3.1. Headspace Gas Composition
Packaging cherries with a Topaz film creates a modified internal atmosphere (reduced O
2 and increased CO
2 content) via the metabolism of the fruit. The Life
+ packaged cherries reached a steady-state atmosphere (14 kPa for O
2 and 8 kPa for CO
2) after just one day. The same trend was reported in a previous study by Caner et al. (2008), in which the observed steady-state O
2 concentration was above that recommended for O
2 (3–10 kPa) for MAP cherries [
24]. In the case of cherries, the observed CO
2 levels fell within the recommended levels (8–10 kPa) in MAP storage conditions [
25], a result that is attributed to the ability of the film to regulate O
2 and CO
2 concentrations inside the packaging. By comparison, no O
2 or CO
2 changes were detected in the control packages due to the presence of holes on the container.
The O
2 concentrations inside all the Life
+ packaged strawberries decreased rapidly during the initial days of storage, and a steady-state concentration was established between the second and third day. According to Chen et al. [
26], MAP packaging takes approximately three days to generate a steady-state atmosphere passively through produce respiration. In this study, the O
2 concentration fell from 21 kPa to a steady-state value of about 12 kPa, whereas the CO
2 concentration hovered around 10 kPa in both treatments following an initial rapid increase in CO
2 concentration from 0 kPa to 6 kPa inside all the packages during the first day. Overall, the study values were similar to previous strawberry research [
27,
28]. As expected, the O
2 and CO
2 values were unchanged in the clamshell packages due to the presence of holes on the containers.
3.2. Weight Loss
The weight lost from the loss of water in the cherries increased during storage in both treatments (
Table 1). In general, losses were significantly smaller (below 0.12%) after 15 days of storage with the use of the innovative packaging. The decreasing water losses during storage effectively limited undesirable wilting, shriveling, and color changes. Moreover, it is assumed that the Life
+ system maintained a higher relative humidity in the packs due to the heat sealing that reduced the vapor pressure deficit and, consequently, lowered the transpiration.
The weight loss of the strawberry samples in storage under passive EMAP and the control is shown in
Table 2. When fruits like strawberries are stored in MAP, their weight loss is usually very low (0.2%–2%) [
27,
28,
29,
30]. Life
+ creates a modified atmosphere with higher CO
2 and lower O
2 concentrations around the produce, which slows metabolic processes and transpiration. Moreover, due to an adequate water vapor barrier inside the packaging, the samples yielded an extremely small weight loss compared to the control. By the end of the storage, the cumulative weight losses in the Life
+ samples averaged <0.1%, compared to an average of 3.5% for the control fruit.
3.3. Quality Parameter
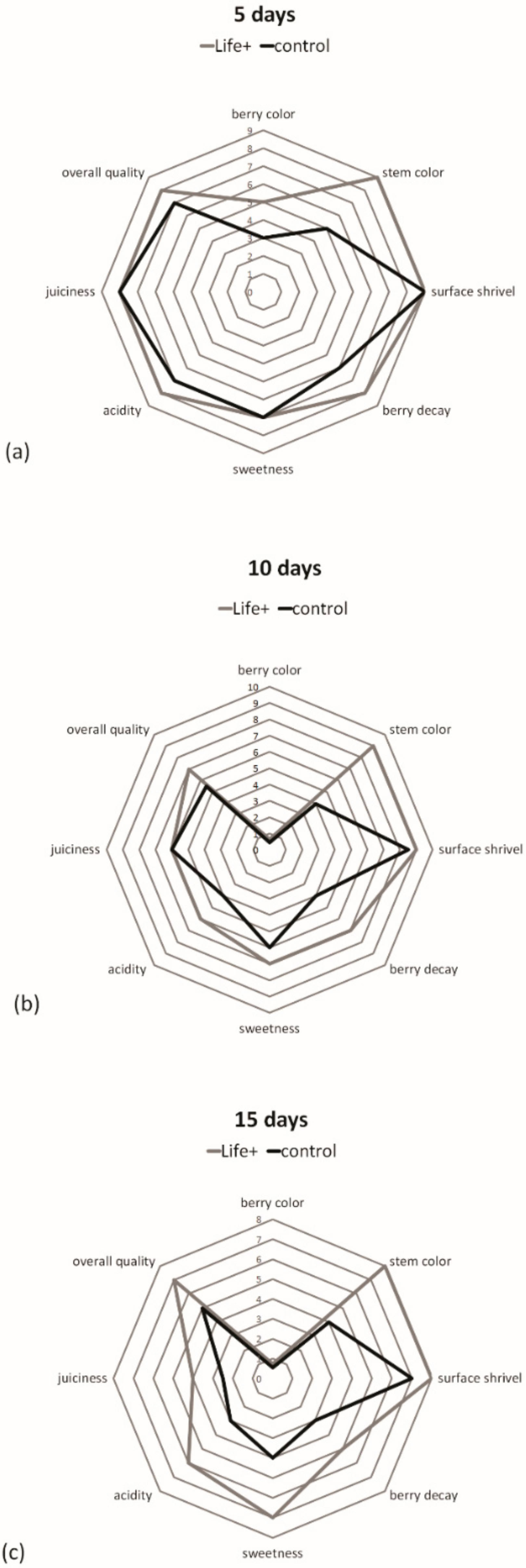
At harvest, the chroma index (color intensity or saturation) for cherries was 17.71, which diminished significantly during storage in both treatments. Despite the trend, the Life
+ packaged cherries had significantly higher values throughout storage (
Table 3), which likely indicates that the fruits stored with EMAP retained their vivid color longer during storage. Fruit lightness (or brightness), which measures the ratio of white (100) to black (0) color, decreased significantly during storage. On the other hand, the L * value differences among the treatments were not significant, with the exception of Life
+ fruits after 10 days of storage. In this case, the cherries had a more brilliant and lighter color. In all cases, the study results aligned with those of Serrano et al. and Valero et al. [
31,
32].
