Fabrication of Co-Based Cladding Layer by Microbeam Plasma and Its Corrosion Mechanism to Molten Salt
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
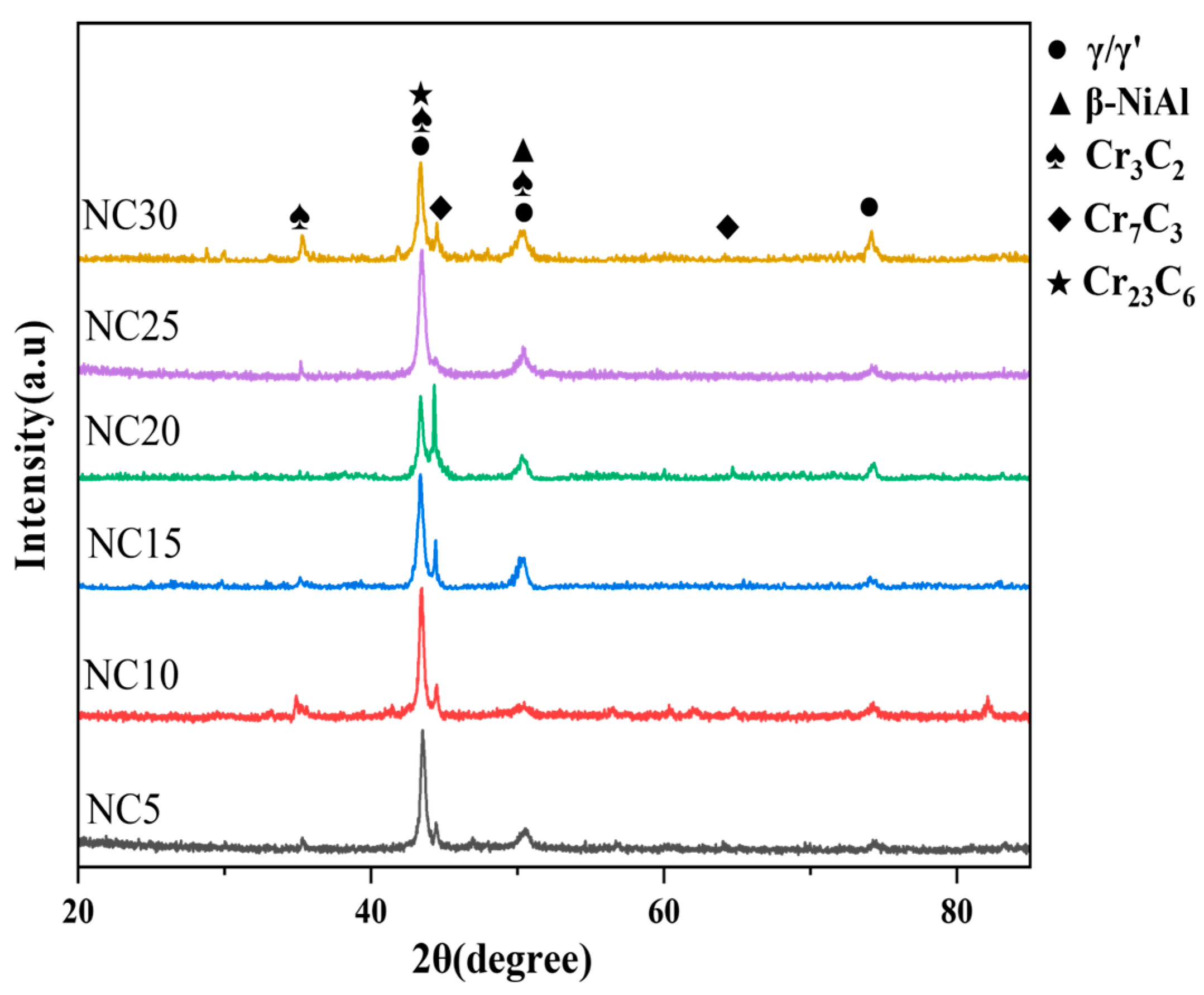
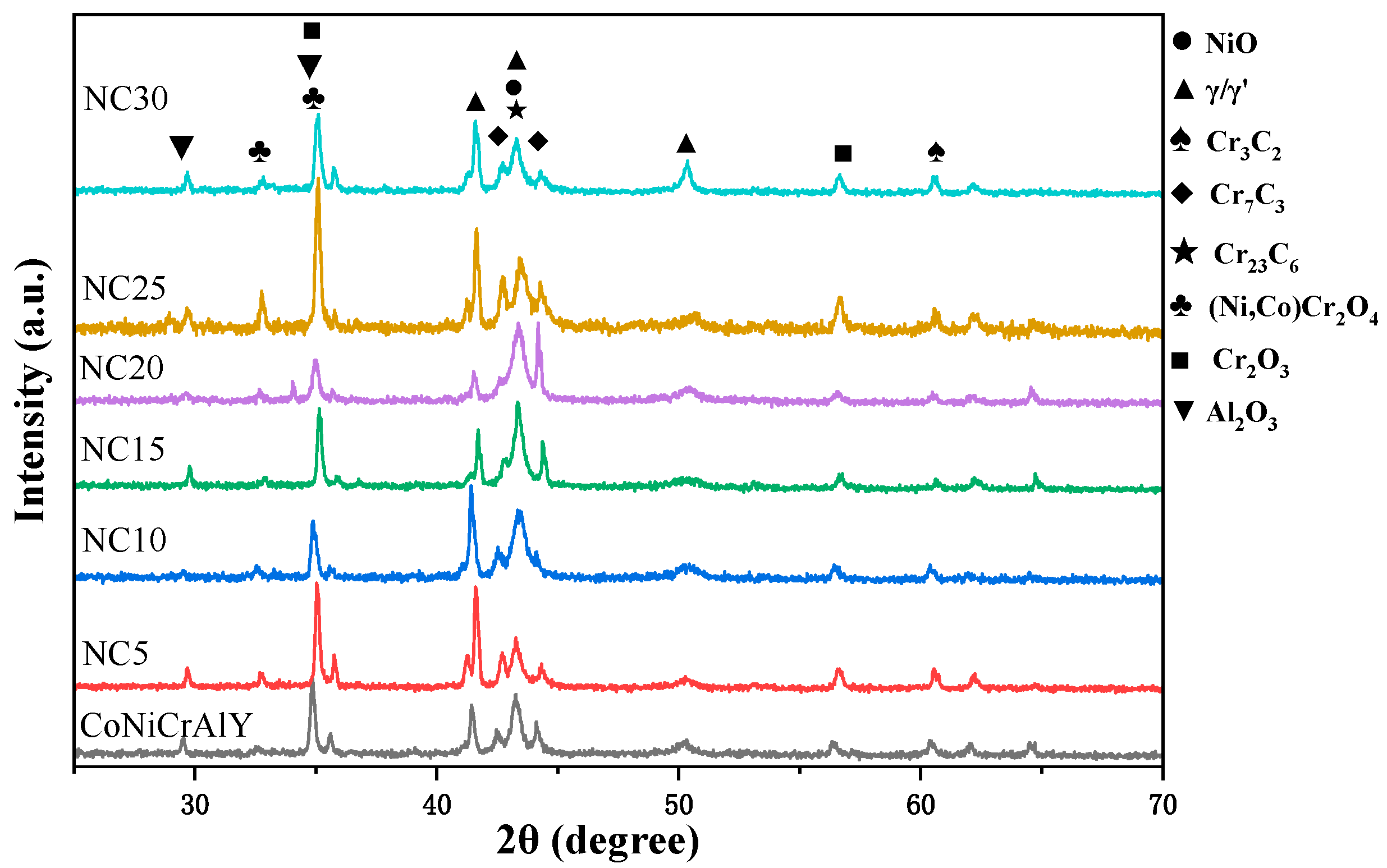
