Potassium Citrate Supplementation Decreases the Biochemical Markers of Bone Loss in a Group of Osteopenic Women: The Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Supplements
2.3. Blood Analyses
2.4. Urine Analyses
2.5. Immunoenzymatic Assay of BTM
2.6. Calculations and Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Participants at Baseline
3.2. K Citrate Supplementation and Changes in Urinary Parameters
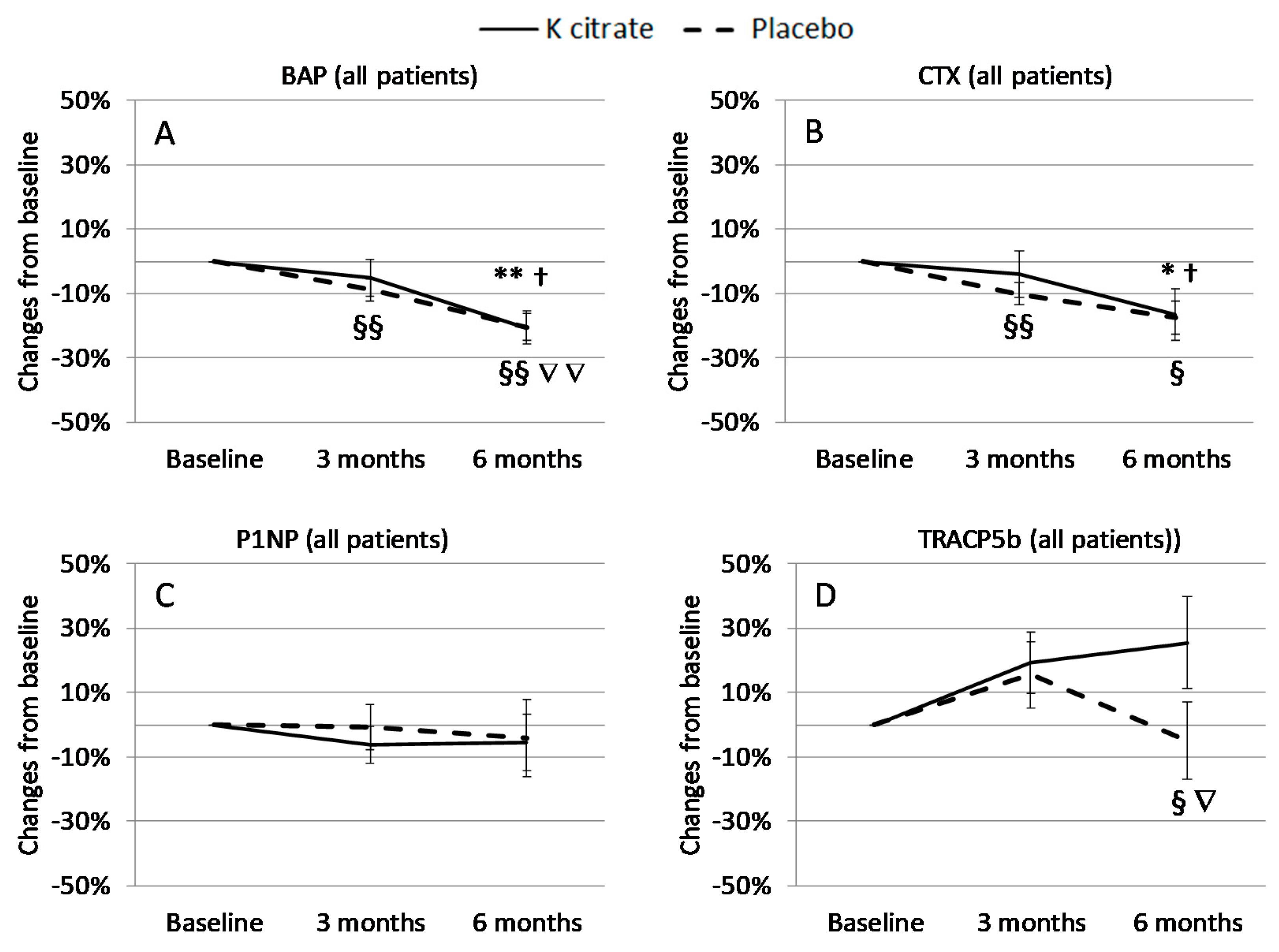
