Development of Robust PEBAX-Based Angiographic Catheter: Design and In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
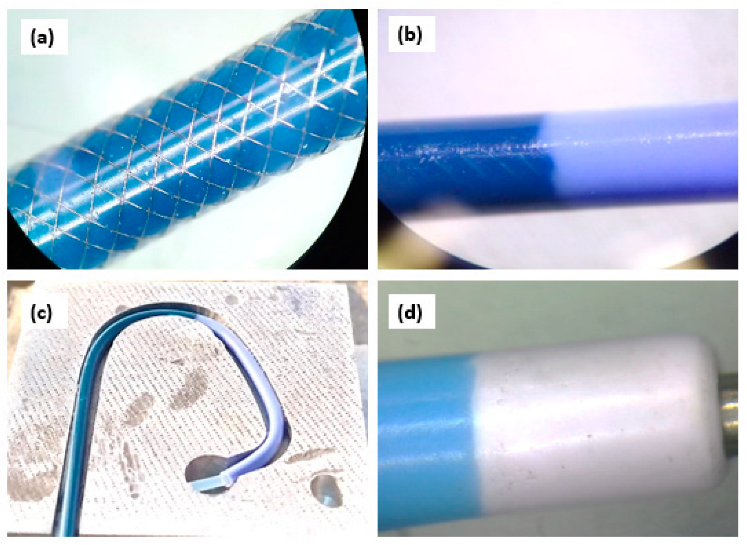
2.2. Development of PEBAX®-Based Diagnostic Angiographic Catheter and Description of Prototype
Description of Tests Related to ISO 10555-1:2013/Amd 1:2017
