Antidiabetic Effects of Hydroxytyrosol: In Vitro and In Vivo Evidence
Abstract
1. Introduction
2. In Vitro Evidence: Antidiabetic Effects of Hydroxytyrosol (HT)
2.1. Effects of Hydroxytyrosol (HT) on Skeletal Muscle Cells
2.2. Effects of Hydroxytyrosol (HT) on Adipocytes
2.3. Effects of Hydroxytyrosol (HT) on Hepatocytes
2.4. Effects of Hydroxytyrosol (HT) on Pancreatic Cells
3. In Vivo Evidence: Antidiabetic Effect of Hydroxytyrosol
3.1. Effect of Hydroxytyrosol (HT) on Alloxan-Induced Diabetes in Rodents
3.2. Effect of Hydroxytyrosol (HT) on Streptozotocin-Induced Diabetes in Rodents
3.3. Effect of Hydroxytyrosol (HT) on Genetically-Induced Diabetes in Rodents
3.4. Effect of Hydroxytyrosol (HT) on Diet-Induced Diabetes in Rodents
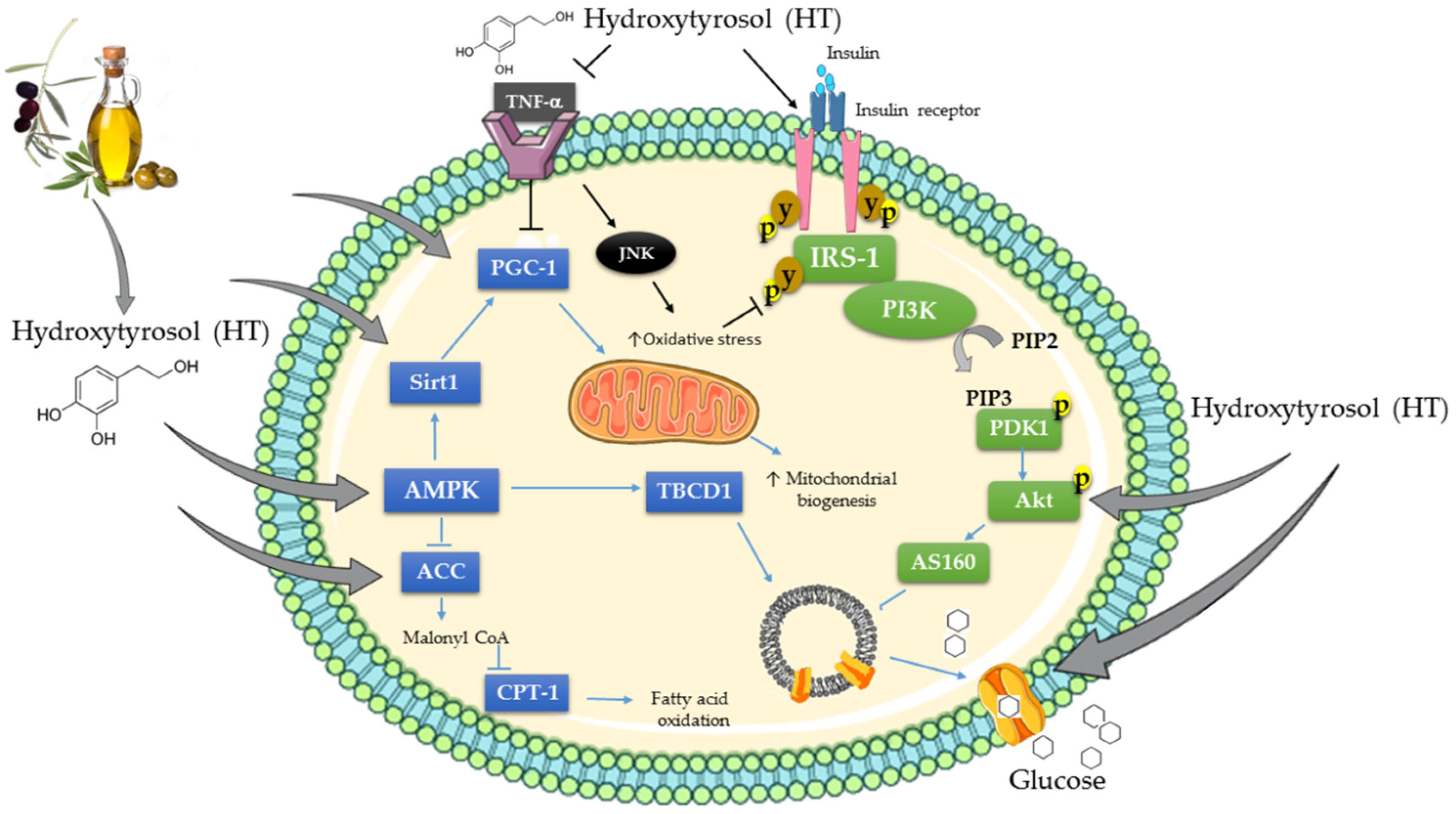
4. Effects of Hydroxytyrosol (HT) on Cellular Signaling Cascades
5. Summary, Conclusion and Future Directions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Anderson, J.T.; Keys, A. Dietary protein and the serum cholesterol level in man. Am. J. Clin. Nutr. 1957, 5, 29–34. [Google Scholar] [PubMed]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: II. The effect of cholesterol in the diet. Metab. Clin. Exp. 1965, 14, 759–765. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: III. Differences among individuals. Metab. Clin. Exp. 1965, 14, 766–775. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: IV. Particular saturated fatty acids in the diet. Metab. Clin. Exp. 1965, 14, 776–787. [Google Scholar] [CrossRef]
- Altomare, R.; Cacciabaudo, F.; Damiano, G.; Palumbo, V.D.; Gioviale, M.C.; Bellavia, M.; Tomasello, G.; Lo Monte, A.I. The Mediterranean Diet: A History of Health. Iran. J. Public Health 2013, 42, 449–457. [Google Scholar] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Barak, Y.; Fridman, D. Impact of Mediterranean Diet on Cancer: Focused Literature Review. Cancer Genom. Proteom. 2017, 14, 403–408. [Google Scholar]
- Lăcătușu, C.-M.; Grigorescu, E.-D.; Floria, M.; Onofriescu, A.; Mihai, B.-M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Berry, E.M.; Arnoni, Y.; Aviram, M. The Middle Eastern and biblical origins of the Mediterranean diet. Public Health Nutr. 2011, 14, 2288–2295. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive Oil Phenols Are Absorbed in Humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Shamshoum, H.; Vlavcheski, F.; Tsiani, E. Anticancer effects of oleuropein. Biofactors 2017, 43, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Pinya, S.; Del Mar Bibiloni, M.; Tur, J.A.; Pons, A.; Sureda, A. Cardioprotective Effects of the Polyphenol Hydroxytyrosol from Olive Oil. Curr. Drug Targets 2017, 18, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tuomilehto, J. Olive Oil Nutraceuticals in the Prevention and Management of Diabetes: From Molecules to Lifestyle. Int. J. Mol. Sci. 2018, 19, 2024. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Peyrol, J.; Riva, C.; Amiot, M.J. Hydroxytyrosol in the Prevention of the Metabolic Syndrome and Related Disorders. Nutrients 2017, 9, 306. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Gani, A.; Baba, W.N.; Rahmanian, N.; Akhter, R.; Wani, I.A.; Ahmad, M. Olive oil and its principal bioactive compound: Hydroxytyrosol—A review of the recent literature. Trends Food Sci. Technol. 2018, 77, 77–90. [Google Scholar] [CrossRef]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the Olive-Derived Polyphenol Oleuropein on Human Health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, S.; Kitaichi, K.; Hori, A.; Suwa, T.; Horikawa, Y.; Yamamoto, M.; Takeda, J.; Itoh, Y. The evaluation of risk factors associated with adverse drug reactions by metformin in type 2 diabetes mellitus. Biol. Pharm. Bull. 2012, 35, 933–937. [Google Scholar] [CrossRef]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metab. Clin. Exp. 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hopper, I.; Skiba, M.; Krum, H. Dipeptidyl peptidase-4 inhibitors and cardiovascular outcomes: meta-analysis of randomized clinical trials with 55,141 participants. Cardiovasc. Ther. 2014, 32, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Forsmark, C.E. Incretins, Diabetes, Pancreatitis and Pancreatic Cancer: What the GI specialist needs to know. Pancreatology 2016, 16, 10–13. [Google Scholar] [CrossRef]
