Milk Powder Fortified with Potassium and Phytosterols to Decrease the Risk of Cardiovascular Events among the Adult Population in Malaysia: A Cost-Effectiveness Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Stroke and MI Incidence per Annum and Mortality Rates
2.2. Potassium Effect on SBP and SBP Effect on CVD Risk
2.3. Phytosterols Effect on LDL-c and LDL-c Effect on CVD Risk
2.4. Cost and Utilities
3. Results
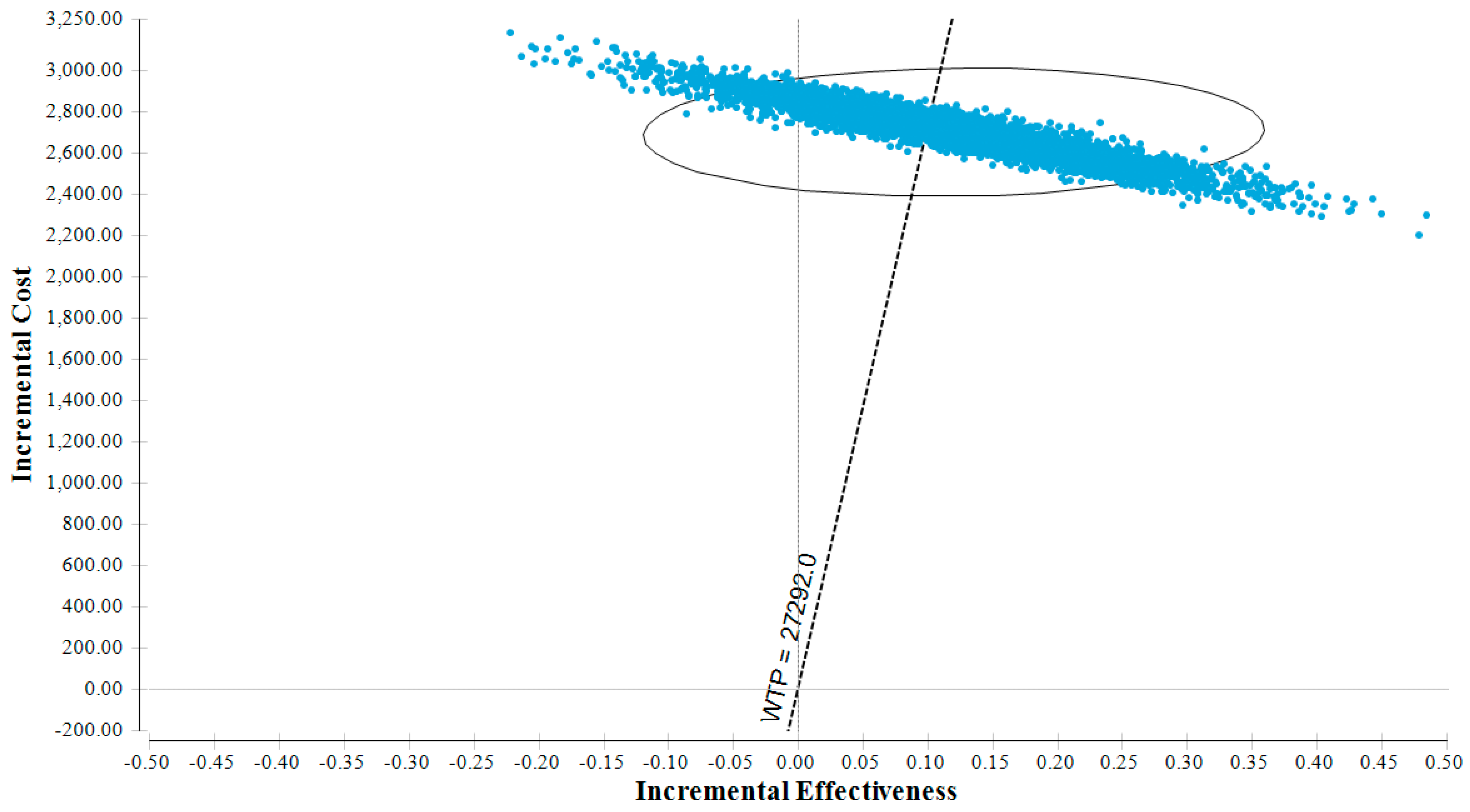
3.1. Base Case Results
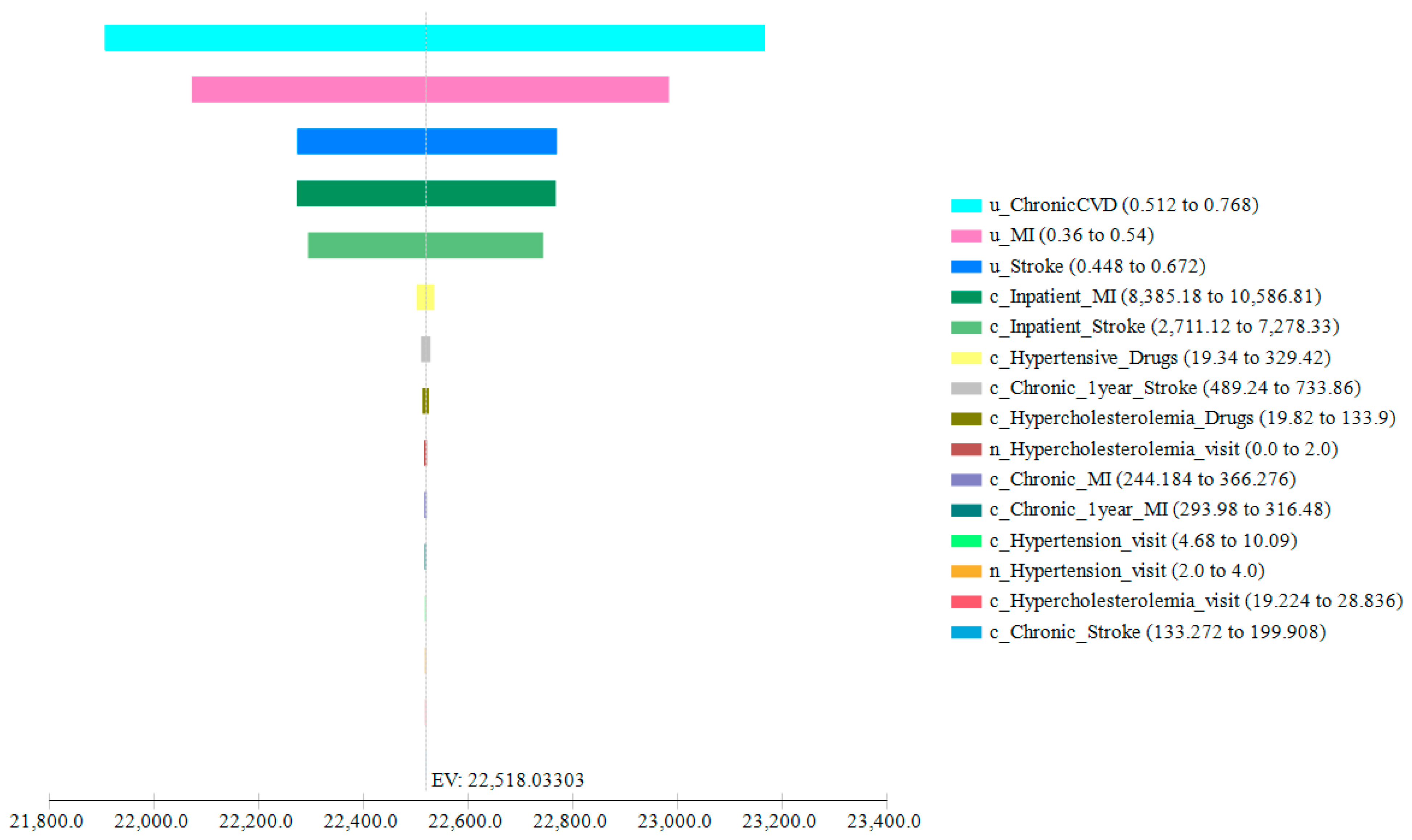
3.2. Sensitivity Analyses
4. Discussion
4.1. Principal Findings
4.2. Public Health Implications
4.3. Comparison with the Literature
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Sharing
References
- Institute for Public Health (IPH)—Ministry of Health. 2016; Determination of Dietary Sodium Intake Among the Ministry of Health Staff 2015 (MySalt 2015). Available online: http://iku.moh.gov.my/images/IKU/Document/Report/MySalt2016/MySaltReport.pdf (accessed on 11 April 2019).