3.1. Microstructures
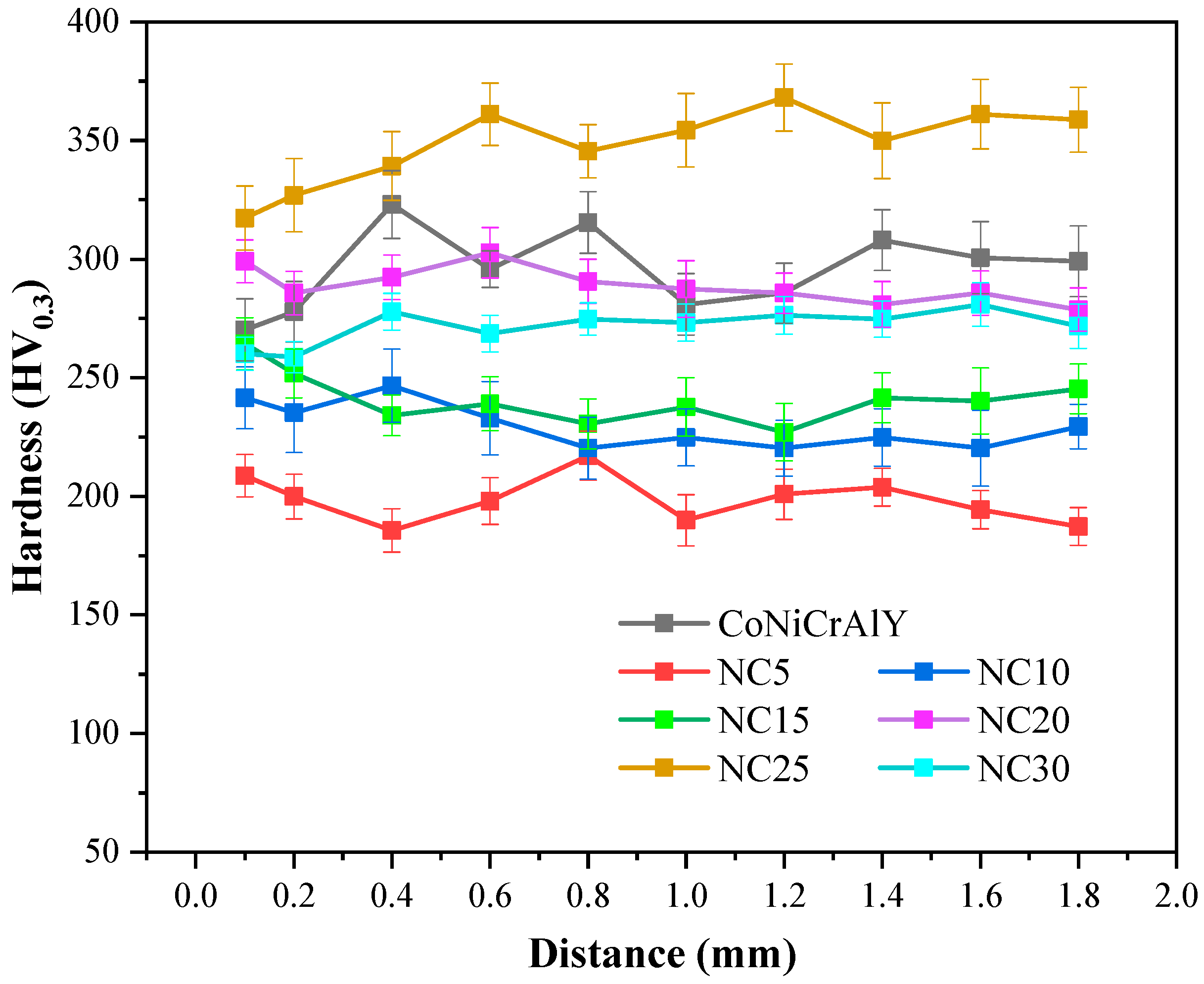
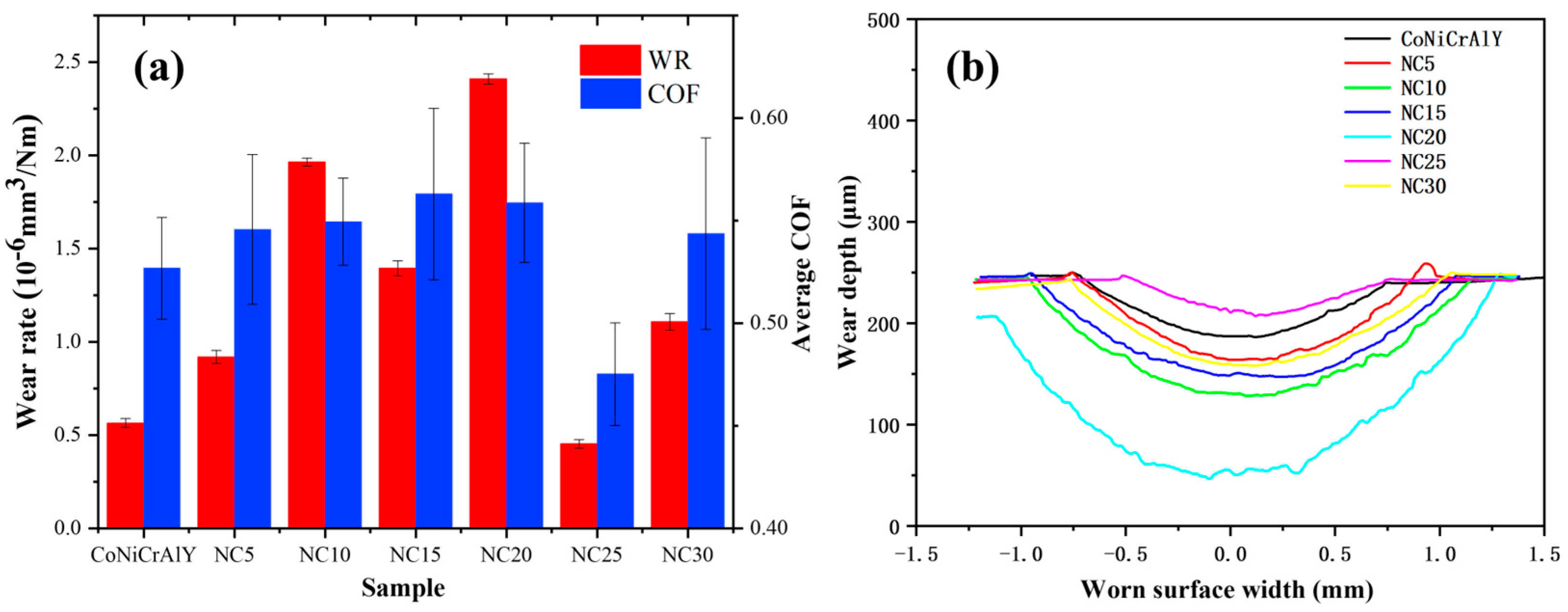
3.2. Hardness and Tribological Properties
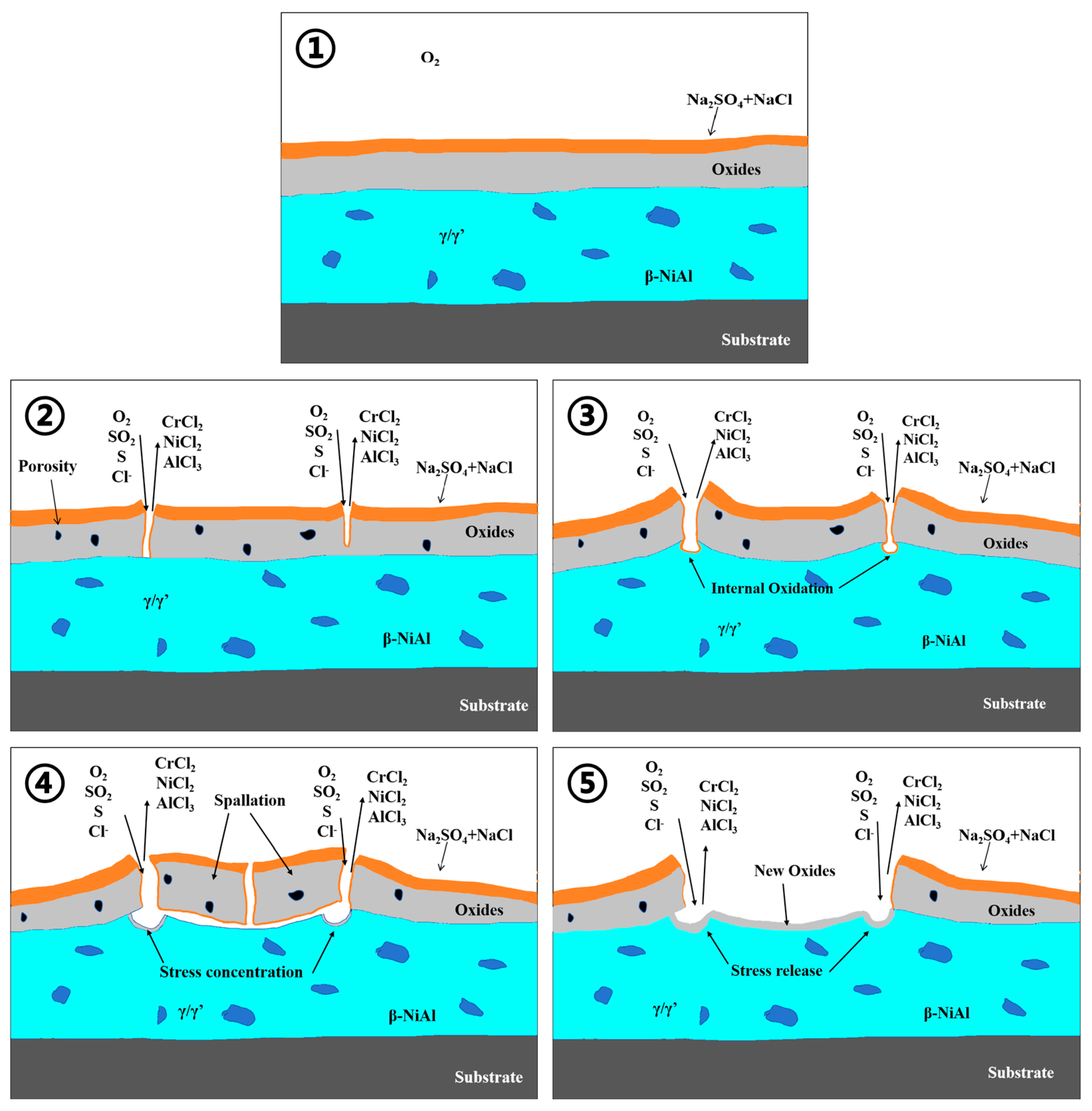
3.3. Molten Salt Corrosion Behavior at 750 °C
