An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline
Abstract
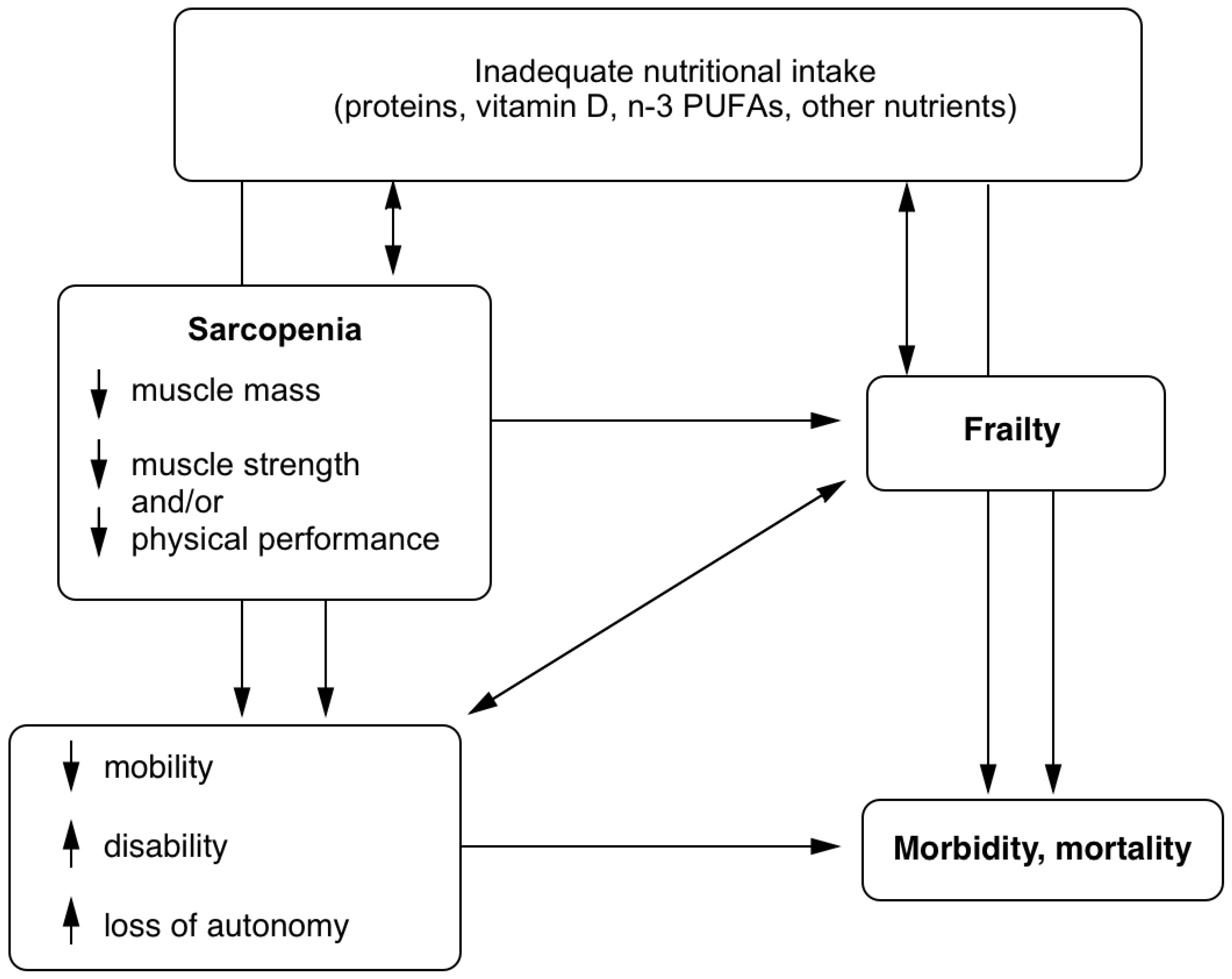
1. Introduction
2. Proteins and Amino Acids
2.1. Total Dietary Protein Intake
2.2. Meal Distribution of Dietary Protein
3. Leucine
4. Vitamin D
4.1. Vitamin D, Physical Function, and Muscle Mass and Strength
4.2. Vitamin D and Fall Prevention
5. N-3 Polyunsaturated Fatty Acids
6. Combined Supplements of Protein, Leucine, Vitamin D, and n-3 PUFA
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MPS | Muscle protein synthesis |
| n-3 PUFA | n-3 polyunsaturated fatty acids |
| RDA | Recommended Dietary Allowance |
| ALM | Appendicular lean mass |
| MyoPS | Myobfibrillar protein synthesis |
| RCT | Randomized controlled trials |
| SPPB | Short Physical Performance Battery |
| VDR | Vitamin D receptor |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| TUG | Timed-Up-and-Go |
| DXA | Dual-energy X-ray absorptiometry |
| BIA | Bioelectrical impedance |
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Woo, T.; Yu, S.; Visvanathan, R. Systematic literature review on the relationship between biomarkers of sarcopenia and quality of life in older people. J. Frailty Aging 2016, 5, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Newman, A.B. Health, Aging and Body Composition Study. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Diabetes, sarcopenia, and frailty. Clin. Geriatr. Med. 2008, 24, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Fox, K.M.; Gandra, S.R.; Delmonico, M.J.; Chiou, C.F.; Anthony, M.S.; Sewall, A.; Goodpaster, B.; Satterfield, S.; Cummings, S.R.; et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J. Am. Geriatr. Soc. 2009, 57, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The healthcare costs of sarcopenia in the united states. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilsirente study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, J.; Volkert, D.; Diekmann, R.; Kaiser, M.J.; Uter, W.; Vidal, K.; Sieber, C.C.; Bauer, J.M. Nutritional status according to the mini nutritional assessment (mna(r)) and frailty in community dwelling older persons: A close relationship. J. Nutr. Health Aging 2013, 17, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Dorner, T.E.; Luger, E.; Tschinderle, J.; Stein, K.V.; Haider, S.; Kapan, A.; Lackinger, C.; Schindler, K.E. Association between nutritional status (mna(r)-sf) and frailty (share-fi) in acute hospitalised elderly patients. J. Nutr. Health Aging 2014, 18, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Saoud, F.; Gray-Donald, K.; Morais, J.A. The physical functional capacity of frail elderly persons undergoing ambulatory rehabilitation is related to their nutritional status. J. Nutr. Health Aging 2008, 12, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.S.; Charlton, K.E.; Maggio, M.; et al. Frequency of malnutrition in older adults: A multinational perspective using the mini nutritional assessment. J. Am. Geriatr. Soc. 2010, 58, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.M.; Tee, A.R. Leucine and mTORC1: A complex relationship. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1329–E1342. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the prot-age study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the espen expert group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; p. 1358. [Google Scholar]
