Abstract
Use of glyphosate and glyphosate-based herbicides is ubiquitous in US agriculture and widespread around the world. Despite marketing efforts to the contrary, numerous studies demonstrate glyphosate toxicity to non-target organisms including animals, primarily focusing on mortality, carcinogenicity, renal toxicity, reproductive, and neurological toxicity, and the biochemical mechanisms underlying these physiological outcomes. Glyphosate toxicity also impacts animal behavior, both in model systems and in agricultural and environmentally relevant contexts. In this review, we examine the effects of glyphosate and glyphosate-based herbicides on animal behaviors, particularly activity, foraging and feeding, anti-predator behavior, reproductive behaviors, learning and memory, and social behaviors. Glyphosate can be detected both in food and in the environment, and avoided through activity and feeding strategies. However, exposure also reduces activity, depresses foraging and feeding, increases susceptibility to predation, interferes with courtship, mating, fertility and maternal behaviors, decreases learning and memory capabilities, and disrupts social behaviors. Changes in animal behavior as a result of glyphosate toxicity are important because of their sometimes severe effects on individual fitness, as well as ecosystem health. Implications for human behavior are also considered.
1. Introduction
Glyphosate-based herbicides (GBHs), including brands such as Roundup®, are the most used pesticides in the United States and for the world as a whole [1]. Commercial farmers spray GBHs in three main application contexts: (1) as a pre-planting herbicide, reducing competition for seedlings and young plants; (2) to reduce competition throughout the growing season on Roundup® Ready crops, including most of the corn, sugar beets, soy, and canola, and smaller portions of zucchini, alfalfa, and other crops, grown in the US; and (3) to kill leafy vegetation before harvest and ease separation of non-vegetative, commercially important parts of crop plants, used extensively for sugar cane, wheat, oats, and legumes. Perhaps because of the timing of application, this last type of use seems to impact food supplies most greatly, since glyphosate residue concentrations measured in oat- and wheat-based foods are the highest among all the foods tested [2,3]. Yet these crops are not genetically modified to be herbicide-tolerant; though herbicide tolerant soybeans also contain high concentrations of glyphosate residue [4]. This results in continuous, low-concentration human exposure to residues through food [5] and, because of runoff, drift, and overspray, in drinking water and the environment for both humans and other animals that live in or near agricultural areas. In addition, GBHs are used extensively by homeowners to suppress vegetation around driveways, walkways and fence lines, and by public employees to suppress weeds in public spaces like sidewalks, parking lots, playgrounds, and schools. Therefore, during application and subsequent exposure, humans and other animals living in urban, suburban and rural environments can all be exposed sporadically to higher doses of GBHs (e.g., [6]; Table 1). This supposition is borne out by examining glyphosate concentration in tissues (personal observation; [7,8,9,10]), including its prevalence in human urine (reviewed in [11]), related to type of exposure (occupational vs. dietary; [12]) and type of diet (organic vs. conventional; [13]).

Table 1.
Selected types of GBH exposure from application, national standards, water, and food; and the range of concentrations at which they occur.
The marketing of Roundup® focuses on its safety for animals based on two incorrect assumptions. Several different formulations are sold commercially, different formulations are sold in different countries, and those available to the public are slightly different from the formulations marketed for agriculture. To date, the active ingredient of all formulations is glyphosate, though secondary herbicides may also be included. The first argument for the safety of Roundup® for animals is that glyphosate targets the shikimate pathway of amino acid synthesis, which is present in plants and microbes, but not in animals. Of course this does not preclude the possibility that glyphosate also interacts with other molecules to create toxic secondary effects. The second argument depends on experimental results showing that, when tested alone by Roundup® manufacturers or scientists funded by them, glyphosate exhibited low toxicity to mammals. Regulatory agencies have never required testing of so-called inert ingredients within the various formulations, and tests with the whole GBH formulations are not required to get approval for marketing. Other ingredients within the various formulations, particularly the secondary herbicides and surfactants, have proven either to act synergistically, increasing the toxicity of glyphosate, or to be more toxic than glyphosate, e.g., [25,26,27,28]. These effects are difficult to explore fully because the composition of each formulation is considered proprietary and therefore, herbicide manufacturers do not have to disclose the ingredients; and the composition of formulations can differ from country to country. Given the wide variety of animal species and biological systems known to be disrupted by GBH exposure, one or both of these arguments must be incorrect.
Thousands of peer-reviewed articles have been published that demonstrate toxic effects of glyphosate and/or GBH formulations on animals (Table 2). A thorough review of all the toxic effects reported across all animal taxa studied is beyond the scope of any single review article, but particular types of GBH toxicity have been reviewed. Some particularly thorough reviews discuss health risks [29,30,31,32] and ecotoxicology [33], while more focused reviews elaborate on the effects on water fleas [34], on bees [35] and honeybees [36,37], on fish [38], on amphibians [39], in South American agriculture [40], as studied in Brazil [41], in aquatic systems [42,43], and to offspring of exposed mothers [44]. Other reviews focus on particular outcomes of toxicity, including cancer and genotoxicity [45,46,47], pregnancy outcomes [48,49,50], mammalian nervous systems [51], and autism spectrum disorders [52,53,54,55]. Since it is apparent that the effects of glyphosate and of GBH formulations often differ (reviewed in [28,56]), this distinction must be considered when possible.