- World Health Organization. Malaysia Health System Review. 2012. Available online: https://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwixnr326MXhAhVRwcQBHbvXDEsQFjAAegQIAhAC&url=https%3A%2F%2Fapps.who.int%2Firis%2Frest%2Fbitstreams%2F1077991%2Fretrieve&usg=AOvVaw2fn-ilQ8LaxeoW4pKP7CsP (accessed on 11 April 2019).
- Noor, M.I. The Nutrition and Health Transition in Malaysia. Public Health Nutr. 2002, 5, 191–195. [Google Scholar] [CrossRef]
- Institute for Public Health (IPH). The National Health and Morbidity Survey 2015 (NHMS 2015)—Non-Communicable Diseases, Risk Factors & Other Health Problems; Malaysian Ministry of Health: Putrajaya, Malaysia, 2015. Available online: http://www.moh.gov.my/moh/resources/nhmsreport2015vol2.pdf (accessed on 11 April 2019).
- Ministry of Health Malaysia, Public Health Department. National Strategic Plan for Non-Communicable Disease (NSP-NCD) 2016–2025. Available online: http://www.iccp-portal.org/system/files/plans/MYS_B3_NSP%20NCD%202016-2025%2C%20FINAL.pdf (accessed on 11 April 2019).
- Abdullah, W.M.S.W.; Yusoff, Y.S.; Basir, N.; Yusuf, M.M. Mortality rates due to coronary heart disease by specific sex and age groups among Malaysians. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 25–27 October 2017; Available online: http://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwjNxOrmjcrhAhUFPVAKHVO2D_UQFjAAegQIABAC&url=http%3A%2F%2Fwww.iaeng.org%2Fpublication%2FWCECS2017%2FWCECS2017_pp736-741.pdf&usg=AOvVaw0CTYlgmdTbENZckZmLtnqn (accessed on 11 April 2019).
- Aniza, I.; Syafrawati, S.S.; Zafar, M.; Amrizal, M.N.; Ika Fazura, M.N. Developing the cost for Uncomplicated Acute ST Elevated Myocardial Infarction (STEMI Primary Percutaneous Coronary Intervention) Using Step down and Activity Based Costing at UKMMC. Malays. J. Community Health 2011, 17, 26–31. [Google Scholar]
- Yusoff, A.F.; Mustafa, A.N.; Kaur, G.K.; Omar, M.A.; Vos, T.; Rao, V.P.C.; Begg, S. Malaysian Burden of Disease and Injury Study; Institute for Public Health; National Institute of Health: Kuala Lumpur, Malaysia, 2005; pp. 10–16. Available online: https://espace.library.uq.edu.au/view/UQ:172329 (accessed on 9 April 2019).
- Suzana, S.; Azlinda, A.; Hin, S.L.; Khor, W.H.; Zahara, Z.; Sa¨ida Munira, J.; Norliza, M. Influence of food intake on eating habits on hypertension control among outpatients at a Government Health Clinic in Klang Valley, Malaysia. Malays. J. Nutr. 2011, 17, 163–173. [Google Scholar] [PubMed]
- World Health Organization. WHO Issues New Guidance on Dietary Salt and Potassium—Note for the Media: WHO. 2013. Available online: https://www.who.int/mediacentre/news/notes/2013/salt_potassium_20130131/en/ (accessed on 9 April 2019).
- Perez, V.; Chang, E.T. Sodium-to-potassium ratio and blood pressure, hypertension, and related factors—American Society for Nutrition. Adv. Nutr. 2014, 5, 712–741. [Google Scholar] [CrossRef]
- Cook, N.R.; Obarzanek, E.; Cutler, J.A.; Buring, J.E.; Rexrode, K.M.; Kumanyika, S.K.; Appel, L.J.; Whelton, P.K. Trials of Hypertension Prevention Collaborative Research Group Joint effects of sodium and potassium intake on subsequent cardiovascular disease: The Trials of Hypertension Prevention follow-up study. Arch. Intern. Med. 2009, 169, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, T.; Kuklina, E.V.; Flanders, W.D.; Hong, Y.; Gillespie, C.; Chang, M.; Gwinn, M.; Dowling, N.; Khoury, M.J.; et al. Sodium and potassium intake and mortality among US adults: Prospective data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2011, 171, 1183–1191. [Google Scholar] [CrossRef]
- Binia, A.; Jaeger, J.; Hu, Y.; Singh, A.; Zimmermann, D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: A meta-analysis of randomized controlled trials. J. Hypertens. 2015, 33, 1509–1520. [Google Scholar] [CrossRef]
- Mirnalini, K.; Zalilah, M.S.; Safiah, M.Y.; Tahir, A.; Siti Haslinda, M.D.; Siti Rohana, D.; Khairul Zarina, M.Y.; Mohd Hasyami, S.; Normah, H. Energy and nutrient intakes: Findings from the Malaysian Adult Nutrition Survey (MANS). Malays. K. Nutr. 2008, 14, 1–24. [Google Scholar]
- Institute for Public Health (IPH). Key findings of recent food consumption and nutrition surveys in ASEAN—Malaysian Adult Nutrition Survey (MANS) 2014. In Proceedings of the Symposium on Dietary Intakes: Assessing What We Eat, Evaluating Methodologies, Singapore, 26 April 2016; Available online: http://ilsisea-region.org/wp-content/uploads/sites/21/2016/06/07.-Mr.-Mohamad-Hasnan.pdf (accessed on 9 April 2019).
- Rashidah, A.; Yeo, P.S.; Noor Ani, A.; Muhammad Fadhli, M.Y.; Tahir, A.; Feisul Idzwan, M.; Ahmad Ali, Z.; Suhaila, A.G.; Azli, B.; Viola, M.; et al. Sodium intake among normotensive health staff assessed by 24-hour urinary excretion: A cross-sectional study. Malays. J. Nutr. 2014, 20, 317–326. [Google Scholar]
- Aris, T.; Guat, H.T. National Health and Morbidity Survey 2014: Malaysian Adults Nutrition Survey (MANS); Institute for Public Health: Kuala Lumpur, Malaysia, 2014; Available online: https://www.researchgate.net/publication/301203882_National_Health_and_Morbidity_Survey_2014_Malaysian_Adult_Nutrition_Survey_MANS_Vol_II_Survey_Findings (accessed on 11 April 2019).
