The Stability and Activity Changes of Apigenin and Luteolin in Human Cervical Cancer Hela Cells in Response to Heat Treatment and Fe2+/Cu2+ Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Line and Culture Conditions
2.3. Assays of Degradation Rates of the Two Flavones
2.4. Treatments of the Two Flavones for Cell Experiments
2.5. Assay of Growth Inhibition
2.6. Hoechst 33258 Staining
2.7. Assay of Apoptosis Induction
2.8. Assay of Intracellular Reactive Oxygen Species
2.9. Statistical Analysis
3. Results
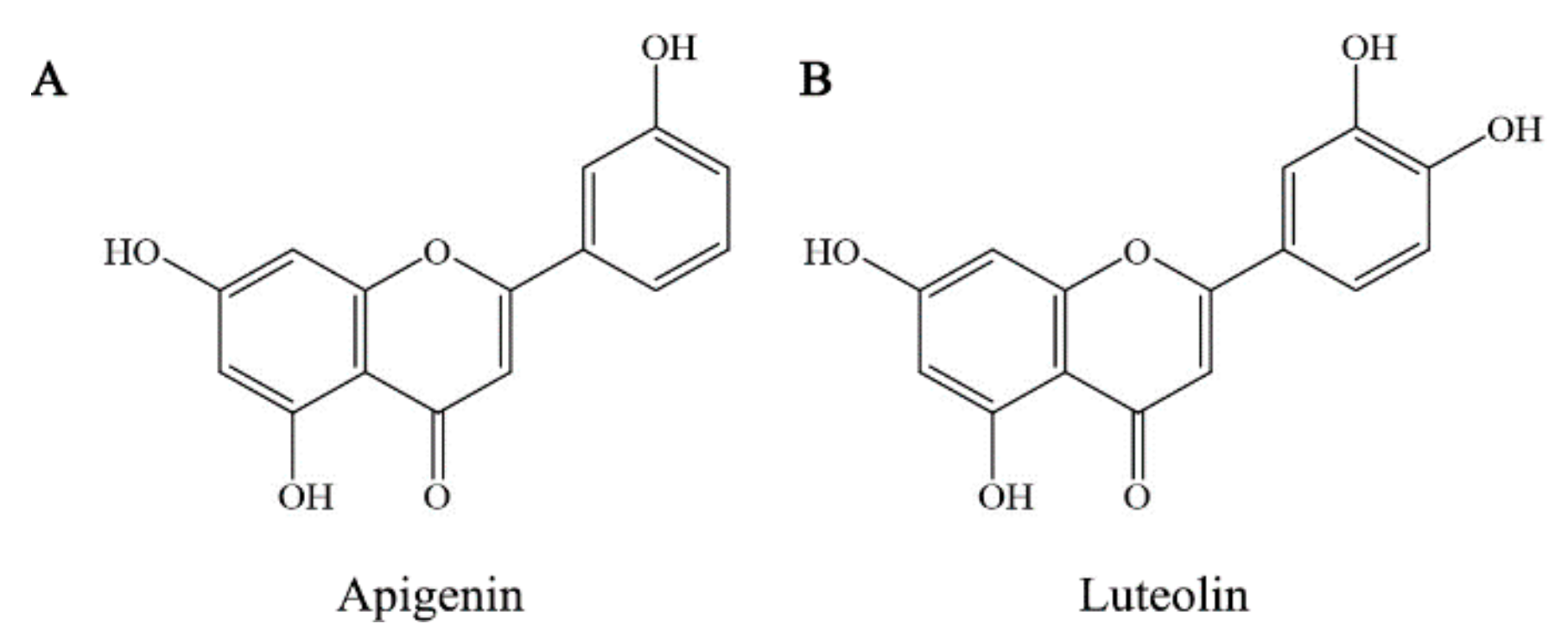
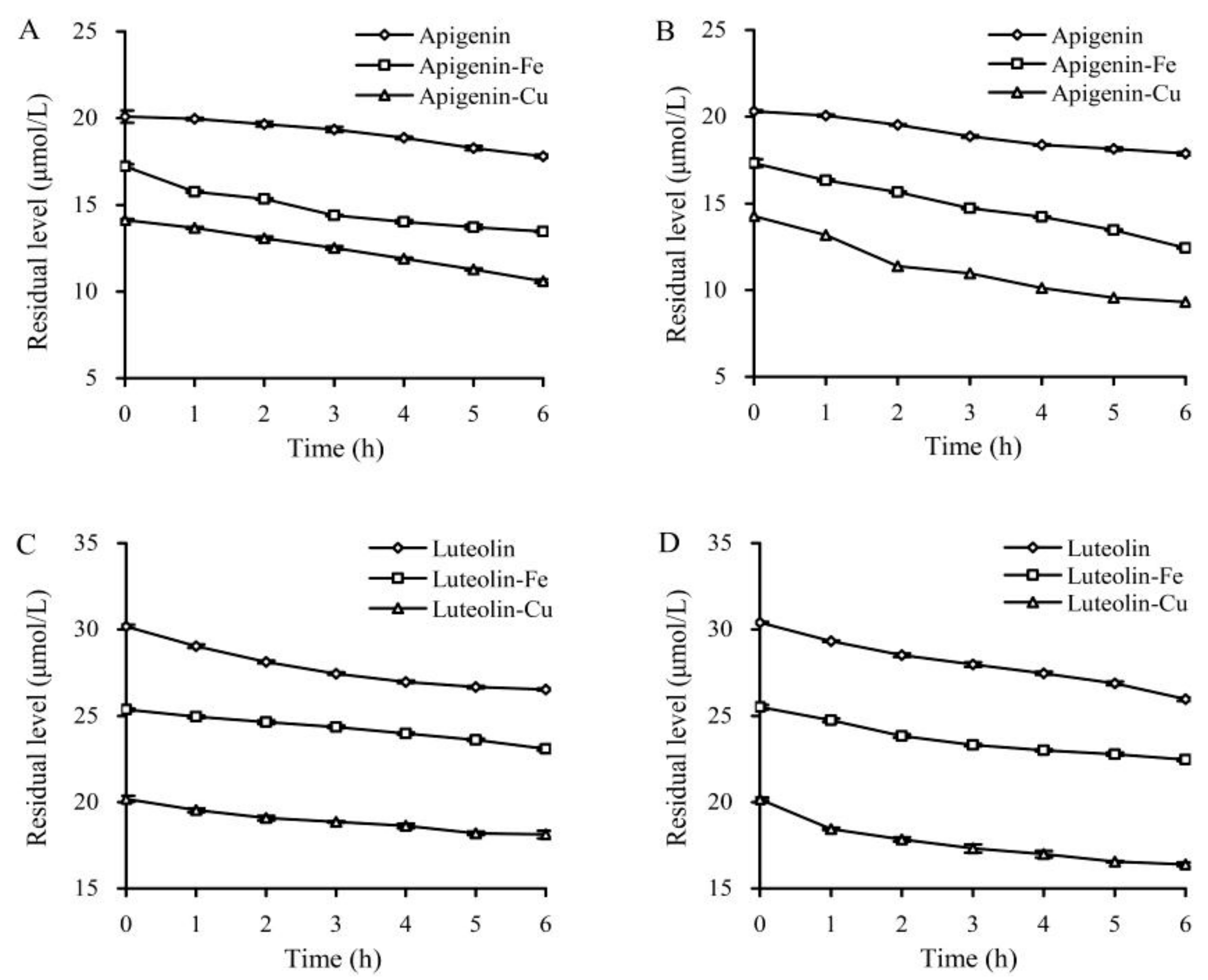
3.1. Instability of Apigenin and Luteolin at Two Temperatures or in the Presence of Fe2+/Cu2+
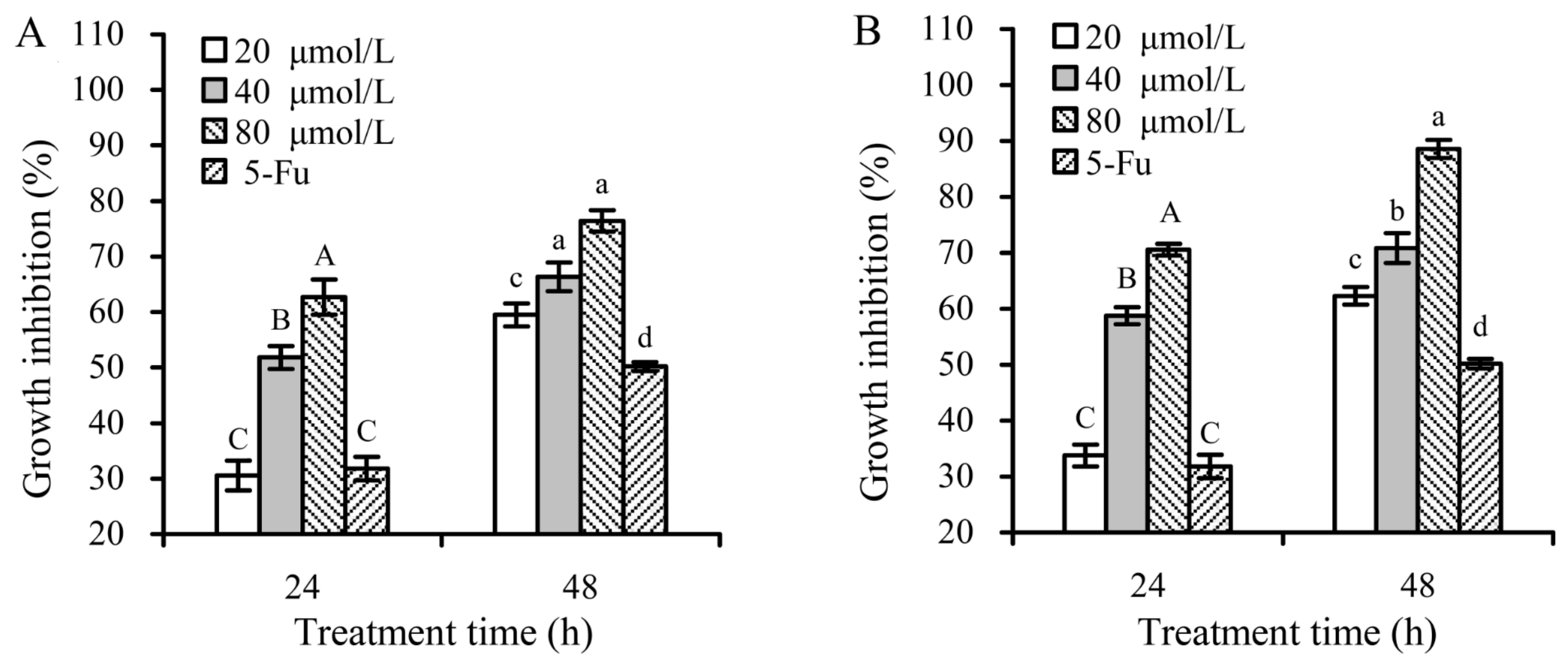
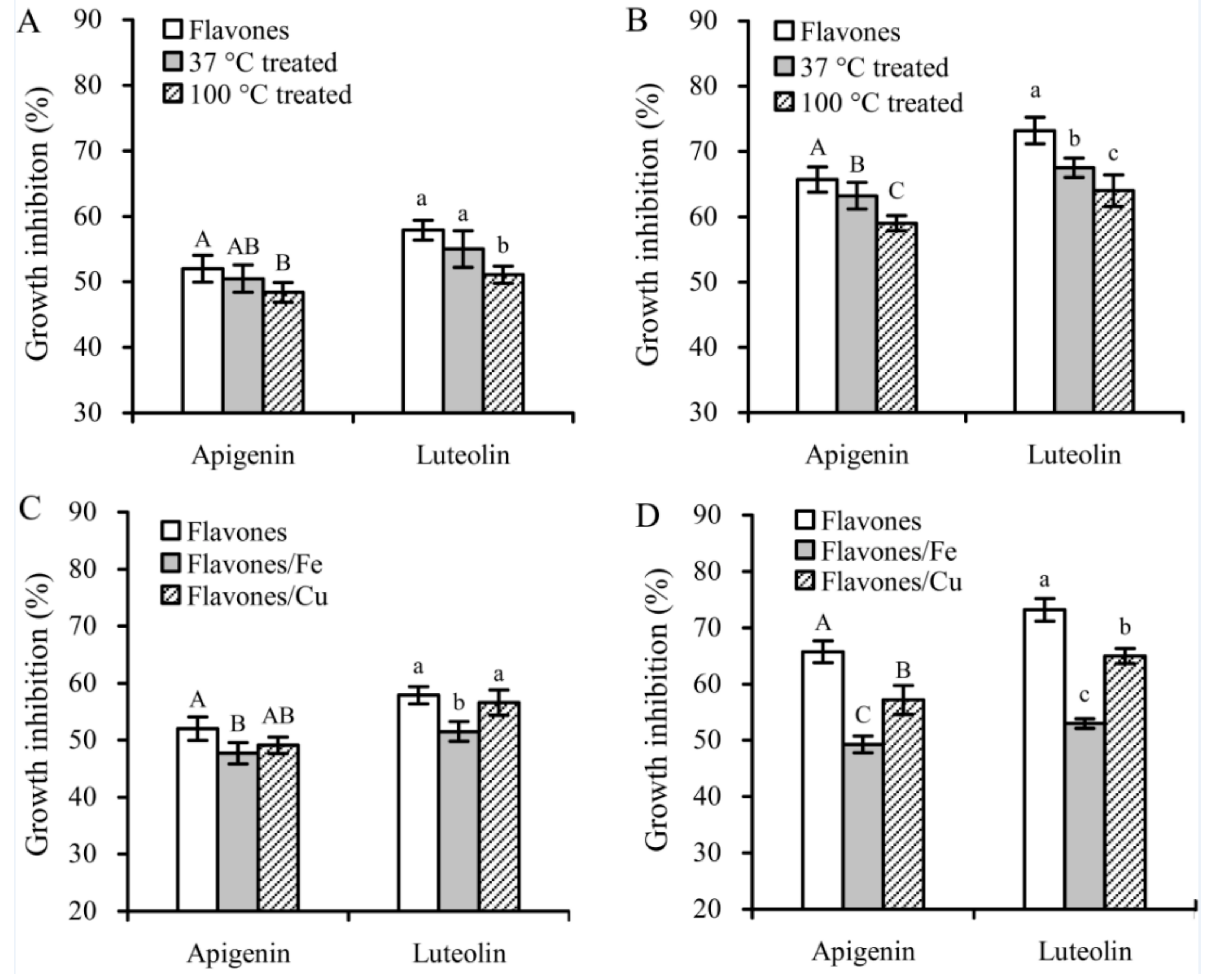
3.2. Growth Inhibition of the Flavone Samples on Hela Cells
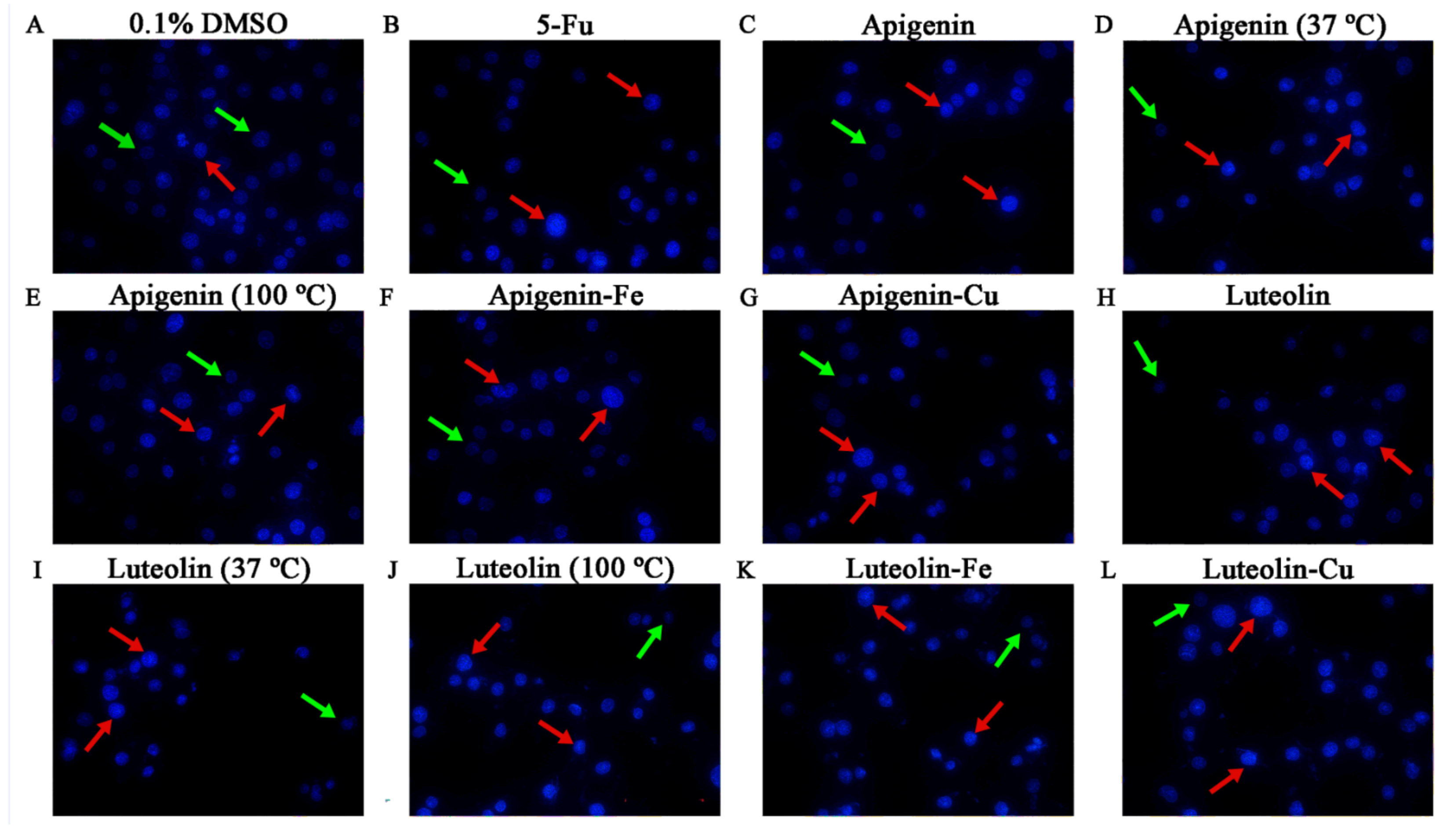
3.3. Morphological Alteration of Hela Cells Treated by the Flavone Samples
3.4. Pro-Oxidation of the Flavone Samples
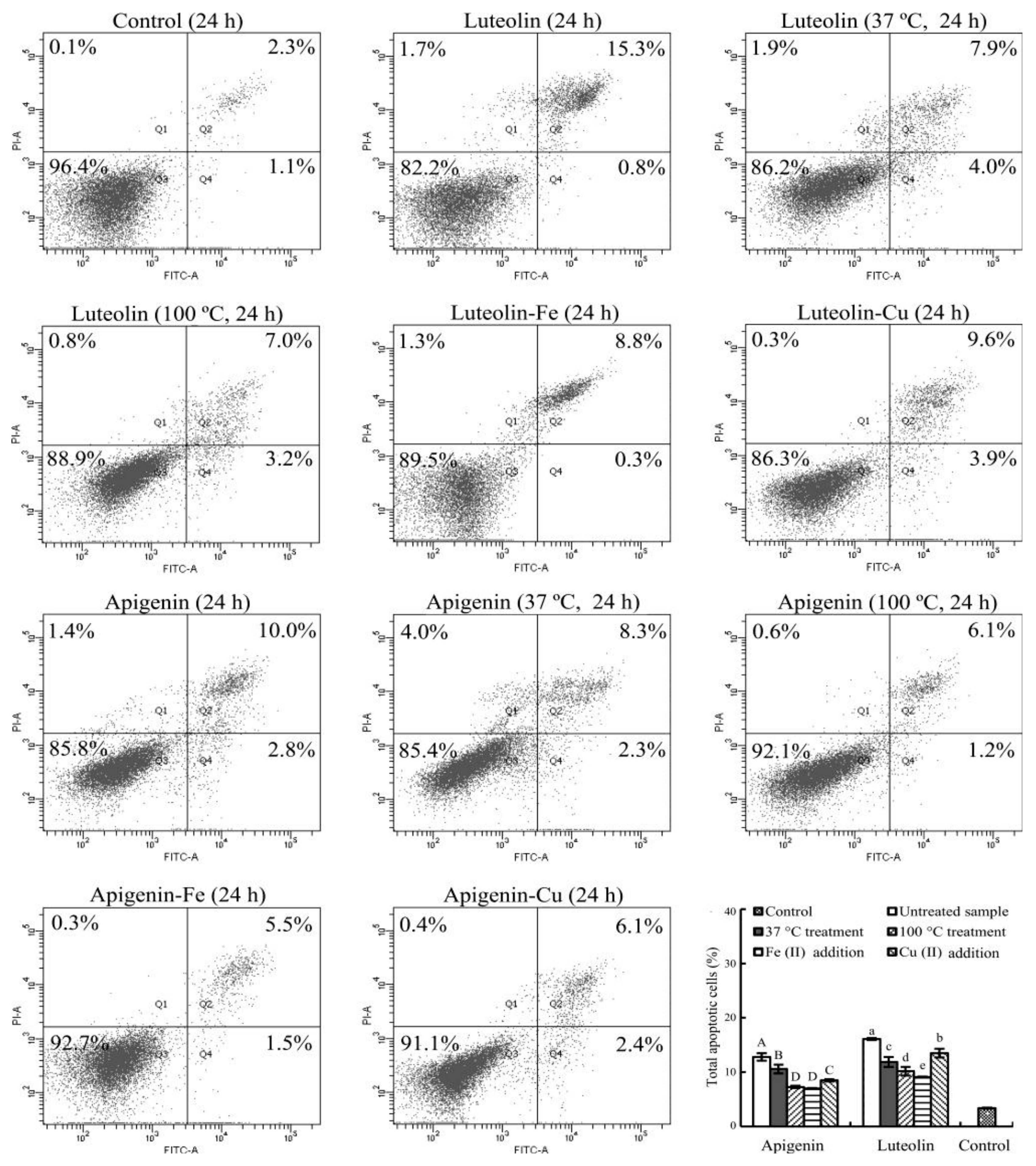
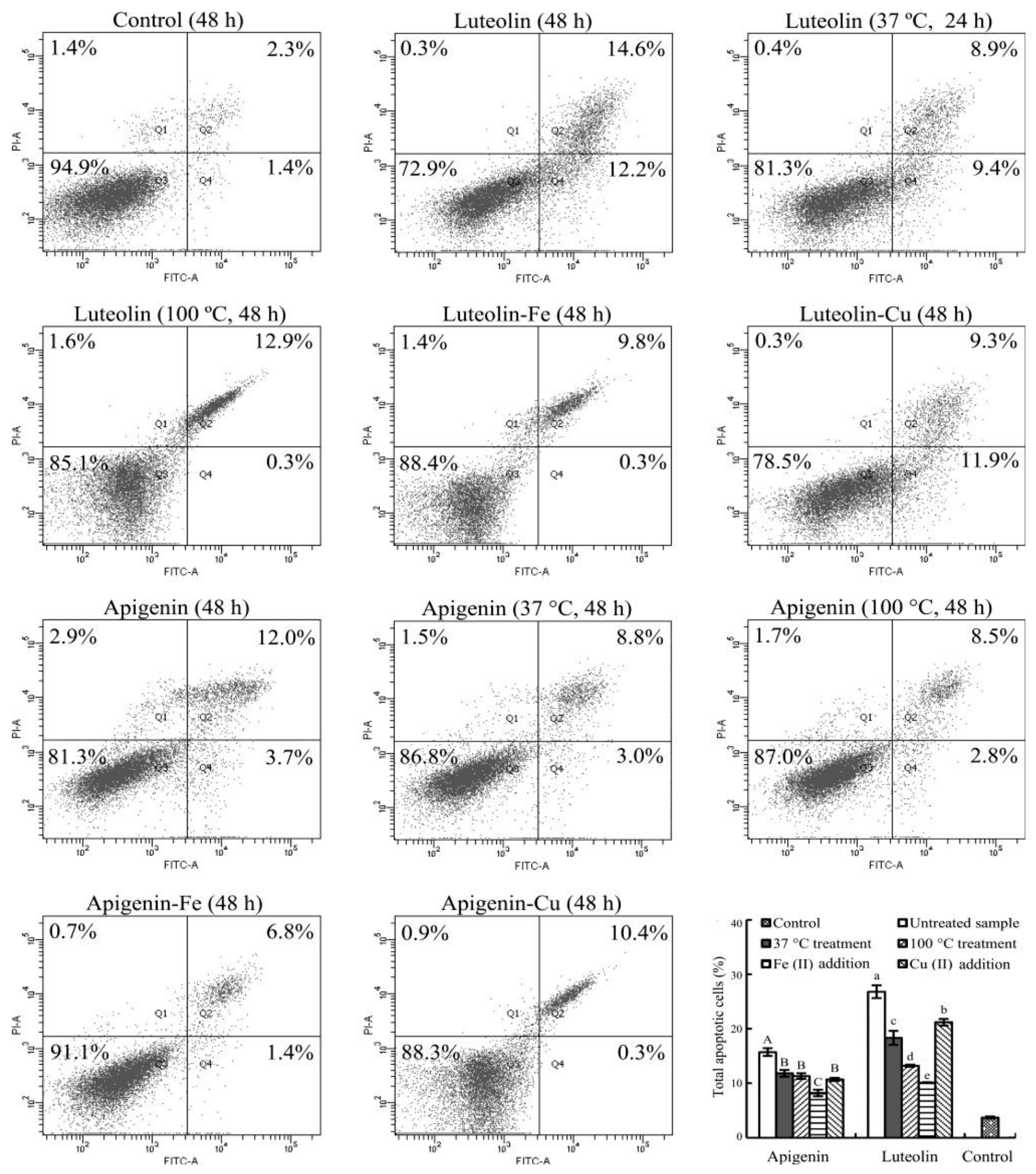
3.5. Apoptosis Induction of the Flavone Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robak, J.; Gryglewski, R.J. Bioactivity of flavonoids. Pol. J. Pharmacol. 1996, 48, 555–564. [Google Scholar] [PubMed]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Han, M.J.; Kim, D.H. In vitro anti-helicobacter pylori activity of some flavonoids and their metabolites. Planta. Med. 1999, 65, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992, 43, 1167–1179. [Google Scholar] [CrossRef]
