Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Production and Maintenance
2.2. Experimental Diet and RJ Treatment
2.3. Physical Performance Tests
2.4. Blood and Tissue Collection
2.6. Histological Analyses
2.7. Measurement of Bone Mineral Density
2.8. Analyses of mRNA Expression
2.9. Statistical Analyses
3. Results
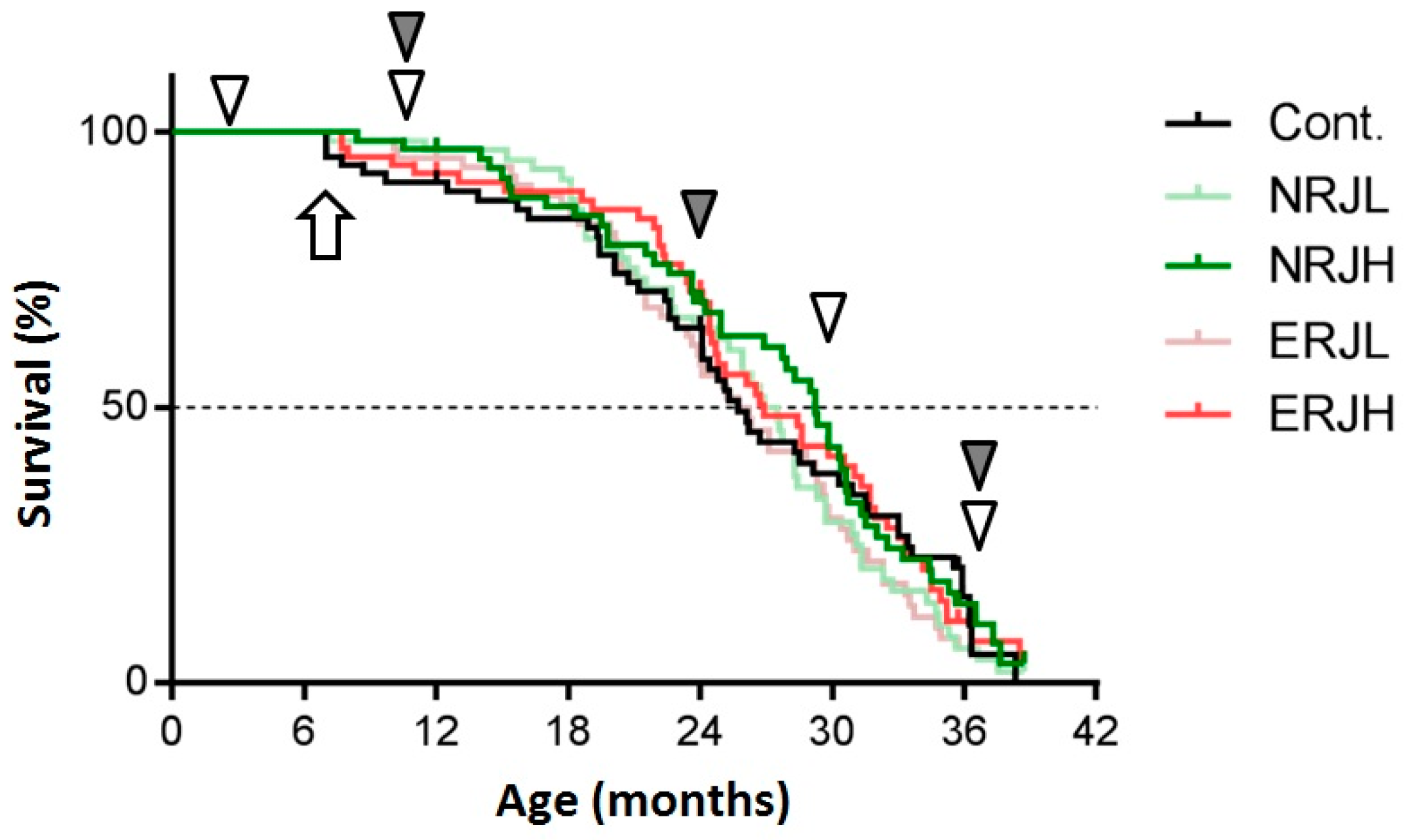
3.1. Food Intake, Body Weight, and Survival
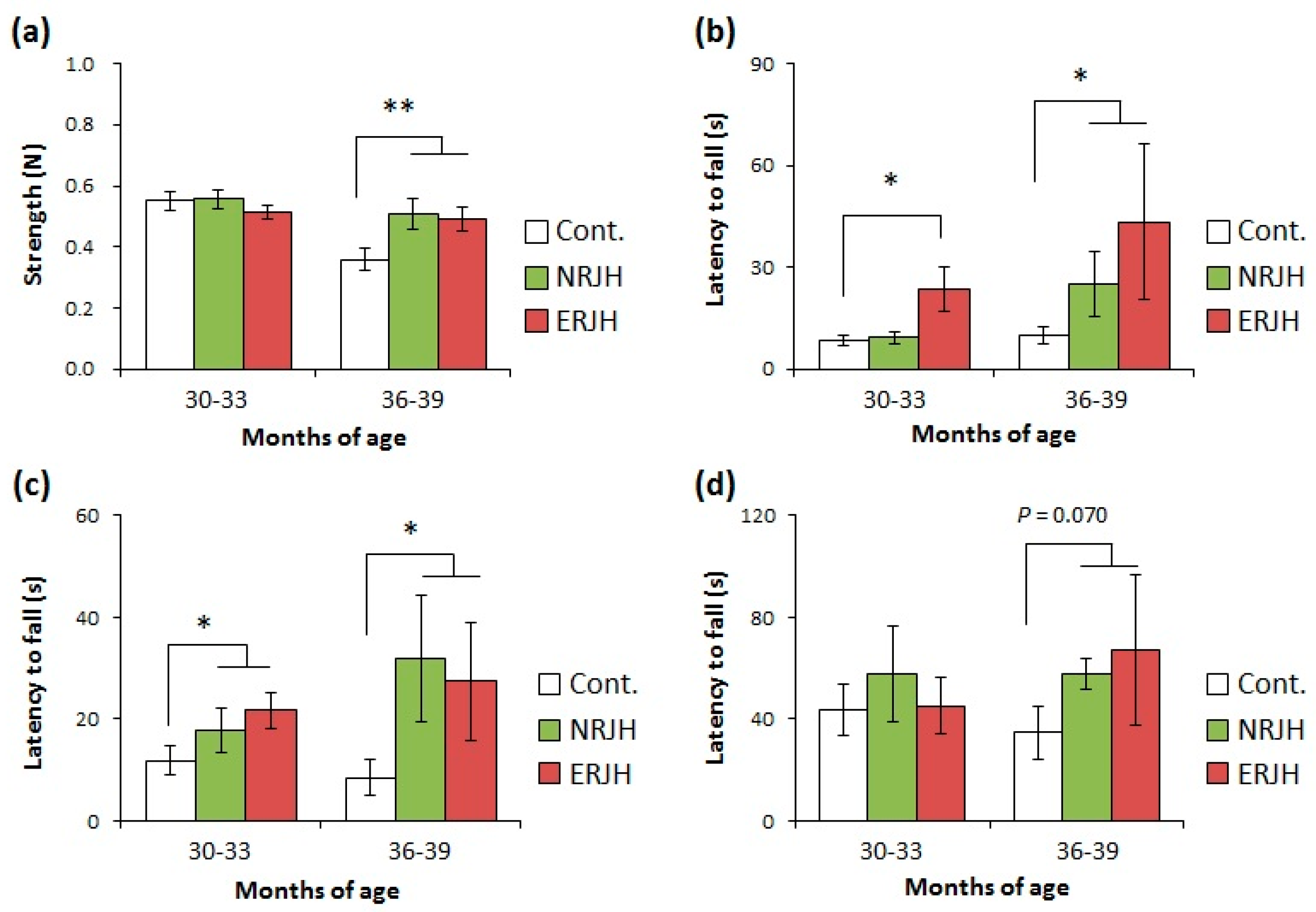
3.2. Effects of RJ on the Age-Dependent Impairment of Physical Performance
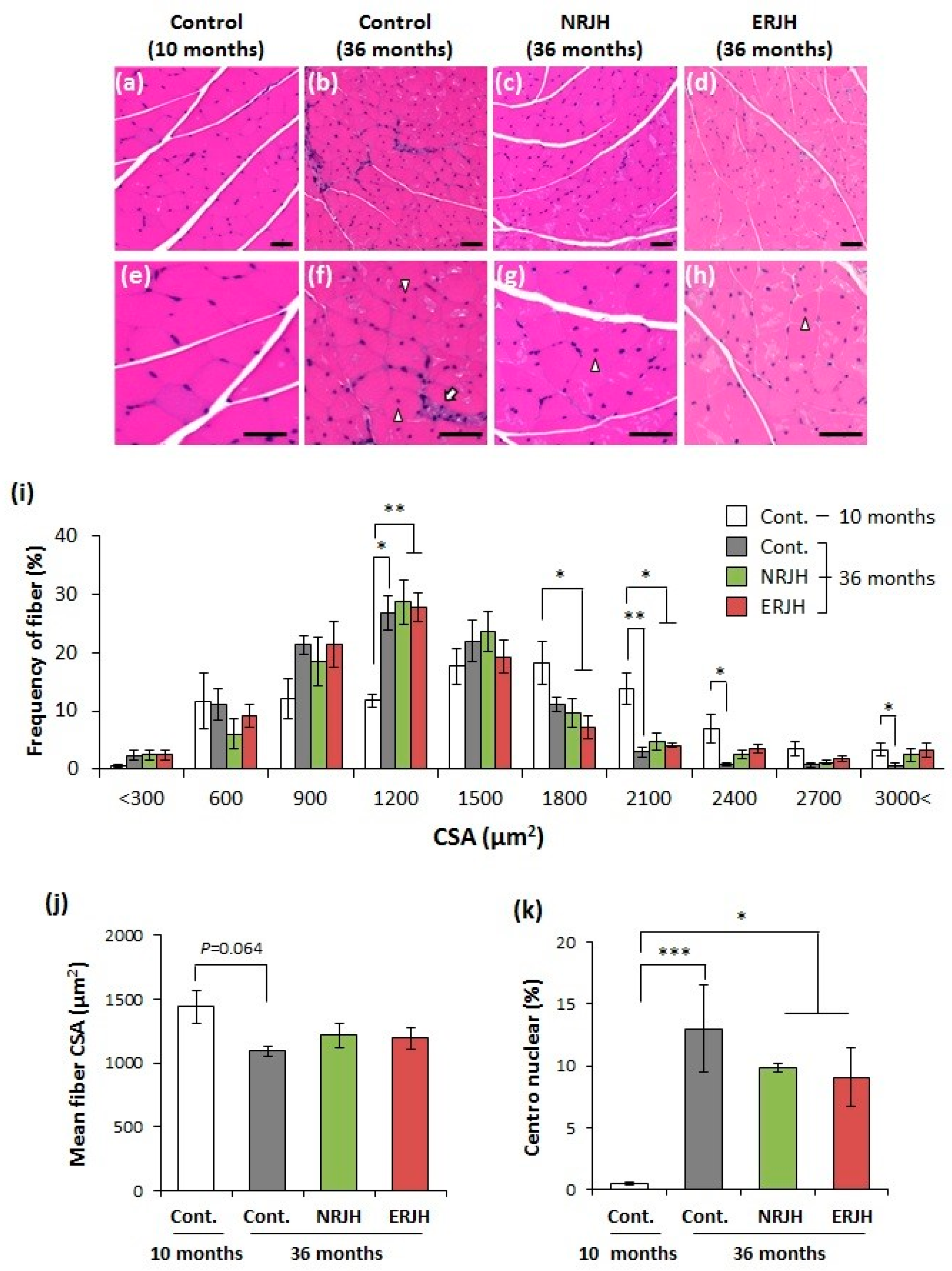
3.3. Effects of RJ on the Age-Related Morphological Changes in Muscle Fiber Quality
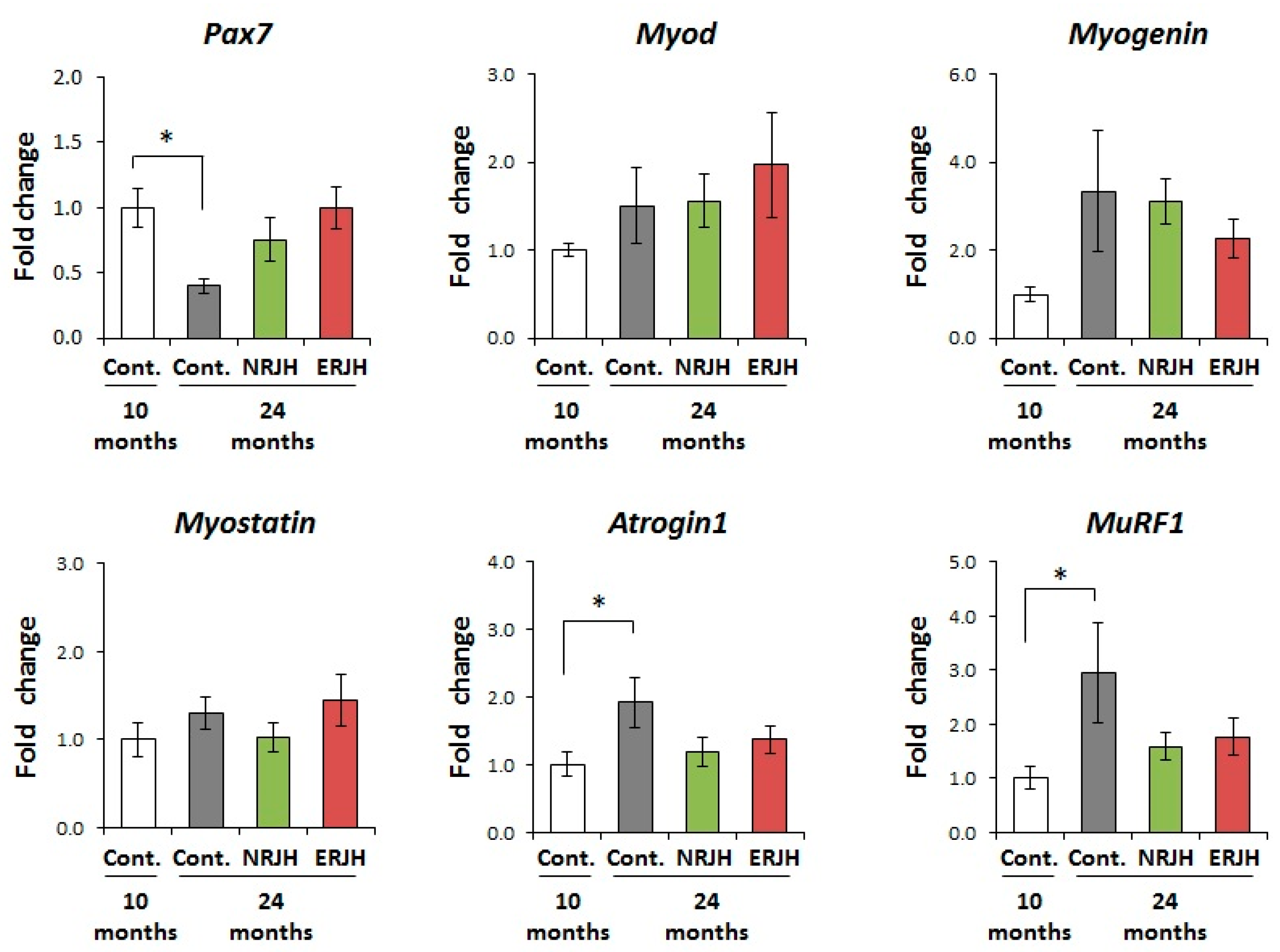
3.4. Effects of RJ on the Age-Related Changes in the mRNA Expression of Muscle Regeneration and Degradation-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- De Luca d’Alessandro, E.; Bonacci, S.; Giraldi, G. Aging populations: The health and quality of life of the elderly. Clin. Ter. 2011, 162, e13–e18. [Google Scholar] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.K.; Chan, P.; Vellas, B.; Cesari, M. Motoric Cognitive Risk Syndrome: Predictor of Dementia and Age-Related Negative Outcomes. Front. Med. (Lausanne) 2017, 4, 166. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del, R.D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Antebi, A.; Bartke, A.; Barzilai, N.; Brown-Borg, H.M.; Caruso, C.; Curiel, T.J.; de Cabo, R.; Franceschi, C.; Gems, D.; et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell 2015, 14, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Spyridopoulos, I.; Haendeler, J. Interventions to slow cardiovascular aging: Dietary restriction, drugs and novel molecules. Exp. Gerontol. 