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S.M. Perspective: Protein requirements and optimal intakes in aging: Are we ready to recommend more than the recommended daily allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Franssen, R.; Dullemeijer, C.; van Dronkelaar, C.; Kyung Kim, H.; Ispoglou, T.; Zhu, K.; Prince, R.L.; van Loon, L.J.C.; de Groot, L. The impact of dietary protein or amino acid supplementation on muscle mass and strength in elderly people: Individual participant data and meta-analysis of rct’s. J. Nutr. Health Aging 2017, 21, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.J.; Milan, A.M.; Mitchell, S.M.; Zeng, N.; Ramzan, F.; Sharma, P.; Knowles, S.O.; Roy, N.C.; Sjodin, A.; Wagner, K.H.; et al. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: A 10-wk randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Cawood, A.L.; Elia, M.; Stratton, R.J. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res. Rev. 2012, 11, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Neelemaat, F.; Bosmans, J.E.; Thijs, A.; Seidell, J.C.; van Bokhorst-de van der Schueren, M.A. Oral nutritional support in malnourished elderly decreases functional limitations with no extra costs. Clin. Nutr. 2012, 31, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.O.; Lee, K.R. Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: A community-based randomized controlled study. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; van de Rest, O.; Dirks, M.L.; van der Zwaluw, N.; Mensink, M.; van Loon, L.J.; de Groot, L.C. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Kerr, D.A.; Meng, X.; Devine, A.; Solah, V.; Binns, C.W.; Prince, R.L. Two-year whey protein supplementation did not enhance muscle mass and physical function in well-nourished healthy older postmenopausal women. J. Nutr. 2015, 145, 2520–2526. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Dardevet, D.; Remond, D.; Peyron, M.A.; Papet, I.; Savary-Auzeloux, I.; Mosoni, L. Muscle wasting and resistance of muscle anabolism: The “anabolic threshold concept” for adapted nutritional strategies during sarcopenia. Sci. World J. 2012, 2012, 269531. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Gougeon, R.; Choong, N.; Lamarche, M.; Morais, J.A. Influence of adiposity in the blunted whole-body protein anabolic response to insulin with aging. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Glynn, E.L.; Timmerman, K.L.; Rasmussen, B.B.; Volpi, E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: Evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia 2009, 52, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Goulet, E.D.B.; Burgos, S.A.; Wykes, L.J.; Morais, J.A. Protein anabolic responses to a fed steady state in healthy aging. J. Gerontol. Ser. A 2011, 66A, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Glover, E.I.; Phillips, S.M.; Oates, B.R.; Tang, J.E.; Tarnopolsky, M.A.; Selby, A.; Smith, K.; Rennie, M.J. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J. Physiol. 2008, 586, 6049–6061. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.; Stokes, K.A.; Churchward-Venne, T.A.; Moore, D.R.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Two weeks of reduced activity decreases leglean mass and induces “anabolic resistance” of myofibrillar protein synthesis n healthy elderly. J. Clin. Endocrinol. Metab. 2013, 98, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y. Fighting sarcopenia in older frail subjects: Protein fuel for strength, exercise for mass. J. Am. Med. Dir. Assoc. 2013, 14, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Mamerow, M.M.; Mettler, J.A.; English, K.L.; Casperson, S.L.; Arentson-Lantz, E.; Sheffield-Moore, M.; Layman, D.K.; Paddon-Jones, D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J. Nutr. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Schutzler, S.; Schrader, A.; Spencer, H.; Kortebein, P.; Deutz, N.E.; Wolfe, R.R.; Ferrando, A.A. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E21–E28. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Curis, E.; Hamon-Vilcot, B.; Nicolis, I.; Chretien, P.; Schauer, N.; Vincent, J.P.; Cynober, L.; Aussel, C. Impact of protein pulse feeding on lean mass in malnourished and at-risk hospitalized elderly patients: A randomized controlled trial. Clin. Nutr. 2013, 32, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, P.; Morais, J.A.; Shatenstein, B.; Gray-Donald, K.; Khalil, A.; Dionne, I.; Ferland, G.; Fulop, T.; Jacques, D.; Kergoat, M.J.; et al. Nutrition as a determinant of successful aging: Description of the Quebec longitudinal study nuage and results from cross-sectional pilot studies. Rejuvenation Res. 2007, 10, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Morais, J.A.; Payette, H.; Gaudreau, P.; Shatenstein, B.; Gray-Donald, K.; Chevalier, S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the nuage study. Am. J. Clin. Nutr. 2016, 104, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Payette, H.; Morais, J.A.; Shatenstein, B.; Gaudreau, P.; Chevalier, S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-y physical function decline, in free-living older adults: The Quebec longitudinal study on nutrition as a determinant of successful aging (nuage study). Am. J. Clin. Nutr. 2017, 106, 113–124. [Google Scholar] [CrossRef] [PubMed]
- ten Haaf, D.; van Dongen, E.; Nuijten, M.; Eijsvogels, T.; de Groot, L.; Hopman, M. Protein intake and distribution in relation to physical functioning and quality of life in community-dwelling elderly people: Acknowledging the role of physical activity. Nutrients 2018, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, A.; Spiegel, A.; Kob, R.; Schoene, D.; Skurk, T.; Hauner, H.; Sieber, C.C.; Volkert, D.; Kiesswetter, E. Amount, distribution, and quality of protein intake are not associated with muscle mass, strength, and power in healthy older adults without functional limitations-an enable study. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.H.; Oikawa, S.Y.; Phillips, S.M. Dietary protein to maintain muscle mass in aging: A case for per-meal protein recommendations. J. Frailty Aging 2016, 5, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Goldman, J.D.; Sahyoun, N.R.; Moshfegh, A.J. Association between dietary protein intake and grip strength among adults aged 51 years and over: What we eat in america, national health and nutrition examination survey 2011-2014. PLoS ONE 2018, 13, e0191368. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Loprinzi, P.D.; Murphy, C.H.; Phillips, S.M. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin. Nutr. 2016, 35, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.J.; Kimball, S.R.; Emmert, S.W.; Anthony, J.C.; Jefferson, L.S. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J. Nutr. 2005, 135, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The rag gtpases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, C.S.; Aarsland, A.; Cree, M.G.; Wolfe, R.R. Muscle protein synthesis and balance responsiveness to essential amino acids ingestion in the presence of elevated plasma free fatty acid concentrations. J. Clin. Endocrinol. Metab. 2009, 94, 2984–2990. [Google Scholar] [CrossRef] [PubMed]