- Ramiro, E.; Franch, A.; Castellote, C.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Castell, M. Effect of theobroma cacao flavonoids on immune activation of a lymphoid cell line. Br. J. Nutr. 2005, 93, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Rotelli, A.E.; Guardia, T.; Juárez, A.O.; de La Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.H.; Wang, Z.J. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem. Toxicol. 2008, 46, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Choi, H.L.; Chen, Z.Y.; Chan, P.S.F.; Huang, Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000, 160, 219–228. [Google Scholar] [CrossRef]
- Frydoonfar, H.R.; Mcgrath, D.R.; Spigelman, A.D. The variable effect on proliferation of a colon cancer cell line by the citrus fruit flavonoid naringenin. Colorectal Dis. 2010, 5, 149–152. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Hajji, H.E.; Nkhili, E.; Tomao, V.; Dangles, O. Interactions of quercetin with iron and copper ions: Complexation and autoxidation. Free Radic. Res. 2006, 40, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Biesaga, M.; Pyrzynska, K. Interaction of quercetin with copper ions: Complexation, oxidation and reactivity towards radicals. Bio. Met. 2010, 24, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.A.; Potapovich, A.I.; Strigunova, E.N.; Kostyuk, T.V.; Afanas’ev, I.B. Experimental evidence that flavonoid metal complexes may act as mimics of superoxide dismutase. Arch. Biochem. Biophys. 