- Blau, J.E.; Tella, S.H.; Taylor, S.I.; Rother, K.I. Ketoacidosis associated with SGLT2 inhibitor treatment: Analysis of FAERS data. Diabetes Metab. Res. Rev. 2017, 33, 10–1002. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Li, S.; Jia, P.; Deng, K.; Chen, W.; Sun, X. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci. Rep. 2017, 7, 2824. [Google Scholar] [CrossRef]
- Friedel, A.; Raederstorff, D.; Roos, F.; Toepfer, C.; Wertz, K. Hydroxytyrosol Benefits Muscle Differentiation and Muscle Contraction and Relaxation. U.S. Patent Application No. 13/550,972, 7 March 2013. [Google Scholar]
- Burattini, S.; Salucci, S.; Baldassarri, V.; Accorsi, A.; Piatti, E.; Madrona, A.; Espartero, J.L.; Candiracci, M.; Zappia, G.; Falcieri, E. Anti-apoptotic activity of hydroxytyrosol and hydroxytyrosyl laurate. Food Chem. Toxicol. 2013, 55, 248–256. [Google Scholar] [CrossRef]
- Drira, R.; Sakamoto, K. Modulation of adipogenesis, lipolysis and glucose consumption in 3T3-L1 adipocytes and C2C12 myotubes by hydroxytyrosol acetate: A comparative study. Biochem. Biophys. Res. Commun. 2013, 440, 576–581. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Zheng, A.; Yang, L.; Liu, J.; Chen, C.; Tang, Y.; Zou, X.; Li, Y.; Long, J.; et al. Mitochondrial dysfunction-associated OPA1 cleavage contributes to muscle degeneration: Preventative effect of hydroxytyrosol acetate. Cell. Death Dis. 2014, 5, e1521. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Shen, W.; Yu, G.; Jia, H.; Li, X.; Feng, Z.; Wang, Y.; Weber, P.; Wertz, K.; Sharman, E.; et al. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J. Nutr. Biochem. 2010, 21, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Warnke, I.; Goralczyk, R.; Fuhrer, E.; Schwager, J. Dietary constituents reduce lipid accumulation in murine C3H10 T1/2 adipocytes: A novel fluorescent method to quantify fat droplets. Nutr. Metab. 2011, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Drira, R.; Chen, S.; Sakamoto, K. Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in 3 T3-L1 cells. Life Sci. 2011, 89, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Drira, R.; Sakamoto, K. Hydroxytyrosol stimulates lipolysis via A-kinase and extracellular signal-regulated kinase activation in 3T3-L1 adipocytes. Eur. J. Nutr. 2014, 53, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Massaro, M.; Carluccio, M.A.; Pellegrino, M.; Wabitsch, M.; Calabriso, N.; Storelli, C.; De Caterina, R. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic Acid and hydroxytyrosol in human adipocytes. PLoS ONE 2015, 10, e0128218. [Google Scholar] [CrossRef] [PubMed]
- Tutino, V.; Orlando, A.; Russo, F.; Notarnicola, M. Hydroxytyrosol Inhibits Cannabinoid CB1 Receptor Gene Expression in 3T3-L1 Preadipocyte Cell Line. J. Cell. Physiol. 2016, 231, 483–489. [Google Scholar] [CrossRef]
- Engeli, S.; Böhnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Bátkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Sharma, A.M.; et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef]
- Stefanon, B.; Colitti, M. Original Research: Hydroxytyrosol, an ingredient of olive oil, reduces triglyceride accumulation and promotes lipolysis in human primary visceral adipocytes during differentiation. Exp. Biol. Med. 2016, 241, 1796–1802. [Google Scholar] [CrossRef]
- Anter, J.; Quesada-Gómez, J.M.; Dorado, G.; Casado-Díaz, A. Effect of Hydroxytyrosol on Human Mesenchymal Stromal/Stem Cell Differentiation into Adipocytes and Osteoblasts. Arch. Med. Res. 2016, 47, 162–171. [Google Scholar] [CrossRef]
