–25. [Google Scholar] [CrossRef] [PubMed]
- López-Expósito, I.; Recio, I. Protective effect of milk peptides: Antibacterial and antitumor properties. In Bioactive Components of Milk; Bösze, Z., Ed.; Springer: New York, NY, USA, 2008; pp. 271–294. ISBN 978-0-387-74087-4. [Google Scholar]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; García-Nebot, M.J.; Fernández-Tomé, S.; Amigo, L.; Recio, I. Dairy protein hydrolysates: Peptides for health benefits. Int. Dairy J. 2014, 38, 82–100. [Google Scholar] [CrossRef]
- Siltari, A.; Vapaatalo, H.; Korpela, R. Milk and milk-derived peptides combat against hypertension and vascular dysfunction: A review. Int. J. Food Sci. Technol. 2019, 54, 1920–1929. [Google Scholar] [CrossRef]
- Ricci, I.; Artacho, R.; Olalla, M. Milk protein peptides with angiotensin I-Converting enzyme inhibitory (ACEI) activity. Crit. Rev. Food Sci. Nutr. 2010, 50, 390–402. [Google Scholar] [CrossRef]
- Wicker, L.; Kim, Y.; Kim, M.-J.; Thirkield, B.; Lin, Z.; Jung, J. Pectin as a bioactive polysaccharide—Extracting tailored function from less. Food Hydrocoll. 2014, 42, 251–259. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef]
- Popov, S.V.; Ovodov, Y.S. Polypotency of the immunomodulatory effect of pectins. Biochemistry 2013, 78, 823–835. [Google Scholar] [CrossRef]
- Van der Gronde, T.; Hartog, A.; van Hees, C.; Pellikaan, H.; Pieters, T. Systematic review of the mechanisms and evidence behind the hypocholesterolaemic effects of HPMC, pectin and chitosan in animal trials. Food Chem. 2016, 199, 746–759. [Google Scholar] [CrossRef]
- Simpson, R.; Morris, G.A. The anti-diabetic potential of polysaccharides extracted from members of the cucurbit family: A review. Bioact. Carbohydr. Diet. Fibre 2014, 3, 106–114. [Google Scholar] [CrossRef]
- Cardarelli, H.R.; Aragon-Alegro, L.C.; Alegro, J.H.A.; de Castro, I.A.; Saad, S.M.I. Effect of inulin and Lactobacillus paracasei on sensory and instrumental texture properties of functional chocolate mousse. J. Sci. Food Agric. 2008, 88, 1318–1324. [Google Scholar] [CrossRef]
- Tárrega, A.; Costell, E. Effect of inulin addition on rheological and sensory properties of fat-free starch-based dairy desserts. Int. Dairy J. 2006, 16, 1104–1112. [Google Scholar] [CrossRef]
- Aragon-Alegro, L.C.; Alarcon Alegro, J.H.; Roberta Cardarelli, H.; Chih Chiu, M.; Isay Saad, S.M. Potentially probiotic and synbiotic chocolate mousse. LWT—Food Sci. Technol. 2007, 40, 669–675. [Google Scholar] [CrossRef]
- Torkova, A.A.; Ryazantseva, K.A.; Agarkova, E.Y.; Kruchinin, A.G.; Tsentalovich, M.Y.; Fedorova, T.V. Rational design of enzyme compositions for the production of functional hydrolysates of cow milk whey proteins. Appl. Biochem. Microbiol. 2017, 53, 669–679. [Google Scholar] [CrossRef]
- Torkova, A.A.; Lisitskaya, K.V.; Filimonov, I.S.; Glazunova, O.A.; Kachalova, G.S.; Golubev, V.N.; Fedorova, T.V. Physicochemical and functional properties of Cucurbita maxima pumpkin pectin and commercial citrus and apple pectins: A comparative evaluation. PLoS ONE 2018, 13, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Araujo, M.C.; Melo, R.L.; Cesari, M.H.; Juliano, M.A.; Juliano, L.; Carmona, A.K. Peptidase specificity characterization of C- and N-Terminal catalytic sites of angiotensin I-converting enzyme. Biochemistry 2000, 39, 8519–8525. [Google Scholar] [CrossRef]
