Evaluation of the Larvicidal Potential of the Essential Oil Pogostemon cablin (Blanco) Benth in the Control of Aedes aegypti
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Essential Oils
2.3. Chemical Analysis
2.4. Larvicidal Activity
2.5. Determination of Antioxidant Activity
- %AA—percentage of antioxidant activity
- Abssample—Sample absorbance
- Abswhite— White absorbance
- Abscontrol—Control absorbance
2.6. Antimicrobial Activity
2.6.1. Bacterial Strains and Culture Conditions
2.6.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.7. Cytotoxic Activity
2.8. Statistical Analysis
3. Results
3.1. Chemical Analysis
3.2. Larvicidal Activity
3.3. Antioxidant Activity
3.4. Antimicrobial Activity
3.5. Cytotoxic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malan, D.F.; Neuba, D.F.R.; Kouakou, L. Medicinal plants and traditional healing practices in ehotile people, around the aby lagoon (eastern littoral of Côte d’Ivoire). J. Ethnobiol. Ethomed. 2015, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.S.; Rodrigues, A.B.L.; Almeida, S.S.M.S. Preliminary Study of the Extract of the Barks of Licania macrophylla Benth: Phytochemicals and Toxicological Aspects. Biota Amazônia 2014, 94–99. [Google Scholar] [CrossRef]
- Swamy, M.K.; Balasubramanya, S.; Anuradha, M. In vitro multiplication of Pogostemon cablin Benth. Through direct regeneration. Afr. J. Biotechnol. 2010, 9, 2069–2075. [Google Scholar]
- Kusuma, H.S.; Mahfud, M. Microwave-assisted Hydrodistillation for Extraction of Essential Oil from Patchouli (Pogostemon cablin) Leaves. Period. Polytech. Chem. 2017, 61, 82–92. [Google Scholar] [CrossRef]
- Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch. Biochem. Biophys. 2006, 454, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, P.; Venkatesh, K.; Singh, B.C.S.; Jyothi, B.A.; Kumar, P.; Amareshwari, P.; Roja, A.R. Phytochemical, pharmacological importance of Patchouli (Pogostemon cablin (Blanco) Benth) an aromatic medicinal plant. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 7–15. [Google Scholar]
- Liu, X.R.; Fan, R.; Zhang, Y.Y.; Zhu, M.J. Study on antimicrobial activities of extracts from Pogestemon cablin (Blanco) Benth. Food Sci. Technol. 2009, 24, 220–227. [Google Scholar]
- Dongare, P.; Dhande, S.; Kadam, V. A Review on Pogostemon patchouli. Res. J. Pharmacogn. Phytochem. 2014, 691, 41–47. [Google Scholar]
- Beek, T.A.V.; Joulain, D. The essential oil of patchouli, Pogostemon cablin: A review. Flavour Fragr. J. 2018, 33, 6–51. [Google Scholar] [CrossRef]
- Wan, F.; Peng, F.; Xiong, L.; Chen, J.; Peng, C.; Dai, M. In vitro and in vivo antibacterial activity of Patchouli Alcohol from Pogostemon cablin. Chin. J. Integr. Med. 2016, 22, 1–6. [Google Scholar] [CrossRef]
- Kumara, S.M.; Anuradha, M. Analysis of genetic variability in Patchouli cultivars (Pogostemon cablin Benth.) using RAPD Markers. Res. Biotechnol. 2011, 2, 64–71. [Google Scholar]
- Marcombe, S.; Chonephetsarath, S.; Thammavong, P.; Brey, P.T. Alterative insecticides for larval control of the dengue vector Aedes aegypti in Lao PDR: Insecticide resistance and semi-field trial study. Parasites Vectors 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Erlanger, T.E.; Keiser, J.; Utzinger, J. Effect of degue vector control interventions on entomological parameters in developing countries: A systematic review and meta-analysis. Med. Vet. Entomol. 2008, 22, 203–221. [Google Scholar] [CrossRef]
- Zhu, F.; Lavine, L.; O´Neal, S.; Lavine, M.; Foss, C.; Walsh, D. Insecticide Resistance and Management Strategies in Urban Ecosystems. Insects 2016, 2, 2. [Google Scholar] [CrossRef]