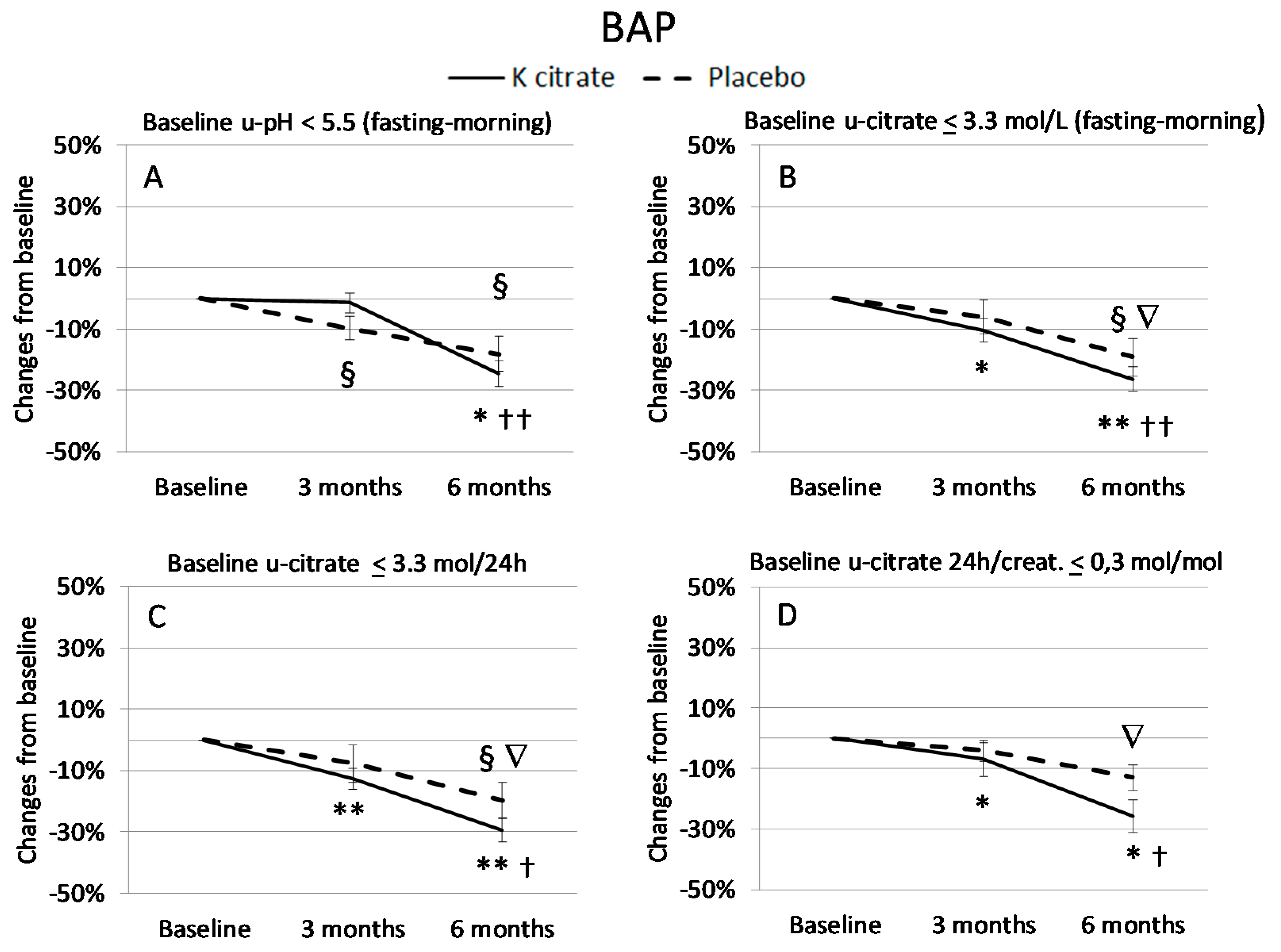
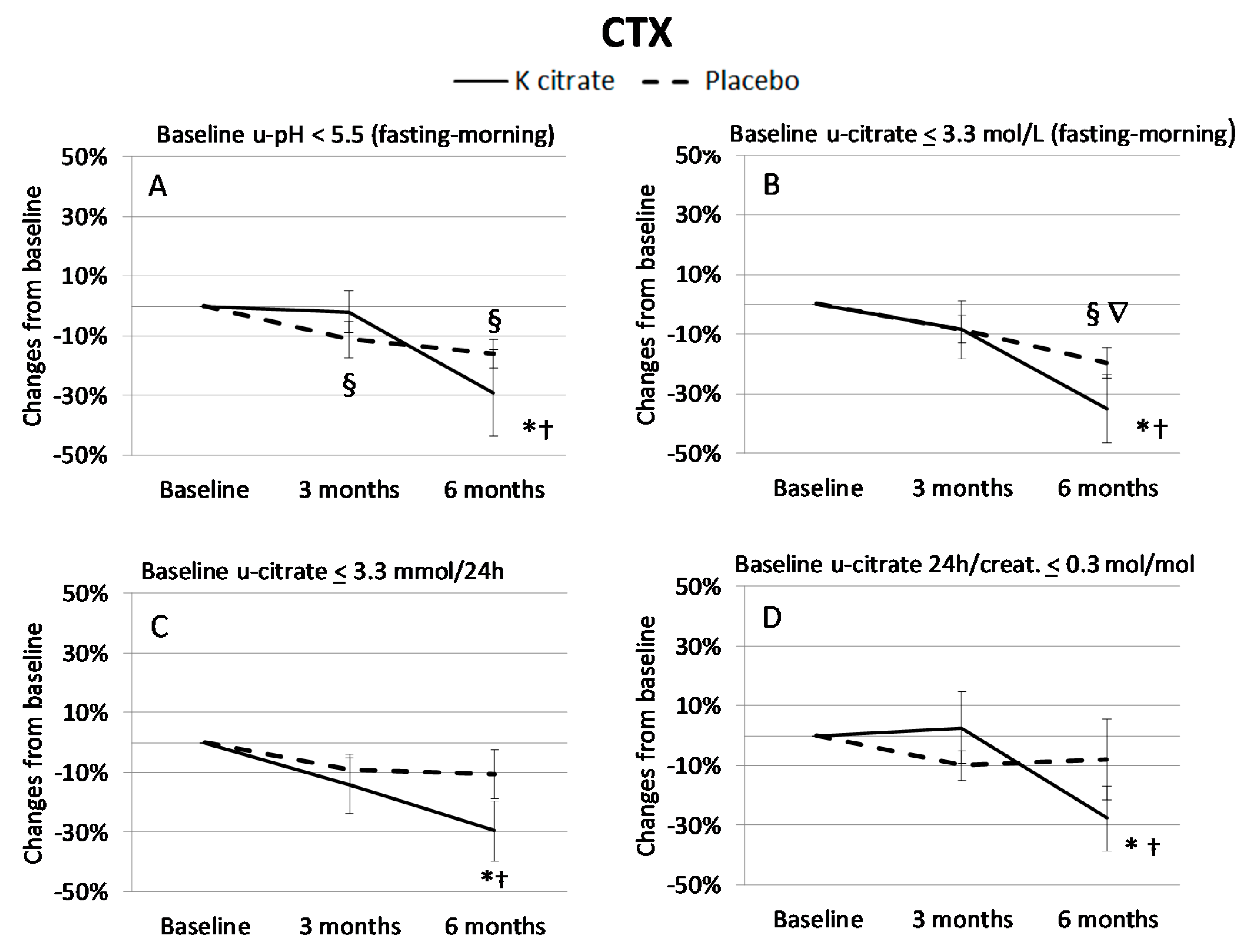
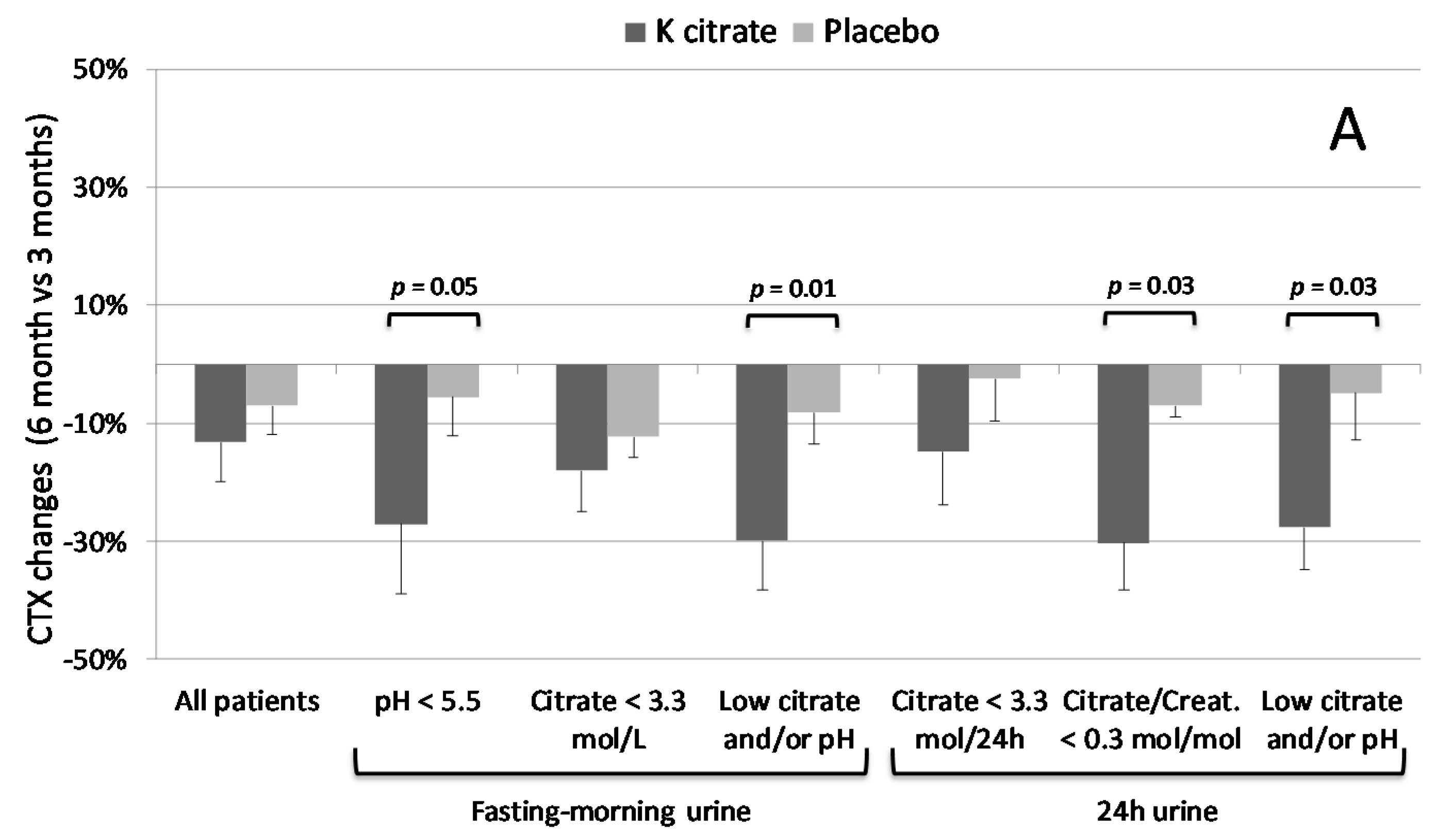
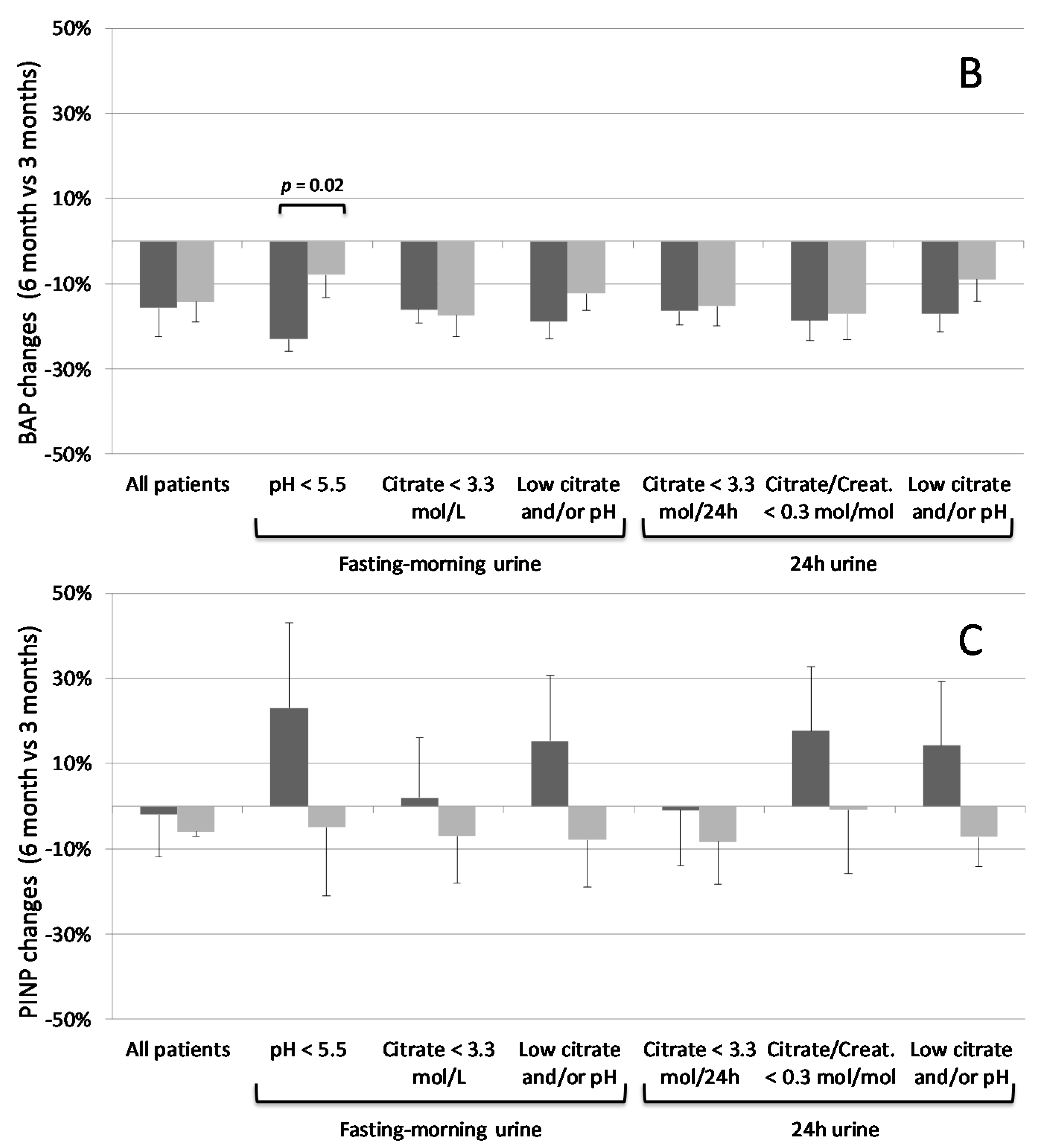
3.3. K Citrate Supplementation and Changes in BTM