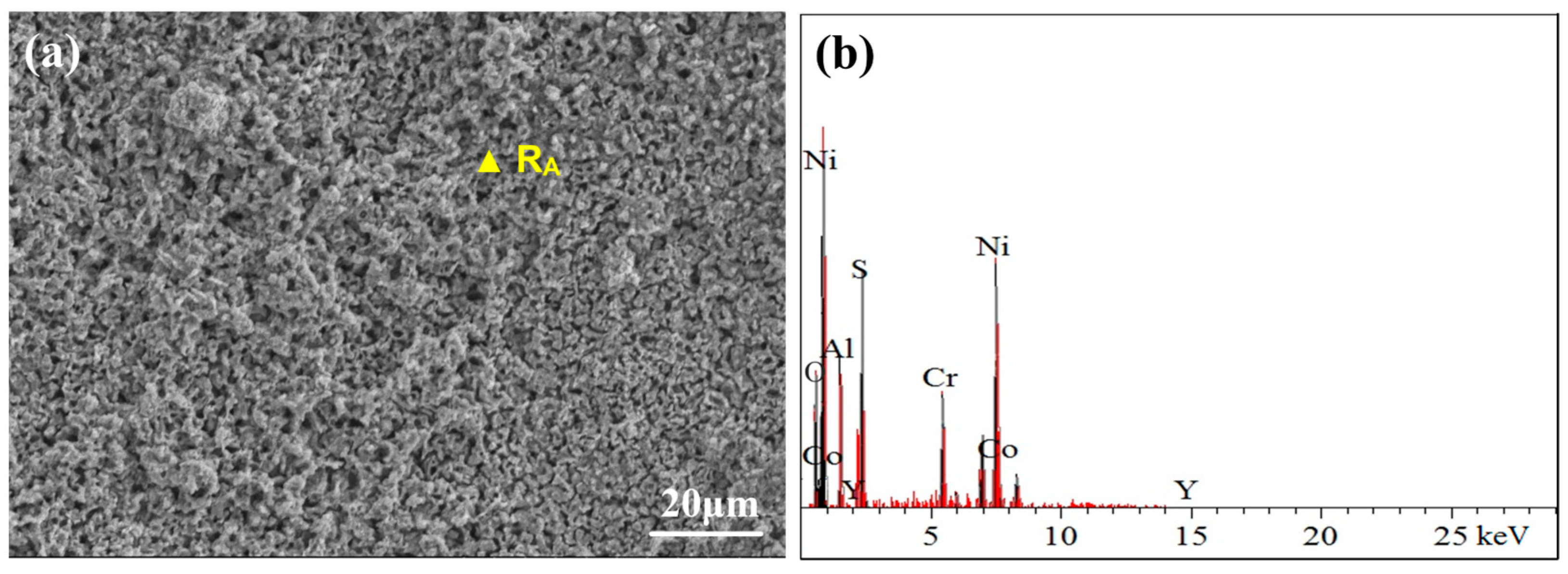
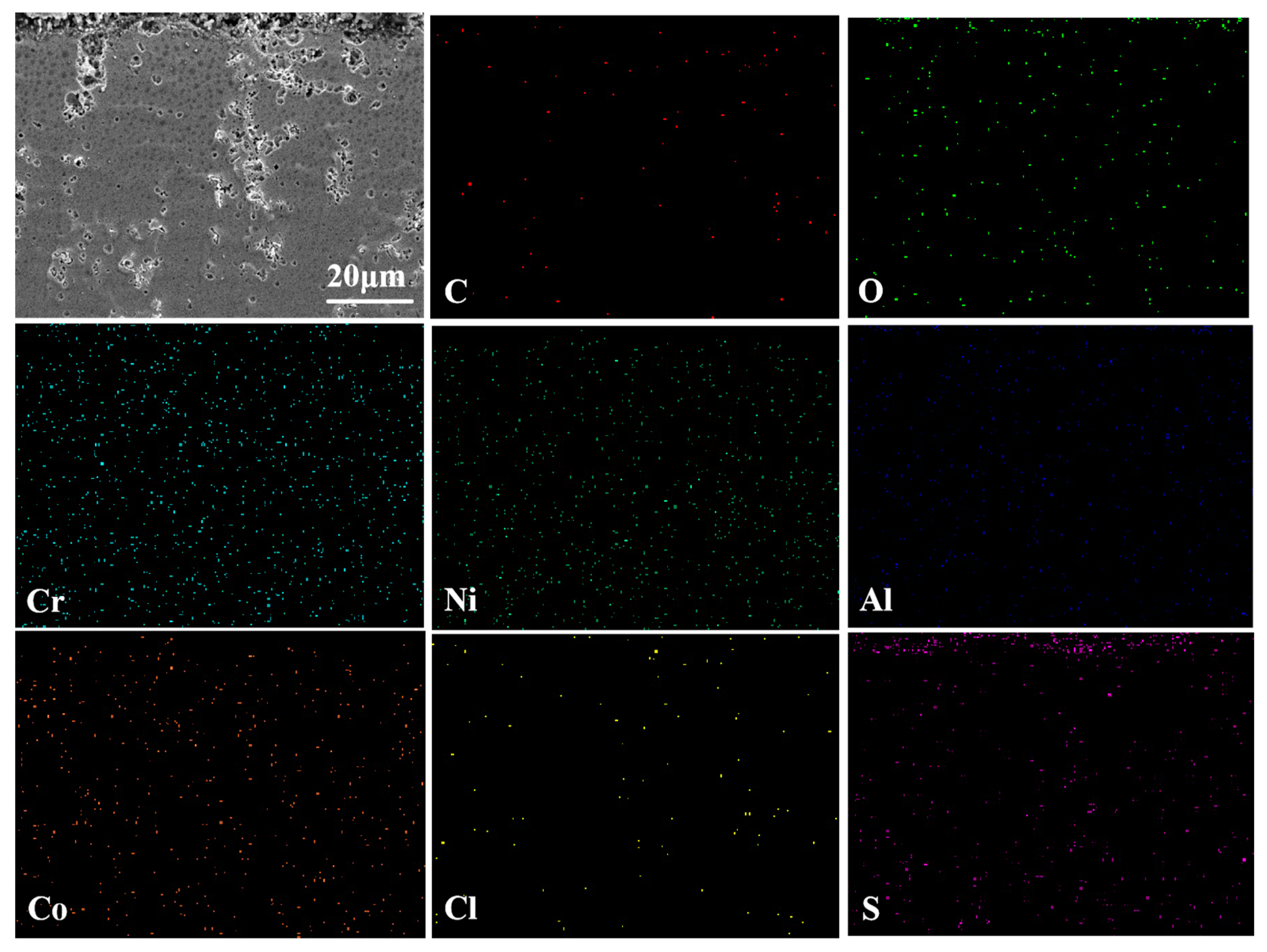
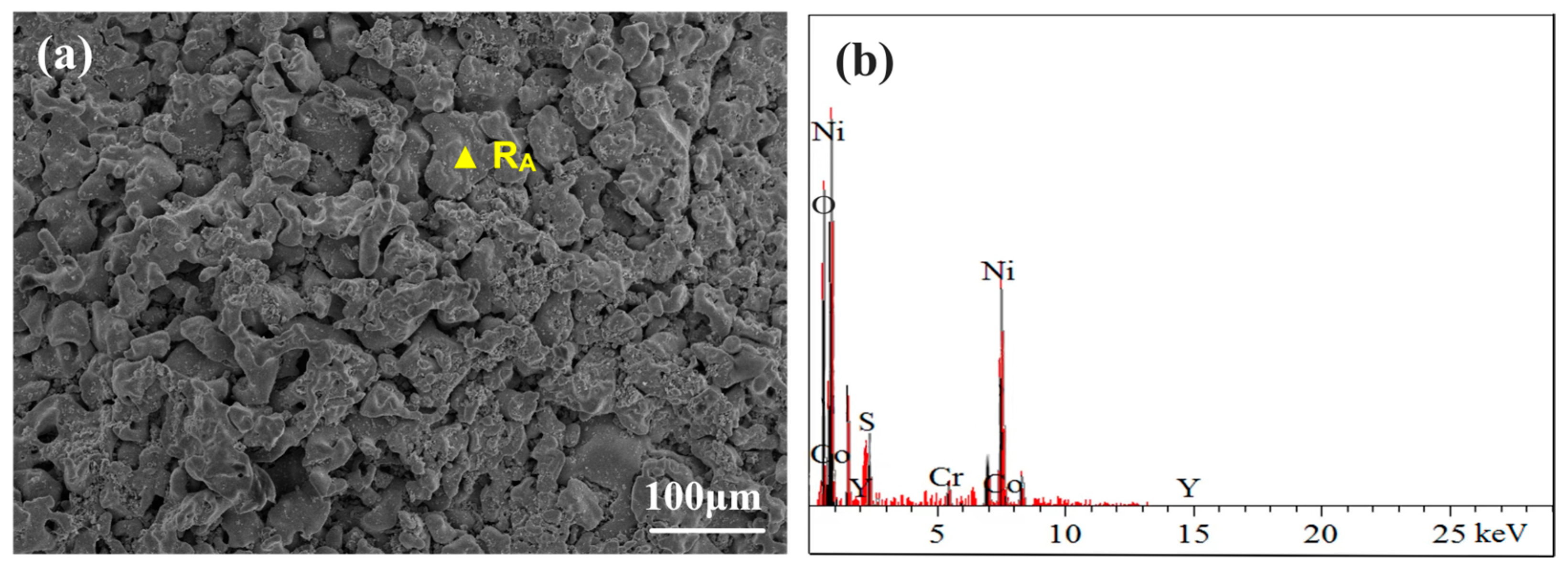
3.3.1. Surface Phase and Morphology Analysis at 750 °C
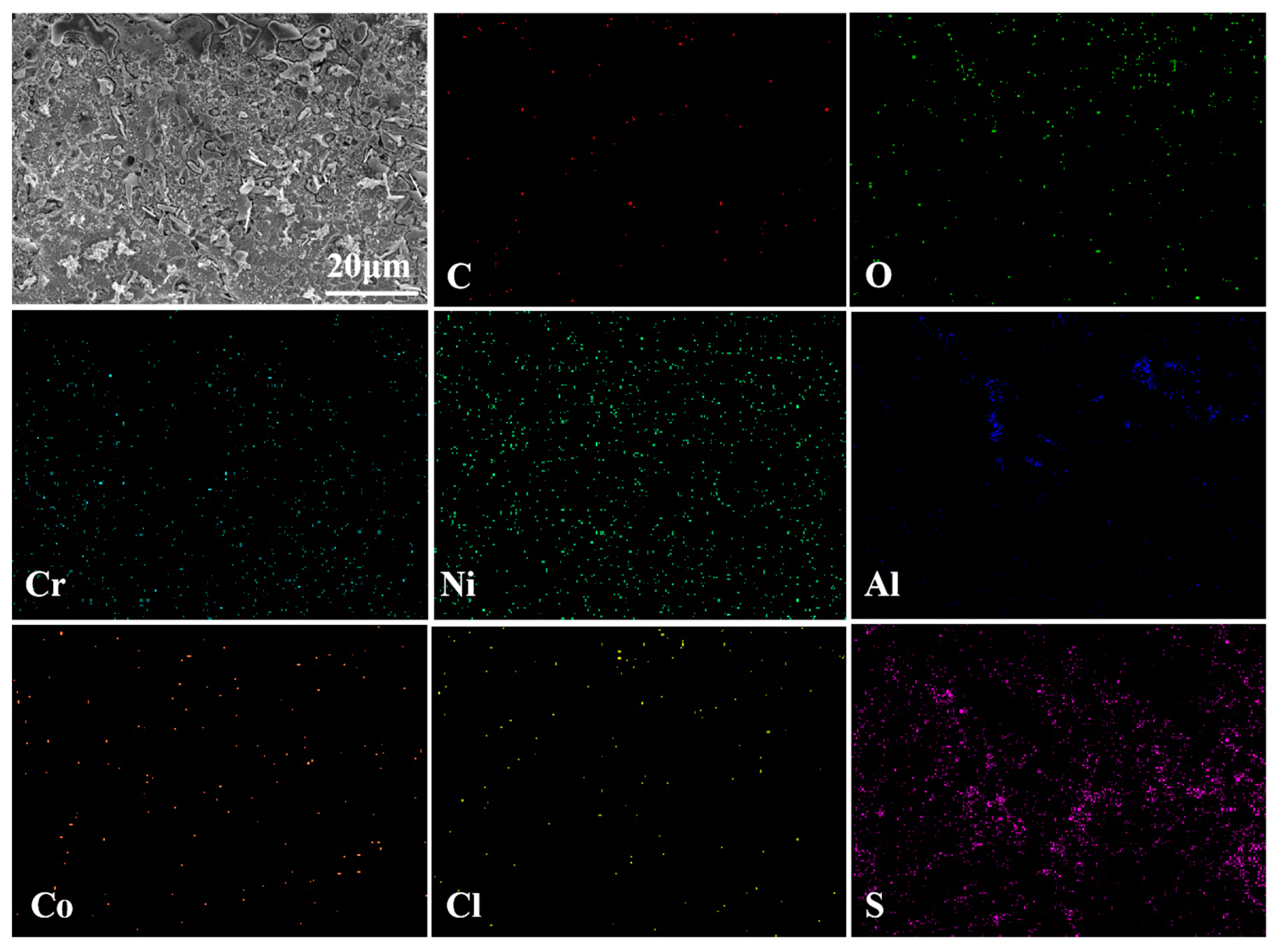
