Potential of Cooperage Byproducts Rich in Ellagitannins to Improve the Antioxidant Activity and Color Expression of Red Wine Anthocyanins
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples
2.3. Phenolic Extraction
2.4. Preparation of Model Solutions
2.5. Total Phenolic Content
2.6. Individual Phenolic Profile
2.7. Antioxidant Activity
2.8. Color and Copigmentation Analyses
2.9. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Composition of Oak Phenolic Extracts
3.2. Antioxidant Activity of Oak Phenolic Extracts
3.3. Composition and Antioxidant Activity of Skins Anthocyanin Extract
3.4. Antioxidant Activity of Model Solutions
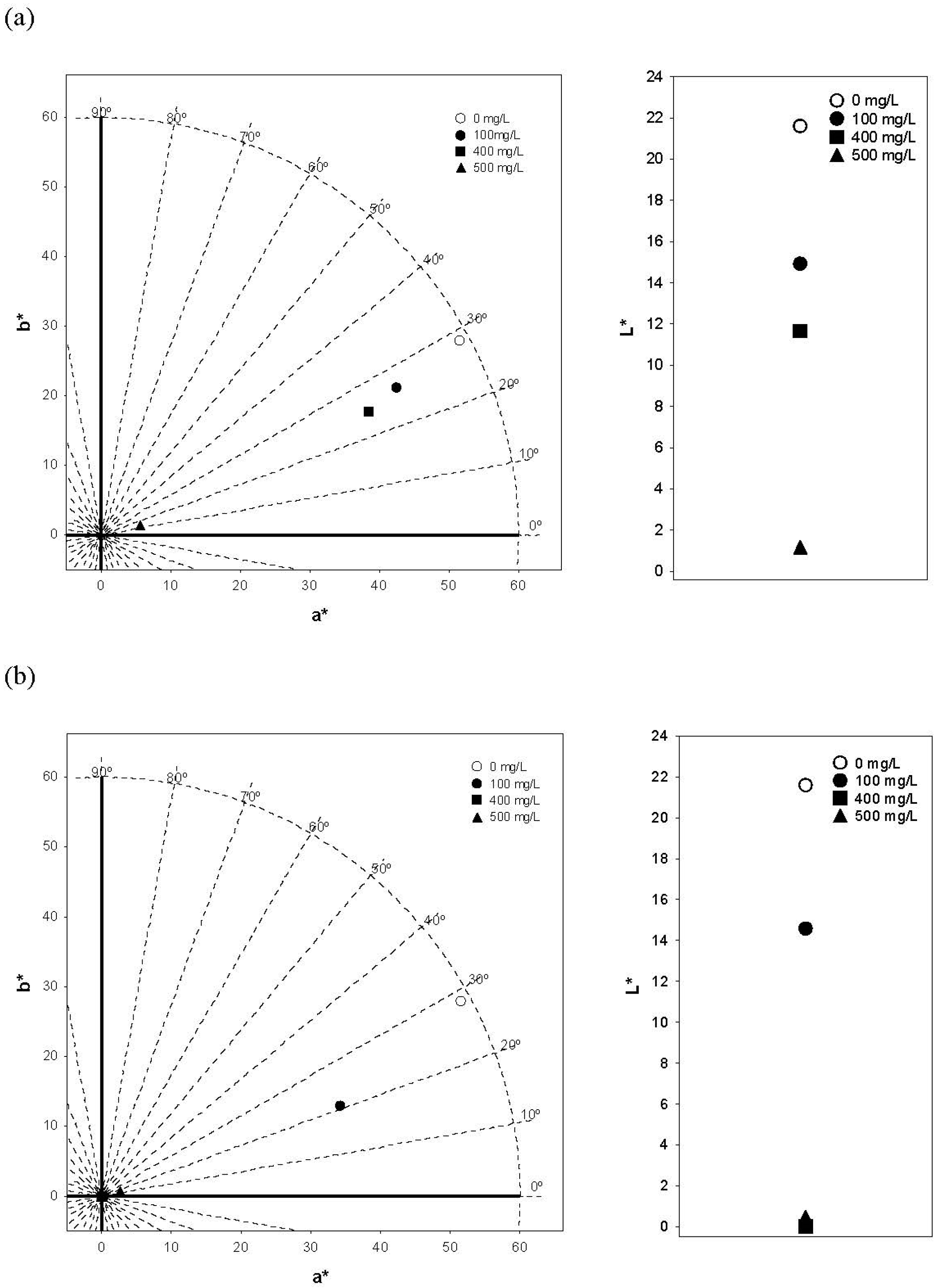
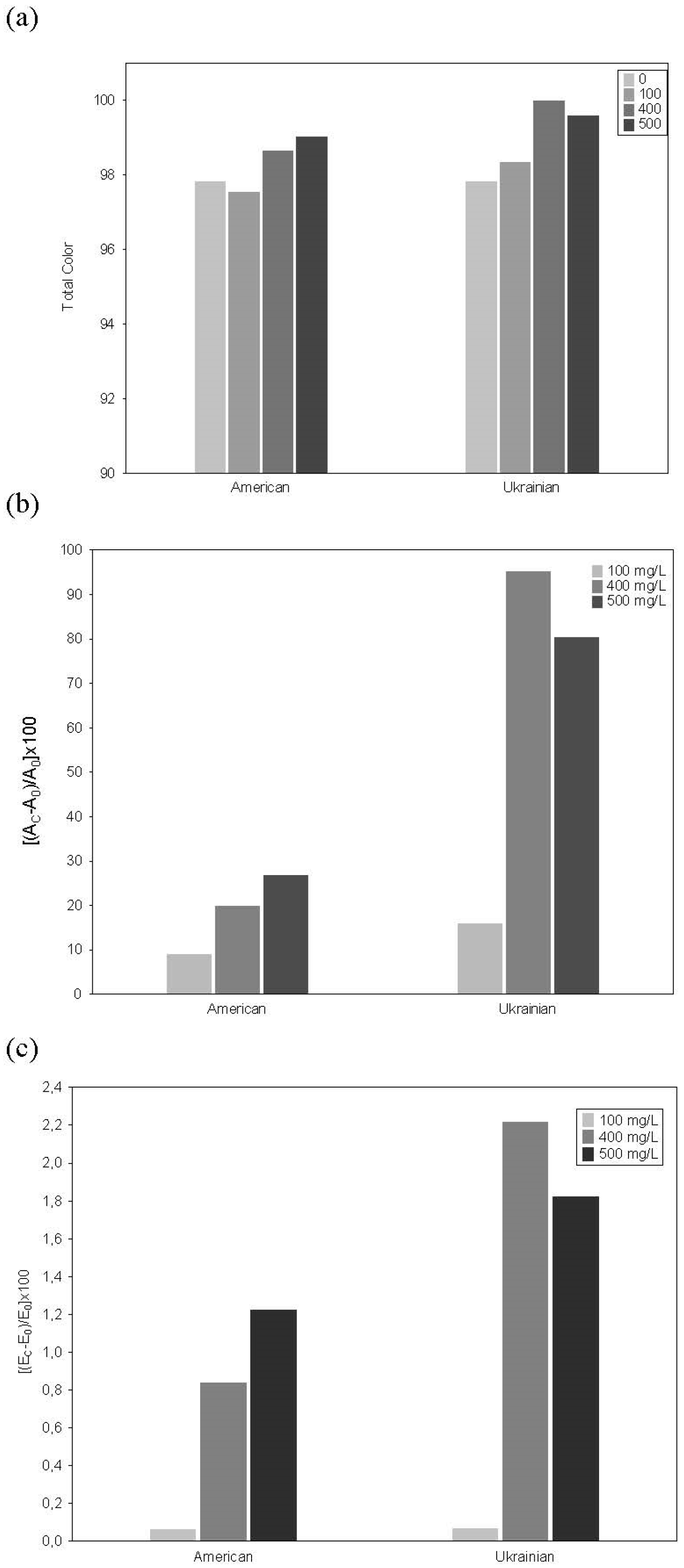
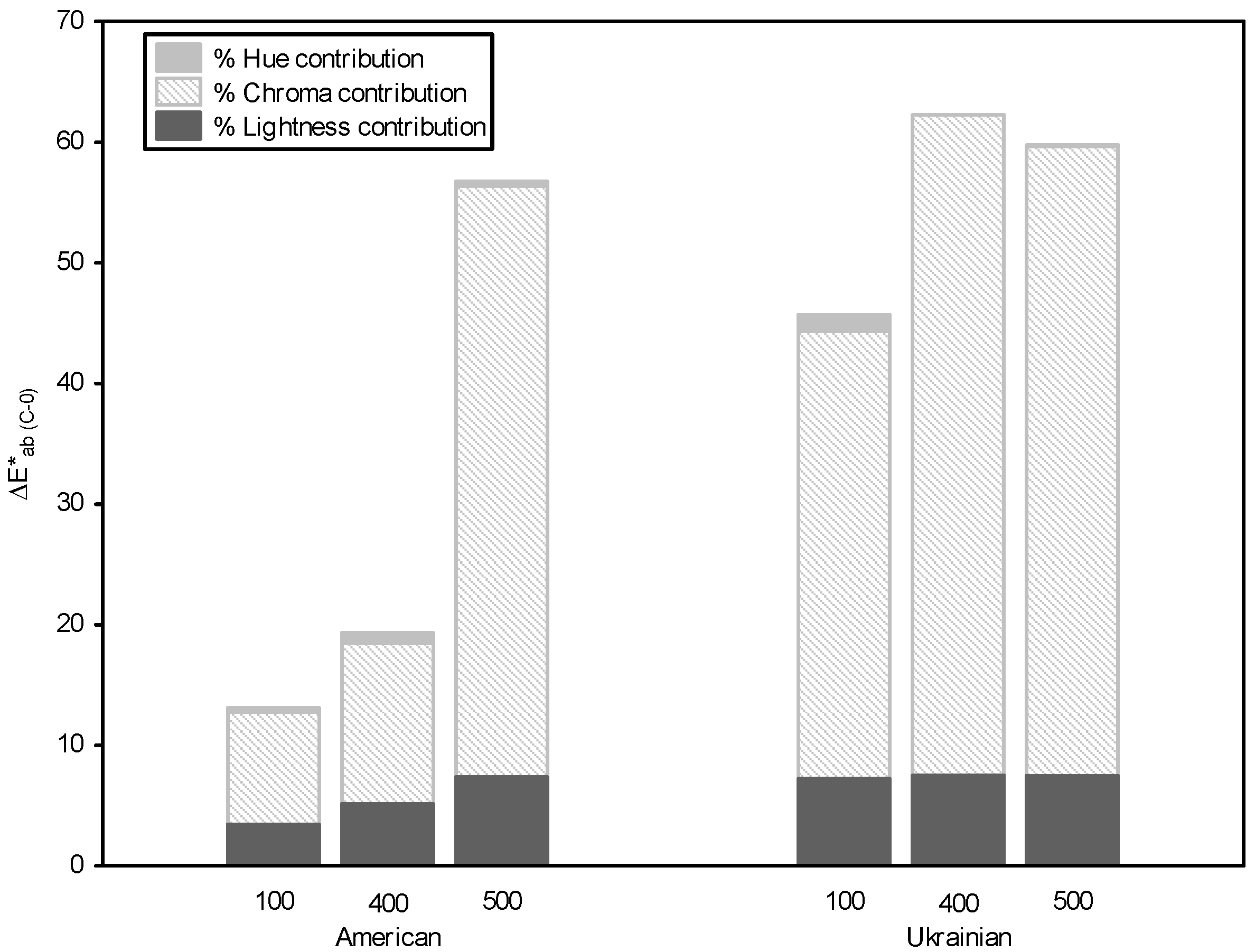
3.5. Color and Copigmentation Effect in Model Solutions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopez, M.I.; Sanchez, M.T.; Diaz, A.; Ramirez, P.; Morales, J. Influence of a deficit irrigation regime during ripening on berry composition in grapevines (Vitis vinifera L.) grown in semi-arid areas. Int. J. Food Sci. Nutr. 2007, 58, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Mira de Orduña, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Gordillo, B.; Rodríguez-Pulido, F.J.; Escudero-Gilete, M.L.; González-Miret, M.L.; Heredia, F.J. Comprehensive colorimetric study of anthocyanic copigmentation in model solutions. Effects of pH and molar ratio. J. Agric. Food Chem. 2012, 60, 2896–2905. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Bastante, M.J.; Rodríguez-Morgado, B.; Jara-Palacios, M.J.; Rivas-Gonzalo, J.C.; Parrado, J.; Heredia, F.J. Pre-fermentative addition of an enzymatic grape seed hydrolysate in warm climate winemaking. Effect on the differential colorimetry, copigmentation and polyphenolic profiles. Food Chem. 2016, 209, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, B.; Cejudo-Bastante, M.J.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; Heredia, F.J. Application of the differential colorimetry and polyphenolic profile to the evaluation of the chromatic quality of Tempranillo red wines elaborated in warm climate. Influence of the presence of oak wood chips during fermentation. Food Chem. 2013, 141, 2184–2190. [Google Scholar] [CrossRef]
- Pedroza, M.A.; Carmona, M.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. Pre-bottling use of dehydrated waste grape skins to improve colour, phenolic and aroma composition of red wines. Food Chem. 2013, 136, 224–236. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Rodríguez-Pulido, F.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Determination of phenolic substances of seeds skins and stems from white grape marc by near-infrared hyperspectral imaging. Aust. J. Grape Wine Res. 2016, 22, 11–15. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. The use of grape seed by-products rich in flavonoids to improve the antioxidant potential of red wines. Molecules 2016, 21, 1526. [Google Scholar] [CrossRef]