The total soluble solid (TSS) content is related to the taste for a variety of fruit, including strawberries and cherries, and is commonly used to establish the optimal harvest and postharvest quality. The initial TSS (17.5%) of fresh cherries indicated good maturity, as described by Kappel et al. [
33]. In this study, the TSS values for the Life
+ samples initially decreased and then increased slightly during storage as a result of ripening (
Table 4). The control cherry TSS values did not change during storage.
Storage time caused a significant decrease in the acidity of cherry samples (
Table 4). Among treatments, TA was significantly higher with the Life
+ system than with the control. This effect is considered important because the preservation of TA during storage maintains the aroma and taste of freshly-harvested cherries. A decrease in TA is a natural trend during fruit ripening. However, higher values found in active packaging result in higher quality during storage. Caner et al. [
10] reported the same results in a study where the equilibrium of the atmosphere reached in the packaging affected the senescence and significantly reduced the loss of quality.
The pH level increased slightly during storage under both treatments (
Table 4). At the end of storage, significant differences were detected between the EMAP and the control, which is similar to other results [
34,
35,
36]. The equilibrium-modified-atmosphere inside the Life+ packaging had no significant effect on pH.
The Vitamin C content in the cherry samples was measured on day 0 and day 15 of cold storage. Significant differences were observed between the control and treatment (
Table 4). Vitamin C content losses in the control were significantly higher than those in the Life
+ samples. Vitamin C declines rapidly after harvest and during storage. In general, MAP-treated cherries maintained their initial level of Vitamin C content during storage by establishing an atmosphere (appropriate CO
2 and low O
2 concentrations) slowed fruit respiration and decreased senescence. Qian et al. [
37] described how an appropriate concentration of CO
2 prevents declines in vitamin C due to the reduction of enzymatic synthesis.
The lightness (L * value) and intensity of color (C * value) of “Marmolada” strawberries are displayed in
Table 5. The chroma index (C *) reflects color saturation; higher values correspond to the development of an intense red color. In this case, the higher values of C * measured on day 5 and 10 in the Life
+-packaged fruit indicated that the red color of the strawberries was purer and more vivid than that of the control.
The L * values significantly decreased during storage as a consequence of the pink-red color that naturally develops when strawberries ripen. In this study, the L * value of the actively-packaged fruit remained higher than that of the control packages. The same trend was observed for ‘Selva’ strawberries during another postharvest study [
38].
The results from the evaluation of strawberry quality during storage are summarized in
Table 6. In this study, the TSS of the ‘Marmolada’ strawberries in EMAP was relatively stable throughout storage, whereas TSS declined significantly in the control samples. The lower values in the control can be explained by the use of sugars for respiration during storage [
36].
As with TSS, the total acid content (TA) of the control strawberries decreased significantly during storage (
Table 6), probably due to greater respiration and higher amounts of organic acids that were used as substrates in this process. Specifically, a comparison of the TA postharvest quality results of the control-packaged fruit to the Life
+ system-packaged fruit showed sizeable decreases in the former and only a slight decrease in the latter packaging over time.
Measurements of pH in both the control and Life
+ fruit were close to 4.5. No significant variation in pH was detected during storage for either treatment (
Table 6). Previous studies [
39,
40], have also found the pH to be unaffected by storage or MAP packaging.
3.4. Decay Incidence
The effect of packaging on cherry decay is shown in
Table 7. Fruit was considered unacceptable when one disorder, mainly fungal spoilage, was visible, according to the parameters established by Sanz et al. [
41]. Disorders were evident on the fifth day of storage. The number of infected fruit grew significantly with storage for both treatments. The percentage of decay was lower in the Life
+ samples than in the control, and a difference was significant at every time of measurement. After 15 days, the control exhibited the greatest level of visible disorders with about 5% of the cherries having infections (
Table 7). These data support the Life
+ system claims that their system protects fruit against fungal and microbial attack, and that this effect persists throughout storage, with a positive and effective impact on waste reduction at the retail and consumer levels.
This study demonstrated that the decay incidence in strawberries was significantly affected by treatment. Five percent of the control berries showed fungal development after five days, and this percentage grew during storage. On the other hand, the Life+ system fruit’s decay was delayed and significantly less significant. Five days after storage, no disorders were observed. By the end of storage, the number of infected strawberries in the active packaging reached 8%, compared with 30% in the control. The effect of the pad on decay reduction was clearly observed in the active packaging.