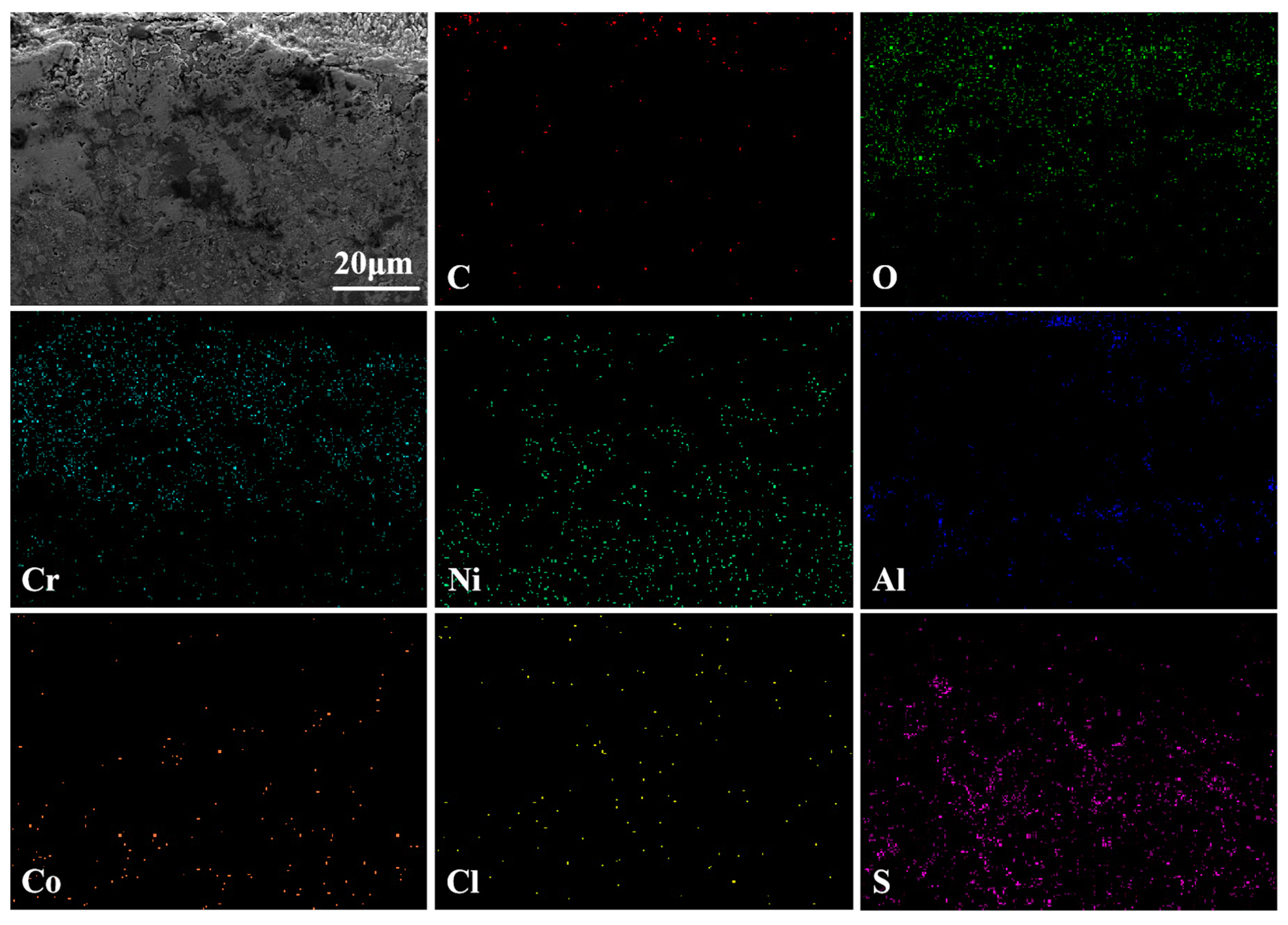
3.3.2. Cross-Sectional Morphology Analysis at 750 °C
3.3.3. Molten Salt Corrosion Kinetics at 750 °C
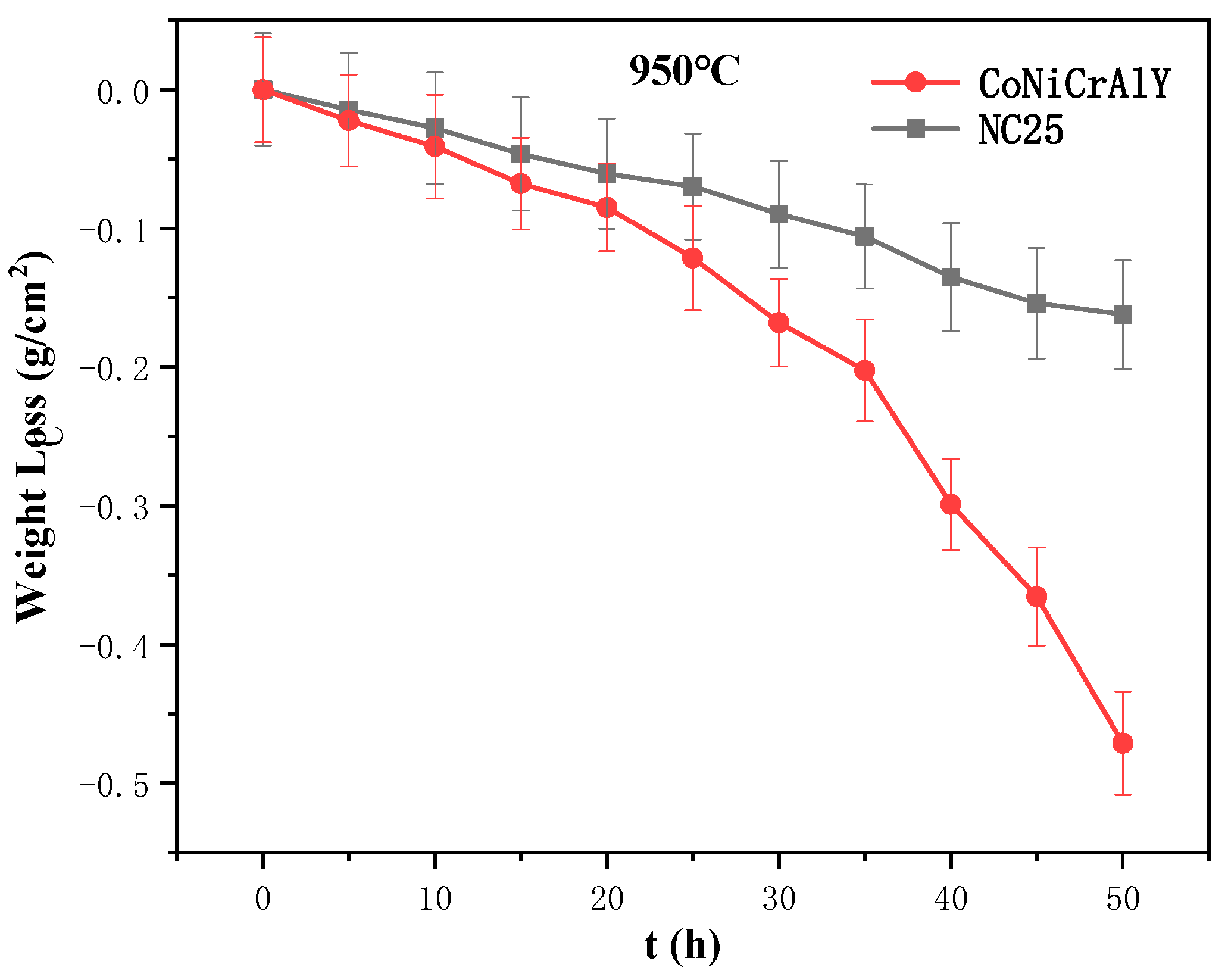
3.4. Molten Salt Corrosion Behavior at 950 °C
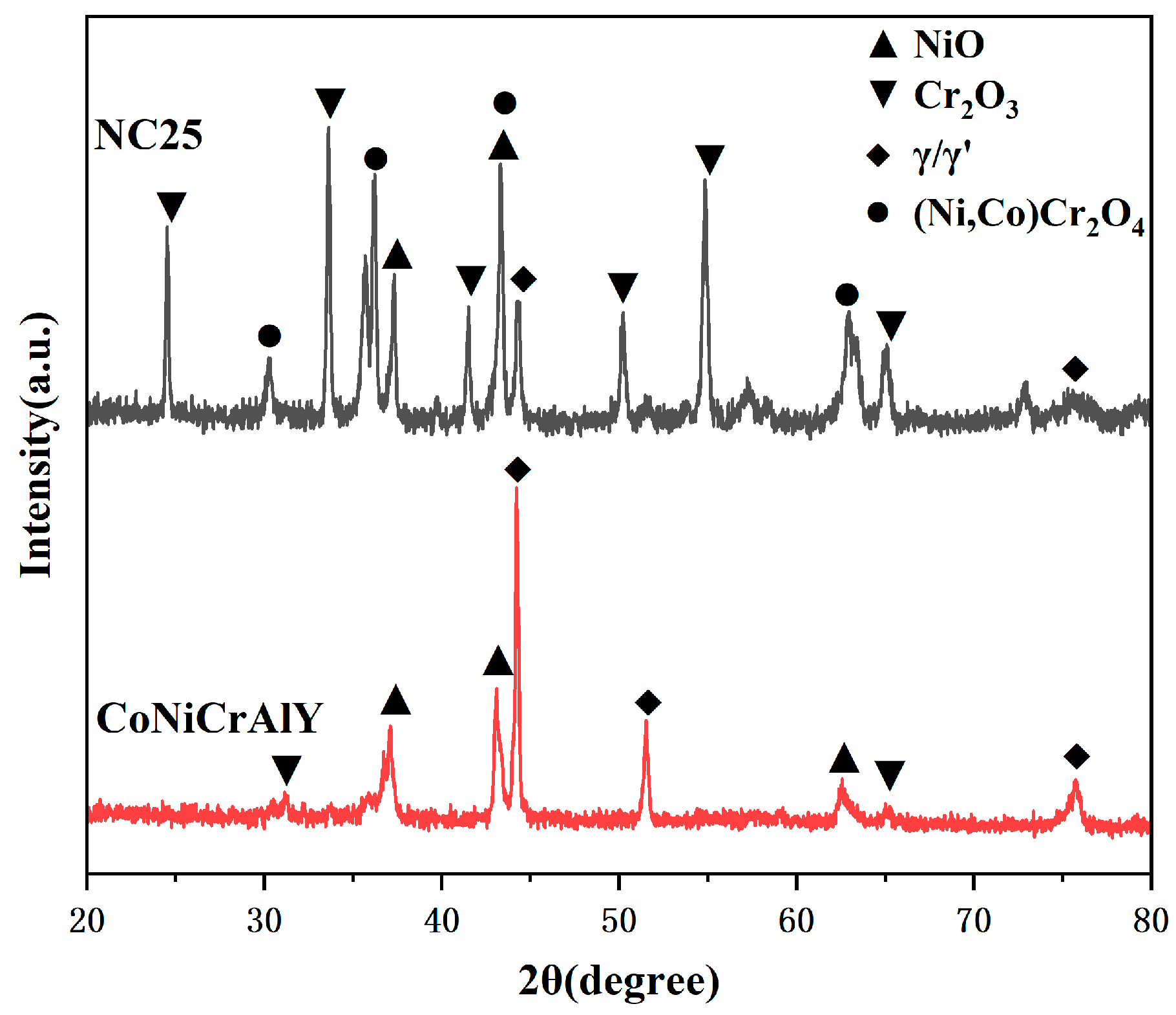