- Adaszynska-Skwirzynska, M.; Smist, M.; Swarcewicz, M. Comparison of extraction methods for the determination of essential oil content and composition of lavender leaves. Chem. Chem. Technol. 2013, 5, 21–23. [Google Scholar]
- Martins, R.L.; Simões, R.C.; Rabelo, E.M.; Farias, A.L.F.; Rodrigues, A.B.L.; Ramos, R.S.; Fernandes, J.B.; Santos, L.S.; Almeida, S.S.M.S. Chemical Composition, an Antioxidant, Cytotoxic and Microbiological Activity of the Essential Oil from the Leaves of Aeollanthus suaveolens Mart. ex Spreng. PLoS ONE 2016, 11, e0166684. [Google Scholar] [CrossRef]
- Adams, R.P. Review of Identification of essential oil components by gas chromatography mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 4, 803–806. [Google Scholar] [CrossRef]
- Van den dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005; pp. 1–39. [Google Scholar]
- Chen, Z.; Berlin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef]
- Lopez-Lutz, D.; Alvino, D.S.; Alvino, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oil. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Souza, C.R.F.; Georgetti, S.R.; Salvador, M.J.; Fonseca, M.J.V.F.; Oliveira, W.P.O. Antioxidant activity and physical-chemical properties of spray and spouted bed dried extracts of Bauhinia forficata. Braz. J. Pharm. Sci. 2009, 45, 209–218. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibily Tests for Bacteria that Grow Aerobically, 11th ed.; Clinical and Laboratory Standards: Pennsylvania, PA, USA, 2018. [Google Scholar]
- Araújo, M.G.F.; Cunha, W.R.; Veneziani, R.C.S. Structures of steroidal alkaloid oligoglycosides, robeneosides A and B, and antidiabetogenic constituents from the Brazilian medicinal plant Solanum lycocarpum. J. Basic Appl. Pharm. Sci. 2010, 31, 205–209. [Google Scholar]
- Milhem, M.M.; Al-Hiyasat, A.S.; Darmani, H. Toxicity Testing of Restorative Dental Materials Using Brine Shrimp Larvae (Artemia salina). J. Appl. Oral Sci. 2008, 18, 297–301. [Google Scholar] [CrossRef]
- Cheng, S.S.; Chang, H.T.; Chang, S.T.; Tsai, K.H.; Chen, W.J. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour. Technol. 2003, 89, 99–102. [Google Scholar] [CrossRef]
- Ghosh, A.; Chowdhury, N.; Chandra, G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012, 134, 581–598. [Google Scholar]
- Albuquerque, E.L.D.; Lima, J.K.A.; Souza, F.H.O.; Silva, I.M.A.; Santos, A.A.; Araújo, A.A.; Blank, A.F.; Lima, R.N.; Alves, P.B.; Bacci, L. Insecticidal and repellence activity of the essential oil of Pogostemon cablin against urban ants species. Acta Trop. 2013, 127, 181–186. [Google Scholar] [CrossRef]
- Rocha, A.G.; Oliveira, B.M.S.; Melo, C.R.; Sampaio, T.S.; Blank, A.F.; Lima, A.D.; Nunes, R.S.; Araújo, A.P.A.; Cristaldo, P.F.; Bacc, L. Lethal effect and Behavioral Responses of Leaf-Cutting Ants to Essential oil of Pogostemon Cablin (Lamiaceae) and Its Nanoformulation. Neotrop. Entomol. 2018, 47, 769–779. [Google Scholar] [CrossRef]
- Orcutt, D.M.; Nilsen, E.T. Physiology of Plants under Stress: Soil and Biotic Factors; Virginia Polytechnic Institute and State University: Black Castle, VA, USA, 2000. [Google Scholar]
- Maciel, M.V.; Morais, S.M.; Bevilaqua, C.M.L.; Silva, R.A.; Barros, R.S.; Sousa, R.N.; Sousa, L.C.; Brito, E.S.; Souza-Neto, M.A. Chemical composition of Eucalyptus spp. Essential oils and their insecticidal effects on Lutzomyia longipalpis. Vet. Parasitol. 2010, 167, 1–7. [Google Scholar] [CrossRef]
- Nasir, S.; Batool, M.; Hussain, S.M.; Nasir, I.; Hafeez, F.; Debboun, M. Bioactivity of Oils from Medicinal Plants against Immature Stages of Dengue Mosquito Aedes aegypti (Diptera: Culicidae). Int. J. Agric. Biol. 2015, 17, 1814–9596. [Google Scholar]