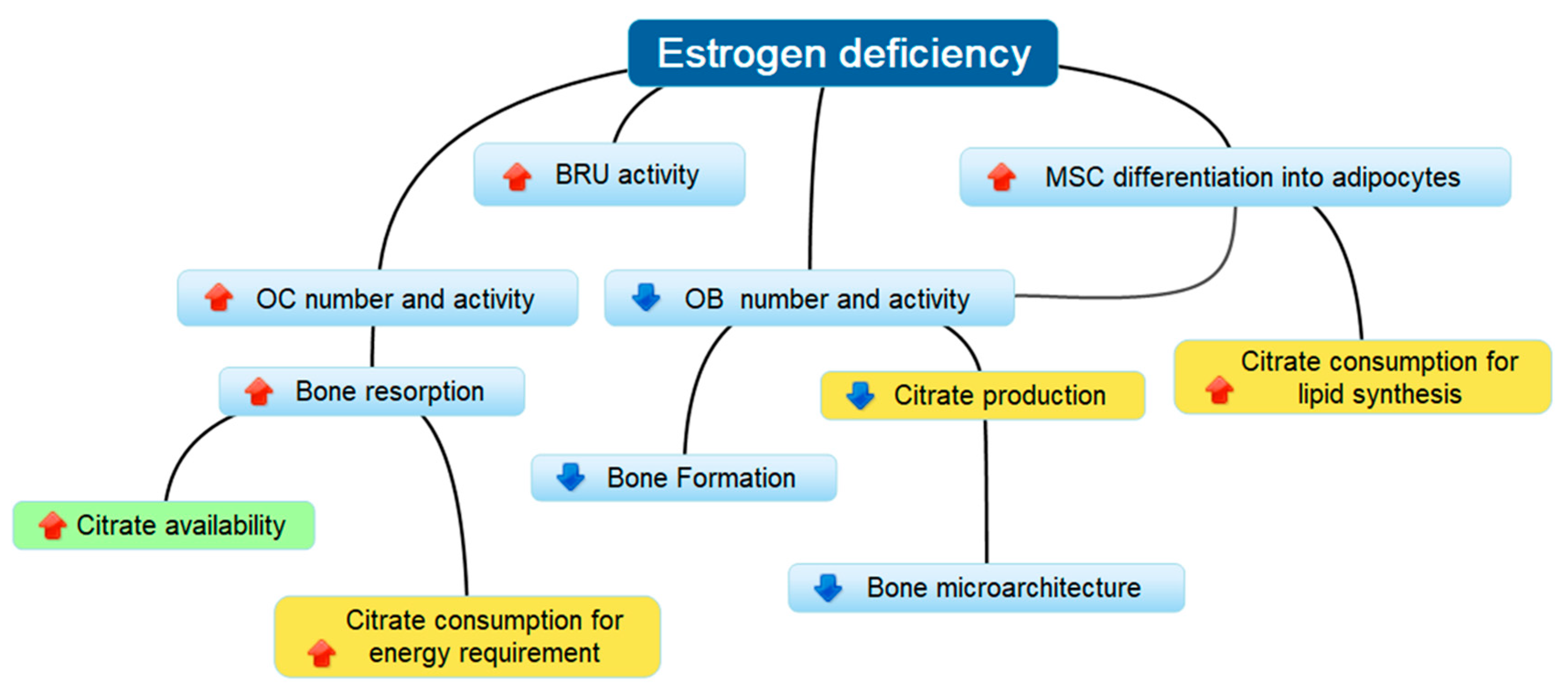
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Safi, Z.; Santoro, N. The Postmenopausal Woman. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279131/ (accessed on 26 July 2018).