2004, 428, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.S.; Foss, F.W.; Schug, K.A. Thermally accelerated oxidative degradation of quercetin using continuous flow kinetic electrospray-ion trap-time of flight mass spectrometry. J. Am. Soc. Mass Spectrom. 2013, 24, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.M.; Onofrei, C.; Turturică, M.; Bahrim, G.; Râpeanu, G.; Stănciuc, N. The kinetics of thermal degradation of polyphenolic compounds from elderberry (Sambucus nigra L.) extract. Food Sci. Technol. Int. 2018, 24, 361–369. [Google Scholar] [CrossRef]
- De Paepe, D.; Valkenborg, D.; Coudijzer, K.; Noten, B.; Servaes, K.; De Loose, M.; Voorspoels, S.; Diels, L.; Van Droogenbroeck, B. Thermal degradation of cloudy apple juice phenolic constituents. Food Chem. 2014, 162, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Effect of different cooking conditions on phenolic compounds and antioxidant capacity of some selected brazilian bean (Phaseolus vulgaris L.) cultivars. J. Agric. Food Chem. 2009, 57, 5734–5742. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Zhao, X.H. In vitro activities of the four structurally similar flavonols weakened by the prior thermal and oxidative treatments to a human colorectal cancer line. J. Food Biochem. 2016, 41, e12310. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, X.H. Four in vitro activities of apigenin to human colorectal carcinoma cells susceptible to air-oxidative and heating treatments. Emir. J. Food Agri. 2017, 29, 69–77. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Sergediene, E.; Jönsson, K.; Szymusiak, H.; Tyrakowska, B.; Rietjens, I.M.; Cenas, N. Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: Description of quantitative structure-activity relationships. FEBS Lett. 1999, 462, 392–396. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.R.; Sharma, S.; Mandal, S.; Goswami, A.; Mukhopadhyay, S.; Majumder, H.K. Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem. J. 2002, 366, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Xu, S.Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Lou, J.L.; Chu, G.H.; Zhou, G.J.; Jiang, J.; Huang, F.F.; Xu, J.J.; Zheng, S.; Jiang, W.; Lu, Y.Z.; Li, X.X.; et al. Comparison between two kinds of cigarette smoke condensates (CSCs) of the cytogenotoxicity and protein expression in a human B-cell lymphoblastoid cell line using CCK-8 assay, comet assay and protein microarray. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2010, 697, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Haanen, C.; Steffensnakken, H.; Reutelingsperger, C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Li, J.; Tang, Q.; Li, Y.; Hu, B.; Ming, Z.; Fu, Q.; Qian, J.Q.; Xiang, J.Z. Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta Pharmacol. Sin. 2010, 27, 1078–1084. [Google Scholar] [CrossRef]
- Sólyom, K.; Solá, R.; Cocero, M.J.; Mato, R.B. Thermal degradation of grape marc polyphenols. Food Chem. 2014, 159, 361–366. [Google Scholar] [CrossRef]