- Gan, W.Y.; Boo, S.; Seik, M.Y.; Khoo, H.E. Comparing the nutritional status of vegetarians and non-vegetarians from a Buddhist Organisation in Kuala Lumpur, Malaysia—Nutritional status, dietary intake and Body composition. Malays. J. Nutr. 2018, 24, 89. [Google Scholar]
- Department of Statistics Malaysia. Statistics Yearbook Malaysia 2016; Department of Statistics Malaysia: Putrajaya, Malaysia; Volume 9–11. Available online: https://www.dosm.gov.my (accessed on 12 September 2018).
- Ministry of Health Malaysia, National Coordinating Committee on Food and Nutrition (NCCFN). National Plan of Action for Nutrition of Malaysia III 2016-2025—Malaysian Ministry of Health Press. 2016. Available online: http://nutrition.moh.gov.my/wp-content/uploads/2016/12/NPANM_III.pdf (accessed on 11 April 2019).
- Ministry of Health Malaysia, National Coordinating Committee on Food and Nutrition (NCCFN). Recommended Nutrient Intakes for Malaysia—A Report of the Technical Working Group on Nutritional Guidelines—Malaysian Ministry of Health Press. 2017. Available online: http://nutrition.moh.gov.my/wp-content/uploads/2017/05/FA-Buku-RNI.pdf (accessed on 11 April 2019).
- Thomsen, A.B.; Hansen, H.B.; Christiansen, C.; Green, H.; Berger, A. Effect of free plant sterols in low-fat milk on serum lipid profile in hypercholesterolemic subjects. Eur. J. Clin. Nutr. 2004, 58, 860. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gage, H.; Jackson, D.; Raats, M. The effectiveness and cost-effectiveness of plant sterol or stanol-enriched functional foods as a primary prevention strategy for people with cardiovascular disease risk in England: A modeling study. Eur. J. Health Econ. 2018, 19, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. Ldl-Cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef]
- Law, M.R.; Wald, N.J.; Thompson, S.G. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? Br. Med. J. 1994, 308, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jan, S.; Yan, L.L.; Hayes, A.; Chu, Y.; Wang, H.; Feng, X.; Niu, W.; He, F.J.; Ma, J.; et al. Cost and cost-effectiveness of a schoolbased education program to reduce salt intake in children and their families in China. PLoS ONE 2017, 12, e0183033. [Google Scholar]
- Yang, Z.J.; Liu, J.; Ge, J.P.; Chen, L.; Zhao, Z.G.; Yang, W.Y. Prevalence of cardiovascular disease risk factor in the Chinese population: The 2007–2008 China National Diabetes and Metabolic Disorders Study. Eur. Heart J. 2011, 33, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Zuhdi, A.S.; Mariapun, J.; Hairi, N.N.; Ahmad, W.A.W.; Abidin, I.Z.; Undock, A.W.; Ismail, M.D.; Sim, K. Young coronary artery disease in patients undergoing percutaneous coronary intervention. Ann. Saudi Med. 2013, 33, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Zuhdi, A.S.M.; Ahmad, W.A.W.; Zaki, R.A.; Mariapun, J.; Ali, R.M.; Sari, N.M.; Ismail, M.D.; Hian, S.K. Acute coronary syndrome in the elderly: The Malaysian National Cardiovascular Disease Database-Acute Coronary Syndrome registry. Singap. Med. J. 2016, 57, 191. [Google Scholar] [CrossRef]
- Venkatason, P.; Zubairi, Y.Z.; Hafidz, I.; Ahmad, W.A.W.; Zuhdi, A.S. Trends in evidence-based treatment and mortality for ST elevation myocardial infarction in Malaysia from 2006 to 2013: Time for real change. Ann. Saudi Med. 2016, 36, 184. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6074549/ (accessed on 11 April 2019). [CrossRef] [PubMed]
- Kooi, C.W.; Peng, H.C.; Aziz, Z.A.; Looi, I. A review of stroke research in Malaysia from 2000–2014. Med. J. Malays. 2016, 71, 58–69. [Google Scholar]
- Wan-Arfah, N.; Hafiz, H.M.; Naing, N.N.; Muzaimi, M.; Shetty, H.J.M. Short-term and long-term survival probabilities among first-ever ischaemic and haemorrhagic stroke patients at a hospital in the suburban east coast of Peninsular Malaysia. Health Sci. Rep. 2018, 1, e27. [Google Scholar] [CrossRef]
- Buenaflor, F.G. Recurrence rate of ischemic stroke: A single center experience. J. Neurol. Sci. 2017, 381, 399. [Google Scholar] [CrossRef]
- Sun, Y.; Lee, S.H.; Heng, B.H.; Chin, V.S. 5-year survival and rehospitalization due to stroke recurrence among patients with hemorrhagic or ischemic strokes in Singapore. BMC Neurol. 2013, 13, 133. [Google Scholar] [CrossRef]
- Khor, G.L. Cardiovascular epidemiology in the Asia–Pacific region. Asia Pac. J. Clin. Nutr. 2001, 10, 76–80. [Google Scholar] [CrossRef]
- Selvarajah, S.; Fong, A.Y.; Selvaraj, G.; Haniff, J.; Hairi, N.N.; Bulgiba, A.; Bots, M.L. Impact of cardiac care variation on ST-elevation myocardial infarction outcomes in Malaysia. Am. J. Cardiol. 2013, 111, 270–1276. [Google Scholar] [CrossRef]
- Chin, S.P.; Jeyaindran, S.; Azhari, R.; Wan Azman, W.A.; Omar, I.; Robaayah, Z.; Sim, K.H. Acute coronary syndrome (ACS) registry-leading the charge for National Cardiovascular Disease (NCVD) Database. Med. J. Malays. 2008, 63, 29–36. [Google Scholar]
- Kiu-Hian, S.; Zambahari, R.; Simmandurai, J.T.S.; Rosmau, H.A.; Mohd Ali, R.; Azman, W. National Cardiovascular Disease Database (NCVD), ACS Registry, PCI Registry. In Proceedings of the NHAM ASM, Kuala Lumpur, Malaysia, 17 April 2009; Available online: http://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&cad=rja&uact=8&ved=2ahUKEwjq98qe3MfhAhX3QhUIHUtYC50QFjACegQIBRAC&url=http%3A%2F%2Fwww.acrm.org.my%2Fncvd%2Fdocuments%2Fpresentations%2FNHAM%252013th%2520ASM_NCVD%2520by%2520SIM.pps&usg=AOvVaw0-0iPb2JFUTu-Ywj-23Jhw (accessed on 11 April 2019).
- Ahmad, W.A.W.; Sim, K.H. Annual Report of the NCVD-PCI Registry, 2007–2009; National Cardiovascular Disease Database: Kuala Lumpur, Malaysia, 2011; Available online: http://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwjdjOzz3MfhAhWLSBUIHdejA78QFjAAegQIAhAC&url=http%3A%2F%2Fwww.acrm.org.my%2Fncvd%2Fdocuments%2Freport%2FpciReport_07-09%2FfullReport.pdf&usg=AOvVaw0o23A7sWxHKGleYJKUjeI- (accessed on 11 April 2019).