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Delaisse, J.M. The reversal phase of the bone-remodeling cycle: Cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2014, 3, 561. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P. Osteoporosis: From osteoscience to neuroscience and beyond. Mech. Ageing Dev. 2015, 145, 26–38. [Google Scholar] [CrossRef] [PubMed]
- International Osteoporosis Foundation. Facts and Statistics. Available online: https://www.iofbonehealth.org/facts-statistics (accessed on 26 July 2018).
- Abrahamsen, B.; van Staa, T.; Ariely, R.; Olson, M.; Cooper, C. Excess mortality following hip fracture: A systematic epidemiological review. Osteoporos. Int. 2009, 20, 1633–1650. [Google Scholar] [CrossRef] [PubMed]
- Siris, E.S.; Chen, Y.T.; Abbott, T.A.; Barrett-Connor, E.; Miller, P.D.; Wehren, L.E.; Berger, M.L. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch. Intern. Med. 2004, 164, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Lello, S.; Sorge, R.; Surico, N.; OMERO Study Group. Osteoporosis’s Menopausal Epidemiological Risk Observation (O.M.E.R.O.) study. Gynecol. Endocrinol. 2015, 31, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; McCloskey, E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 2008, 19, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.; McLaren Howard, J. The acid-ash hypothesis revisited: A reassessment of the impact of dietary acidity on bone. J. Bone Miner. Metab. 2014, 32, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Arnett, T.R. Acidosis, hypoxia and bone. Arch. Biochem. Biophys. 2010, 503, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lencel, P.; Magne, D. Inflammaging: The driving force in osteoporosis? Med. Hypotheses 2011, 76, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Carnauba, R.A.; Baptistella, A.B.; Paschoal, V.; Hubscher, G.H. Diet-Induced low-grade metabolic acidosis and clinical outcomes: A review. Nutrients 2017, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Jassal, S.K.; von Muhlen, D.; Barrett-Connor, E. Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: The Rancho Bernardo study. J. Bone Miner. Res. 2007, 22, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Tough, S.C.; Lyon, A.W.; Eliasziw, M.; Hanley, D.A. Causal assessment of dietary acid load and bone disease: A systematic review & meta-analysis applying Hill’s epidemiologic criteria for causality. Nutr. J. 2011, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Lyon, A.W.; Eliasziw, M.; Tough, S.C.; Hanley, D.A. Meta-analysis of the effect of the acid-ash hypothesis of osteoporosis on calcium balance. J. Bone Miner. Res. 2009, 24, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, G.; Trinchieri, A. Recent advances in managing and understanding nephrolithiasis/nephrocalcinosis. F1000Res 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.S.; Bushinsky, D.A. The relation between bone and stone formation. Calcif. Tissue Int. 2013, 93, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Arrabal-Polo, M.A.; Cano-Garcia Mdel, C.; Canales, B.K.; Arrabal-Martin, M. Calcium nephrolithiasis and bone demineralization: Pathophysiology, diagnosis, and medical management. Curr. Opin. Urol. 2014, 24, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Caudarella, R.; Ripamonti, C.; Spinnato, P.; Bazzocchi, A.; Torreggiani, E.; Massa, A.; Baldini, N. Association between markers of bone loss and urinary lithogenic risk factors in osteopenic postmenopausal women. J. Biol. Regul. Homeost. Agents 2016, 30, 145–151. [Google Scholar] [PubMed]
- Esche, J.; Johner, S.; Shi, L.; Schonau, E.; Remer, T. Urinary citrate, an index of acid-base status, predicts bone strength in youths and fracture risk in adult females. J. Clin. Endocrinol. Metab. 2016, 101, 4914–4921. [Google Scholar] [CrossRef] [PubMed]
- Mizunashi, K.; Furukawa, Y.; Katano, K.; Abe, K. Effect of omeprazole, an inhibitor of H+, K (+)-ATPase, on bone resorption in humans. Calcif. Tissue Int. 1993, 53, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.A.; Leslie, W.D.; Targownik, L.E.; Papaioannou, A.; Adachi, J.D.; CaMos Research Group. The effect of proton pump inhibitors on fracture risk: Report from the Canadian Multicenter Osteoporosis Study. Osteoporos. Int. 2013, 24, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Lambert, H.; Frassetto, L.; Moore, J.B.; Torgerson, D.; Gannon, R.; Burckhardt, P.; Lanham-New, S. The effect of supplementation with alkaline potassium salts on bone metabolism: A meta-analysis. Osteoporos. Int. 2015, 26, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Vescini, F.; Buffa, A.; La Manna, G.; Ciavatti, A.; Rizzoli, E.; Bottura, A.; Stefoni, S.; Caudarella, R. Long-term potassium citrate therapy and bone mineral density in idiopathic calcium stone formers. J. Endocrinol. Investig. 2005, 28, 218–222. [Google Scholar] [CrossRef]
- Granchi, D.; Torreggiani, E.; Massa, A.; Caudarella, R.; Di Pompo, G.; Baldini, N. Potassium citrate prevents increased osteoclastogenesis resulting from acidic conditions: Implication for the treatment of postmenopausal bone loss. PLoS ONE 2017, 12, e0181230. [Google Scholar] [CrossRef] [PubMed]
- Rivers, K.; Shetty, S.; Menon, M. When and how to evaluate a patient with nephrolithiasis. Urol. Clin. N. Am. 2000, 27, 203–213. [Google Scholar] [CrossRef]
- Marangella, M.; Di Stefano, M.; Casalis, S.; Berutti, S.; D’Amelio, P.; Isaia, G.C. Effects of potassium citrate supplementation on bone metabolism. Calcif. Tissue Int. 2004, 74, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic Medical Laboratories. Endocrinology Catalog Bone/Minerals. Available online: https://Endocrinology.Testcatalog.Org/Search?Q=Mml-Bone-Minerals&Sort=Alpha (accessed on 26 July 2018).