2.3. Test for Corrosion Resistance
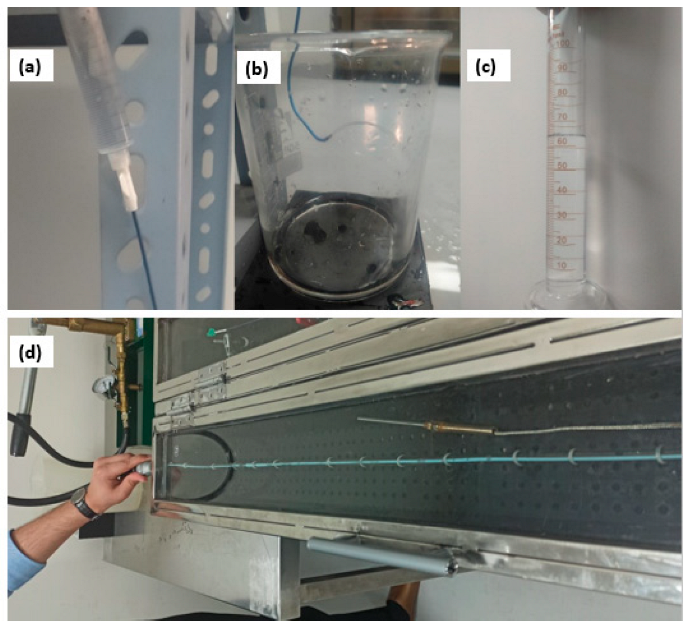
2.3.1. Liquid Leakage under Pressure
2.3.2. Determination of the Flow Rate through the Catheter
2.3.3. Test for Burst Pressure under Static Conditions
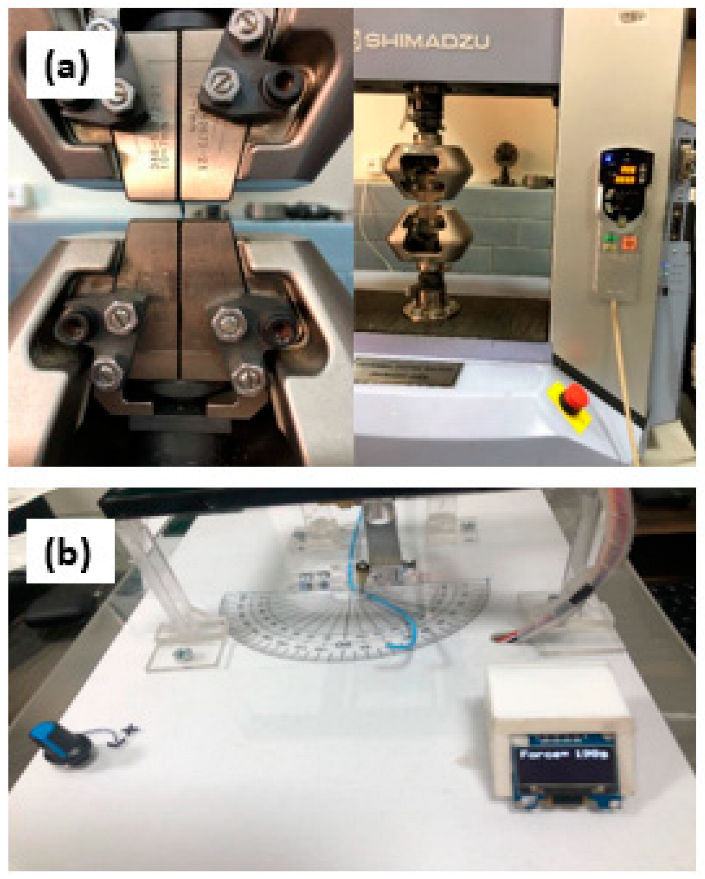