- Ahmad, W.A.W.; Sim, K.H. (Eds.) Annual Report of the NCVD-ACS Registry, 2009 & 2010; National Cardiovascular Disease Database: Kuala Lumpur, Malaysia, 2013. Available online: https://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwj97PmP3cfhAhXZRhUIHSFQDqUQFjAAegQIABAC&url=https%3A%2F%2Fwww.crc.gov.my%2Fwp-content%2Fuploads%2Fdocuments%2Freport%2Freport_NCVD_Acute_Coronary_Syndrome_Registry_09_10.pdf&usg=AOvVaw3uTC9FfabwZK28yYFuKV-g (accessed on 11 April 2019).
- Wan Ahmad, W.A. Annual Report of the NCVD-ACS Registry, 2014–2015; National Cardiovascular Disease Database: Kuala Lumpur, Malaysia, 2017. Available online: http://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwj2u-6q3cfhAhWDSxUIHchMBIAQFjAAegQIBhAC&url=http%3A%2F%2Fwww.crc.gov.my%2Fwp-content%2Fuploads%2Fdocuments%2Freport%2Freport_NCVD_2014_2015.pdf&usg=AOvVaw3yrpsnEGL1JTznIsFfm4f1 (accessed on 11 April 2019).
- Aziz, Z.A.; Lee, Y.Y.; Ngah, B.A.; Sidek, N.N.; Looi, I.; Hanip, M.R.; Basri, H.B. Acute stroke registry Malaysia, 2010–2014: Results from the National Neurology Registry. J. Stroke Cerebrovasc. Dis. 2015, 24, 2701–2709. [Google Scholar] [CrossRef]
- Ministry of Health Malaysia. Consensus Statement on the Management of Ischemic Stroke. 2000. Available online: http://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwin5KiB1MfhAhWPr6QKHT8XBzsQFjAAegQIBBAC&url=http%3A%2F%2Fwww.acadmed.org.my%2Fview_file.cfm%3Ffileid%3D213&usg=AOvVaw3uGlwf8jBTaGgTcxniWJl2 (accessed on 12 September 2018).
- Ministry of Health Malaysia. Clinical Practice Guidelines, Management of Stroke, MOH/P/PAK/113.06(GU). 2006. Available online: https://www.malaysianheart.org/files/41722642648ff4e8ee2c09.pdf (accessed on 12 September 2018).
- Ministry of Health Malaysia, Clinical Practice Guidelines, UA/NSTEMI. MOH/P/PAK/219.11(GU). 2011. Available online: http://www.acadmed.org.my/view_file.cfm?fileid=414 (accessed on 23 July 2018).
- Ministry of Health Malaysia. Clinical Practice Guidelines, Management of Ischaemic Stroke, 2nd ed.; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2012. Available online: http://www.acadmed.org.my/view_file.cfm?fileid=501 (accessed on 23 July 2018).
- Ministry of Health Malaysia. Clinical Practice Guidelines, Management of Acute ST Segment Elevation Myocardial Infarction (STEMI), 3rd ed.; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2014. Available online: http://www.acadmed.org.my/view_file.cfm?fileid=683 (accessed on 23 July 2018).
- Ministry of Health Malaysia. Clinical Practice Guidelines, Management of Dyslipidaemia, 5th ed.; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2017. Available online: http://www.acadmed.org.my/view_file.cfm?fileid=849 (accessed on 26 July 2018).
- Department of Statistics Malaysia. Press Release: Statistics on Causes of Death, Malaysia. 2017. Available online: https://www.dosm.gov.my/v1/index.php?r=column/pdfPrev&id=Y3psYUI2VjU0ZzRhZU1kcVFMMThGUT09 (accessed on 12 September 2018).
- Dainelli, L.; Xu, T.; Li, M.; Zimmermann, D.; Fang, H.; Wu, Y.; Detzel, P. Cost-effectiveness of milk powder fortified with potassium to decrease blood pressure and prevent cardiovascular events among the adult population in China: A Markov model. BMJ Open 2017, 7, e017136. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Urso, R.; Cicero, A.F. Renin-angiotensin system at the crossroad of hypertension and hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 115–120. [Google Scholar] [CrossRef]
- Whelton, P.K.; He, J.; Cutler, J.A.; Brancati, F.L.; Appel, L.J.; Follmann, D.; Klag, M.J. Effects of oral potassium on blood pressure: Meta-analysis of randomized controlled clinical trials. Jama 1997, 277, 1624–1632. Available online: https://jamanetwork.com/journals/jama/article-abstract/416446 (accessed on 11 April 2019). [CrossRef] [PubMed]
- Houston, M.C. The importance of potassium in managing hypertension. Curr. Hypertens. Rep. 2011, 13, 309–317. Available online: https://link.springer.com/article/10.1007/s11906-011-0197-8 (accessed on 11 April 2019). [CrossRef] [PubMed]
- Lewington, S. Prospective studies collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Hunt, B.D.; Cappuccio, F.P. Potassium intake and stroke risk: A review of the evidence and practical considerations for achieving a minimum target. Stroke 2014, 45, 1519–1522. Available online: https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.113.004282 (accessed on 11 April 2019). [CrossRef]
- Van Mierlo, L.A.J.; Greyling, A.; Zock, P.L.; Kok, F.J.; Geleijnse, J.M. Suboptimal potassium intake and potential impact on population blood pressure. Arch. Intern. Med. 2010, 170, 1501–1502. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Barba, G.; Cappuccio, F.P.; Strazzullo, P. Potassium intake, stroke, and cardiovascular disease: A meta-analysis of prospective studies. J. Am. Coll. Cardiol. 2011, 57, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; He, J.; Wu, X.; Duan, X.; Whelton, P.K. Effect of potassium supplementation on blood pressure in Chinese: A randomized, placebo-controlled trial. J. Hypertens. 2001, 19, 1325–1331. [Google Scholar] [CrossRef]
- Liu, K.; Dyer, A.R.; Cooper, R.S.; Stamler, R.; Stamler, J. Can overnight urine replace 24-hour urine collection to asses salt intake? Hypertension 1979, 1, 529–536. [Google Scholar] [CrossRef]
- Siani, A.; Iacoviello, L.; Giorgione, N.; Iacone, R.; Strazzullo, P. Comparison of variability of urinary sodium, potassium, and calcium in free-living men. Hypertension 1989, 13, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Lerchl, K.; Rakova, N.; Dahlmann, A.; Rauh, M.; Goller, U.; Basner, M.; Dinges, D.F.; Beck, L.; Agureev, A.; Larina, I. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension 2015, 66, 850–857. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Campbell, N.R.C. Population dietary salt reduction and the risk of cardiovascular disease: A commentary on recent evidence. J. Clin. Hypertens. 2017, 19, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ 2013, 346, f1378. [Google Scholar] [CrossRef]
- Mendis, S.; Puska, P.; Norrving, B. Global atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland, 2011; Available online: https://www.who.int/cardiovascular_diseases/publications/atlas_cvd/en/ (accessed on 11 April 2019).