- Zhou, Q.M.; Chen, M.H.; Li, X.H.; Peng, C.; Lin, D.S.; Li, X.N.; He, Y.; Xiong, L.J. Absolute configurations and bioactivities of Guiaiene-Type sesquiterpenoids isolated from Pogostemon cablin. J. Nat. Prod. 2018, 81, 1919–1927. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, Q.M.; Peng, C.; Chen, M.H.; Li, X.N.; Lin, D.S.; Xiong, L. Pocahemiketals A and B, two new hemiketals with unprecedented sesquiterpenoid skeletons from Pogostemon cablin. Fitoter 2017, 120, 67–71. [Google Scholar] [CrossRef]
- Autran, E.S.; Neves, A.; Silva, C.S.B.; Santos, G.K.N.; Câmara, C.A.G.; Navarro, D.M.A.F. Chemical composition, oviposition deterrent and larvicidal activities against Aedes aegypti of essential oils from Piper marginatun Jacq. (Piperaceae). Bioresour. Technol. 2009, 100, 2284–2288. [Google Scholar] [CrossRef]
- Paulraj, S. Larvicidal efficacy of plant oils against the dengue vector Aedes aegypti (L.) (Diptera: Culicidae). World J. Pharm. Res. 2018, 7, 1131–1136. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Iqbal, T.; Bhatti, I.A. Antioxidant attributes of four lamiaceae essential oils. Pak. J. Bot. 2011, 43, 1315–1321. [Google Scholar]
- Beatović, D.; Krstić-Milošević, D.; Trifunović, S.; Šiljegović, J.; Glamočlija, J.; Ristić, M.; Jelačić, S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod. 2015, 9, 62–75. [Google Scholar]
- Adhavan, P.; Kaur, G.; Princy, A.; Murugan, R. Essential oil nanoemulsions of wild patchouli attenuate multi-drug resistant gram-positive, gram-negative and Candida albicans. Ind. Crop. Prod. 2017, 100, 106–116. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yang, S.; Liu, W. Evaluation of the antibacterial activity of Patchouli oil. Iran J. Pharm. Res. 2013, 12, 307–316. [Google Scholar]
- Pattnaik, S.; Subramanyam, V.R.; Kole, C. Antibacterial and antifungal activity of ten essential oils in vitro. Microbios 1996, 86, 237–246. [Google Scholar]
- Pereira, E.J.P.; Silva, H.C.; Holanda, C.L.; Menezes, J.E.S.A.; Siqueira, S.M.C.; Rodrigues, T.H.S.; Fontenelle, R.O.S.; Vale, J.P.C.; Silva, P.T.; Santiago, G.M.P.; et al. Chemical Composition, cytotoxicity and larvicidal activity against Aedes aegypti of essential oils from Vitex gardineriana Schauer. Bol. Latinoam. Caribe Plant. Med. Aromat. 2018, 17, 302–308. [Google Scholar]
- Powers, C.N.; Osier, J.L.; McFeeters, R.L.; Brazell, C.B.; Olsen, E.L.; Moriarity, D.M.; Satyal, P.; Setzer, W.N. Antifungal and cytotoxic activities of sixty commercially-available essential oil. Molecules 2018, 23, 1549. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R.A. Comprehensive Review on the Phytochemical Constituents and Pharmacological Activities of Pogostemon cablin Benth.: An Aromatic Medicinal Plant of Industrial Importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef]
- Bacci, L.; Lima, J.K.A.; Araujo, A.P.A.; Blank, A.F.; Silva, I.M.A.; Santos, A.A.; Santos, A.C.C.; Alves, P.B.; Picanço, M.C. Toxicity, behavior impairment, and repellence of essential oils from pepper-rosmarin and patchouli to termites. Entomol. Exp. Appl. 2015, 156, 66–76. [Google Scholar] [CrossRef]
 ), BHI with 2% DMSO (
), BHI with 2% DMSO ( ), and Amoxiline (
), and Amoxiline ( ). *** p < 0.001 statistically significant in relation to the negative control, # p < 0.001 statistically significant in relation to the positive control.
). *** p < 0.001 statistically significant in relation to the negative control, # p < 0.001 statistically significant in relation to the positive control.