- Krupp, D.; Shi, L.; Remer, T. Longitudinal relationships between diet-dependent renal acid load and blood pressure development in healthy children. Kidney Int. 2014, 85, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar] [CrossRef]
- Moseley, K.F.; Weaver, C.M.; Appel, L.; Sebastian, A.; Sellmeyer, D.E. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J. Bone Miner. Res. 2013, 28, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhong, B. Last observation carry-forward and last observation analysis. Stat. Med. 2003, 22, 2429–2441. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Trials 2010, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.Y.; Resnick, M.I. Medical therapy and new approaches to management of urolithiasis. Urol. Clin. N. Am. 2000, 27, 243–253. [Google Scholar] [CrossRef]
- Lucato, P.; Trevisan, C.; Stubbs, B.; Zanforlini, B.M.; Solmi, M.; Luchini, C.; Girotti, G.; Pizzato, S.; Manzato, E.; Sergi, G.; et al. Nephrolithiasis, bone mineral density, osteoporosis, and fractures: A systematic review and comparative meta-analysis. Osteoporos. Int. 2016, 27, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Domrongkitchaiporn, S.; Stitchantrakul, W.; Kochakarn, W. Causes of hypocitraturia in recurrent calcium stone formers: Focusing on urinary potassium excretion. Am. J. Kidney Dis. 2006, 48, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Sellmeyer, D.E.; Schloetter, M.; Sebastian, A. Potassium citrate prevents increased urine calcium excretion and bone resorption induced by a high sodium chloride diet. J. Clin. Endocrinol. Metab. 2002, 87, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Sakhaee, K.; Maalouf, N.M.; Abrams, S.A.; Pak, C.Y. Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J. Clin. Endocrinol. Metab. 2005, 90, 3528–3533. [Google Scholar] [CrossRef] [PubMed]
- Jehle, S.; Zanetti, A.; Muser, J.; Hulter, H.N.; Krapf, R. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J. Am. Soc. Nephrol. 2006, 17, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; Black, A.J.; Aucott, L.; Duthie, G.; Duthie, S.; Sandison, R.; Hardcastle, A.C.; Lanham New, S.A.; Fraser, W.D.; Reid, D.M. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: A randomized controlled trial. Am. J. Clin. Nutr. 2008, 88, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Karp, H.J.; Ketola, M.E.; Lamberg-Allardt, C.J. Acute effects of calcium carbonate, calcium citrate and potassium citrate on markers of calcium and bone metabolism in young women. Br. J. Nutr. 2009, 102, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Jehle, S.; Hulter, H.N.; Krapf, R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: A randomized controlled trial. J. Clin. Endocrinol. Metab. 2013, 98, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.S.; Kumar, R.; Stein, E.M.; Alexander, E.; Christos, P.; Bockman, R.S.; Rodman, J.S. Potassium citrate decreases bone resorption in postmenopausal women with osteopenia: A randomized, double-blind clinical trial. Endocr. Pract. 2015, 21, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Pigott, T.; Gossiel, F.; Naylor, K.E.; Walsh, J.S.; Peel, N.F.A. Diagnosis of endocrine disease: Bone turnover markers: Are they clinically useful? Eur. J. Endocrinol. 2018, 178, R19–R31. [Google Scholar] [CrossRef] [PubMed]
- Diez-Perez, A.; Naylor, K.E.; Abrahamsen, B.; Agnusdei, D.; Brandi, M.L.; Cooper, C.; Dennison, E.; Eriksen, E.F.; Gold, D.T.; Guanabens, N.; et al. International osteoporosis foundation and european calcified tissue society working group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos. Int. 2017, 28, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Krege, J.H.; Lane, N.E.; Harris, J.M.; Miller, P.D. PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos. Int. 2014, 25, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Goulis, D.G.; Polyzos, S.A.; Gerou, S.; Koukoulis, G.N.; Efstathiadou, Z.; Kita, M.; Avramidis, A. Head-to-head comparison of risedronate vs. teriparatide on bone turnover markers in women with postmenopausal osteoporosis: A randomised trial. Int. J. Clin. Pract. 2008, 62, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Strontium ranelate: A physiological approach for optimizing bone formation and resorption. Bone 2006, 38, S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.P. Nutritional disturbance in acid-base balance and osteoporosis: A hypothesis that disregards the essential homeostatic role of the kidney. Br. J. Nutr. 2013, 110, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.M.; Assimos, D.G. Hypocitraturia: Pathophysiology and medical management. Rev. Urol. 2009, 11, 134–144. [Google Scholar] [PubMed]