- Rivero, F.J.; Gordillo, B.; Jara-Palacios, M.J.; González-Miret, M.L.; Heredia, F.J. Effect of addition of overripe seeds from white grape by-products during red wine fermentation on wine colour and phenolic composition. LWT-Food Sci. Technol. 2017, 84, 544–550. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Gordillo, B.; González-Miret, M.L.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Comparative study of the enological potential of different winemaking Byproducts: Implications in the antioxidant activity and color expression of red wine anthocyanins in a model solution. J. Agric. Food Chem. 2014, 62, 6975–6983. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ortin, A.B.; Martinez-Cutillas, A.; Ros-Garcia, J.M.; Lopez-Roca, J.M.; Gomez-Plaza, E. Improving colour extraction and stability in red wines: The use of maceration enzymes and enological tannins. Int. J. Food Sci. Technol. 2005, 40, 867–878. [Google Scholar] [CrossRef]
- Vivas, N.; Glories, Y. Role of oak wood ellagitannins in the oxidation process of red wines during aging. Am. J. Enol. Vitic. 1996, 47, 103–107. [Google Scholar]
- Baca-Bocanegra, B.; Nogales-Bueno, J.; Hernández-Hierro, J.M.; Heredia, F.J. Evaluation of extractable polyphenols released to wine from cooperage byproduct by near infrared hyperspectral imaging. Food Chem. 2018, 244, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Baca-Bocanegra, B.; Nogales-Bueno, J.; García-Estévez, I.; Escribano-Bailón, M.T.; Hernández-Hierro, J.M.; Heredia, F.J. Screening