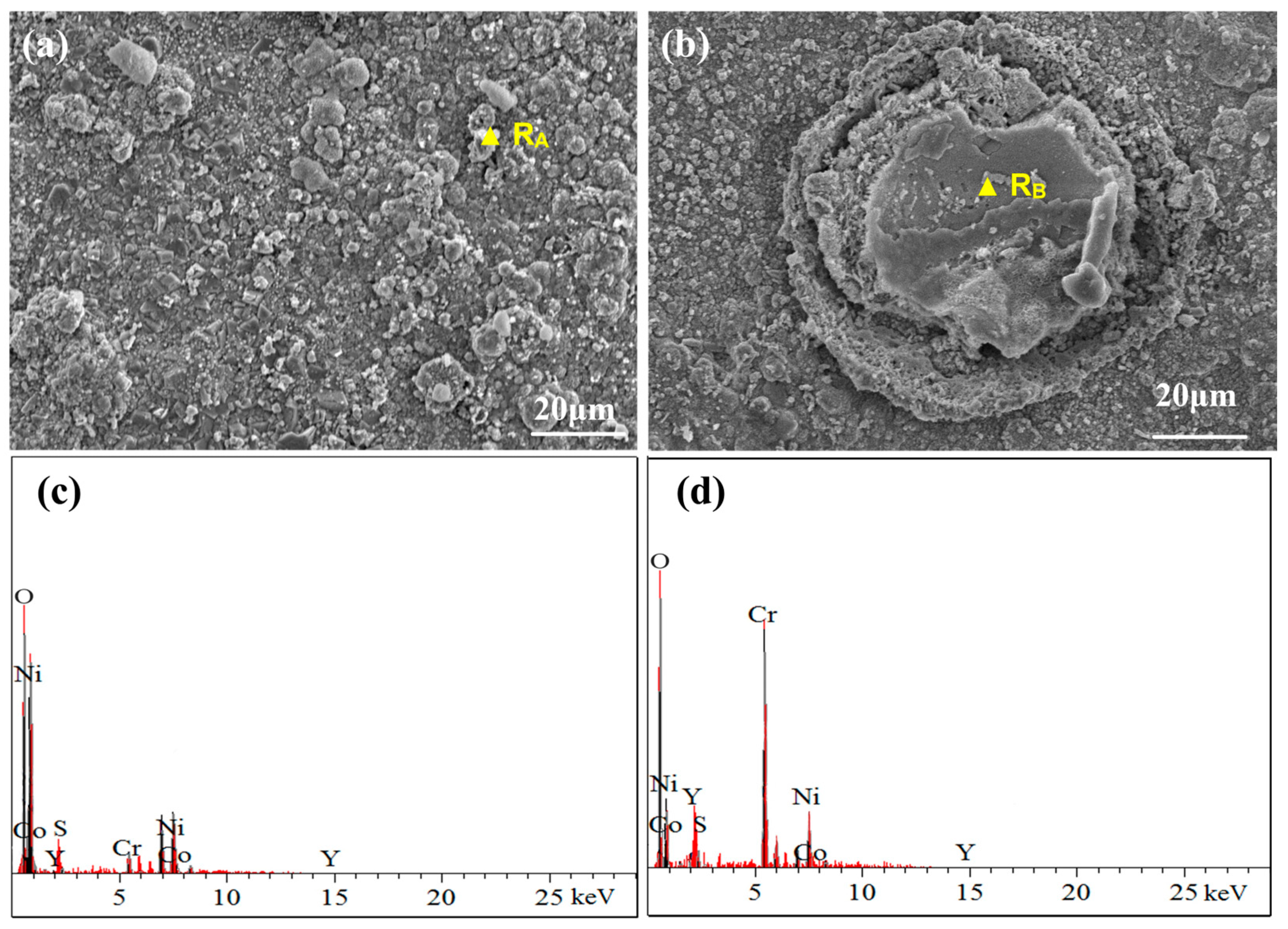
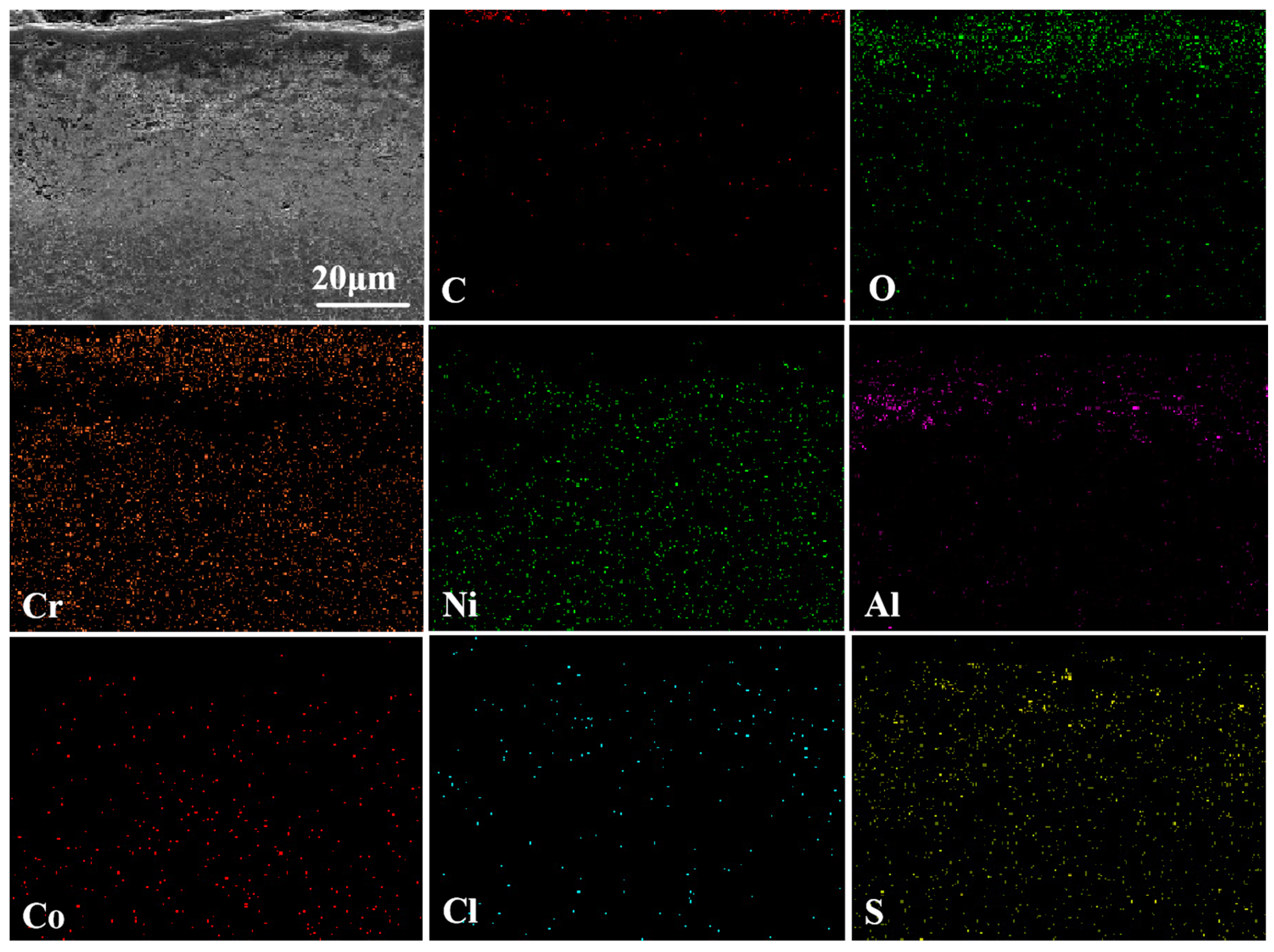
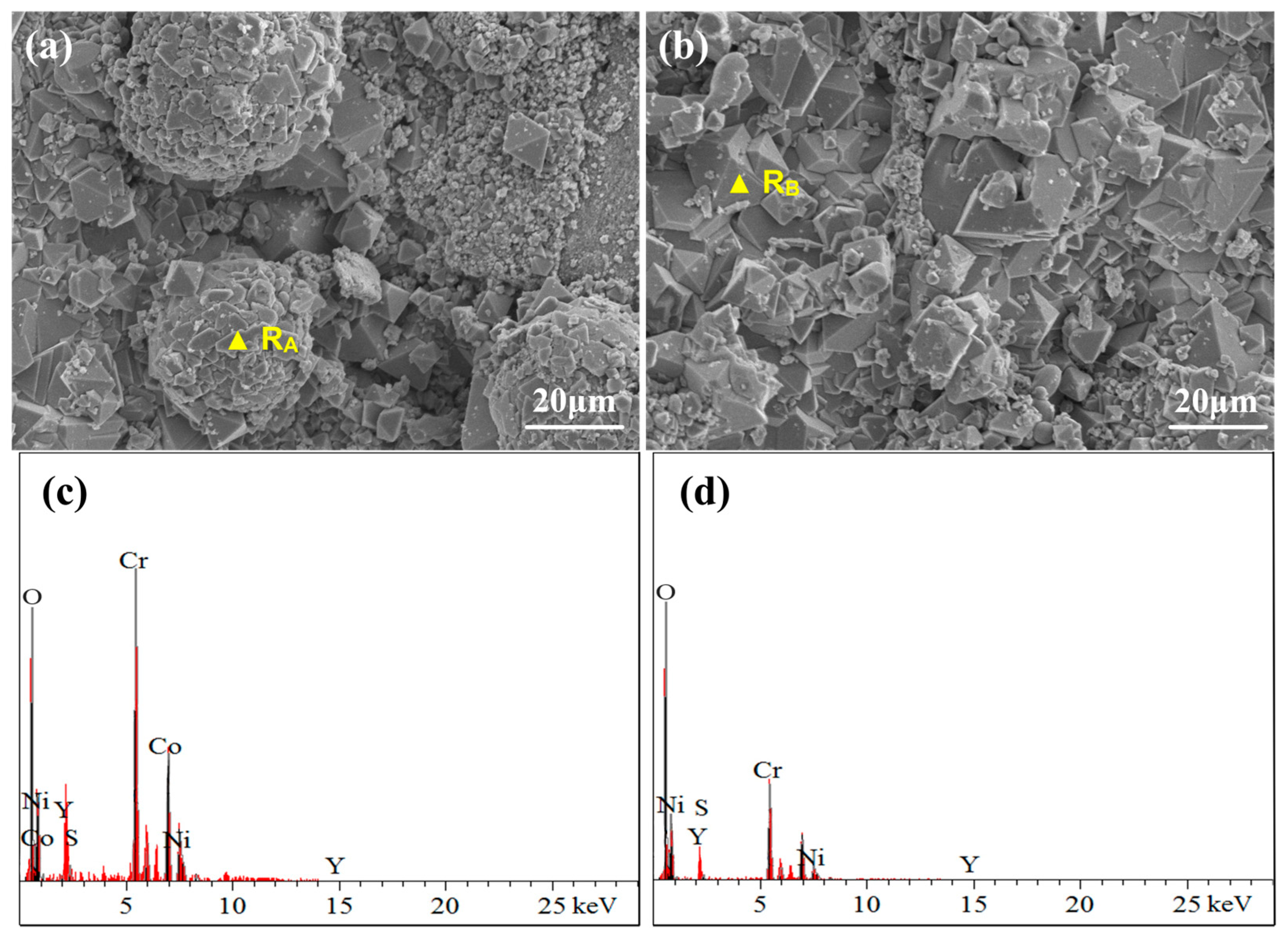
3.4.1. Surface Phase and Morphology Analysis at 950 °C
3.4.2. Cross-Sectional Morphology Analysis at 950 °C
3.4.3. Molten Salt Corrosion Kinetics at 950 °C
4. Conclusions
- With the addition of NiCr-Cr3C2, the layer grain size firstly increases and then decreases, and the dispersion and granular width of the β phase are promoted.