- Perna, S.; Avanzato, I.; Nichetti, M.; D’Antona, G.; Negro, M.; Rondanelli, M. Association between dietary patterns of meat and fish consumption with bone mineral density or fracture risk: A systematic literature. Nutrients 2017, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Banerjee, T.; Powe, N.; Sebastian, A. Acid Balance, Dietary acid load, and bone effects-a controversial subject. Nutrients 2018, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Caudarella, R.; Vescini, F.; Buffa, A.; Stefoni, S. Citrate and mineral metabolism: Kidney stones and bone disease. Front. Biosci. 2003, 8, S1084–S1106. [Google Scholar] [PubMed]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The Pathophysiology and Treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Chellaiah, M.; Zou, J.; Reynolds, M.A.; Costello, L.C. Evidence that Osteoblasts are Specialized Citrate-producing Cells that Provide the citrate for incorporation into the structure of bone. Open Bone J. 2014, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Jeong, D.; Kang, H.K.; Jung, S.Y.; Kang, S.S.; Min, B.M. Osteoclast precursors display dynamic metabolic shifts toward accelerated glucose metabolism at an early stage of RANKL-stimulated osteoclast differentiation. Cell. Physiol. Biochem. 2007, 20, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Lemma, S.; Sboarina, M.; Porporato, P.E.; Zini, N.; Sonveaux, P.; Di Pompo, G.; Baldini, N.; Avnet, S. Energy metabolism in osteoclast formation and activity. Int. J. Biochem. Cell Biol. 2016, 79, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Y.; Dai, H.; Tian, X.; Cui, Z.K.; Chen, Z.; Hu, L.; Song, Q.; Liu, A.; Zhang, Z.; et al. Bone and plasma citrate is reduced in osteoporosis. Bone 2018, 114, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.G.; Carter, R.E.; Nietert, P.J.; Stewart, P.W. Recommendations for planning pilot studies in clinical and translational research. Clin. Transl. Sci. 2011, 4, 332–337. [Google Scholar] [CrossRef] [PubMed]


| K Citrate (n = 20) | Placebo (n = 20) | |
|---|---|---|
| Age | 60.8 ± 1.0 (52.0–69.0; 62.0) | 58.2 ± 1.1 (48.0–70.0; 57.0) |
| Years post-menopause | 11.5 ± 1.4 (5.0–31.0; 9.0) | 8.3 ± 0.9 (5.0–20.0; 7.5) |
| BMI (kg m−2) | 23.7 ± 1.0 (18.7–37.0; 22.7) | 22.9 ± 0.8 (18.3–31.2; 21.8) |
| T-score | ||
| Femoral neck L2–L4 | −1.6 ± 0.1 (−2.4 to −0.6; −1.7) −1.7 ± 0.1 (−2.3 to −0.6; −1.9) | −1.7 ± 0.1 (−2.4 to −0.5; −1.8) −1.5 ± 0.1 (−2.4 to − 0.1; −1.5) |
| FRAX | ||
| Major osteoporotic risk Minor osteoporotic risk | 5.7 ± 0.8 (2.2–19.0; 4.9) 1.1 ± 0.2 (0–3.1; 0.9) | 4.8 ± 0.3 (2.4–8.6; 4.7) 1.0 ± 0.1 (0.1–1.9; 1.0) |
| Analyte (Unit) | Reference Values | Baseline | 3 Months | 6 Months | |||
|---|---|---|---|---|---|---|---|
| K Citrate | Placebo | K Citrate | Placebo | K Citrate | Placebo | ||
| Creatinine (mg dL−1) | 0.5–1.2 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0 |
| Calcium (mg dL−1) | 8.6–10.5 | 9.6 ± 0.1 | 9.6 ± 0.1 | 9.5 ± 0.1 | 9.6 ± 0.1 | 9.6 ± 0.1 | 9.7 ± 0.1 |
| Phosphorus (mg dL−1) | 2.5–4.5 | 3.6 ± 0.1 | 3.7 ± 0.1 | 3.7 ± 0.1 | 3.8 ± 0.1 | 3.7 ± 0.1 | 3.8 ± 0.1 |
| Magnesium (mg dL−1) | 1.6–2.6 | 2.2 ± 0.0 | 2.1 ± 0.0 | 2.2 ± 0.0 | 2.1 ± 0.0 | 2.2 ± 0.0 | 2.2 ± 0.0 |
| Sodium (mg dL−1) | 136.0–145.0 | 142.2 ± 0.5 | 142.3 ± 0.4 | 141.2 ± 0.5 | 141.4 ± 0.5 | 140.1 ± 0.6 | 140.6 ± 0.5 |
| Potassium (mg dl−1) | 3.5–5.3 | 4.5 ± 0.1 | 4.5 ± 0.1 | 4.5 ± 0.1 | 4.4 ± 0.1 | 4.4 ± 0.1 | 4.4 ± 0.1 |
| PTH (pg mL−1) | 12.0–88.0 | 46.5 ± 3.9 | 47.0 ± 3.3 | 48.0 ± 4.6 | 44.2 ± 2.6 | 44.2 ± 4.9 | 47.0 ± 4.3 |
| 25OH Vitamin D3 (ng mL−1) | <20 failure 20–100 sufficiency >100 potential toxicity | 31.6 ± 2.3 | 32.1 ± 1.6 | 31.0 ± 2.4 | 34.1 ± 1.9 | 32.1 ± 1.9 | 34.9 ± 1.3 |
| Analyte (Unit) | Reference Values | Baseline | 3 Months | 6 Months | |||
|---|---|---|---|---|---|---|---|
| K Citrate | Placebo | K Citrate | Placebo | K Citrate | Placebo | ||
| 24 h urine | |||||||
| Diuresis (mL) | 1724.0 ± 155.0 | 1702.0 ± 150.0 | 1763.0 ± 181.0 | 1721.0 ± 163.0 | 1899.0 ± 179.0 | 1853.0 ± 192.0 | |
| pH | 5.5–7 | 6.1 ± 0.1 | 6.0 ± 0.2 | 6.5 ± 0.2 | 6.3 ± 0.1 | 6.6 ± 0.1 | 6.2 ± 0.1 a |
| Creatinine (g day−1) | 1.3–1.8 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Citrate (mmol day−1) | >3.3 | 3.0 ± 0.4 | 3.5 ± 0.4 | 4.0 ± 0.5 | 3.2 ± 0.3 | 4.0 ± 0.4 | 3.2 ± 0.3 |