of wine extractable total phenolic and ellagitannin contents in revalorized cooperage by products: Evaluation by Micro-NIRS technology. Food Bioprocess Technol. 2019, 12, 477–485. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Gordon, M.H.; Pérez-Coello, M.S. A study of the antioxidant capacity of oak wood in wine ageing and the correlation with polyphenol composition. Food Chem. 2011, 128, 997–1002. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Hermosín-Gutiérrez, I.; Gordon, M.H.; Pérez-Coello, M.S. Antioxidant capacity and phenolic composition of different woods used in cooperage. Food Chem. 2011, 129, 1584–1590. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Jara-Palacios, M.J.; Goncalves, S.; Hernanz, D.; Heredia, F.J.; Romano, A. Effects of in vitro gastrointestinal digestion on phenolic compounds and antioxidant activity of different white winemaking byproducts extracts. Food Res. Int. 2018, 109, 433–439. [Google Scholar] [CrossRef]
- García-Estévez, I.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Alcalde-Eon, C. Validation of a mass spectrometry method to quantify oak ellagitannins in wine samples. J. Agric. Food Chem. 2012, 60, 1373–1379. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Heredia, F.J.; Álvarez, C.; González-Miret, M.L.; Ramírez, A. CromaLab®, Análisis de Color; Registro General de la Propiedad Intelectual: Sevilla, Spain, 2004.