2018, 109, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Remolina, S.C.; Hughes, K.A. Evolution and mechanisms of long life and high fertility in queen honey bees. Age (Dordr) 2008, 30, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chittka, A.; Chittka, L. Epigenetics of royalty. PLoS Biol. 2010, 8, e1000532. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, R.; Maleszka, J.; Foret, S.; Maleszka, R. Nutritional control of reproductive status in honeybees via DNA methylation. Science 2008, 319, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Spannhoff, A.; Kim, Y.K.; Raynal, N.J.; Gharibyan, V.; Su, M.B.; Zhou, Y.Y.; Li, J.; Castellano, S.; Sbardella, G.; Issa, J.P.; et al. Histone deacetylase inhibitor activity in royal jelly might facilitate caste switching in bees. EMBO Rep. 2011, 12, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Fujita, Y.; Maruyama, H.; Araki, Y.; Ichihara, K.; Sato, A.; Kojima, T.; Tanaka, M.; Nozawa, Y.; Ito, M.; Honda, S. Lifespan-extending effects of royal jelly and its related substances on the nematode Caenorhabditis elegans. PLoS ONE 2011, 6, e23527. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-decenoic Acid, the major lipid component of royal jelly, extends the lifespan of Caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging. Res. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cook, L.F.; Grasso, L.M.; Cao, M.; Dong, Y. Royal jelly-mediated prolongevity and stress resistance in Caenorhabditis elegans is possibly modulated by the interplays of DAF-16, SIR-2.1, HCF-1, and 14-3-3 Proteins. J. Gerontol. A. Biol. Sci. Med. Sci. 2015, 70, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Detienne, G.; De Haes, W.; Ernst, U.R.; Schoofs, L.; Temmerman, L. Royalactin extends lifespan of Caenorhabditis elegans through epidermal growth factor signaling. Exp. Gerontol. 2014, 60, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Koya-Miyata, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly prolongs the life span of C3H/HeJ mice: Correlation with reduced DNA damage. Exp. Gerontol. 