- Asia Pacific Cohort Studies Collaboration (APCSC). Blood pressure and cardiovascular disease in the Asia Pacific region. J. Hypertens. 2003, 21, 707–716. [Google Scholar] [CrossRef]
- Lawes, C.M.M.; Hoorn, S.V.; Law, M.R.; Elliott, P.; MacMahon, S.; Rogers, A. High blood pressure. In Comparative Quantification of Health Risks; Ezzati, M., Lopez, A.D., Rodgers, A.A., Murray, C., Eds.; World Health Organisation: Geneva, Switzerland, 2004; Available online: https://www.who.int/publications/cra/chapters/volume1/0281-0390.pdf?ua=1 (accessed on 12 September 2018).
- McDonald, T.J.; Oram, R.A.; Vaidya, B. Investigating hyperkalaemia in adults. BMJ 2015, 351, h4762. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Plant Sterols and Blood Cholesterol-Scientific substantiation of a health claim related to plant sterols and lower/reduced blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2008, 6, 781. [Google Scholar] [CrossRef]
- Lampi, A.M.; Piironen, V.; Toivo, J. Analysis of phytosterols in foods. In Phytosterols as Functional Food Components and Nutraceuticals; Dutta, P.C., Ed.; Marcel Dekker: New York, NY, USA, 2004; pp. 33–73. [Google Scholar]
- Talati, R.; Sobieraj, D.M.; Makanji, S.S.; Phung, O.J.; Coleman, C.I. The comparative efficacy of plant sterols and stanols on serum lipids: A systematic review and meta-analysis. J. Am. Diet Assoc. 2010, 110, 719–726. [Google Scholar] [CrossRef]
- Christiansen, L.I.; Lähteenmäki, P.L.A.; Mannelin, M.R.; Hiltunen, R.V.K.; Yiliruusi, J.K. Cholesterol-lowering effect of spreads enriched with microcrystalline plant sterols in hypercholesterolemic subjects. Eur. J. Nutr. 2001, 40, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mijares, A.; Banuls, C.; Rocha, M.; Morillas, C.; Martínez-Triguero, M.-L.; Victor, V.M.; Lacomba, R.; Alegría, A.; Barberá, R.; Farré, R.; et al. Effects of phytosterol ester-enriched low-fat milk on serum lipoprotein profile in mildly hypercholesterolaemic patients are not related to dietary cholesterol or saturated fat intake. Br. J. Nutr. 2010, 104, 1018–1025. [Google Scholar] [CrossRef]
- Abumweis, S.S.; Barake, R.; Jones, P.J.H. Plant sterols/stanols as cholesterol lowering agents: A meta-analysis of randomized controlled trials. Food Nutr. Res. 2008, 52, 1811. [Google Scholar] [CrossRef]
- Nestel, P.; Cehun, M.; Pomeroy, S.; Abbey, M.; Weldon, G. Cholesterol-lowering effects of plant sterol esters and non-esterified stanols in margarine, butter and low-fat foods. Eur. J. Clin. Nutr. 2001, 55, 1084. [Google Scholar] [CrossRef] [PubMed]
- Noakes, M.; Clifton, P.M.; Doornbos, A.M.; Trautwein, E.A. Plant sterol ester–enriched milk and yoghurt effectively reduce serum cholesterol in modestly hypercholesterolemic subjects. Eur. J. Nutr. 2005, 44, 214–222. [Google Scholar] [CrossRef]
- Katan, M.B.; Grundy, S.M.; Jones, P.; Law, M.; Miettinen, T.; Paoletti, R.; Stresa Workshop Participants. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 2003, 78, 965–978. [Google Scholar] [CrossRef]
- Law, M. Plant sterol and stanol margarines and health. BMJ 2000, 320, 861. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, J.R.; Richard, E. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef] [PubMed]
- International Monetary Fund. International Financial Statistics and Data Files. 2018. Available online: https://www.imf.org/en/Data (accessed on 12 September 2018).
- World Bank. PPP Conversion Factor, GDP (LCU per International $) in 2016—World Bank. 2018. Available online: https://data.worldbank.org/indicator/pa.nus.ppp (accessed on 11 April 2019).
- Lee, Y.K.; Nam, H.S.; Chuang, L.H.; Kim, K.Y.; Yang, H.K.; Kwon, I.S.; Kind, P.; Kweon, S.S.; Kim, Y.T. South Korean time trade-off values for EQ-5D health states: Modeling with observed values for 101 health states. Value Health 2009, 12, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Luo, N.; Shaw, J.W.; Kind, P.; Coons, S.J. Valuations of EQ-5D health states: Are the United States and United Kingdom different? Med. Care 2005, 221–228. [Google Scholar] [CrossRef]
- Weinstein, M.C.; Torrance, G.; McGuire, A. QALYs: The basics. Value Health 2009, 12, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.E.; Noor Siah, A.A.; Zaidah, Z.; Teoh, K.H.; Gurbinder, J.S.; Ismail, M.S.; Choy, Y.C.; Mazeni, A. Quality of life amongst post coronary artery bypass patients at the National Heart Institute, Malaysia. Med. Health 2010, 5, 77–85. [Google Scholar]
- Nor Azlin, M.N.; Rizal, A.M.; Wei Bi, L. Health related quality of life (HRQOL) among stroke survivors attending rehabilitation centres in Selangor. Malays. J. Community Health 2009, 15, 83–90. [Google Scholar]
- Wan-Fei, K.; Hassan, S.T.S.; Latiff, L.A. Physical activity and quality of life of hypertensive patients with and without diabetes: A cross-sectional study. Int. J. Public Health Res. 2017, 4, 76–88. [Google Scholar]
- Azmi, S.; Goh, A.; Fong, A.; Anchah, L. Quality of Life among patients with acute coronary syndrome in Malaysia. Value Health Reg. Issues 2015, 6, 80–83. [Google Scholar] [CrossRef][Green Version]
- Tan-Torres Edejer, T.; Baltussen, R.; Adam, T.; Hutubessy, R.; Acharya, A.; Evans, D.B.; Murray, C.J.L. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis; World Health Organization: Geneva, Switzerland, 2003; Available online: https://www.who.int/choice/publications/p_2003_generalised_cea.pdf (accessed on 11 April 2019).
- Aizuddin, A.N.; Amrizal, M.N.; Kang, T.W.; Rafidah, A.R.; Geok Hong, Y.; Adibah, A.; Ismail, A.; Wan Puteh, E.S.; Abdul Manaf, M.R. Cost analysis of hypertension management in an urban primary medical centre kuala lumpur. Malays. J. Public Health Med. 2014, 14, 18–23. [Google Scholar]
- Chong, H.Y.; Mohamed, Z.; Tan, L.L.; Wu, D.B.C.; Shabaruddin, F.H.; Dahlui, M.; Apalasamy, Y.D.; Snyder, S.R.; Williams, M.S.; Hao, J.; et al. Is universal HLA-B* 15:02 screening a cost-effective option in an ethnically diverse population? A case study of Malaysia. Br. J. Dermatol. 2017, 177, 1102–1112. [Google Scholar] [CrossRef]
- Suhil, M.A.; Hassali, M.A.A.; Ibrahim, M.I.M. Evaluation of direct medical cost in treating hypertension in a Malaysian public university. Asian J. Pharm. 2010. [Google Scholar]
- Yong, Y.V.; Shafie, A.A. How Much Does Management of an asthma-related event cost in a Malaysian suburban hospital? Value Health Reg. Issues 2018, 15, 6–11. [Google Scholar] [CrossRef]
- Azzeri, A.; Shabaruddin, F.H.; Mohamed, R.; McDonald, S.A.; Tan, S.S.; Kamarulzaman, A.; Dahlui, M. Cost of Treatment for Chronic Hepatitis C Infection at a National Tertiary-Care Referral Centre in an Asian Middle-Income Country. Value Health 2017, 20, A633. [Google Scholar] [CrossRef]
- Rohana, D.; Wan Norlida, W.I.; Nor Azwany, Y.; Mazlan, A.; Zawiya, D.; Che Kamaludin, C.A.; Cs, C.G. Economic Evaluation of Type 2 Diabetes Management at the Malaysian Ministry of Health Primary Care Clinics, in Machang, Kelantan. Am. J. Public Health Med. 2007, 7, 5–13. [Google Scholar]
- Ministry of Health Malaysia. Clinical Practice Guidelines, Primary and Secondary Prevention of Cardiovascular Disease; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2017. Available online: http://www.moh.gov.my/moh/resources/Penerbitan/CPG/CARDIOVASCULAR/6.pdf (accessed on 23 July 2018).