2.3.4. Determination of Peak Tensile Force
2.3.5. Flexural Rigidity
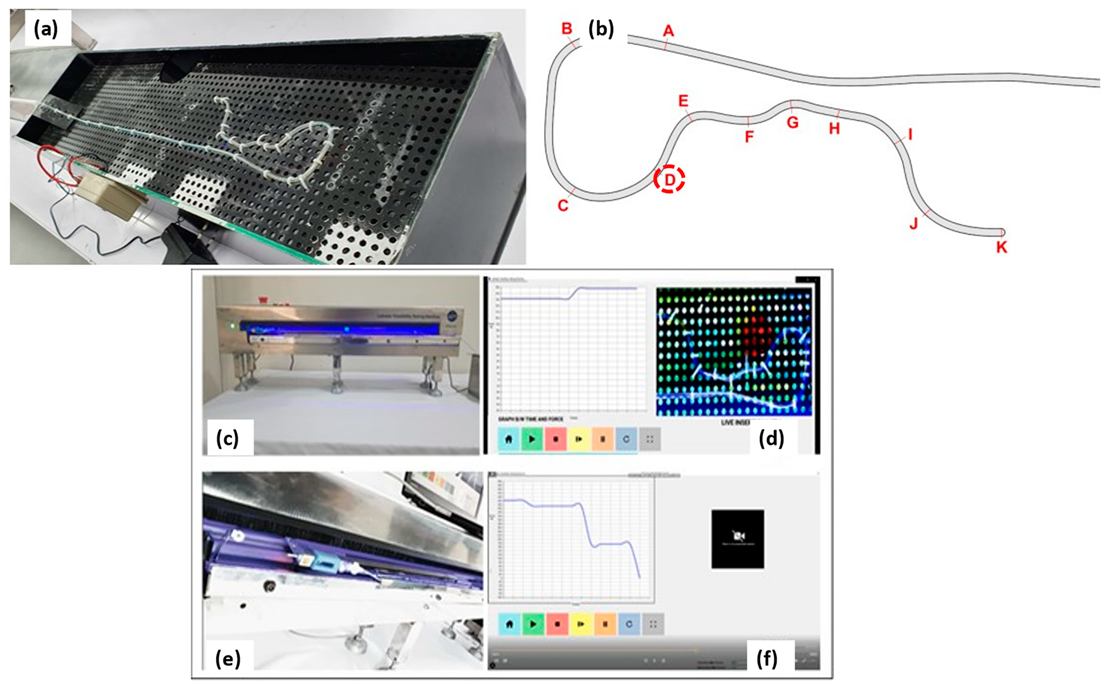
2.3.6. Catheter Trackability and Pushability Testing
2.3.7. Statistical Analysis
3. Results
3.1. Corrosion Resistance Test
3.2. Liquid Leakage under Leak Pressure