 ), BHI with 2% DMSO (
), BHI with 2% DMSO ( ), and Amoxiline (
), and Amoxiline ( ). *** p < 0.001 statistically significant in relation to the negative control, # p < 0.001 statistically significant in relation to the positive control.
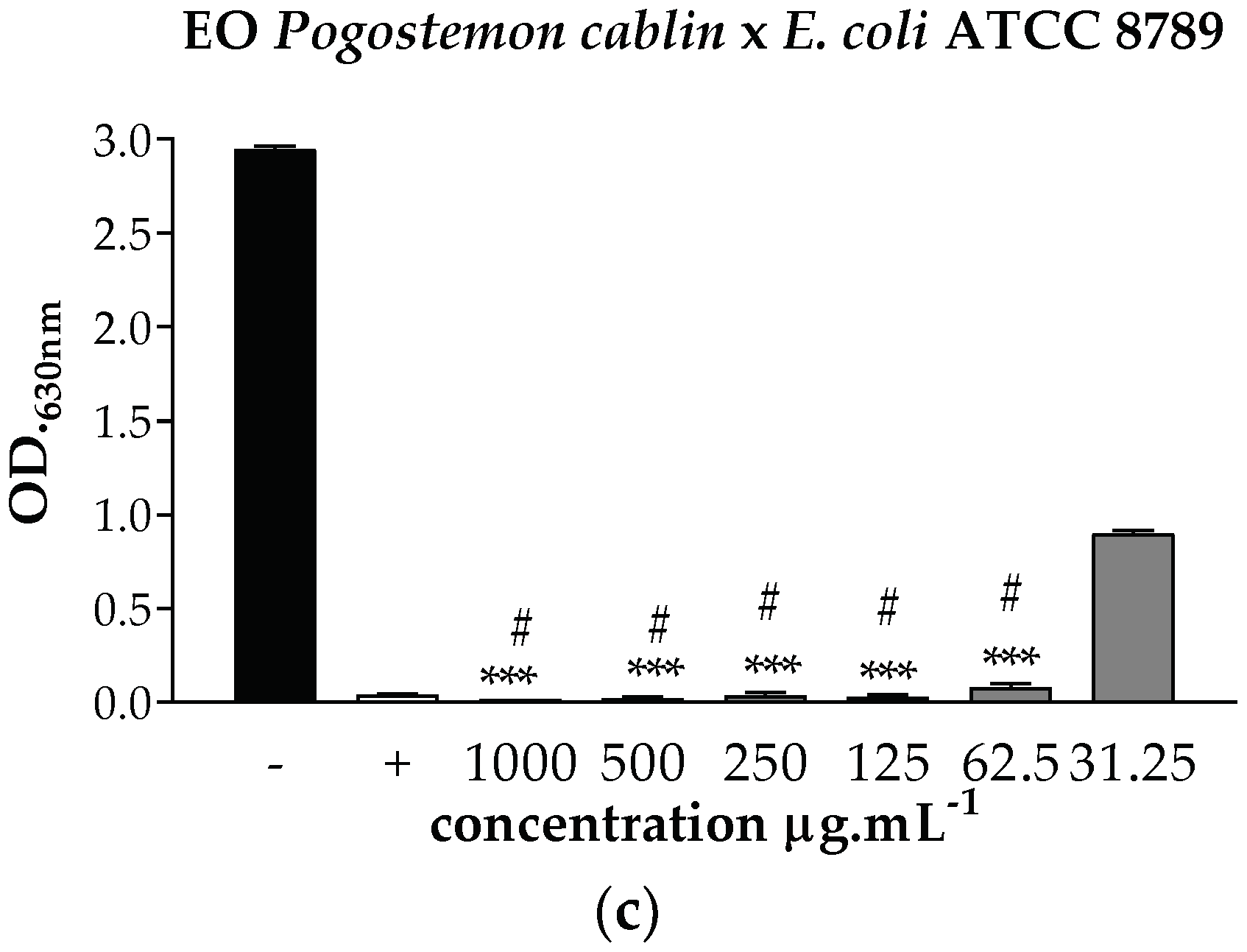
). *** p < 0.001 statistically significant in relation to the negative control, # p < 0.001 statistically significant in relation to the positive control.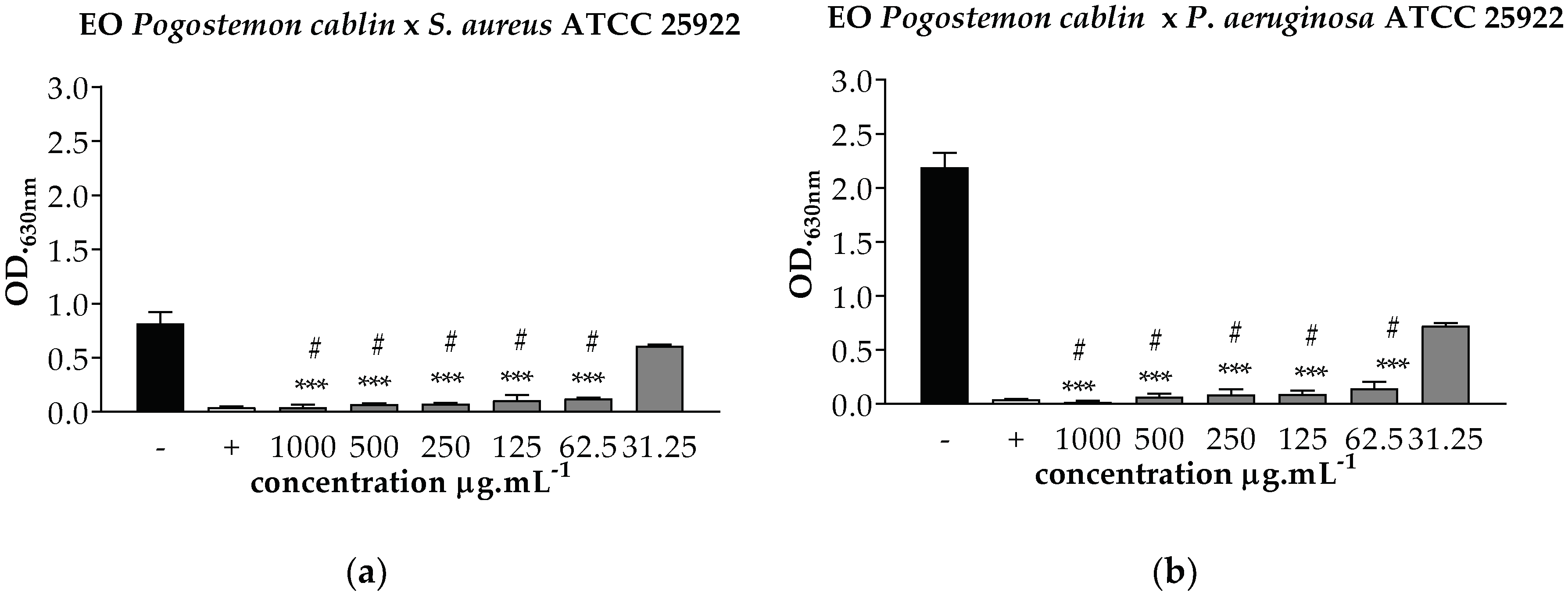
| Peak * | RT (min) | RI | Compounds | Relative Percentage |
|---|---|---|---|---|
| 1 | 23.942 | 1382 | β-patchoulene | 0.46% |
| 2 | 24.183 | 1387 | β-elemene | 0.88% |
| 3 | 25.275 | 1412 | Cycloseychellene | 0.65% |
| 4 | 25.467 | 1417 | (E)-caryophyllene | 0.65% |
| 5 | 26.217 | 1434 | α-guaiene | 2.99% |
| 6 | 26.817 | 1448 | Seychellene | 6.12% |
| 7 | 27.042 | 1453 | α-humulene | 0.42% |
| 8 | 27.292 | 1459 | α-patchoulene | 3.59% |
| 9 | 27.558 | 1465 | 9-epi-(E)-caryophyllene | 1.24% |
| 10 | 27.875 | 1473 | β-chamigrene | 0.14% |
| 11 | 28.450 | 1486 | β-selinene | 0.19% |
| 12 | 28.750 | 1493 | Aciphyllene | 0.54% |
| 13 | 28.875 | 1496 | Viridiflorene | 0.57% |
| 14 | 29.042 | 1500 | α-bulnesene | 4.11% |
| 15 | 29.708 | 1516 | 7-epi-α-selinene | 0.15% |
| 16 | 31.042 | 1549 | Elemol | 0.16% |
| 17 | 31.942 | 1571 | Norpatchoulenol | 5.72% |
| 18 | 32.342 | 1580 | Caryophyllene oxide | 3.86% |
| 19 | 32.458 | 1583 | Globulol | 1.79% |