- The NC25 cladding layer had the highest average hardness at 348.2 HV0.3, as well as the lowest average friction coefficient and wear rate at 0.4751 and 0.4528 × 10−6 mm3/N·m, respectively. The wear mechanism is the surface of the cladding layer undergoing oxidative wear during friction with the formation of a dense protective Al2O3 film, together with Cr2O3 and (Ni,Co)Cr2O4. Additionally, Cr2O3 has certain lubricative function, which improves the layer’s tribological performance.
- Under molten salt corrosion at 750 °C, the cladding layer exhibited typical low-temperature hot corrosion characteristics, with pitting holes extending inward on the surface due to the rapid corrosion by Na2SO4. The rapid formation of Cr2O3 and (Ni,Co)Cr2O4 in the NC25 cladding layer effectively inhibited the formation of low-melting-point eutectic Na2SO4-CoSO4, which reduced the average weight loss rate by 32.7%.
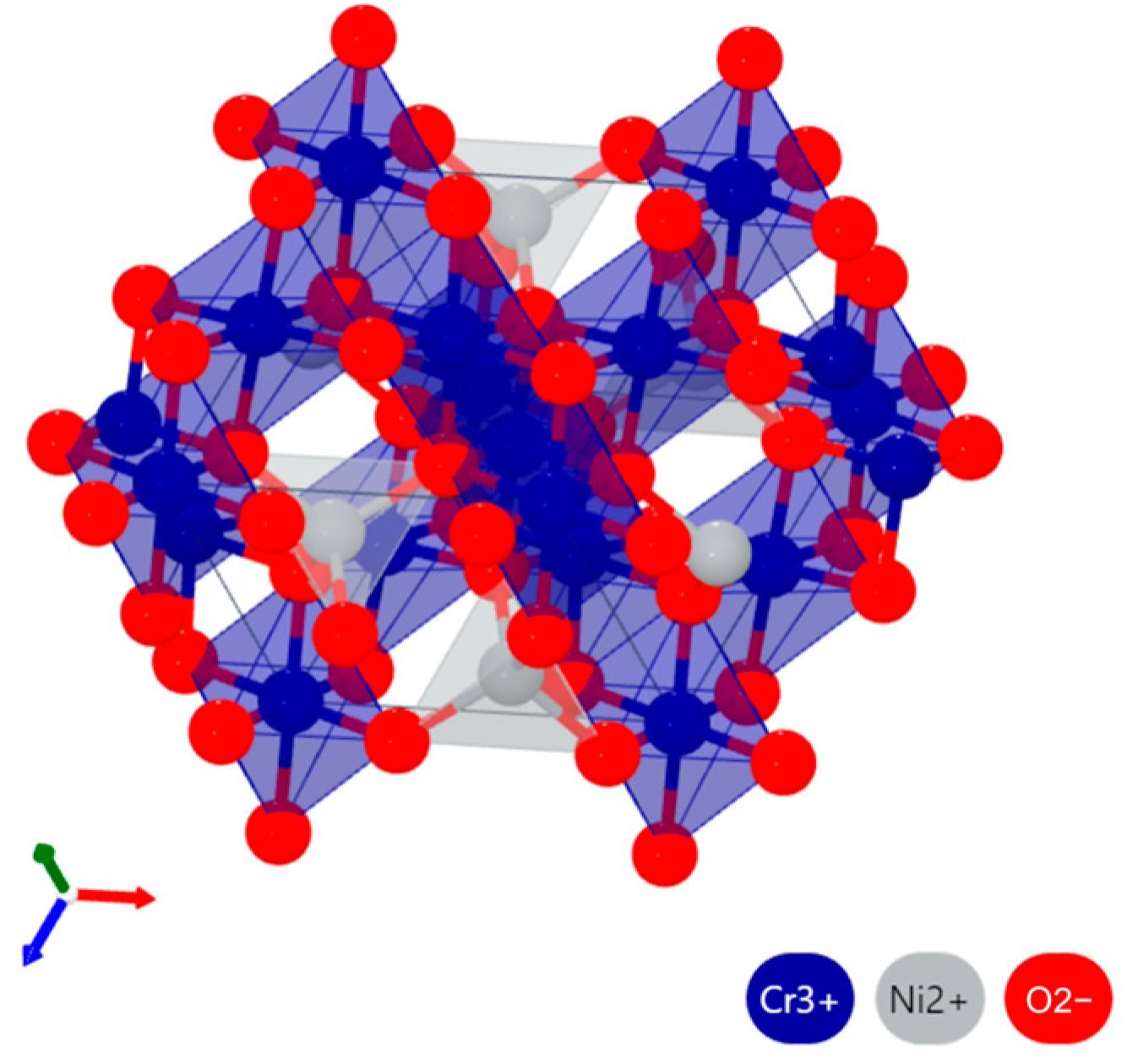
- Under molten salt corrosion at 950 °C, the cladding layer exhibited typical high-temperature hot corrosion characteristics, with local Al depletion and sulfide formation. The high content of Cr2O3 in the NC25 cladding layer promoted the formation of the spinel phase (Ni,Co)Cr2O4, inhibiting the corrosion effect of Na2SO4 and ion diffusion, significantly improving the stability of the oxide film, which reduced the average weight loss rate by 65.6%. Moreover, at 950 °C, the improvement in the corrosion resistance of the NC25 cladding layer compared with the matrix was far greater than at 750 °C, indicating it is more suitable for service at 950 °C.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Singh, G.; Sidhu, H.S. Investigations on hot corrosion resistance of bare and nickel chromium coated superni 76 super alloy. Mater. Today Proc. 2022, 48, 1582–1586. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, C. Microstructure and Characteristics of Co-based Alloy Coating Produced by Plasma Cladding Process. J. Mater. Eng. 2009, 457 (Suppl. S2), 193–195. [Google Scholar]
- Liu, N.; Liu, Q.; Li, Z.; Bai, Y.; Sun, Y.W.; Li, Z.D.; Bao, M.Y.; Zhan, H.; Guo, D.G.; Ma, Y.S. Tribological behavior of plasma-sprayed metal based solid self-lubricating coatings under heavy load. Wear 2021, 486–487, 204108. [Google Scholar] [CrossRef]
- Rana, N.; Shuklaa, V.N.; Jayaganthan, R.; Prakash, S. Degradation Studies of Micro and Nanocrystalline NiCrAlY coatings for High Temperature Corrosion Protection. Procedia Eng. 2014, 75, 118–122. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Tang, H.P.; Li, W.J.; Han, C. Oxidation behavior and mechanism of porous nickel-based alloy between 850 and 1000 °C. T. Nonferr. Metal. Soc. 2017, 27, 1558–1568. [Google Scholar] [CrossRef]
- Shuklaa, V.N.; Jayaganthan, R.; Tewari, V.K. Degradation Behavior of HVOF-Sprayed Cr3C2-25% NiCr Cermet Coatings Exposed to High Temperature Environment. Mater. Today Proc. 2015, 2, 1805–1813. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zeng, D.C.; Wang, G.; Zhang, Y.S. High Temperature Oxidation and Tribological Behavior of Cr3C2-25CoNiCrAlY and Cr3C2-25NiCr Coatings Prepared by AC-HVAF. China Surf. Eng. 2017, 30, 102–109. [Google Scholar]
- Kumar, D.; Pandey, K.N.; Das, D.K. Microstructure studies of air-plasma-spray-deposited CoNiCrAlY coatings before and after thermal cyclic loading for high-temperature application. Int. J. Min. Met. Mater. 2016, 23, 934–942. [Google Scholar] [CrossRef]
- Marginean, G.; Utu, D. Cyclic oxidation behaviour of different treated CoNiCrAlY coatings. Appl. Surf. Sci. 2012, 258, 8307–8311. [Google Scholar] [CrossRef]
- Liang, G.Y.; Zhu, C.; Wu, X.Y.; Wu, Y. The formation model of Ni-Cr oxides on NiCoCrAlY-sprayed coating. Appl. Surf. Sci. 2011, 257, 6468–6473. [Google Scholar] [CrossRef]
- Liu, X.B.; Liu, H.Q.; Liu, Y.F.; He, X.M.; Sun, C.F.; Wang, M.D.; Yang, H.B.; Qi, L.H. Effects of temperature and normal load on tribological behavior of nickel-based high temperature self-lubricating wear-resistant composite coating. Compos. Part B Eng. 2013, 53, 347–354. [Google Scholar] [CrossRef]
- Matthews, S.; James, B.; Hyland, M. High temperature erosion-oxidation of Cr3C2-NiCr thermal spray coatings under simulated turbine conditions. Corros. Sci. 2013, 70, 203–211. [Google Scholar] [CrossRef]
- Goyal, D.K.; Singh, H.; Kumar, H.; Sahni, V. Erosive Wear Study of HVOF Spray Cr3C2-NiCr Coated CA6NM Turbine Steel. J. Tribol. 2014, 136, 216–223. [Google Scholar] [CrossRef]
- Cai, J.; Zu, Z.; Li, C.; Lyu, P.; Guan, Q.; Li, Y. Hot Corrosion Behavior of Arc Ion Plating NiCoCrAlYSiHf Coating Via High-Current Pulsed Electron Beam. Oxid. Met. 2020, 94, 569–586. [Google Scholar] [CrossRef]
- He, H.; Liu, Z.; Wang, W.; Zhou, C. Microstructure and hot corrosion behavior of Co-Si modified aluminide coating on nickel based superalloys. Corros. Sci. 2015, 100, 466–473. [Google Scholar] [CrossRef]
- Xia, M.; Gu, T.F.; Jia, C.L.; Ge, C.C. Hot corrosion behavior of the spray-formed nickel-based superalloy. Chin. Phys. B 2019, 43, 183–200. [Google Scholar] [CrossRef]