- Pan, S.; Liu, L.; Pan, H.; Ma, Y.; Wang, D.; Kang, K.; Wang, J.; Sun, B.; Sun, X.; Jiang, H. Protective effects of hydroxytyrosol on liver ischemia/reperfusion injury in mice. Mol. Nutr. Food Res. 2013, 57, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Priore, P.; Siculella, L.; Gnoni, G.V. Extra virgin olive oil phenols down-regulate lipid synthesis in primary-cultured rat-hepatocytes. J. Nutr. Biochem. 2014, 25, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Senent, F.; de Roos, B.; Duthie, G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Inhibitory and synergistic effects of natural olive phenols on human platelet aggregation and lipid peroxidation of microsomes from vitamin E-deficient rats. Eur. J. Nutr. 2015, 54, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Jardine, D.; Antolovich, M.; Prenzler, P.D.; Robards, K. Liquid chromatography-mass spectrometry (LC-MS) investigation of the thiobarbituric acid reactive substances (TBARS) reaction. J. Agric. Food Chem. 2002, 50, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Gómez-Carretero, A.; Maya, I.; Fernández-Bolaños, J.; Duthie, G.G.; de Roos, B. Selenium and sulphur derivatives of hydroxytyrosol: Inhibition of lipid peroxidation in liver microsomes of vitamin E-deficient rats. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009, 180, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Velander, P.; Liu, D.; Xu, B. Olive Component Oleuropein Promotes β-Cell Insulin Secretion and Protects β-Cells from Amylin Amyloid-Induced Cytotoxicity. Biochemistry 2017, 56, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, C.; Haataja, L.; Gurlo, T.; Butler, A.E.; Rizza, R.A.; Butler, P.C. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 2007, 56, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Gurlo, T.; Ryazantsev, S.; Huang, C.; Yeh, M.W.; Reber, H.A.; Hines, O.J.; O’Brien, T.D.; Glabe, C.G.; Butler, P.C. Evidence for proteotoxicity in beta cells in type 2 diabetes: Toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am. J. Pathol. 2010, 176, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Mulder, H. Transcribing β-cell mitochondria in health and disease. Mol. Metab. 2017, 6, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, V.R.; de la Puerta, R.; Catalá, A. The effect of tyrosol, hydroxytyrosol and oleuropein on the non-enzymatic lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 2001, 217, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Stupans, I.; Kirlich, A.; Tuck, K.L.; Hayball, P.J. Comparison of Radical Scavenging Effect, Inhibition of Microsomal Oxygen Free Radical Generation, and Serum Lipoprotein Oxidation of Several Natural Antioxidants. J. Agric. Food Chem. 2002, 50, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Jemai, H.; El Feki, A.; Sayadi, S. Antidiabetic and Antioxidant Effects of Hydroxytyrosol and Oleuropein from Olive Leaves in Alloxan-Diabetic Rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Allouche, N.; Jouadi, B.; El-Fazaa, S.; Gharbi, N.; Carreau, S.; Damak, M.; Elfeki, A. Inhibitory action of purified hydroxytyrosol from stored olive mill waste on intestinal disaccharidases and lipase activities and pancreatic toxicity in diabetic rats. Food Sci. Biotechnol. 2010, 19, 439. [Google Scholar] [CrossRef]
- Feher, J. 8.5-Digestion and Absorption of the Macronutrients. In Quantitative Human Physiology, 2nd ed.; Feher, J., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 821–833. [Google Scholar]
- Ristagno, G.; Fumagalli, F.; Porretta-Serapiglia, C.; Orrù, A.; Cassina, C.; Pesaresi, M.; Masson, S.; Villanova, L.; Merendino, A.; Villanova, A.; et al. Hydroxytyrosol attenuates peripheral neuropathy in streptozotocin-induced diabetes in rats. J. Agric. Food Chem. 2012, 60, 5859–5865. [Google Scholar] [CrossRef]
- López-Villodres, J.A.; Abdel-Karim, M.; De La Cruz, J.P.; Rodríguez-Pérez, M.D.; Reyes, J.J.; Guzmán-Moscoso, R.; Rodriguez-Gutierrez, G.; Fernández-Bolaños, J.; González-Correa, J.A. Effects of hydroxytyrosol on cardiovascular biomarkers in experimental diabetes mellitus. J. Nutr. Biochem. 2016, 37, 94–100. [Google Scholar] [CrossRef]