| 20 | 32.950 | 1595 | Fokienol | 0.67% |
| 21 | 33.442 | 1607 | Humulene epoxide II | 0.72% |
| 22 | 34.267 | 1629 | Junenol | 1.87% |
| 23 | 34.717 | 1640 | allo-aromandendrene epoxide | 1.82% |
| 24 | 35.742 | 1666 | Pogostol | 6.33% |
| 25 | 36.308 | 1681 | Patchouli alcohol | 33.25% |
| 26 | 37.083 | 1701 | Thujopsenal | 2.06% |
| 27 | 37.758 | 1719 | Z-α-atlantone | 1.44% |
| 28 | 38.225 | 1732 | Isobicyclogermacrenal | 0.77% |
| 29 | 39.925 | 1777 | Squamulosone | 0.97% |
| Compounds identified | 84.3% | |||
| Unidentified compounds | 15.87% | |||
| Concentrations | Larvicidal Activity (%) | |
|---|---|---|
| (µg·mL−1) | 24 h | 48 h |
| Control (−) | 0.0 | 0.0 |
| 20 | 38.0 | 70.66 |
| 40 | 52.0 | 89.33 |
| 60 | 92.0 | 97.33 a |
| 80 | 94.66 a | 98.66 a |
| 100 | 96.0 a | 98.66 a |
| LC50 (EO) | 28.43 μg·mL−1 | |
| LC50 (Control +) | 0.0034 μg·mL−1 | |
| Concentrations (µg·mL−1) | Mortality (%) |
|---|---|
| Control negative | 0.0% a |
| 1 | 3.3% a |
| 10 | 20.0% a |
| 20 | 45.0% b |
| 30 | 56.6% c |
| 40 | 76.6% d |
| LC50(EO) | 24.25 μg·mL−1 |
| LC50 (K2Cr2O7) | 12.60 μg·mL−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima Santos, L.; Barreto Brandão, L.; Lopes Martins, R.; de Menezes Rabelo, E.; Lobato Rodrigues, A.B.; da Conceição Vieira Araújo, C.M.; Fernandes Sobral, T.; Ribeiro Galardo, A.K.; Moreira da Silva de Ameida, S.S. Evaluation of the Larvicidal Potential of the Essential Oil Pogostemon cablin (Blanco) Benth in the Control of Aedes aegypti. Pharmaceuticals 2019, 12, 53. https://doi.org/10.3390/ph12020053