- Risso-Gill, I.; Balabanova, D.; Majid, F.; Ng, K.K.; Yusoff, K.; Mustapha, F.; Kuhlbrandt, C.; Nieuwlaat, R.; Schwalm, J.D.; McCready, T. Understanding the modifiable health system barriers to hypertension management in Malaysia: A multi-method heath systems appraisal approach. MC Health Serv. Res. 2015, 15, 254. [Google Scholar] [CrossRef]
- Nordin, N.; Aljunid, S.; Aziz, N.; Muhammad, A.; Sulong, S. Direct medical cost of stroke: Findings from a tertiary hospital in Malaysia. Malays. J. Med. Sci. 2012, 67. [Google Scholar]
- Aljunid, S.M. The Collaborative Funding Program for for Southeast Asia Tobacco Control Research—Health Care Costs of Smoking in Malaysia; SEATCA: Bangkok, Thailand, 2007; Available online: https://seatca.org/dmdocuments/10_health_care_costs_of_smoking_in_malaysia.pdf (accessed on 12 September 2018).
- Mustapha, F.I.; Azmi, S.; Manaf, M.R.A.; Hussein, Z.; Mahir, J.N.; Ismail, F.; Aizuddin, A.N.; Goh, A. What are the direct medical costs of managing Type 2 Diabetes Mellitus in Malaysia? Med. J. Malays. 2017, 72, 271–277. [Google Scholar]
- Aznida, F.A.A.; Azlin, N.M.; Amrizal, M.N.; Saperi, S.; Aljunid, S.M. The cost of treating an acute ischaemic stroke event and follow-up at a teaching hospital in Malaysia: A Casemix costing analysis. BMC Health Serv. Res. 2012, 12, 6. Available online: https://link.springer.com/article/10.1186/1472-6963-12-S1-P6 (accessed on 11 April 2019). [CrossRef]
- WHO. Available online: https://www.who.int/countries/mys/en/ (accessed on 28 May 2019).
- Lovegrove, J.A.; Hobbs, D.A. New perspectives on dairy and cardiovascular health. Proc. Nutr. Soc. 2016, 75, 247–258. [Google Scholar] [CrossRef]
- Rampal, L.; Rampal, S.; Azhar, M.Z.; Rahman, A.R. Prevalence, awareness, treatment and control of hypertension in Malaysia: A national study of 16,440 subjects. Public Health 2008, 122, 11–18. [Google Scholar] [CrossRef]
- Ministry of Health. Malaysia National Health Accounts Health Expenditure Report 1997–2015; Malaysia National Health Accounts: Putrajaya, Malaysia, 2016. Available online: http://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwiGgMSajMrhAhWK-aQKHcvHBkYQFjAAegQIAxAC&url=http%3A%2F%2Fjknjohor.moh.gov.my%2Fbmv%2Fuploads%2Fpenerbitan%2FMNHA19972015.pdf&usg=AOvVaw152Ks-bW58wTaeAVEQKt9Q (accessed on 11 April 2019).
- Hu, D.; Huang, J.; Wang, Y.; Zhang, D.; Qu, Y. Dairy foods and risk of stroke: A meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 460–469. [Google Scholar] [CrossRef]
- Talaei, M.; Pan, A.; Yuan, J.M.; Koh, W.P. Dairy Food Intake Is Inversely Associated with Risk of Hypertension: The Singapore Chinese Health Study. J. Nutr. 2016, 147, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Malaysia. Health Facts 2017 (Reference Data for 2016). Available online: http://www.aidsdatahub.org/sites/default/files/publication/Malaysia_Health_Facts_2017.pdf (accessed on 12 September 2018).
- Nestle Malaysia. Nestlé data from the Malaysian market. In Nestlé in Society 2017; Nestle Malaysia: Petaling Jaya, Malaysia, 2017. [Google Scholar]
- Syed, A.J. Primary Health Care and Health Information Needs in Malaysia. 2009. Available online: http://www.whofic-apn.com/pdf_files/4th_17_35p.pdf (accessed on 12 September 2018).
- Loganathan, T.; Ng, C.W.; Lee, W.S.; Jit, M. The hidden health and economic burden of Rotavirus gastroenteritis in Malaysia. Pediatr. Infect. Dis. J. 2016, 35, 601–606. Available online: https://www.ingentaconnect.com/content/wk/inf/2016/00000035/00000006/art00006 (accessed on 11 April 2019). [CrossRef] [PubMed]
- Pérez-Cueto, F.J.; Aschemann-Witzel, J.; Shankar, B.; Brambila-Macias, J.; Bech-Larsen, T.; Mazzocchi, M.; Capacci, S.; Saba, A.; Turrini, A.; Niedwiedzka, B. Assessment of evaluations made to healthy eating policies in Europe: A review within the EATWELL Project. Public Health Nutr. 2012, 15, 1489–1496. Available online: https://www.cambridge.org/core/journals/public-health-nutrition/article/assessment-of-evaluations-made-to-healthy-eating-policies-in-europe-a-review-within-the-eatwell-project/B9CD3B11FDE3F68CFEE0D7E374AB1DBF (accessed on 11 April 2019). [CrossRef] [PubMed]
- Irz, X.; Leroy, P.; Réquillart, V.; Soler, L.G. Economic assessment of nutritional recommendations. J. Health Econ. 2015, 39, 188–210. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0167629614001155 (accessed on 11 April 2019). [CrossRef]