- Komar, B.; Schwingshackl, L.; Hoffmann, G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: A systematic review and meta-analysis. J. Nutr. Health Aging 2015, 19, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Tan, Z.J.; Zhang, Q.; Gui, Q.F.; Yang, Y.M. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; McGlory, C.; Bolster, D.R.; Kamil, A.; Rahn, M.; Harkness, L.; Baker, S.K.; Phillips, S.M. Leucine, not total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. J. Nutr. 2018, 148, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; McGlory, C.; Bolster, D.R.; Kamil, A.; Rahn, M.; Harkness, L.; Baker, S.K.; Phillips, S.M. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: A randomized, controlled trial. Am. J. Clin. Nutr. 2018, 107, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.H.; Saddler, N.I.; Devries, M.C.; McGlory, C.; Baker, S.K.; Phillips, S.M. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: A parallel-group crossover study. Am. J. Clin. Nutr. 2016, 104, 1594–1606. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef] [PubMed]
- van Schoor, N.M.; Lips, P. Worldwide vitamin D status. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.U.; Thomas, G.A.; Arnold, A.J. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J. Biol. Chem. 1985, 260, 8882–8891. [Google Scholar] [PubMed]
- Dirks-Naylor, A.J.; Lennon-Edwards, S. The effects of vitamin D on skeletal muscle function and cellular signaling. J. Steroid Biochem. Mol. Biol. 2011, 125, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011, 14, 938–939. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Tooze, J.A.; Davis, C.C.; Chaves, P.H.; Hirsch, C.H.; Robbins, J.A.; Arnold, A.M.; Newman, A.B.; Kritchevsky, S.B. Serum 25-hydroxyvitamin D and physical function in older adults: The cardiovascular health study all stars. J. Am. Geriatr. Soc. 2011, 59, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Cesari, M.; Ferrucci, L.; Cherubini, A.; Maggio, D.; Bartali, B.; Johnson, M.A.; Schwartz, G.G.; Kritchevsky, S.B. Association between vitamin D status and physical performance: The inCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Hu, F.B.; Zhang, Y.; Karlson, E.W.; Dawson-Hughes, B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am. J. Clin. Nutr. 2004, 80, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Tooze, J.A.; Neiberg, R.H.; Hausman, D.B.; Johnson, M.A.; Cauley, J.A.; Bauer, D.C.; Cawthon, P.M.; Shea, M.K.; Schwartz, G.G.; et al. 25-hydroxyvitamin D status and change in physical performance and strength in older adults: The health, aging, and body composition study. Am. J. Epidemiol. 2012, 176, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Toffanello, E.D.; Perissinotto, E.; Sergi, G.; Zambon, S.; Musacchio, E.; Maggi, S.; Coin, A.; Sartori, L.; Corti, M.C.; Baggio, G.; et al. Vitamin D and physical performance in elderly subjects: The pro.V.A study. PLoS ONE 2012, 7, e34950. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.-T.L.; von Mühlen, D.; Barrett-Connor, E.L. Sex specific association of serum 25-hydroxyvitamin D levels with physical function in older adults. Osteoporos. Int. 2009, 20, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Mowe, M.; Haug, E.; Bohmer, T. Low serum calcidiol concentration in older adults with reduced muscular function. J. Am. Geriatr. Soc. 1999, 47, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Zoico, E.; Tosoni, P.; Zivelonghi, A.; Bortolani, A.; Maggi, S.; Di Francesco, V.; Bosello, O. Relation between vitamin D, physical performance, and disability in elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M7-11. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Beauchet, O.; Berrut, G.; Fantino, B.; Bonnefoy, M.; Herrmann, F.R.; Schott, A.M. Is there an association between serum 25-hydroxyvitamin D concentration and muscle strength among older women? Results from baseline assessment of the epidos study. J. Nutr. Health Aging 2009, 13, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Henni, S.; Walrand, S.; Montero-Odasso, M.; Duque, G.; Duval, G.T. Vitamin D and walking speed in older adults: Systematic review and meta-analysis. Maturitas 2017, 106, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Vaes, A.M.M.; Brouwer-Brolsma, E.M.; Toussaint, N.; de Regt, M.; Tieland, M.; van Loon, L.J.C.; de Groot, L. The association between 25-hydroxyvitamin D concentration, physical performance and frailty status in older adults. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, P.; Liu, P.; Hao, Q.; Chen, S.; Dong, B.; Wang, J. Association of vitamin D deficiency and frailty: A systematic review and meta-analysis. Maturitas 2016, 94, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sohl, E.; de Jongh, R.T.; Heijboer, A.C.; Swart, K.M.; Brouwer-Brolsma, E.M.; Enneman, A.W.; de Groot, C.P.; van der Velde, N.; Dhonukshe-Rutten, R.A.; Lips, P.; et al. Vitamin D status is associated with physical performance: The results of three independent cohorts. Osteoporos. Int. 2013, 24, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Neiberg, R.H.; Tooze, J.A.; Hausman, D.B.; Johnson, M.A.; Cauley, J.A.; Bauer, D.C.; Shea, M.K.; Schwartz, G.G.; Williamson, J.D.; et al. Low 25-hydroxyvitamin D predicts the onset of mobility limitation and disability in community-dwelling older adults: The health abc study. J. Gerontol. A Biol. Sci. Med. Sci 2013, 68, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wicherts, I.S.; van Schoor, N.M.; Boeke, A.J.; Visser, M.; Deeg, D.J.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Deeg, D.J.; Lips, P.; Longitudinal Aging Study, A. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The longitudinal aging study amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- Verreault, R.; Semba, R.D.; Volpato, S.; Ferrucci, L.; Fried, L.P.; Guralnik, J.M. Low serum vitamin D does not predict new disability or loss of muscle strength in older women. J. Am. Geriatr. Soc. 2002, 50, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Hill, T.R.; Davies, K.; Jagger, C.; Adamson, A.; Siervo, M.; Kirkwood, T.B.; Mathers, J.C.; Sayer, A.A. Vitamin D status, muscle strength and physical performance decline in very old adults: A prospective study. Nutrients 2017, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Schott, A.M.; Berrut, G.; Fantino, B.; Beauchet, O. Vitamin D-related changes in physical performance: A systematic review. J. Nutr. Health Aging 2009, 13, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Muir, S.W.; Montero-Odasso, M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2011, 59, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl-Riise, H.; Spielau, U.; Ranhoff, A.H.; Gudbrandsen, O.A.; Dierkes, J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyere, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Glendenning, P.; Zhu, K.; Inderjeeth, C.; Howat, P.; Lewis, J.R.; Prince, R.L. Effects of three-monthly oral 150,000 iu cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: A randomized controlled trial. J. Bone Miner. Res. 2012, 27, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, S.; Kidgell, D.J.; Daly, R.M. Effects of vitamin D supplementation on neuroplasticity in older adults: A double-blinded, placebo-controlled randomised trial. Osteoporos. Int. 2015, 26, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Orav, E.; Staehelin, H.B.; Meyer, O.W.; Theiler, R.; Dick, W.; Willett, W.C.; Egli, A. Monthly high-dose vitamin D treatment for the prevention of functional decline: A randomized clinical trial. JAMA Intern. Med. 2016, 176, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 10 Leading Causes of Injury Deaths by Age Group Highlighting Unintentional Injury Deaths, United States—2016. Available online: https://www.cdc.gov/injury/images/lc-charts/leading_causes_of_death_highlighting_unintentional_2016_1040w800h.gif (accessed on 8 August 2018).