- Reyes, J.J.; Villanueva, B.; López-Villodres, J.A.; De La Cruz, J.P.; Romero, L.; Rodríguez-Pérez, M.D.; Rodriguez-Gutierrez, G.; Fernández-Bolaños, J.; González-Correa, J.A. Neuroprotective Effect of Hydroxytyrosol in Experimental Diabetes Mellitus. J. Agric. Food Chem. 2017, 65, 4378. [Google Scholar] [CrossRef]
- González-Correa, J.A.; Rodríguez-Pérez, M.D.; Márquez-Estrada, L.; López-Villodres, J.A.; Reyes, J.J.; Rodriguez-Gutierrez, G.; Fernández-Bolaños, J.; De La Cruz, J.P. Neuroprotective Effect of Hydroxytyrosol in Experimental Diabetic Retinopathy: Relationship with Cardiovascular Biomarkers. J. Agric. Food Chem. 2018, 66, 637–644. [Google Scholar] [CrossRef]
- Wang, W.; Shang, C.; Zhang, W.; Jin, Z.; Yao, F.; He, Y.; Wang, B.; Li, Y.; Zhang, J.; Lin, R. Hydroxytyrosol NO regulates oxidative stress and NO production through SIRT1 in diabetic mice and vascular endothelial cells. Phytomedicine 2019, 52, 206–215. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Jemai, H.; Bouaziz, M.; Fki, I.; El Feki, A.; Sayadi, S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Biol. Interact. 2008, 176, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, M.; Sarriá, B.; Largo, C.; Martínez-López, S.; Madrona, A.; Espartero, J.L.; Bravo, L.; Mateos, R. Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct. 2014, 5, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.; Ribot, J.; Sabater, A.G.; Palou, A.; Bonet, M.L.; Klaus, S. Identification of Mest/Peg1 gene expression as a predictive biomarker of adipose tissue expansion sensitive to dietary anti-obesity interventions. Genes Nutr. 2015, 10, 27. [Google Scholar] [CrossRef]
- Pirozzi, C.; Lama, A.; Simeoli, R.; Paciello, O.; Pagano, T.B.; Mollica, M.P.; Di Guida, F.; Russo, R.; Magliocca, S.; Canani, R.B.; et al. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J. Nutr. Biochem. 2016, 30, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Lemonakis, N.; Efentakis, P.; Gikas, E.; Halabalaki, M.; Andreadou, I.; Skaltsounis, L.; Brown, L. Hydroxytyrosol ameliorates metabolic, cardiovascular and liver changes in a rat model of diet-induced metabolic syndrome: Pharmacological and metabolism-based investigation. Pharmacol. Res. 2017, 117, 32–45. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Ma, Y.; Wen, D. Hydroxytyrosol ameliorates insulin resistance by modulating endoplasmic reticulum stress and prevents hepatic steatosis in diet-induced obesity mice. J. Nutr. Biochem. 2018, 57, 180–188. [Google Scholar] [CrossRef]
- Echeverría, F.; Valenzuela, R.; Espinosa, A.; Bustamante, A.; Álvarez, D.; Gonzalez-Mañan, D.; Ortiz, M.; Soto-Alarcon, S.A.; Videla, L.A. Reduction of high-fat diet-induced liver proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J. Nutr. Biochem. 2018, 63, 35–43. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, Y.; Chen, Z.; Lu, W.; Li, N.; Wang, Q.; Shao, L.; Li, Y.; Yang, G.; Bian, X. A new multifunctional hydroxytyrosol-fenofibrate with antidiabetic, antihyperlipidemic, antioxidant and antiinflammatory action. Biomed. Pharmacother. 2017, 95, 1749–1758. [Google Scholar] [CrossRef]
- Xie, Y.-D.; Chen, Z.-Z.; Li, N.; Lu, W.-F.; Xu, Y.-H.; Lin, Y.-Y.; Shao, L.-H.; Wang, Q.-T.; Guo, L.-Y.; Gao, Y.-Q.; et al. Hydroxytyrosol nicotinate, a new multifunctional hypolipidemic and hypoglycemic agent. Biomed. Pharmacother. 2018, 99, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-D.; Chen, Z.-Z.; Shao, L.-H.; Wang, Q.-T.; Li, N.; Lu, W.-F.; Xu, Y.-H.; Gao, Y.-Q.; Guo, L.-Y.; Liu, H.-L.; et al. A new multifunctional hydroxytyrosol-clofibrate with hypolipidemic, antioxidant, and hepatoprotective effects. Bioorg. Med. Chem. Lett. 2018, 28, 3119–3122. [Google Scholar] [CrossRef] [PubMed]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Auñon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxytyrosol. Food Chem. Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033.