- Khuwijitjaru, P.; Plernjit, J.; Suaylam, B.; Samuhaseneetoo, S.; Pongsawatmanit, R.; Adachi, S. Degradation kinetics of some phenolic compounds in subcritical water and radical scavenging activity of their degradation products. Can. J. Chem. Eng. 2013, 92, 810–815. [Google Scholar] [CrossRef]
- Ramanouskaya, T.V.; Smolnykova, V.V.; Grinev, V.V. Relationship between structure and antiproliferative, proapoptotic, and differentiation effects of flavonoids on chronic myeloid leukemia cells. Anticancer Drugs 2009, 20, 573–583. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Flavonoids as anticancer agents: Structure-activity relationship study. Curr. Med. Chem. Anticancer Agents 2002, 2, 691–714. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, M.; Mustapha, N.; Mokdad-Bzeouich, I.; Chaaban, H.; Abed, B.; Iaonnou, I.; Ghedira, K.; Ghoul, M.; Ghedira, L.C. Thermal treatment of luteolin-7-O-β-glucoside improves its immunomodulatory and antioxidant potencies. Cell Stress Chaperones 2017, 22, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Rossiter, J.T. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (quercetin 3-o-rhamnosylglucoside) in aqueous model systems. J. Agric. Food Chem. 2000, 48, 3830–3838. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid-metal ion complexes: A novel class of therapeutic agents. Med. Res. Rev. 2014, 34, 677–702. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.F.V.; Sussuchi, E.M.; De Giovani, W.F. Synthesis, Electrochemical, Spectral, and Antioxidant Properties of Complexes of Flavonoids with Metal Ions. Redox Rep. 2003, 33, 1125–1144. [Google Scholar] [CrossRef]