- Deandrea, S.; Lucenteforte, E.; Bravi, F.; Foschi, R.; La Vecchia, C.; Negri, E. Risk factors for falls in community-dwelling older people: A systematic review and meta-analysis. Epidemiology 2010, 21, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Reid, I.R. Differences in overlapping meta-analyses of vitamin D supplements and falls. J. Clin. Endocrinol. Metab. 2014, 99, 4265–4272. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Reid, I.R. Vitamin D supplements and the risk of falls. JAMA Intern. Med. 2015, 175, 1723–1724. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Gamble, G.D.; Reid, I.R. Vitamin D supplementation and falls: A trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014, 2, 573–580. [Google Scholar] [CrossRef]
- Guirguis-Blake, J.M.; Michael, Y.L.; Perdue, L.A.; Coppola, E.L.; Beil, T.L. Interventions to prevent falls in older adults: Updated evidence report and systematic review for the us preventive services task force. JAMA 2018, 319, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- U.S. Preventive Services Task Force. Interventions to prevent falls in community-dwelling older adults: Us preventive services task force recommendation statement. JAMA 2018, 319, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men*. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Marino, A.; Cuzzocrea, S. N-3 fatty acids: Role in neurogenesis and neuroplasticity. Curr. Med. Chem. 2013, 20, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Sullenbarger, B.; Prakash, R.; McDaniel, J.C. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Lemaitre, R.N.; King, I.B.; Song, X.; Huang, H.; Sacks, F.M.; Rimm, E.B.; Wang, M.; Siscovick, D.S. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: A cohort study. Ann. Intern. Med. 2013, 158, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Vannice, G.; Rasmussen, H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, Y.; Brooks, J.; Reider, C.; Fulgoni, V.L., III. U.S. Adults are not meeting recommended levels for fish and omega-3 fatty acid intake: Results of an analysis using observational data from nhanes 2003–2008. Nutr. J. 2014, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Almasud, A.; Mazurak, V.C. Role of n-3 fatty acids in muscle loss and myosteatosis. Appl. Physiol. Nutr. Metab. 2014, 39, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.R.; Mittendorfer, B. Fish oil-derived n-3 polyunsaturated fatty acids for the prevention and treatment of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Lalia, A.Z.; Dasari, S.; Robinson, M.M.; Abid, H.; Morse, D.M.; Klaus, K.A.; Lanza, I.R. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging 2017, 9, 1096–1129. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Jameson, K.A.; Batelaan, S.F.; Martin, H.J.; Syddall, H.E.; Dennison, E.M.; Cooper, C.; Sayer, A.A.; Hertfordshire Cohort Study, G. Diet and its relationship with grip strength in community-dwelling older men and women: The hertfordshire cohort study. J. Am. Geriatr. Soc. 2008, 56, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.H.; Kleppinger, A.; Kenny, A.M. Self-reported dietary intake of omega-3 fatty acids and association with bone and lower extremity function. J. Am. Geriatr. Soc. 2009, 57, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Plourde, M.; Stark, K.D.; Jones, P.J.; Lin, Y.H. Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am. J. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Abbatecola, A.M.; Cherubini, A.; Guralnik, J.M.; Andres Lacueva, C.; Ruggiero, C.; Maggio, M.; Bandinelli, S.; Paolisso, G.; Ferrucci, L. Plasma polyunsaturated fatty acids and age-related physical performance decline. Rejuvenation Res. 2009, 12, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Frison, E.; Boirie, Y.; Peuchant, E.; Tabue-Teguo, M.; Barberger-Gateau, P.; Feart, C. Plasma fatty acid biomarkers are associated with gait speed in community-dwelling older adults: The three-city-bordeaux study. Clin. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Fougere, B.; de Souto Barreto, P.; Goisser, S.; Soriano, G.; Guyonnet, S.; Andrieu, S.; Vellas, B.; Group, M.S. Red blood cell membrane omega-3 fatty acid levels and physical performance: Cross-sectional data from the mapt study. Clin. Nutr. 2018, 37, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Reinders, I.; Song, X.; Visser, M.; Eiriksdottir, G.; Gudnason, V.; Sigurdsson, S.; Aspelund, T.; Siggeirsdottir, K.; Brouwer, I.A.; Harris, T.B.; et al. Plasma phospholipid pufas are associated with greater muscle and knee extension strength but not with changes in muscle parameters in older adults. J. Nutr. 2015, 145, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Reinders, I.; Murphy, R.A.; Song, X.; Visser, M.; Cotch, M.F.; Lang, T.F.; Garcia, M.E.; Launer, L.J.; Siggeirsdottir, K.; Eiriksdottir, G.; et al. Polyunsaturated fatty acids in relation to incident mobility disability and decline in gait speed; the age, gene/environment susceptibility-reykjavik study. Eur. J. Clin. Nutr. 2015, 69, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Fougere, B.; Goisser, S.; Cantet, C.; Soriano, G.; Guyonnet, S.; De Souto Barreto, P.; Cesari, M.; Andrieu, S.; Vellas, B.; Group, M.S. Omega-3 fatty acid levels in red blood cell membranes and physical decline over 3 years: Longitudinal data from the mapt study. Geroscience 2017. [Google Scholar] [CrossRef] [PubMed]
- Hutchins-Wiese, H.L.; Kleppinger, A.; Annis, K.; Liva, E.; Lammi-Keefe, C.J.; Durham, H.A.; Kenny, A.M. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J. Nutr. Health Aging 2013, 17, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 pufa therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.L.; Spriet, L.L. Omega-3 fatty acid supplementation for 12 weeks increases resting and exercise metabolic rate in healthy community-dwelling older females. PLoS ONE 2015, 10, e0144828. [Google Scholar] [CrossRef] [PubMed]
- Krzyminska-Siemaszko, R.; Czepulis, N.; Lewandowicz, M.; Zasadzka, E.; Suwalska, A.; Witowski, J.; Wieczorowska-Tobis, K. The effect of a 12-week omega-3 supplementation on body composition, muscle strength and physical performance in elderly individuals with decreased muscle mass. Int J. Environ. Res. Public Health 2015, 12, 10558–10574. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Liu, C.; Ji, Z.; Yang, R.; An, Q.; Zhang, X.; You, J.; Duan, D.; Sun, Y.; Zhu, Y.; et al. A high whey protein, vitamin D and e supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chanet, A.; Verlaan, S.; Salles, J.; Giraudet, C.; Patrac, V.; Pidou, V.; Pouyet, C.; Hafnaoui, N.; Blot, A.; Cano, N.; et al. Supplementing breakfast with a vitamin D and leucine-enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J. Nutr. 2017, 147, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.E.; Snijders, T.; Zulyniak, M.; Kumbhare, D.; Parise, G.; Chabowski, A.; Phillips, S.M. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: A randomized controlled trial. PLoS ONE 2017, 12, e0181387. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the provide study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Hangartner, T.N.; Warner, S.; Braillon, P.; Jankowski, L.; Shepherd, J. The official positions of the international society for clinical densitometry: Acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J. Clin. Densitom. 2013, 16, 520–536. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessier, A.-J.; Chevalier, S. An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline. Nutrients 2018, 10, 1099. https://doi.org/10.3390/nu10081099
Tessier A-J, Chevalier S. An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline. Nutrients. 2018; 10(8):1099. https://doi.org/10.3390/nu10081099
Chicago/Turabian StyleTessier, Anne-Julie, and Stéphanie Chevalier. 2018. "An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline" Nutrients 10, no. 8: 1099. https://doi.org/10.3390/nu10081099
APA StyleTessier, A.-J., & Chevalier, S. (2018). An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline. Nutrients, 10(8), 1099. https://doi.org/10.3390/nu10081099