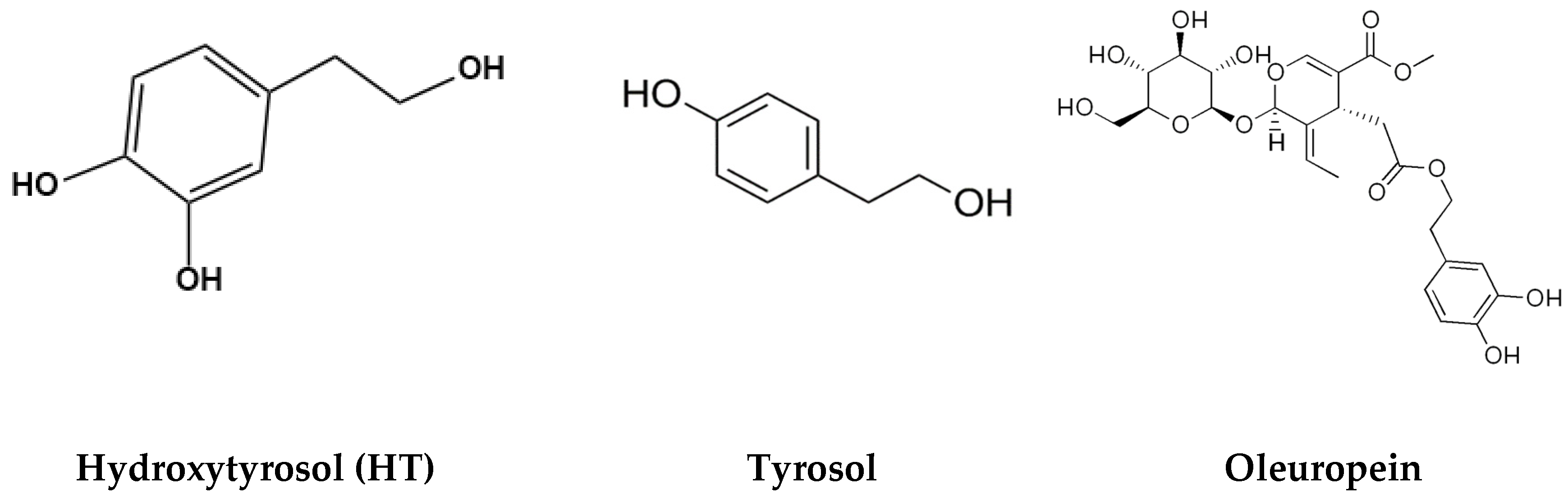
| Polyphenol | Quantity | Olive Oil Type | Source |
|---|---|---|---|
| Hydroxytyrosol (3,4-dihydroxyphenyl ethanol) (HT) | 0.93–14.64 mg/kg | Olive oil (various brands) | [21] |
| Tyrosol | 0.25–14.97 mg/kg | Olive oil (various brands) | [21] |
| Oleuropein | 0.0–4.7 mg/kg | Virgin olive oil | [22] |
| Antidiabetic Agent | Target Tissues | Target Pathways | Effect | Side Effects |
|---|---|---|---|---|
| Biguanides metformin | Liver, fat, muscle | ↑ AMPK activity ↓ Complex I of the respiratory chain | ↑ glucose uptake (fat and muscle) ↓ hepatic glucose production ↑ glucose tolerance ↑ insulin sensitivity | lactic acidosis GI problems (camps, nausea, vomiting, diarrhea) |
| Thiazolidinediones (TZD) glitazones: rosiglitazone rioglitazone | Liver, fat, muscle | ↑ PPARγ activity | ↑ adipocyte lipid storage ↓ circulating FFA ↓ ectopic lipid accumulation ↑ glucose uptake (fat and muscle) ↑ insulin sensitivity ↑ β-cell function | bladder cancer heart failure hepatitis bone fractures weight gain edema |
| Sulfonylureas glinides: glyburide, glipizide glinepiride meglitinides: repaglinide | pancreas fat, muscle | ↑ intracellular potassium concentration leading to depolarization of pancreatic β cells | ↑ glucose- mediated insulin release ↓ blood glucose | hypoglycemia weight gain hunger skin reactions |
| α-glucosidase-inhibitors: acarbose, voglibose, miglitol | small intestine | Competitive inhibition of enzymes vital for carbohydrate digestion | ↓ carbohydrate absorption ↓ blood glucose | abdominal pain bloating, nausea, vomiting diarrhea flatulence |
| Dipeptidyl peptide 4 (DDP-4) inhibitors) gliptins: sitagliptin, saxagliptin vidagliptin, linagliptin alogliptin | pancreas | ↓ DDP-4 activity ↑ incretin levels (GLP-1 and GIP) | ↓ glucagon secretion ↑ insulin release ↓ blood glucose | heart failure pancreatitis pancreatic cancer prostate cancer GI problems flu-like symptoms |
| Incretin mimetics (glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide, exenatide dulaglutide Gastric inhibitory polypeptide (GIP) | direct effect on pancreas, stomach and brain indirect on liver and muscle | ↑ activation of GLP-1 receptor | ↓ glucagon secretion ↑ insulin release ↓ β-cell apoptosis ↓ gastric emptying ↓ appetite ↓ blood glucose | pancreatic cancer pancreatitis heart failure prostate cancer abdominal pain nausea, vomiting |
| Sodium–glucose cotransporter 2 (SGLT2) inhibitors gliflozins: canagliflozin capagliflozin | kidneys | ↓ SGLT2 action in the proximal convoluted tubule | ↓ reabsorption of glucose ↑facilitate excretion in urine | Hypotension urinary tract infections ketoacidosis hyperkalemia kidney failure bone fractures |
| Cell Type | Hydroxytyrosol Concentration/Duration | Effect | Source |
|---|---|---|---|
| C2C12 myoblasts | 1–50 µM for 30 min; TNFα for 4–5 days | ↑ muscle cell differentiation (↑ creatine kinase & myosin heavy chain) ↑ PGC1α ↑ mitochondrial biogenesis | [29] |
| C2C12 myoblasts | 5 or 20 µM HT for 3 h with 1 mM H2O2 | ↓ H2O2-induced apoptosis ↓ morphology changes ↓ oxidative stress | [30] |