3.3. The Flow Rate through the Catheter
3.4. Test for Burst Pressure under Static Conditions
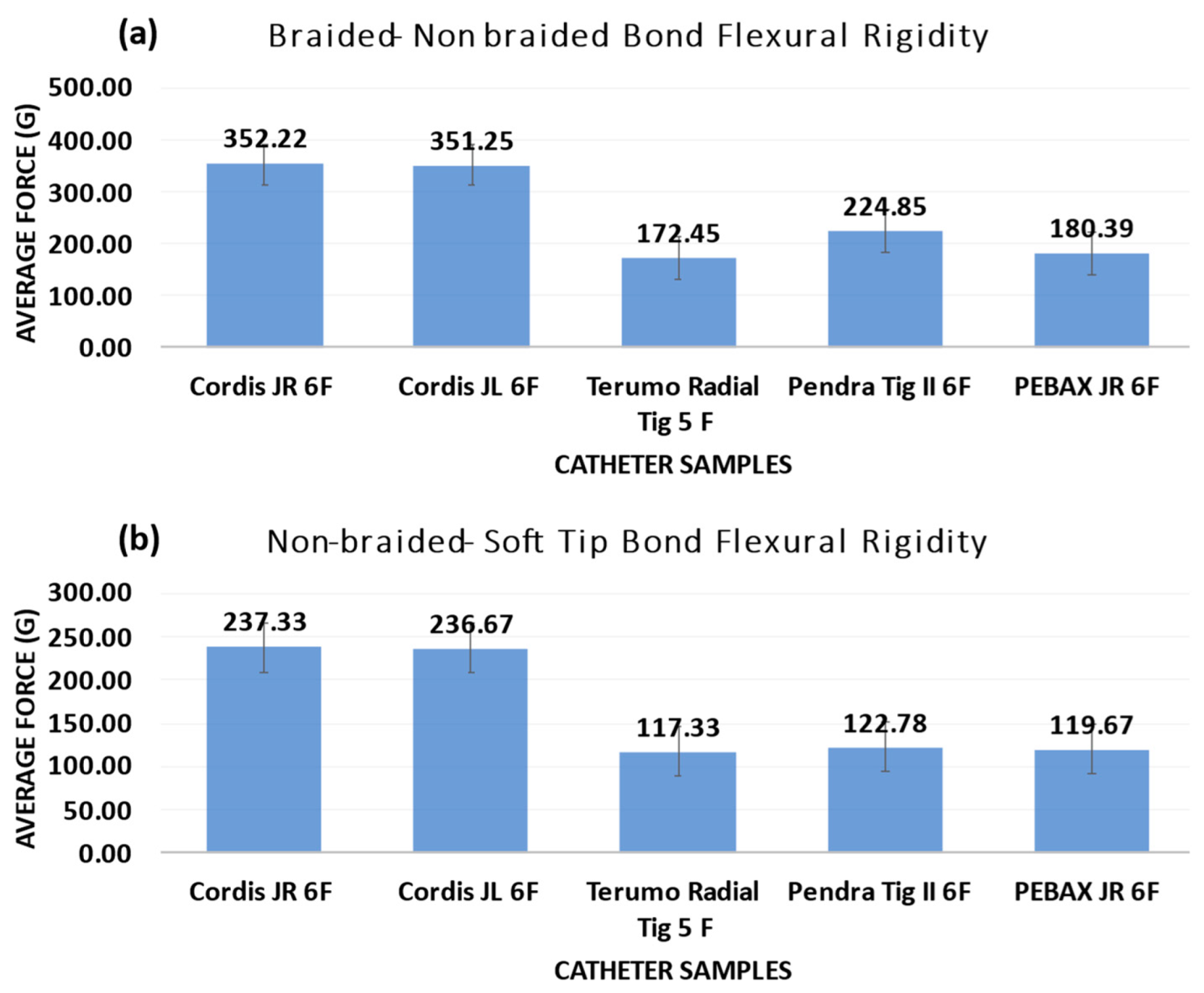
3.5. Flexural Rigidity
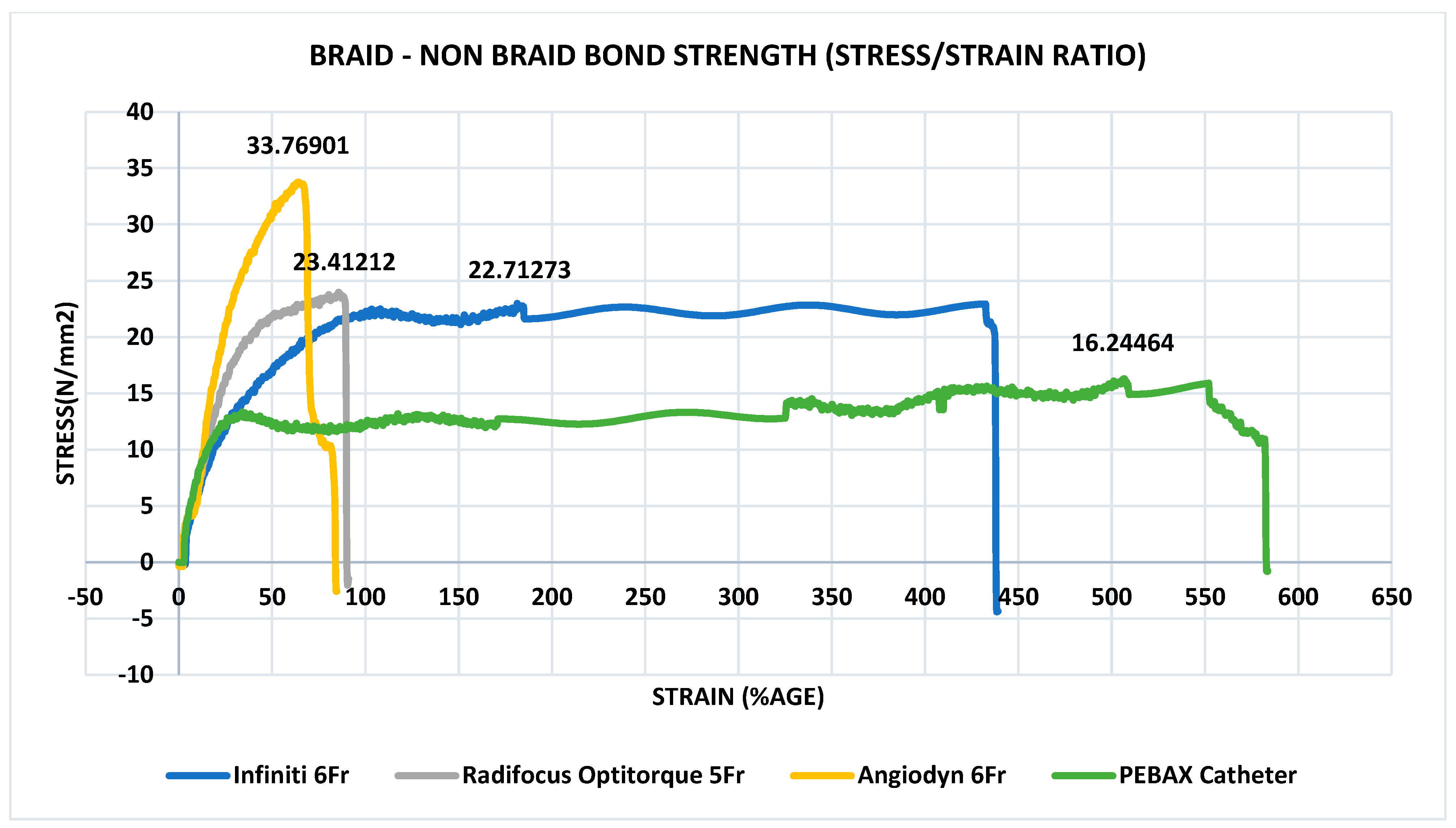
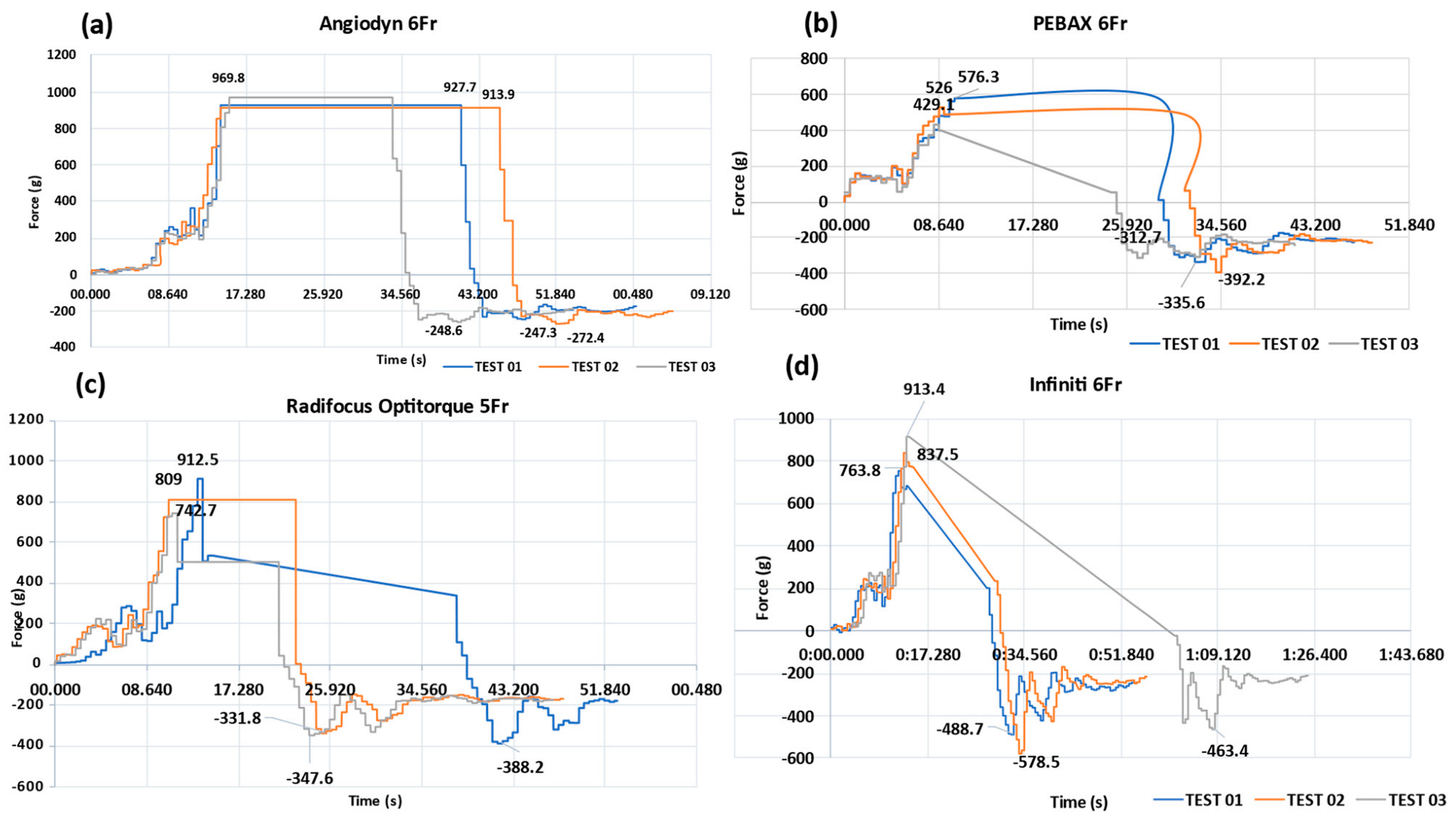
3.6. Determination of Peak Tensile Force
3.7. Quantitative Tracking Analysis: PEBAX®-Based Braided Catheter vs. Commercially Available Nylon- and Polyurethane-Based Catheters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization: WHO. Cardiovascular Diseases (CVDs). (11 June 2021). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 19 August 2024).