- CIE. Technical Report Colorimetry; Commission Internationale de l’Eclairage Central Bureau: Vienna, Austria, 2004. [Google Scholar]
- Boulton, R. A method for the assessment of copigmentation in red wines. In Proceedings of the Conference of the 47th Annual Meeting of the American Society for Enology and Viticulture, Reno, NV, USA, 26–28 June 1996. [Google Scholar]
- StatSoft Inc. STATISTICA (Data Analysis Software System), V 8.0; StatSoft Inc.: Tulsa, OK, USA, 2007. [Google Scholar]
- Prida, A.; Puech, J.L. Influence of geographical origin and botanical species on the content of extractives in American, French, and East European oak woods. J. Agric. Food Chem. 2006, 54, 8115–8126. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, I.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Alcalde-Eon, C. Development of a fractionation method for the detection and identification of oak ellagitannins in red wines. Anal. Chim. Acta 2010, 660, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Glabasnia, A.; Hofmann, T. Sensory-directed identification of taste-active ellagitannins in American (Quercus alba L.) and European oak wood (Quercus robur L.) and quantitative analysis in bourbon whiskey and oak-matured red wines. J. Agric. Food Chem. 2006, 54, 3380–3390. [Google Scholar] [CrossRef]
- Viriot, C.; Scalbert, A.; Herve du Penhoat, C.L.M.; Moutounet, M. Ellagitannins in woods of sessile oak and sweet chestnut dimerization and hydrolysis during wood ageing. Phytochemistry 1994, 36, 1253–1260. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Jara-Palacios, M.J.; Hernández-Hierro, J.M.; Heredia, F.J. Evaluation of the influence of white grape seed extracts as copigment sources on the anthocyanin extraction from grape skins previously classified by near infrared hyperspectral tools. Food Chem. 2017, 221, 1685–1690. [Google Scholar] [CrossRef]
- López de Lerma, N.; Peinado, J.; Peinado, R.A. In vitro and in vivo antioxidant activity of musts and skin extracts from off-vine dried Vitis vinifera cv. “Tempranillo” grapes. J. Funct. Foods 2013, 5, 914–922. [Google Scholar] [CrossRef]
- Martínez, J.A.; Melgosa, M.; Pérez, M.M.; Hita, E.; Negueruela, A.I. Visual and instrumental color evaluation in red wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Chassaing, S.; Lefeuvre, D.; Jacquet, R.; Jourdes, M.; Ducasse, L.; Galland, S.; Grelard, A.; Saucier, C.; Teissedre, P.L.; Dangles, O. Physicochemical studies of new anthocyano-ellagitannin hybrid pigments: About the origin of the influence of oak C-glycosidic ellagitannins on wine color. Eur. J. Org. Chem. 2010, 2010, 55–63. [Google Scholar] [CrossRef]
- Kunsagí-Máté, S.; Ortmann, E.; Kollár, L.; Kikfardjam, M.P. Effect of the solvatation shell exchange on the formation of malvidin-3-O-glucoside-ellagic acid complexes. J. Phys. Chem. B 2007, 111, 11750–11755. [Google Scholar] [CrossRef] [PubMed]
- Kunsagí-Máté, S.; Ortmann, E.; Kollár, L.; Kikfardjam, M.P. Entropy-driven complex formation of malvidin-3-O-glucoside with common polyphenols in ethanol-water binary solutions. Spectrochim. Acta Part A 2008, 70, 860–865. [Google Scholar] [CrossRef]
- Zhang, B.; He, F.; Liu, Y.; Cai, J.; Duan, C.Q. Impact of adding ellagic acid to red grapes on the phenolic composition and chromatic quality of cabernet sauvignon wines from warm climate. J. Food Process. Preserv. 2017, 41, e13080. [Google Scholar] [CrossRef]