| C2C12 myotubes | Hydroxytyrosol-acetate 0–75 µM for 12 h | ↑ glucose uptake | [31] |
| C2C12 myotubes | 1–50 µM for 24 h; 100 µM t-BHP | ↑ cell viability ↓ mitochondrial dysfunction (↑ ATP production and activity of complex I, II and V) ↓ muscle cell degeneration (OPA cleavage) ↑ myosin heavy chain expression | [32] |
| Cell Type | Hydroxytyrosol Concentration/Duration | Effect | Source |
|---|---|---|---|
| 3T3-L1 adipocytes | 0.1–10 µM for 24–72 h | ↑ mitochondrial biogenesis and O2 consumption ↑ mitochondrial complexes I, II, III and V ↓ fatty acid content ↑ CPT-1, PPARα, PPARγ | [33] |
| C3H10 T1/T2 preadipocytes | 25 µM for 4 or 7 days | ↓ lipid differentiation and accumulation ↓ lipid droplet size and number ↓ adipogenesis-related genes (PPARγ and C/EBPα) ↓ differentiation markers (aP2 and adiponectin) ↓ GLUT4 gene expression | [34] |
| 3T3-L1 preadipocytes | 100 or 150 µM for 0–8 days | ↓ cell division and lipid accumulation ↓ mitotic clonal expansion ↓ adipogenesis marker genes (PPARg, SREBP-1c, C/EBPα, GLUT4, CD36 and FAS) | [35] |
| 3T3-L1 adipocytes | Hydroxytyrosol-acetate 25–75 µM for 12 h | ↓ lipid accumulation ↓ adipogenesis (PPARγ, SREBP-1c, C/EBPα, GLUT4, CD36, and FAS) ↑lipolysis and glycerol release ↑ HSL | [31] |
| 3T3-L1 adipocytes | 0–150 µM for 24–72 h | ↑ lipolysis and glycerol release ↓ triglyceride accumulation ↑ HSL, ERK, perilipin phosphorylation ↓ ATGL, HSL, C/EBPα | [36] |
| 3T3-L1 adipocytes SGBS adipocytes | 0.1–20 µM with 10 ng/mL TNFα | ↓ adiponectin suppression ↓ PPARγ suppression ↓ JNK phosphorylation | [37] |
| 3T3-L1 preadipocytes | 10–100 µM for 24–48 h 24 h only | ↓ cell proliferation ↓ CBI receptor ↑ FAS, lipoprotein lipase (LPS) ↓ PPARγ | [38] |
| Primary human visceral preadipocytes | 5–70 µg/mL for 20 days | ↓ triglyceride accumulation ↑ apoptosis, lipolysis, glycerol release ↑ adipogenesis inhibition markers (GATA2, GATA3, WNT3A, SFRP5, HES1, and SIRT1) ↓ adipogenesis promoting genes (LEP, FGF1, CCND1, and SREBF1) | [40] |
| Human bone marrow MSC adipocytes | 1 or 100 µM for 7–14 days | ↑ adipogenesis markers PPARγ, FABP4 ↑ fat vesicle formation ↓ LPS | [41] |
| Cell Type | Hydroxytyrosol Concentration/Duration | Effect | Source |
|---|---|---|---|
| Mouse hepatocytes | 100 µM for 4 h (hypoxia); followed by reoxygenation | ↓ cell apoptosis ↑ hepatocyte viability ↑ SOD1, SOD2, CAT activity | [42] |
| Rat hepatocytes | 25 µM for 2 h | ↓ lipid synthesis (fatty acid, cholesterol and triglyceride) ↓ ACC, diacylglycerol acyltransferase, 3-hydroxy-3-methyl-glutaryl-CoA reductase ↑ AMPK and ACC phosphorylation | [43] |
| Vit. E-deficient rat liver microsomes | 0.05–2 mM for 30 min | ↓ lipid peroxidation, TBARS | [44] |
| Vit. E-deficient rat liver microsomes | 0.05–0.25 mM for 20 min | ↓ lipid peroxidation, TBARS | [46] |
| Cell Type | Hydroxytyrosol Concentration/Duration | Effect | Source |
|---|---|---|---|
| Rat pancreatic tissue | 50 µg/mL for 0–40 min with 4 g/L glucose | ↓ decline in insulin secretion induced by hyperglycemia | [47] |
| Rat INS-1 β cells | 0.1–30 µM 3-HT; 11 mM glucose for 1 h 3-HT:amylin ratio of 10:1 M for 0–40 h | ↔ insulin secretion ↓ amylin amyloids | [48] |