- Lenoir-Wijnkoop, I.; Nuijten, M.J.C.; Gutiérrez-Ibarluzea, I.; Hutton, J.; Poley, M.J.; Segal, L.; Bresson, J.L.; van Ganse, E.; Jones, P.; Moreno, L. Workshop Report: Concepts and methods in the economics of nutrition–gateways to better economic evaluation of nutrition interventions. Br. J. Nutr. 2012, 108, 1714–1720. Available online: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/workshop-report-concepts-and-methods-in-the-economics-of-nutrition-gateways-to-better-economic-evaluation-of-nutrition-interventions/81CA3798CCAF4627EE710E7A8CB431C2 (accessed on 11 April 2019). [CrossRef]
- Gandjour, A.; Stock, S. A national hypertension treatment program in Germany and its estimated impact on costs, life expectancy, and cost-effectiveness. Health Policy 2007, 83, 257–267. [Google Scholar] [CrossRef]
- Gu, D.; Kelly, T.N.; Wu, X.; Chen, J.; Duan, X.; Huang, J.F.; Chen, J.C.; Whelton, P.K.; He, J. Blood pressure and risk of cardiovascular disease in Chinese men and women. Am. J. Hypertens. 2008, 21, 265–272. [Google Scholar] [CrossRef]
- Gerber, A.; Evers, T.; Haverkamp, H.; Lauterbach, K.W. Cost-benefit analysis of a plant sterol containing low-fat margarine for cholesterol reduction. Eur. J. Health Econ. 2006, 7, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.R.; Feenstra, T.L.; Toxopeus, I.B.; Hoekstra, J.; Klungel, O.H.; Verhagen, H.; van Kranen, H.J.; Rompelberg, C.J.M. Costs and health effects of adding functional foods containing phytosterols/-stanols to statin therapy in the prevention of cardiovascular disease. Eur. J. Pharmacol. 2011, 668, S91–S100. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Macgregor, G.A. Beneficial effects of potassium on human health. Physiol. Plant 2008, 133, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef]
- Oberleithner, H.; Callies, C.; Kusche-Vihrog, K.; Schillers, H.; Shahin, V.; Riethmüller, C.; MacGregor, G.A.; de Wardener, H.E. Potassium softens vascluar endothelium and increases nitric oxide release. Proc. Natl. Acad. Sci. USA 2009, 106, 2829–2834. [Google Scholar] [CrossRef] [PubMed]
- Lambert, H.; Frassetto, L.; Moore, J.B.; Torgerson, D.; Gannon, R.; Burckhardt, P.; Lanham-New, S. The effect of supplementation with alkaline potassium salts on bone metabolism: A meta-analysis. Osteoporos. Int. 2015, 26, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiao, Y.; Xie, B.; Zhang, Q.; Ma, X.; Li, N.; Liu, M.; Zhang, Q. Effect of milk powder supplementation with different calcium contents on bone mineral density of postmenopausal women in Northern China: A randomized controlled double-blind trial. Calcif. Tissue Int. 2016, 98, 60–66. [Google Scholar] [CrossRef]
- Van den Heuvel, E.G.H.M.; Steijns, J.M.J.M. Dairy products and bone health: How strong is the scientific evidence? Nutr. Res. Rev. 2018, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, T.A. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff. 2007, 26, 13–24. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Kypridemos, C.; Collins, B.; Mozaffarian, D.; Huang, Y.; Bandosz, P.; Capewell, S.; Whitsel, L.; Wilde, P.; O’Flaherty, M.; Micha, R. Estimating the health and economic effects of the proposed US Food and Drug Administration voluntary sodium reformulation: Microsimulation cost-effectiveness analysis. PLoS Med. 2018, 15, e1002551. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the safety of stigmasterol-rich plant sterols as food additive. EFSA J. 2012, 10, 2659. [Google Scholar] [CrossRef]
- Capewell, S.; O’Flaherty, M. Can dietary changes rapidly decrease cardiovascular mortality rates? Eur. Heart J. 2011, 32, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.O.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD003177. [Google Scholar] [PubMed]
- Shafie, A.A.; Lim, Y.W.; Chua, G.N.; Ahmad Hassali, M.A. Exploring the willingness to pay for a quality-adjusted life-year in the state of Penang, Malaysia. Clin. Out. 2014, 6, 473–481. [Google Scholar] [CrossRef] [PubMed]
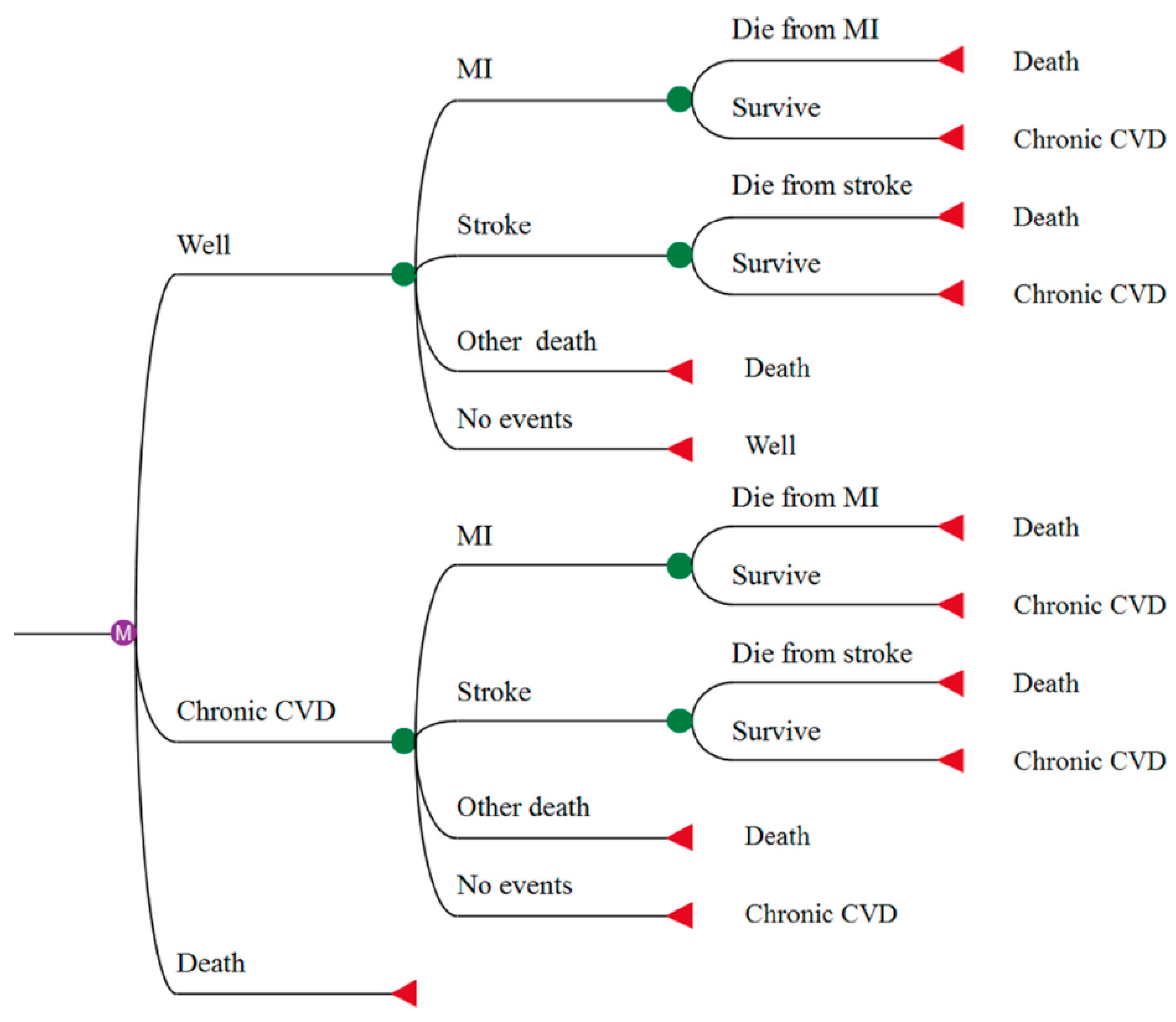
| Age Group | Stroke Incidence [32,35,38,45] | 28-day Mortality Risk of Stroke (%) [33,43] | MI Incidence [39,42] | 30-day Mortality Risk of MI (%) [30,42] | Non-CVD Mortality [20,50] | ||
| Well | 35–39 | 425/100,000 | 17.80 | 11.4/100,000 | 7.10 | 74.6/100,000 | |
| 40–44 | 850/100,000 | 18.52 | 43.7/100,000 | 12.55 | 74.6/100,000 | ||
| 45–49 | 850/100,000 | 19.27 | 43.7/100,000 | 12.55 | 2365.2/100,000 | ||
| 50–54 | 1700/100,000 | 20.05 | 101.2/100,000 | 12.55 | 2365.2/100,000 | ||
| 55–59 | 1700/100,000 | 20.86 | 101.2/100,000 | 12.55 | 4655.8/100,000 | ||
| 60–64 | 2000/100,000 | 21.70 | 141.8/100,000 | 31.35 | 4655.8/100,000 | ||