- Okamoto, K.; Aoki, K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963, 27, 282–293. [Google Scholar] [CrossRef]
- Conceição-Vertamatti, A.G.; Borghi, F.; Canova, F.; Grassi-Kassisse, D.M. History of vascular reactivity models and their involvement in hypertension pathogenesis. Vasa 2017, 46, 431–439. [Google Scholar] [CrossRef]
- Rosa, C.P.; Brancaglion, G.A.; Miyauchi-Tavares, T.M.; Corsetti, P.P.; de Almeida, L.A. Antibiotic-induced dysbiosis effects on the murine gastrointestinal tract and their systemic repercussions. Life Sci. 2018, 207, 480–491. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; O’Cuinn, G. Enzymatic debittering of food protein hydrolysates. Biotechnol. Adv. 2006, 24, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, N.; Ono, I.; Kato, K.; Shigenaga, T.; Shinoda, I.; Okai, H.; Fukui, S. Role of the hydrophobic amino acid residue in the bitterness of peptides. Agric. Biol. Chem. 1988, 52, 91–94. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef]
- Zeeb, B.; Yavuz-Düzgun, M.; Dreher, J.; Evert, J.; Stressler, T.; Fischer, L.; Özcelik, B.; Weiss, J. Modulation of the bitterness of pea and potato proteins by a complex coacervation method. Food Funct. 2018, 9, 2261–2269. [Google Scholar] [CrossRef]
- Kiani, H.; Mousavi, M.E.; Razavi, H.; Morris, E.R. Effect of gellan, alone and in combination with high-methoxy pectin, on the structure and stability of doogh, a yogurt-based Iranian drink. Food Hydrocoll. 2010, 24, 744–754. [Google Scholar] [CrossRef]
- Walkenström, P.; Kidman, S.; Hermansson, A.-M.; Rasmussen, P.B.; Hoegh, L. Microstructure and rheological behaviour of alginate/pectin mixed gels. Food Hydrocoll. 2003, 17, 593–603. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Córdoba, B. Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef]
- Wu, J.; Liao, W.; Udenigwe, C.C. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci. Technol. 2017, 69, 214–219. [Google Scholar] [CrossRef]
- Coates, D. The angiotensin converting enzyme (ACE). Int. J. Biochem. Cell Biol. 2003, 35, 769–773. [Google Scholar] [CrossRef]
- Kim, W.-S.; Rahman, M.M.; Kumura, H.; Shimazaki, K. Comparison of growth promoting effects on bifidobacterium spp. by bovine lactoferrin hydrolysates. Biosci. Microflora 2005, 24, 119–123. [Google Scholar] [CrossRef][Green Version]
- Yu, Y.J.; Amorim, M.; Marques, C.; Calhau, C.; Pintado, M. Effects of whey peptide extract on the growth of probiotics and gut microbiota. J. Funct. Foods 2016, 21, 507–516. [Google Scholar] [CrossRef]
- Bergamini, C.V.; Hynes, E.R.; Palma, S.B.; Sabbag, N.G.; Zalazar, C.A. Proteolytic activity of three probiotic strains in semi-hard cheese as single and mixed cultures: Lactobacillus acidophilus, Lactobacillus paracasei and Bifidobacterium lactis. Int. Dairy J. 2009, 19, 467–475. [Google Scholar] [CrossRef]
- Shihata, A.; Shah, N.P. Proteolytic profiles of yogurt and probiotic bacteria. Int. Dairy J. 2000, 10, 401–408. [Google Scholar] [CrossRef]