| Study Model | Hydroxytyrosol Concentration/Duration | Effect | Source |
|---|---|---|---|
| Alloxan-Induced Diabetes Model | |||
| Alloxan-induced diabetic male Wistar rats | 20 mg/kg for 2 months; intraperitoneal injection | ↓ blood glucose levels ↓ liver TBARS, bilirubin, fatty cysts ↑ hepatic glycogen, HDL ↑ SOD, CAT, GPX in liver/kidney ↓ β cell damage | [47] |
| Alloxan-induced diabetic male Wistar rats | 8 or 16 mg/kg orally for 4 weeks; | ↓ blood glucose levels ↓ TC ↓ hepatic oxidative damage (TBARS) ↑ hepatic glycogen ↑ antioxidant enzymes (SOD, CAT) | [55] |
| Streptozotocin-Induced Diabetes Model | |||
| STZ-induced diabetic male Wistar rats | 20 mg/kg/day orally for 2 months | ↓ blood glucose, HDL ↓ LDL cholesterol, TG ↓ intestinal enzymes (maltase, lactase and sucrose, lipase) ↑ pancreas SOD, CAT, GSH, GPX ↓ pancreas TBARS, AGE, LDH | [56] |
| STZ-induced male diabetic Sprague-Dawley rats | 10 or 100 mg/kg/day for 6 weeks via gavage | ↓ plasma TBARS ↑ NCV, thermal nociception Na+/K+-ATPase activity | [58] |
| STZ-induced male diabetic Wistar rats | 0.5-10 mg/kg/day orally for 7 days prior to STZ and 2 months thereafter | ↓ nitrosative, oxidative stress ↓ inflammation, IL-1β ↓ platelet aggregation ↓ aortic wall area | [59] |
| STZ-induced diabetic male Wistar rats | 1, 5, or 10 mg/kg/day orally for 7 days prior to STZ and 2 months thereafter | ↓ brain lipid peroxides, ↓ nitrosative stress, cell death ↓ brain inflammation, IL-1β, prostaglandin E2 | [60] |
| STZ-induced diabetic male Wistar rats | 5 mg/kg/day via endogastric cannula for 7 days prior to STZ and 2 months thereafter | ↑ retinal ganglion cell number ↓ retinal thickness, cell size | [61] |
| STZ-induced diabetic KM mice | 77 mg/kg/day HT or HT-NO via gavage for 4 weeks | ↓ plasma glucose levels ↓ oxidative stress (↓ serum NO, MDA, ↑ SOD) ↑ SIRT1 expression in aorta and HUVEC) | [62] |
| Genetic Diabetes Model | |||
| Male db/db C57BL/6J mice | 10 mg/kg/day via gavage for 8 weeks | ↓ fasting glucose levels ↓ serum lipids ↓ oxidative damage in liver and muscle | [63] |
| Male db/db C57BL/6J mice | 10 or 50 mg/kg/day orally for 8 weeks | ↑ mitochondrial complex I/II/IV expression (brain) ↑ activity complex I (brain) ↓ oxidative stress (↑ p62, HO-1) SOD1, SOD2) ↓ protein oxidation in brain ↑ AMPK, SIRT1, PPARγ -1α activation in brain | [64] |
| Study Model | Hydroxytyrosol Concentration/Duration | Effect | Source |
|---|---|---|---|
| Diet-induced hypercholesterolemic male Wistar rats | Olive leaf hydrolysate extract for 3 weeks (3 mg/kg b. w. orally containing HT (1.4 g/100 g dry weight), oleuropein | ↓ serum TC, TG, LDL ↑ serum HDL ↓ TBARS (heart, liver, kidney) ↑ serum antioxidant capacity ↑ liver CAT, SOD activity | [65] |
| Diet-induced diabetic/obese male C57BL/6 mice | 10 or 50 mg/kg/day via gavage for 17 weeks | ↓ serum glucose, insulin ↓ serum IL-6, CRP, TG, leptin ↓ LDL/HDL ratio ↓ lipid content (liver, muscle) ↓ liver SREBP-1c, FAS ↓ liver protein carbonyls, MDA ↑ liver GST, SOD activity | [63] |