| 65–69 | 3000/100,000 | 22.55 | 141.8/100,000 | 31.35 | 6946.4/100,000 | ||
| 70–74 | 3500/100,000 | 23.42 | 173.2/100,000 | 31.35 | 6946.4/100,000 | ||
| 75–79 | 7800/100,000 | 24.33 | 173.2/100,000 | 31.35 | 6946.4/100,000 | ||
| Chronic | Age group | Stroke incidence | 28-day mortality risk of stroke (%) | MI incidence | 30-day mortality risk of MI (%) | Stroke: non-CVD mortality | MI: non-CVD mortality |
| 35–39 | 13,000/100,000 | 21.43 | 24,400/100,000 | 53 | 7870.9/100,000 | 5260/100,000 | |
| 40–44 | 13,000/100,000 | 22.30 | 24,400/100,000 | 54.08 | 7870.9/100,000 | 5260/100,000 | |
| 45–49 | 14,560/100,000 | 23.20 | 24,400/100,000 | 54.08 | 8107/100,000 | 5417.8/100,000 | |
| 50–54 | 16,307/100,000 | 24.13 | 24,400/100,000 | 55.16 | 8107/100,000 | 5417.8/100 000 | |
| 55–59 | 18,264/100,000 | 25.11 | 24,400/100,000 | 55.16 | 8350.2/100,000 | 5580.3/100,000 | |
| 60–64 | 20,446/100,000 | 26.13 | 24,400/100,000 | 56.92 | 8350.2/100,000 | 5580.9/100,000 | |
| 65–69 | 22,910/100,000 | 27.14 | 36,700/100,000 | 56.92 | 8600.7/100,000 | 5747.7/100,000 | |
| 70–74 | 25,659/100,000 | 28.19 | 36,700/100,000 | 58.05 | 8600.7/100,000 | 5747.7/100,000 | |
| 75–79 | 28,739/100,000 | 29.29 | 36,700/100,000 | 58.05 | 8600.7/100,000 | 5747.7/100,000 | |
| Age Group | RR Reduction Due to A 4.81% Decrease in LDL-c Levels [4,24] | RR Reduction Due to A 3.86 mmHg Decrease in SBP [66] | Compounded RR Reduction Due to Decreased LDL-c and SBP [4,24,66] | Compounded RR Ratio Due to Decreased LDL-c and SBP [4,24,66] | |
|---|---|---|---|---|---|
| Stroke | 35–44 | 1.96 | 20 | 21.96 | 78.04 |
| 45–54 | 2.60 | 20 | 22.60 | 77.40 | |
| 55–64 | 2.61 | 16.5 | 19.11 | 80.89 | |
| 65–74 | 2.46 | 11.10 | 13.56 | 86.44 | |
| 75+ | 2.34 | 9 | 11.34 | 88.66 | |
| MI | 35–44 | 1.96 | 16 | 17.96 | 82.04 |
| 45–54 | 2.60 | 16 | 18.60 | 81.40 | |
| 55–64 | 2.61 | 12.5 | 15.11 | 84.89 | |
| 65–74 | 2.46 | 7.5 | 9.96 | 90.04 | |
| 75+ | 2.34 | 6 | 8.34 | 91.66 |
| Outpatient Costs | Value (Range) |
|---|---|
| Yearly mean anti-hypercholesterolemic drug cost [90] | 76.86 (21.86–133.86) |
| Yearly mean anti-hypertensive drug cost [90] | 174.38 (19.38–329.38) |
| Screening visit cost, hypercholesterolemia [91] | 24.03 |
| Screening visit cost, hypertension [91,92,93,94,95] | 7.38 (4.68–10.09) |
| Yearly number of screening visits, hypercholesterolemia [96] | 1 |
| Yearly number of screening visits, hypertension [97] | 3 |
| Inpatient costs and days | |
| Inpatient MI [82] | 9491.00 (8395.18–10586.81) |
| Inpatient stroke [98] | 4994.96 (2711.12–7278.33) |
| MI inpatient days, mean [7,99] | 5.3 |
| Stroke inpatient days, mean [98] | 6.4 |
| Chronic costs | |
| Chronic cost for the rest of year 1 MI [95,99,100] | 305.23 (293.98–316.48) |
| Chronic cost after year 1 MI [95,99,100] | 305.23 |
| Chronic costs for the rest of year 1 stroke [101] | 611.55 |
| Chronic costs after year 1 stroke [101] | 166.59 |
| Price of the milk powder product per portion/day | 0.5 |
| Discount (%) | |
| Annual discount rate for costs and QALYs [89] | 3 |
| Utilities (QALY) | |
| MI [85] | 0.45 |
| Stroke [86] | 0.56 |
| Chronic CVD [87] | 0.64 |
| Well [88] | 1 |
| Death [88] | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandola, A.E.; Dainelli, L.; Zimmermann, D.; Dahlui, M.; Detzel, P. Milk Powder Fortified with Potassium and Phytosterols to Decrease the Risk of Cardiovascular Events among the Adult Population in Malaysia: A Cost-Effectiveness Analysis. Nutrients 2019, 11, 1235. https://doi.org/10.3390/nu11061235
Gandola AE, Dainelli L, Zimmermann D, Dahlui M, Detzel P. Milk Powder Fortified with Potassium and Phytosterols to Decrease the Risk of Cardiovascular Events among the Adult Population in Malaysia: A Cost-Effectiveness Analysis. Nutrients. 2019; 11(6):1235. https://doi.org/10.3390/nu11061235
Chicago/Turabian StyleGandola, Anita E., Livia Dainelli, Diane Zimmermann, Maznah Dahlui, and Patrick Detzel. 2019. "Milk Powder Fortified with Potassium and Phytosterols to Decrease the Risk of Cardiovascular Events among the Adult Population in Malaysia: A Cost-Effectiveness Analysis" Nutrients 11, no. 6: 1235. https://doi.org/10.3390/nu11061235
APA StyleGandola, A. E., Dainelli, L., Zimmermann, D., Dahlui, M., & Detzel, P. (2019). Milk Powder Fortified with Potassium and Phytosterols to Decrease the Risk of Cardiovascular Events among the Adult Population in Malaysia: A Cost-Effectiveness Analysis. Nutrients, 11(6), 1235. https://doi.org/10.3390/nu11061235