- Chen, L.; Lan, H.; Huang, C.; Yang, B.; Du, L.; Zhang, W. Hot corrosion behavior of porous nickel-based alloys containing molybdenum in the presence of NaCl at 750 °C. Eng. Fail. Anal. 2017, 79, 245–252. [Google Scholar] [CrossRef]
- Eliaz, N.; Shemesh, G.; Latanision, R.M. Hot corrosion in gas turbine components. Eng. Fail. Anal. 2002, 9, 31–43. [Google Scholar] [CrossRef]
- Lutz, B.S.; Alvarado, J.M.; Garcia, L.; Meier, G.H. Na2SO4-Deposit-Induced Corrosion of Mo-Containing Alloys. Oxid. Met. 2017, 88, 599–620. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, Y.; Lou, Z.; Wang, Y. Enhanced Tribological Performance of Micro-Beam Plasma-Cladded Ni60 Coatings with Addition of Mo and Ag Lubricants in a Wide Temperature Range. Coatings 2023, 13, 1996. [Google Scholar] [CrossRef]
- Sadri, E.; Ashrafizadeh, F. Self-Lubrication Mechanism of Plasma-Sprayed Cr2O3-Ag Nanocomposite Coatings at Room to Moderate Temperatures. J. Therm. Spray Technol. 2021, 30, 1595–1614. [Google Scholar] [CrossRef]
- Sreedhar, G.; Raja, V.S. Hot corrosion of YSZ/Al2O3 dispersed NiCrAlY plasma-sprayed coatings in Na2SO4-10 wt.% NaCl melt. Corros. Sci. 2010, 52, 2592–2602. [Google Scholar] [CrossRef]
- Wortman, D.J.; Fryxell, R.E.; Luthra, K.L.; Bergman, P.A. Mechanism of Low Temperature Hot Corrosion: Burner Rig Studies. Thin Solid Films 1979, 64, 281–288. [Google Scholar] [CrossRef]
- Balashadehi, M.M.; Nourpour, P.; Aghdam, A.S.R.; Allahyarzadeh, M.H.; Heydarzadeh, A.; Hamdi, M. The formation, microstructure and hot corrosion behaviour of slurry aluminide coating modified by Ni/Ni-Co electrodeposited layer on Ni-base superalloy. Surf. Coat. Technol. 2020, 402, 126283. [Google Scholar] [CrossRef]
- Jafari, R.; Saadeghi, E. High-temperature corrosion performance of HVAF-sprayed NiCr, NiAl, and NiCrAlY coatings with alkali sulfate/chloride exposed to ambient air. Corros. Sci. 2019, 160, 108066. [Google Scholar] [CrossRef]
- Zahs, A.; Spiegel, M.; Grabke, H.J. Chloridation and oxidation of iron, chromium, nickel and their alloys in chloridizing and oxidizing atmospheres at 400–700 °C. Corros. Sci. 2000, 42, 1093–1122. [Google Scholar] [CrossRef]
- Simons, E.L.; Browning, G.V.; Liebhafsky, H.A. Sodium Sulfate in Gas Turbines. Corrosion 1955, 11, 17. [Google Scholar] [CrossRef]
- Masrour, R.; Jabar, A.; Hlil, E.K.; Hamedoun, M.; Benyoussef, A.; Hourmatallah, A.; Rezzouk, A.; Bouslykhane, K.; Benzakour, N. First principle and series expansions calculations of electronic and magnetic properties of Co(Ni)Cr2O4 spinels. J. Magn. Magn. Mater. 2017, 430, 89–93. [Google Scholar] [CrossRef]
- Ye, D.; Hu, J. Practical Handbook of Thermodynamic Data for Inorganic Substances, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2002; pp. 725–726. [Google Scholar]
- Goebel, J.A.; Pettit, F.S.; Goward, G.W. Mechanisms for the Hot Corrosion of Nickel-Base Alloys. Metall. Trans. 1973, 4, 261–278. [Google Scholar] [CrossRef]
- Stringer, J. High-temperature corrosion of superalloys. Mater. Sci. Technol. 1987, 3, 482–493. [Google Scholar] [CrossRef]




| Element | Co | Ni | Cr | Al | Y |
|---|---|---|---|---|---|
| Composition | 38.5 | 32 | 21 | 8 | 0.5 |
| No. | CoNiCrAlY | NiCr-Cr3C2 |
|---|---|---|
| CoNiCrAlY | 100 | 0 |
| NC5 | 95 | 5 |
| NC10 | 90 | 10 |
| NC15 | 85 | 15 |
| NC20 | 80 | 20 |
| NC25 | 75 | 25 |
| NC30 | 70 | 30 |
| Current I/A | Voltage U/V | Welding Velocity v/(mm·s−1) | Plasma Gas Pressure P/(MPa) | Shielding Gas Flow F/(L·min−1) | Plasma Arc Height (mm) | Nozzle Diameter (mm) |
|---|---|---|---|---|---|---|
| 22.5 | 20 | 0.6 | 0.4 | 8 | 4 | 2.4 |
| Temperature (°C) | Applied Load (N) | Frequency (Hz) | Stroke Length (mm) | Testing Time (min) |
|---|---|---|---|---|
| 25 | 20 | 5 | 10 | 30 |
| γ/γ′ | β-NiAl | Cr3C2 | Cr7C3 | Cr23C6 | |
|---|---|---|---|---|---|
| NC5 | 54.6 | 1.4 | 17.9 | 7.4 | 18.7 |
| NC10 | 46.3 | 7.3 | 19.3 | 9.5 | 17.6 |
| NC15 | 46.6 | 7.6 | 16.5 | 14.7 | 14.6 |
| NC20 | 38.4 | 12.2 | 13.5 | 26.2 | 9.6 |
| NC25 | 41.7 | 15.8 | 24.6 | 10.2 | 7.7 |
| NC30 | 49.6 | 6.7 | 16.9 | 16.4 | 10.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Zhang, Y.; Wang, Y.; Ye, F. Fabrication of Co-Based Cladding Layer by Microbeam Plasma and Its Corrosion Mechanism to Molten Salt. Materials 2024, 17, 4249. https://doi.org/10.3390/ma17174249
Sun K, Zhang Y, Wang Y, Ye F. Fabrication of Co-Based Cladding Layer by Microbeam Plasma and Its Corrosion Mechanism to Molten Salt. Materials. 2024; 17(17):4249. https://doi.org/10.3390/ma17174249
Chicago/Turabian StyleSun, Kaiqi, Yufeng Zhang, Yingfan Wang, and Fuxing Ye. 2024. "Fabrication of Co-Based Cladding Layer by Microbeam Plasma and Its Corrosion Mechanism to Molten Salt" Materials 17, no. 17: 4249. https://doi.org/10.3390/ma17174249
APA StyleSun, K., Zhang, Y., Wang, Y., & Ye, F. (2024). Fabrication of Co-Based Cladding Layer by Microbeam Plasma and Its Corrosion Mechanism to Molten Salt. Materials, 17(17), 4249. https://doi.org/10.3390/ma17174249