| Diet-induced hypercholesterolemic male Wistar rats | 0.04% of diet with added HT, HT-Ac, or HT-Et for 8 weeks | ↓ serum glucose, insulin ↓ serum leptin, MDA ↓ serum IL-1β, TNFα ↑ serum antioxidant activity ↓ VAT MCP-1, IL-1β ↓ serum TC, LDL (HT-Ac only) | [66] |
| Male C57BL/6J mice with diet-induced metabolic syndrome | 20 mg/kg/day orally for 3 weeks | ↓ serum glucose, Mest expression ↓ weight gain, visceral fat ↓ inguinal WAT ↔ serum insulin | [67] |
| Male Sprague-Dawley rats with diet-induced NAFLD | 10 mg/kg/day via gavage for 6 weeks | ↑ glucose tolerance ↓ serum glucose, insulin ↓ serum AST, ALT, TC ↑ hepatic PPARα, CPT1a, ACC ↓ liver TNFα, IL-6, COX-2 ↓ intestinal barrier damage ↓ liver ROS, MDA, RNS damage | [68] |
| Male Wistar rats with diet-induced metabolic syndrome | 20 mg/kg/day via gavage for 8 weeks | ↑ glucose tolerance ↓ serum insulin ↓ weight gain and fat mass ↓ liver steatosis, ventricular fibrosis ↓ plasma ALT, AST activity ↔ serum lipids | [69] |
| Diet-induced obese male ICR mice | 20 mg/kg/day via gavage for 10 weeks | ↓ fasting glucose, insulin ↑ glucose and insulin tolerance ↑ GLUT4 (adipocytes, myocytes) ↓ phospho-IRS-1 (Ser307) ↑ phospho-Akt (Ser473) ↓ liver, adipose tissue TNFα, IL-1β ↓ serum CRP, IL-6 ↓ hepatic steatosis, TG, ER stress ↓ liver SREBP-1 ↔ adiposity, adiponectin ↔ serum lipids, liver enzymes | [70] |
| Diet-induced obese male C57BL/6J mice | 5 mg/kg/day orally for 12 weeks | ↓ weight gain, insulin resistance ↓ serum glucose, insulin ↓ serum FFA, TAG, TC, LDL ↓ hepatic steatosis, FFA, TC ↓ liver TNFα, IL-1β/IL-6 ↑ hepatic EPA, DHA ↔ serum AST | [71] |
| STZ-induced diabetic male ICR miceTriton WR-1339 induced hyperlipidemic mice | 36 µmol/kg/day FF-HT via gavage for 11 weeks 36 µmol/kg/day FF-HT via gavage for 7 days | ↓ plasma glucose, lipids ↓ hepatic lipids ↓ TNFα, CRP ↑ glucose tolerance, antioxidants ↓ plasma TG, TC, MDA, atherosclerotic index (AI) | [72] |
| STZ-induced diabetic male KM mice Triton WR-1339 induced hyperlipidemic mice | 0.38 mmol/kg/day via gavage for 4 weeks 0.38 mmol/kg/day via gavage for 7 days prior to Triton WR | ↓ blood glucose, lipids ↑ plasma SOD, CAT, GSH-PX ↓ plasma TG, TC, MDA | [73] |
| Triton WR-1339 induced hyperlipidemic mice | 240 µmol/kg/day for 7 days | ↓ plasma TG, TC, MDA ↓ AST, ALT, TBIL, ALP, hepatic GSSH ↑ serum SOD, CAT ↑ hepatic GSH | [74] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlavcheski, F.; Young, M.; Tsiani, E. Antidiabetic Effects of Hydroxytyrosol: In Vitro and In Vivo Evidence. Antioxidants 2019, 8, 188. https://doi.org/10.3390/antiox8060188
Vlavcheski F, Young M, Tsiani E. Antidiabetic Effects of Hydroxytyrosol: In Vitro and In Vivo Evidence. Antioxidants. 2019; 8(6):188. https://doi.org/10.3390/antiox8060188
Chicago/Turabian StyleVlavcheski, Filip, Mariah Young, and Evangelia Tsiani. 2019. "Antidiabetic Effects of Hydroxytyrosol: In Vitro and In Vivo Evidence" Antioxidants 8, no. 6: 188. https://doi.org/10.3390/antiox8060188
APA StyleVlavcheski, F., Young, M., & Tsiani, E. (2019). Antidiabetic Effects of Hydroxytyrosol: In Vitro and In Vivo Evidence. Antioxidants, 8(6), 188. https://doi.org/10.3390/antiox8060188