2003, 38, 965–969. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Alvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Minami, A.; Matsushita, H.; Ieno, D.; Matsuda, Y.; Horii, Y.; Ishii, A.; Takahashi, T.; Kanazawa, H.; Wakatsuki, A.; Suzuki, T. Improvement of neurological disorders in postmenopausal model rats by administration of royal jelly. Climacteric 2016, 19, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Kawashima, M.; Hisamura, R.; Imada, T.; Izuta, Y.; Nakamura, S.; Ito, M.; Tsubota, K. Clinical Evaluation of a Royal Jelly Supplementation for the restoration of dry eye: A prospective randomized double blind placebo controlled study and an experimental mouse model. PLoS ONE 2017, 12, e0169069. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Kaede, R.; Nagaya, K.; Aoyama, J.; Saito, M.; Okamatsu-Ogura, Y.; Kimura, K.; Terao, A. Royal jelly ameliorates diet-induced obesity and glucose intolerance by promoting brown adipose tissue thermogenesis in mice. Obes. Res. Clin. Pract. 2017, 12, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Guo, H.; Guo, Y.; Ebihara, S.; Asada, M.; Ohrui, T.; Furukawa, K.; Ichinose, M.; Yanai, K.; Kudo, Y.; et al. Royal jelly prevents the progression of sarcopenia in aged mice in vivo and in vitro. J. Gerontol. A. Biol. Sci. Med. Sci. 2013, 68, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Yanagihara, M.; Yano, E.; Kimura, G.; Seishima, M.; Tani, H.; Kanno, T.; Nakamura-Hirota, T.; Hashimoto, K.; Tatefuji, T.; et al. Hypoallergenicity and immunological characterization of enzyme-treated royal jelly from Apis mellifera. Biosci. Biotechnol. Biochem. 2013, 77, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Ladiges, W.; Van, R.H.; Strong, R.; Ikeno, Y.; Treuting, P.; Rabinovitch, P.; Richardson, A. Lifespan extension in genetically modified mice. Aging Cell 2009, 8, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Burke, D.; Nadon, N. Announcement: Four-way cross mouse stocks: A new, genetically heterogeneous resource for aging research. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, B358–B360. [Google Scholar] [CrossRef] [PubMed]
- Warner, H.R. NIA’s intervention testing program at 10 years of age. Age (Dordr) 2015, 37, 22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.A.; Astle, C.M.; Baur, J.A.; de Cabo, R.; Fernandez, E.; Guo, W.; Javors, M.; Kirkland, J.L.; Nelson, J.F.; et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Strong, R.; Miller, R.A.; Antebi, A.; Astle, C.M.; Bogue, M.; Denzel, M.S.; Fernandez, E.; Flurkey, K.; Hamilton, K.L.; Lamming, D.W.; et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 2016, 15, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Bildsoe, H.; Hughes, S.M. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J. Cell. Sci. 2005, 118, 4813–4821. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell. Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Camporez, J.P.; Petersen, M.C.; Abudukadier, A.; Moreira, G.V.; Jurczak, M.J.; Friedman, G.; Haqq, C.M.; Petersen, K.F.; Shulman, G.I. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc. Natl. Acad. Sci. USA 2016, 113, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Baur, J.A.; Boyd, A.R.; de Cabo, R.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nelson, J.F.; et al. Rapamycin, But Not Resveratrol or Simvastatin, Extends Life Span of Genetically Heterogeneous Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Fernandez, E.; Flurkey, K.; Han, M.; Javors, M.A.; Li, X.; Nadon, N.L.; Nelson, J.F.; et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 2014, 13, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Always, S.E.; Myers, M.J.; Mohamed, J.S. Regulation of satellite cell function in sarcopenia. Front. Aging Neurosci. 