- Schmidt, W.E.A. Trackability, crossability, and pushability of coronary stent systems-an experimental approach. Biomed. Tech. Biomed. Eng. 2002, 47, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Brinza, C.; Floria, M.; Popa, I.; Burlacu, A. The Prognostic Performance of Ferritin in Patients with Acute Myocardial Infarction: A Systematic Review. Diagnostics 2022, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Falk, E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br. Heart J. 1983, 50, 127–134. [Google Scholar] [CrossRef]
- Friedman, M.; van den Bovenkamp, G. Role of thrombus in plaque formation in the human diseased coronary artery. Br. J. Exp. Pathol. 1966, 47, 550–557. [Google Scholar] [PubMed]
- Davies, M. A macro and micro view of coronary vascular insult in ischemic heart disease. Circulation 1990, 82, 34–46. [Google Scholar]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef]
- UKN. Cardiac Catheterisation and CORONARY Angiography. NHS, 12 February 2018. Available online: https://www.nhs.uk/conditions/coronary-angiography/ (accessed on 23 August 2021).
- Park, J.-U.; Urtnasan, E.; Kim, S.-H.; Lee, K.-J. A Prediction Model of Incident Cardiovascular Disease in Patients with Sleep-Disordered Breathing. Diagnostics 2021, 11, 2212. [Google Scholar] [CrossRef]
- Mayo Clinic. Coronary Angiogram. 2021. Available online: https://www.mayoclinic.org/tests-procedures/coronary-angiogram/about/pac-20384904#:~:text=A%20coronary%20angiogram%20is%20a,as%20heart%20(cardiac)%20catheterizations (accessed on 23 August 2021).
- Calmac, L.; Popa-Fotea, N.-M.; Bataila, V.; Ploscaru, V.; Turea, A.; Tache, I.; Stoian, D.; Itu, L.; Badila, E.; Scafa-Udriste, A.; et al. Importance of Visual Estimation of Coronary Artery Stenoses and Use of Functional Evaluation for Appropriate Guidance of Coronary Revascularization—Multiple Operator Evaluation. Diagnostics 2021, 11, 2241. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Kalantzis, C.; Katsianos, E.; Sanoudou, D.; Vavuranakis, M.; Tousoulis, D. Personalized Assessment of the Coronary Atherosclerotic Arteries by Intravascular Ultrasound Imaging: Hunting the Vulnerable Plaque. J. Pers. Med. 2019, 9, 8. [Google Scholar] [CrossRef]
- Stefanadis, C.; Antoniou, C.-K.; Tsiachris, D.; Pietri, P. Coronary Atherosclerotic Vulnerable Plaque: Current Perspectives. J. Am. Heart Assoc. 2017, 6, e005543. [Google Scholar] [CrossRef]
- Catheter Jacketing. Zeus Industrial Products, Inc., 2021. Available online: https://www.zeusinc.com/applications/catheters/jacketing/ (accessed on 18 August 2021).
- Golub, C. PEBAX® 101 Blog. Saint-Gobain Medical Components, 22 January 2021. Available online: https://www.medical.saint-gobain.com/blog/pebax-101-blog (accessed on 18 August 2021).
- Clarizia, G.; Bernardo, P. Review of the Recent Progress in the Development of Nanocomposites Based on Poly(ether-block-amide) Copolymers as Membranes for CO2 Separation. Polymers 2022, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Arkema, Pebax® Thermoplastic Elastomer Family—Energizing, Lightweight Resins. 2021. Available online: https://www.extremematerials-arkema.com/en/product-families/pebax-elastomer-family/ (accessed on 24 August 2021).