- Liepke, C.; Adermann, K.; Raida, M.; Mägert, H.J.; Forssmann, W.G.; Zucht, H.D. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur. J. Biochem. 2002, 269, 712–718. [Google Scholar] [CrossRef]
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Sato, T.; Xiao, J.Z.; Abe, F.; Iwatsuki, K. Isolation of a bifidogenic peptide from the pepsin hydrolysate of bovine lactoferrin. Appl. Environ. Microbiol. 2013, 79, 1843–1849. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2014, 10, 323–335. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Chen, J.; Liang, R.; Liu, W.; Li, T.; Liu, C.; Wu, S.; Wang, Z. Pectic-oligosaccharides prepared by dynamic high-pressure microfluidization and their in vitro fermentation properties. Carbohydr. Polym. 2013, 91, 175–182. [Google Scholar] [CrossRef]
- Wei, Y.; Gong, J.; Zhu, W.; Tian, H.; Ding, C.; Gu, L.; Li, N.; Li, J. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiol. 2016, 16, 1–9. [Google Scholar] [CrossRef]
| Ingredient | Amount (g/100 g of Product) | |
|---|---|---|
| Series I | Series II | |
| Low fat quark | 40.0 | 40.0 |
| Cream (10% fat) | 11.65 | 11.65 |
| Cow’s milk (2.5% fat) | 34.14 | 34.14 |
| Sugar | 10.7 | 10.7 |
| Skimmed milk powder | 2.0 | 2.0 |
| Gelatin | 0.83 | 0.83 |
| Guar gum | 0.42 | 0.21 |
| Pumpkin pectin | - | 0.21 |
| Acidity regulator | 0.26 | 0.26 |
| Property | Series I (100% Guar Gum) | Series II (50% Guar Gum: 50% Pectin) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Percent of Cow’s Milk Replaced by Whey Protein Hydrolysate (WPH) | Percent of Cow’s Milk Replaced by WPH | |||||||||
| 0 | 25 | 50 | 75 | 100 | 0 | 25 | 50 | 75 | 100 | |
| AOX **, μM TE/g of protein | 198.5 | 219.5 | 219.5 | 234.1 | 247.4 | 214.8 | 231.4 | 232.7 | 232.9 | 238.4 |
| ACEI activity (IC50 ***), mg of protein/mL | 19.06 | 15.85 | 12.02 | 10.0 | 7.56 | 23.99 | 6.92 | 6.03 | 3.98 | 2.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarkova, E.Y.; Kruchinin, A.G.; Glazunova, O.A.; Fedorova, T.V. Whey Protein Hydrolysate and Pumpkin Pectin as Nutraceutical and Prebiotic Components in a Functional Mousse with Antihypertensive and Bifidogenic Properties. Nutrients 2019, 11, 2930. https://doi.org/10.3390/nu11122930
Agarkova EY, Kruchinin AG, Glazunova OA, Fedorova TV. Whey Protein Hydrolysate and Pumpkin Pectin as Nutraceutical and Prebiotic Components in a Functional Mousse with Antihypertensive and Bifidogenic Properties. Nutrients. 2019; 11(12):2930. https://doi.org/10.3390/nu11122930
Chicago/Turabian StyleAgarkova, Evgeniya Yu., Alexandr G. Kruchinin, Olga A. Glazunova, and Tatyana V. Fedorova. 2019. "Whey Protein Hydrolysate and Pumpkin Pectin as Nutraceutical and Prebiotic Components in a Functional Mousse with Antihypertensive and Bifidogenic Properties" Nutrients 11, no. 12: 2930. https://doi.org/10.3390/nu11122930
APA StyleAgarkova, E. Y., Kruchinin, A. G., Glazunova, O. A., & Fedorova, T. V. (2019). Whey Protein Hydrolysate and Pumpkin Pectin as Nutraceutical and Prebiotic Components in a Functional Mousse with Antihypertensive and Bifidogenic Properties. Nutrients, 11(12), 2930. https://doi.org/10.3390/nu11122930