2014, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Aare, S.; Spendiff, S.; Vuda, M.; Elkrief, D.; Perez, A.; Wu, Q.; Mayaki, D.; Hussain, S.N.; Hettwer, S.; Hepple, R.T. Failed reinnervation in aging skeletal muscle. Skelet. Muscle. 2016, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wei, M.; Kang, X.; Deng, H.; Lu, Z. A novel method developed for acetylcholine detection in royal jelly by using capillary electrophoresis coupled with electrogenerated chemiluminescence based on a simple reaction. Electrophoresis 2009, 30, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kagota, S.; Maruyama, K.; Oonishi, Y.; Miyauchi-Wakuda, S.; Ito, Y.; Yamada, S.; Shinozuka, K. Royal jelly increases peripheral circulation by inducing vasorelaxation through nitric oxide production under healthy conditions. Biomed. Pharmacother. 2018, 106, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Wang, H.; Pei, Y.; Li, Y.; Wu, H.; Song, Y.; Guo, Q.; Guo, H.; Fukushima, S.; Tatefuji, T.; et al. Effects of protease-treated royal jelly on muscle strength in elderly nursing home residents: A. randomized, double-blind, placebo-controlled, dose-response study. Sci. Rep. 2017, 7, 11416. [Google Scholar] [CrossRef] [PubMed]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and skeletal muscle mass: The role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell. 2015, 14, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Wang, Y.X.; Rudnicki, M.A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 2015, 42, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Klose, A.; Forman, S.; Paris, N.D.; Wei-LaPierre, L.; Cortés-Lopéz, M.; Tan, A.; Flaherty, M.; Miura, P.; Dirksen, R.T.; Chakkalakal, J.V. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife 2017, 6, e26464. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.; Doyonnas, R.; Kraft, P.; Vitorovic, S.; Blau, H.M. Self-renewal and expansion of single transplanted muscle stem cells. Nature 2008, 456, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Guasconi, V.; Puri, P.L. Epigenetic drugs in the treatment of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Cavuşoğlu, K.; Yapar, K.; Yalçin, E. Royal jelly (honey bee) is a potential antioxidant against cadmium-induced genotoxicity and oxidative stress in albino mice. J. Med. Food 2009, 12, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid. Med. Cell. Longev. 2011, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, E.; Nejati, V.; Khazaei, M. Antioxidant and protective effects of royal jelly on histopathological changes in testis of diabetic rats. Int. J. Reprod. Biomed. (Yazd) 2016, 14, 519–526. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, K.; Zhang, Y.Z.; Zheng, Y.F.; Hu, F.L. In Vitro Anti-Inflammatory Effects of Three Fatty Acids from Royal Jelly. Mediators Inflamm. 2016, 2016, 11. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T. Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice. Nutrients 2018, 10, 1191. https://doi.org/10.3390/nu10091191
Okumura N, Toda T, Ozawa Y, Watanabe K, Ikuta T, Tatefuji T, Hashimoto K, Shimizu T. Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice. Nutrients. 2018; 10(9):1191. https://doi.org/10.3390/nu10091191
Chicago/Turabian StyleOkumura, Nobuaki, Toshihiko Toda, Yusuke Ozawa, Kenji Watanabe, Tomoki Ikuta, Tomoki Tatefuji, Ken Hashimoto, and Takahiko Shimizu. 2018. "Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice" Nutrients 10, no. 9: 1191. https://doi.org/10.3390/nu10091191
APA StyleOkumura, N., Toda, T., Ozawa, Y., Watanabe, K., Ikuta, T., Tatefuji, T., Hashimoto, K., & Shimizu, T. (2018). Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice. Nutrients, 10(9), 1191. https://doi.org/10.3390/nu10091191