- Lee, K.; Hyun Lee, D.; Jung, Y.J.; Shin, S.Y.; Lee, Y.H. The natural flavone eupatorin induces cell cycle arrest at the G2/M phase and apoptosis in Hela cells. Appl. Biol. Chem. 2016, 59, 193–199. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Lv, Q.; Zhang, J.; Zhu, D. The critical role of quercetin in autophagy and apoptosis in Hela cells. Tumor Biol. 2015, 37, 925–929. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, J.O.; Kim, J.H.; Lee, S.K.; You, G.Y.; Park, S.H.; Park, J.M.; Kim, E.K.; Suh, P.G.; An, J.K.; et al. Quercetin suppresses Hela cell viability via AMPK-induced HSP70 and EGFR down-regulation. J. Cell Physiol. 2010, 223, 408–414. [Google Scholar] [CrossRef]
- Hann, H.W.; Stahlhut, M.W.; Blumberg, B.S. Iron nutrition and tumor growth: Decreased tumor growth in iron-deficient mice. Cancer Res. 1988, 48, 4168–4170. [Google Scholar]
- Yu, Y.; Kovacevic, Z.; Richardson, D.R. Tuning cell cycle regulation with an iron key. Cell Cycle 2007, 6, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.H.; Lee, S.M.; Park, J.W. Enhancement by copper, zinc superoxide dismutase of DNA damage and mutagenicity with hydrogen peroxide. IUBMB Life 2010, 45, 635–642. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I.; Vladykovskaya, E.N.; Korkina, L.G.; Afanas’ev, I.B. Influence of metal ions on flavonoid protection against asbestos-induced cell injury. Arch. Biochem. Biophys. 2001, 385, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M.I. Synthesis, characterization and antioxidant activity copper-quercetin complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 71, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 57, 45853–45877. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O’Brien, P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002, 177, 91–104. [Google Scholar] [CrossRef]
- Stepanic, V.; Gasparovic, A.C.; Troselj, K.G.; Amic, D.; Zarkovic, N. Selected attributes of polyphenols in targeting oxidative stress in cancer. Curr. Top Med. Chem. 2015, 15, 496–509. [Google Scholar] [CrossRef]
- Tao, L.; Park, J.Y.; Lambert, J.D. Differential prooxidative effects of the green tea polyphenol, (-)-epigallocatechin-3-gallate, in normal and oral cancer cells are related to differences in sirtuin 3 signaling. Mol. Nutr. Food Res. 2014, 59, 203–211. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
| Indices | Added Metals (Flavones:Metals 3:1) | Apigenin | Luteolin | |
|---|---|---|---|---|
| Temperature | 37 °C | None | 0.0207 ± 0.0012 F | 0.0214 ± 0.0004 c |
| Fe2+ | 0.0395 ± 0.0011 D | 0.0149 ± 0.0009 f | ||
| Cu2+ | 0.0480 ± 0.0015 C | 0.0176 ± 0.0021 e | ||
| 100 °C | None | 0.0226 ± 0.0001 E | 0.0245 ± 0.0006 b | |
| Fe2+ | 0.0520 ± 0.0002 B | 0.0203 ± 0.0005 d | ||
| Cu2+ | 0.0728 ± 0.0010 A | 0.0317 ± 0.0004 a | ||
| Significance | Temperature | ** | ** | |
| Metals | ** | ** | ||
| Temperature × Metals | ** | ** | ||
| Flavones | Heat Treatment (°C) | Added Ions (Flavones:Metals 3:1) | ROS Levels (% of Control) | |
|---|---|---|---|---|
| 24 h | 48 h | |||
| Apigenin | None | None | 228.6 ± 2.4 A | 262.8 ± 1.5 A |