Lima Santos L, Barreto Brandão L, Lopes Martins R, de Menezes Rabelo E, Lobato Rodrigues AB, da Conceição Vieira Araújo CM, Fernandes Sobral T, Ribeiro Galardo AK, Moreira da Silva de Ameida SS. Evaluation of the Larvicidal Potential of the Essential Oil Pogostemon cablin (Blanco) Benth in the Control of Aedes aegypti. Pharmaceuticals. 2019; 12(2):53. https://doi.org/10.3390/ph12020053
Chicago/Turabian StyleLima Santos, Lizandra, Lethicia Barreto Brandão, Rosany Lopes Martins, Erica de Menezes Rabelo, Alex Bruno Lobato Rodrigues, Camila Mendes da Conceição Vieira Araújo, Talita Fernandes Sobral, Allan Kardec Ribeiro Galardo, and Sheylla Susan Moreira da Silva de Ameida. 2019. "Evaluation of the Larvicidal Potential of the Essential Oil Pogostemon cablin (Blanco) Benth in the Control of Aedes aegypti" Pharmaceuticals 12, no. 2: 53. https://doi.org/10.3390/ph12020053
APA StyleLima Santos, L., Barreto Brandão, L., Lopes Martins, R., de Menezes Rabelo, E., Lobato Rodrigues, A. B., da Conceição Vieira Araújo, C. M., Fernandes Sobral, T., Ribeiro Galardo, A. K., & Moreira da Silva de Ameida, S. S. (2019). Evaluation of the Larvicidal Potential of the Essential Oil Pogostemon cablin (Blanco) Benth in the Control of Aedes aegypti. Pharmaceuticals, 12(2), 53. https://doi.org/10.3390/ph12020053