- Zhang, X.K.; He, F.; Zhang, B.; Reeves, M.J.; Liu, Y.; Zhao, X.; Duan, C.Q. The effect of prefermentative addition of gallic acid and ellagic acid on the red wine color, copigmentation and phenolic profiles during wine aging. Food Res. Int. 2018, 106, 568–579. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, J.; Duan, C.Q.; Reeves, M.J.; He, F. A review of polyphenolics in oak woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef] [PubMed]
| AOW | UOW | |||
| TPC (mg/L) | 556.58 ± 87.94 a | 665.47 ± 80.98 a | ||
| Compound | MW | MS | Relative Proportion (%) | |
| Ellagitanins | ||||
| Monomers | ||||
| Vescalagin | 934 | 933, 466 | 28.5 | 29.2 |
| Castalagin | 934 | 933, 466 | 26.4 | 27.9 |
| Grandinin | 1066 | 1065 | 4.8 | 4.7 |
| Roburin E | 1066 | 1065 | 5.7 | 6.1 |
| Dimers | ||||
| Roburin A | 1851 | 924, 616 | 11.0 | 9.9 |
| Roburin D | 1851 | 924, 616 | 7.4 | 7.2 |
| Roburin B | 1983 | 990, 660 | 9.3 | 8.7 |
| Roburin C | 1983 | 990, 660 | 3.8 | 3.4 |
| Phenolic acids | ||||
| Ellagic acid | 302 | 301 | 0.5 | 0.4 |
| Gallic acid | 170 | 169 | 2.7 | 3.0 |
| AOW | UOW | Skins Anthocyanin Extract | |
|---|---|---|---|
| ABTS (µmol TE/L) | 6733.79 ± 319.35 a | 7713.61 ± 411.34 a | 6919.83 ± 106.29 |
| FRAP (µmol TE/L) | 2385.29 ± 406.12 a | 3809.98 ± 508.80 b | 4950.64 ± 61.56 |
| Anthocyanin | Relative Proportion (%) |
|---|---|
| Delphinidin-3-O-glucoside | 6.1 |
| Cyanidin-3-O-glucoside | 1.2 |
| Petunidin-3-O-glucoside | 9.2 |
| Peonidin-3-O-glucoside | 7.4 |
| Malvidin-3-O-glucoside | 44.7 |
| Petunidin-3-O-acetyl-glucoside | 1.6 |
| Peonidin-3-O-acetyl-glucoside | 1.1 |
| Malvidin-3-O-acetyl-glucoside | 16.4 |
| Petunidin-3-O-p-coumaroyl-glucoside | 1.6 |
| Peonidin-3-O-p-coumaroyl-glucoside | 2.5 |
| Malvidin-3-O-p-coumaroyl-glucoside | 8.1 |
| TAC (mg/L) | 253.79 ± 15.96 |
| AOW | UOW | |||
|---|---|---|---|---|
| ABTS | FRAP | ABTS | FRAP | |
| Control solution | 1300.89 ± 37.33 a | 509.45 ± 41.32 a | 1300.89 ± 37.33 a | 509.45 ± 41.32 a |
| Model solution | ||||
| 100 mg/L | 1489.61 ± 224.16 a | 608.05 ± 2.85 a | 1396.81 ± 33.74 a | 716.63 ± 76.88 a |
| 400 mg/L | 2248.57 ± 0.97 b | 927.43 ± 40.97 b | 2037.66 ± 33.74 b | 847.93 ± 87.92 b |
| 500 mg/L | 1707.33 ± 274.98 b | 851.27 ± 153.28 b | 1839.07 ± 150.59 b | 1006.12 ± 43.28 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero-Gilete, M.L.; Hernanz, D.; Galán-Lorente, C.; Heredia, F.J.; Jara-Palacios, M.J. Potential of Cooperage Byproducts Rich in Ellagitannins to Improve the Antioxidant Activity and Color Expression of Red Wine Anthocyanins. Foods 2019, 8, 336. https://doi.org/10.3390/foods8080336
Escudero-Gilete ML, Hernanz D, Galán-Lorente C, Heredia FJ, Jara-Palacios MJ. Potential of Cooperage Byproducts Rich in Ellagitannins to Improve the Antioxidant Activity and Color Expression of Red Wine Anthocyanins. Foods. 2019; 8(8):336. https://doi.org/10.3390/foods8080336
Chicago/Turabian StyleEscudero-Gilete, María Luisa, Dolores Hernanz, Celia Galán-Lorente, Francisco J. Heredia, and María José Jara-Palacios. 2019. "Potential of Cooperage Byproducts Rich in Ellagitannins to Improve the Antioxidant Activity and Color Expression of Red Wine Anthocyanins" Foods 8, no. 8: 336. https://doi.org/10.3390/foods8080336
APA StyleEscudero-Gilete, M. L., Hernanz, D., Galán-Lorente, C., Heredia, F. J., & Jara-Palacios, M. J. (2019). Potential of Cooperage Byproducts Rich in Ellagitannins to Improve the Antioxidant Activity and Color Expression of Red Wine Anthocyanins. Foods, 8(8), 336. https://doi.org/10.3390/foods8080336