| 37 | None | 211.7 ± 3.8 B | 260.0 ± 6.4 B | |
| 100 | None | 206.9 ± 3.4 C | 245.3 ± 1.6 C | |
| None | Fe2+ | 205.6 ± 3.8 C | 223.6 ± 1.0 E | |
| None | Cu2+ | 212.1 ± 1.6 B | 237.4 ± 1.9 D | |
| Luteolin | None | None | 284.1 ± 8.2 a | 280.9 ± 3.8 a |
| 37 | None | 271.8 ± 5.0 b | 262.9 ± 3.5 b | |
| 100 | None | 234.2 ± 7.7 c | 256.8 ± 2.5 c | |
| None | Fe2+ | 232.1 ± 1.0 c | 225.1 ± 5.7 e | |
| None | Cu2+ | 268.4 ± 2.7 b | 246.4 ± 0.7 d | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-N.; Shi, J.; Fu, Y.; Zhao, X.-H. The Stability and Activity Changes of Apigenin and Luteolin in Human Cervical Cancer Hela Cells in Response to Heat Treatment and Fe2+/Cu2+ Addition. Foods 2019, 8, 346. https://doi.org/10.3390/foods8080346
Liu W-N, Shi J, Fu Y, Zhao X-H. The Stability and Activity Changes of Apigenin and Luteolin in Human Cervical Cancer Hela Cells in Response to Heat Treatment and Fe2+/Cu2+ Addition. Foods. 2019; 8(8):346. https://doi.org/10.3390/foods8080346
Chicago/Turabian StyleLiu, Wan-Ning, Jia Shi, Yu Fu, and Xin-Huai Zhao. 2019. "The Stability and Activity Changes of Apigenin and Luteolin in Human Cervical Cancer Hela Cells in Response to Heat Treatment and Fe2+/Cu2+ Addition" Foods 8, no. 8: 346. https://doi.org/10.3390/foods8080346
APA StyleLiu, W.-N., Shi, J., Fu, Y., & Zhao, X.-H. (2019). The Stability and Activity Changes of Apigenin and Luteolin in Human Cervical Cancer Hela Cells in Response to Heat Treatment and Fe2+/Cu2+ Addition. Foods, 8(8), 346. https://doi.org/10.3390/foods8080346