| Citrate/Creatinine (mol mol−1) | >0.3 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.5 ± 0.1 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 a |
| Potassium (mEq day−1) | 50–100 | 28.6 ± 3.4 | 30.0 ± 2.9 | 43.0 ± 10.6 | 28.5 ± 6.6 | 46.6 ± 4.4 | 35.4 ± 3.4 a |
| Calcium (mg day−1) | <200 | 117.4 ± 16.3 | 128.7 ± 12.4 | 124.4 ± 18.3 | 123.2 ± 13.4 | 142.8 ± 17.8 | 140.9 ± 15.5 |
| Calcium/Creatinine (mg mg−1) | 0.02–0.2 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| Oxalate (mmol day−1) | <0.5 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Oxalate/Creatinine (mmol mol−1) | <50 | 48.9 ± 3.1 | 35.2 ± 2.7 b | 43.0 ± 4.1 | 40.8 ± 4.5 | 33.8 ± 1.9 | 33.9 ± 2.9 |
| Ammonium (mmol day−1) | 20–50 | 20.7 ± 1.0 | 21.2 ± 0.8 | 23.3 ± 2.0 | 20.5 ± 0.7 | 23.2 ± 1.1 | 22.1 ± 1.1 |
| Chloride (mEq day−1) | 140–240 | 121.0 ± 11.4 | 124.1 ± 6.6 | 180.8 ± 7.2 | 126.3 ± 9.1 | 100.9 ± 8.3 | 110.5 ± 9.8 |
| Magnesium (mg day−1) | >50 | 58.2 ± 5.7 | 59.1 ± 4.5 | 61.6 ± 7.6 | 54.1 ± 5.3 | 87.5 ± 13.5 | 70.7 ± 12.9 |
| Phosphate (mmol day−1) | <42 | 13.0 ± 1.2 | 12.1 ± 1.0 | 15.1± 3.1 | 10.0 ± 0.7 | 12.9 ± 1.7 | 9.8 ± 0.7 |
| Sodium (mEq day−1) | 50–200 | 101.5 ± 9.8 | 99.5 ± 5.0 | 97.9 ± 8.1 | 111.8 ± 8.5 | 98.8 ± 8.5 | 107.2 ± 10.7 |
| Sulphate (mmol day−1) | 6–30 | 11.6 ± 1.0 | 12.4 ± 0.9 | 13.8 ± 2.6 | 11.8 ± 0.7 | 13.0 ± 1.4 | 12.3 ± 0.9 |
| Urea (g day−1) | 10–35 | 15.4 ± 0.9 | 8.5 ± 1.3 | 17.4 ± 2.0 | 15.5 ± 0.7 | 17.2 ± 1.3 | 15.8 ± 1.3 |
| Uric Acid (mg day−1) | <600 | 417.6 ± 25.0 | 413.5 ± 20.6 | 499.8 ± 46.0 | 389.7 ± 19.0 a | 461.4 ± 32.9 | 395.5 ± 25.1 |
| Fasting-morning urine | |||||||
| pH | 5.5–7 | 6.1 ± 0.2 | 5.9 ± 0.2 | 6.3 ± 0.2 | 6.0 ± 0.2 | 6.1 ± 0.1 | 5.6 ± 0.2 a |
| Creatinine (mg dL−1) | n.d.2 | 76.4 ± 9.6 | 83.1 ± 12.7 | 78.4 ± 10.4 | 63.0 ± 7.7 | 81.5 ± 11.1 | 97.82± 15.3 |
| Citrate (mmol L−1) | >3.3 | 2.8 ± 0.3 | 3.4 ± 0.5 | 3.4 ± 0.5 | 2.5 ± 0.3 | 3.5 ± 0.5 | 3.3 ± 0.5 |
| Citrate/Creatinine (mol mol−1) | >0.3 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.1 | 0.5 ± 0.0 | 0.5 ± 0.1 | 0.40 ± 0.04 a |
| Calcium/Creatinine (mg mg−1) | 0.02–0.2 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.12 ± 0.02 |
| Uric Acid (mg dL−1) | n.d. 2 | 36.7 ± 3.3 | 37.0 ± 4.0 | 39.3 ± 4.7 | 29.0 ± 3.0 | 35.6 ± 4.5 | 36.0 ± 4.0 |
| Marker | Baseline | 3 Months | 6 Months | |||
|---|---|---|---|---|---|---|
| K Citrate | Placebo | K Citrate | Placebo | K Citrate | Placebo | |
| Carboxy-terminal telopeptide of type I collagen (CTX) (µg L−1) | 0.64 ± 0.08 | 0.64 ± 0.05 | 0.63 ± 0.08 | 0.56 ± 0.05 | 0.53 ± 0.08 | 0.54 ± 0.06 |
| Bone alkaline phosphatase (BAP) (µg L−1) | 21.89 ± 1.67 | 20.36 ± 1.17 | 19.81 ± 1.67 | 18.27 ± 1.00 | 16.83 ± 1.37 | 15.79 ± 1.09 |
| Procollagen type 1 N terminal propeptide (PINP) (pg L−1) | 17.45 ± 1.48 | 18.82 ± 1.73 | 16.24 ± 1.60 | 18.39 ± 1.75 | 14.9 7 ± 1.51 | 16.77 ± 1.89 |
| Tartrate-resistant acid phosphatase 5b (TRACP5b) (U L−1) | 2.35 ± 0.20 | 2.64 ± 0.22 | 2.79 ± 0.27 | 2.85 ± 0.22 | 2.69 ± 0.29 | 2.25 ± 0.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granchi, D.; Caudarella, R.; Ripamonti, C.; Spinnato, P.; Bazzocchi, A.; Massa, A.; Baldini, N. Potassium Citrate Supplementation Decreases the Biochemical Markers of Bone Loss in a Group of Osteopenic Women: The Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2018, 10, 1293. https://doi.org/10.3390/nu10091293
Granchi D, Caudarella R, Ripamonti C, Spinnato P, Bazzocchi A, Massa A, Baldini N. Potassium Citrate Supplementation Decreases the Biochemical Markers of Bone Loss in a Group of Osteopenic Women: The Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients. 2018; 10(9):1293. https://doi.org/10.3390/nu10091293
Chicago/Turabian StyleGranchi, Donatella, Renata Caudarella, Claudio Ripamonti, Paolo Spinnato, Alberto Bazzocchi, Annamaria Massa, and Nicola Baldini. 2018. "Potassium Citrate Supplementation Decreases the Biochemical Markers of Bone Loss in a Group of Osteopenic Women: The Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study" Nutrients 10, no. 9: 1293. https://doi.org/10.3390/nu10091293
APA StyleGranchi, D., Caudarella, R., Ripamonti, C., Spinnato, P., Bazzocchi, A., Massa, A., & Baldini, N. (2018). Potassium Citrate Supplementation Decreases the Biochemical Markers of Bone Loss in a Group of Osteopenic Women: The Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients, 10(9), 1293. https://doi.org/10.3390/nu10091293