- 10555, ISO 10555-1:2013/AMD 1:2017, Intravascular Catheters—Sterile and Single-Use Catheters—Part 1: General Requirements—Amendment 1. 2017. Available online: https://www.iso.org/standard/70319.html (accessed on 25 August 2021).
- PEBAX® Elastomer Family|Arkema High Performance Polymers. (n.d.). Available online: https://hpp.arkema.com/en/product-families/pebax-elastomer-family/ (accessed on 19 August 2024).
- Li, T.; Zhang, Z.; Wang, W.; Mao, A.; Chen, Y.; Xiong, Y.; Gao, F. Simulation and Experimental Investigation of Balloon Folding and Inserting Performance for Angioplasty: A Comparison of Two Materials, Polyamide-12 and Pebax. J. Funct. Biomater. 2023, 14, 312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ojha, V.; Raju, S.N.; Deshpande, A.; Ganga, K.P.; Kumar, S. Catheters in vascular interventional radiology: An illustrated review. Diagn Interv. Radiol. 2023, 29, 138–145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dodge, J.T., Jr.; Brown, B.G.; Bolson, E.L.; Dodge, H.T. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation 1992, 86, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Byron Flagg. New Extrusion Techniques Advance Catheter Design. 21 February 2013. Available online: https://www.mddionline.com/news/new-extrusion-techniques-advance-catheter-design (accessed on 10 March 2022).
- Urodynamic, Urodynamics Catheters and Accessories. 2021. Available online: https://www.medica.it/PDF/Cateteri_URO_ENG_1.16.pdf (accessed on 25 August 2021).
- Ye, J.-Y.; Peng, K.-F.; Zhang, Y.-N.; Huang, S.-Y.; Liang, M. Synthesis and Characterization of N-Substituted Polyether-Block-Amide Copolymers. Materials 2021, 14, 773. [Google Scholar] [CrossRef]
- Sarwar, Z.; Krugly, E.; Danilovas, P.P.; Ciuzas, D.; Kauneliene, V.; Martuzevicius, D. Fabrication and characterization of PEBA fibers by melt and solution electrospinning. J. Mater. Res. Technol. 2019, 8, 6074–6085. [Google Scholar] [CrossRef]
- Sheth, J.; Xu, J.; Wilkes, G. Solid state structure-property behavior of semicrystalline poly(ether-block-amide) PEBAX thermoplastic elastomers. Polymer 2003, 44, 743–756. [Google Scholar] [CrossRef]
- Merit, Angiography. 2014. Available online: https://www.merit.com/wp-content/uploads/2014/09/CI-Angiogrphy.pdf (accessed on 27 January 2022).
- Catheter Angiography. 15 June 2020. Available online: https://radiologyInfo.org (accessed on 1 May 2022).
- Mechanical Properties of Catheters. Acta Radiol. Diagn. 1996, 4, 11–22.
- Flesher, J. Pebax® polyether block amide—A new family of engineering thermoplastic elastomers. In High Performance Polymers: Their Origin and Development; Springer: Dordrecht, The Netherlands, 1986; pp. 401–408. [Google Scholar]
- Farhad Sadeghi, D.L. Characterization of polymeric biomedical balloon: Physical and mechanical properties. J. Polym. Eng. 2021, 41, 799–807. [Google Scholar] [CrossRef]
- Gonzalez Fiol, A.; Horvath, R.; Schoenberg, C.; Ahmed, N.; Dhar, S.K.; Le, V. Comparison of Changes in Tensile Strength in Three Different Flexible Epidural Catheters Under Various Conditions. Anesth. Analg. 2016, 123, 233–237. [Google Scholar] [CrossRef]
- Tokhadzé, N.; Chennell, P.; Pereira, B.; Mailhot-Jensen, B.; Sautou, V. Critical Drug Loss Induced by Silicone and Polyurethane Implantable Catheters in a Simulated Infusion Setup with Three Model Drugs. Pharmaceutics 2021, 13, 1709. [Google Scholar] [CrossRef] [PubMed]
- Putnam Plastics. 2021. Available online: https://www.putnamplastics.com/materials/thermoplastics/?gad_source=1&gclid=CjwKCAjw4ri0BhAvEiwA8oo6F_22LqT4M0mUMqcdZFteSrbC37qIJMHw43iSq64dwegu8FB7gS1aLRoCkl4QAvD_BwE%20Tubing_0.pdf (accessed on 10 July 2024).
- Lunderquist, A.; Ivancev, K.; Wallace, S.; Enge, I.; Laerum, F.; Kolbenstvedt, A.N. The acquisition of skills in interventional radiology by supervised training on animal models: A three-year multicenter experience. Cardiovasc. Intervent. Radiol. 1995, 18, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Hellström, M.; Göthlin, J.; Reznick, R.; Lönn, L. Endovascular training with animals versus virtual reality systems: An economic analysis. J. Vasc. Intervent. Radiol. 2008, 19, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Balcon, R.; Beyar, R.; Chierchia, S.; De Scheerder, I.; Hugenholtz, P.G.; Kiemeneij, F.; Meier, B.; Meyer, J.; Monassier, J.P. Recommendations on stent manufacture, implantation and utilization. Eur. Heart J. 1997, 18, 1536–1547. [Google Scholar] [CrossRef]
- Shah, D.; Lambert, H.; Langenkamp, A.; Vanenkov, Y.; Leo, G.; Gentil-Baron, P.; Walpoth, B. Catheter tip force required for mechanical perforation of porcine cardiac chambers. Europace 2011, 13, 277–283. [Google Scholar] [CrossRef]



| Brand | Catheter | Type | Length of the Catheter (cm) | Gravity Flow Rate (mL/min) |
|---|---|---|---|---|
| Cordis | Infiniti® | Judkins Left | 100.4 | 62.0 |
| Cordis | Infiniti® | Judkins Right | 101.1 | 60.2 |
| BBraun | Angiodyn® | Tig II | 103.2 | 60.2 |
| Terumo | Radifocus Optitorque® | Radial Tig | 100.2 | 60.2 |
| PEBAX | PEBAX®-based braided catheter | Judkins Left and Judkins Right | 100.2 | 63.0 |
| Details of Catheters Tested for Burst Pressure | |||||
|---|---|---|---|---|---|
| Brand | Catheter | French Size | Time (s) | Pressure Generated | Results |
| Cordis | Infiniti® | 6 | 10 | 1210 | No burst or bulging was observed |
| Cordis | Infiniti® | 6 | 10 | 1210 | No burst or bulging was observed |
| BBraun | Angiodyn® | 6 | 11 | 1200 | No burst or bulging was observed |
| Terumo | Radifocus Optitorque® | 6 | 11 | 1200 | No burst or bulging was observed |
| PEBAX | PEBAX®-based Catheter | 6 | 12 | 1220 | No burst or bulging was observed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inam, H.; Ali, M.N.; Jameel, I.R.; Awaiz, D.; Qureshi, Z. Development of Robust PEBAX-Based Angiographic Catheter: Design and In Vitro Study. Materials 2024, 17, 4248. https://doi.org/10.3390/ma17174248
Inam H, Ali MN, Jameel IR, Awaiz D, Qureshi Z. Development of Robust PEBAX-Based Angiographic Catheter: Design and In Vitro Study. Materials. 2024; 17(17):4248. https://doi.org/10.3390/ma17174248
Chicago/Turabian StyleInam, Hafsa, Murtaza Najabat Ali, Ibraheem Raza Jameel, Dil Awaiz, and Zunaira Qureshi. 2024. "Development of Robust PEBAX-Based Angiographic Catheter: Design and In Vitro Study" Materials 17, no. 17: 4248. https://doi.org/10.3390/ma17174248
APA StyleInam, H., Ali, M. N., Jameel, I. R., Awaiz, D., & Qureshi, Z. (2024). Development of Robust PEBAX-Based Angiographic Catheter: Design and In Vitro Study. Materials, 17(17), 4248. https://doi.org/10.3390/ma17174248