Table 2.
Acute mortality induced by glyphosate or GBH from selected studies. Lower LD50/EC50 indicates higher sensitivity to exposure.
Agricultural workers and others living in agricultural areas likely experience particularly high GBH exposure, e.g., [6,12,63]. While it is extremely difficult to study in detail, in human agricultural workers, herbicide exposure is implicated in cases of impaired kidney function [64], altered thyroid and reproductive hormone levels [65], and reduced sperm count [66,67], and increases time to pregnancy [68], chance of short gestation [69], preterm birth [70], and neurobehavioral birth defects in their children [71], perhaps partially by increasing the permeability of the blood–brain barrier and changing the metabolic activity of epithelial cells in the brain [72]. Workers in GBH factories experience increased likelihood of coronary artery disease [73]. Probability of advanced liver fibrosis in patients with fatty liver disease [74] is associated with GBH exposure, as are Parkinson’s Disease and parkinsonism [75,76,77], and autism spectrum disorders [78,79]. Experimentally, GBH exposure decreases human sperm motility [80]; transiently increases genotoxicity [81] via DNA lesions [82] and increases formation of micronuclei in peripheral white blood cells [83]; mimics estradiol in inducing the growth of cholangiocarcinoma cells [84]; induces genomic damage on human lymphocytes [85]; induces breast cancer cell growth through an estrogen receptor pathway [86]; and, at low doses, dysregulates gene expression in breast cancer cell lines, particularly in pathways related to cell cycle and DNA damage repair [87]. Consistent with cancer cell line studies, the development of non-Hodgkin lymphoma [47,88,89] and other cancers [90] have been correlated with GBH exposure. Human suicide attempts using GBH impair cardiac function [91], require attention to airways and cause renal damage [92], and may induce gastrointestinal symptoms and central nervous system complications [93,94] including hippocampal infarction [95].
Following the suggestion of Clotfelter et al. [96] and Zala and Penn [97], who point out the importance of exploring the role of animal behavior when studying toxic chemical pollutants and endocrine disruptors, this review explores the toxic effects of GBHs on the behavior of animals. Based on the research articles available, we focus on the toxic effects of glyphosate and glyphosate-based herbicides across animal taxa on activity level, feeding behavior, anti-predator behavior, reproductive and maternal behavior, learning and memory, and anxiety-like and social behavior.
2. Materials and Methods
The research reviewed in this article was collected in two ways. First, we conducted exhaustive searches using Google Scholar, Science Direct, and Wiley Online Library, using Biological Abstracts and GreenFile for verification in a few searches wherein all articles were identified with the initial three databases. Likewise, we combined each behavior-related search term with “glyphosate”, and for some of these, separately searched “Roundup®” to verify that the same relevant articles were identified. Each of these databases was searched between October 2022 and March 2023 using the search terms listed in Table 3, with the number of hits indicated. From each search, we identified articles based on their title that were about how glyphosate or glyphosate-based herbicides like Roundup® affect behavior. If the title was not sufficient to determine whether each paper focused on an appropriate topic, the abstract was read as well. For each search, once we encountered between five and ten articles in a row that were not relevant to our topic, we stopped reading titles from that search. Only peer-reviewed research articles were included, not review articles, abstracts, conference proceedings, or theses. The resulting set of articles was then sorted into these behavioral categories: activity, feeding behavior, anti-predatory behavior, reproductive and maternal behavior, learning and memory, and anxiety and social behavior.

Table 3.
Number of matches from each search term in each database. The first number is the number of “hits” using each set of search terms, the second is the number the authors examined and assessed for appropriateness, and the third is the number of articles included in the review that were identified via that search. These do not add up to the total number of articles used because of extensive overlap between searches.
The second method we used to identify articles to include in this review was using the bibliographies of the articles identified using the first method, particularly the review articles cited in the introduction. These were sorted into the same behavioral categories.
Some articles included experiments relevant to more than one category, and these were included in both or all, and the resulting number of articles in each behavioral category are distributed by taxa in Table 4. The 128 articles included in our results are distributed by taxa as shown in Figure 1. Each behavioral category was then assigned to one of the authors, who read the articles, compiled the information, and wrote the first draft of the relevant section. Frequent discussions among the authors identified focal topics within and among behavioral categories and key themes for discussion, and every author commented on each section.

Table 4.
Number of research articles used for each section of this review by taxa.
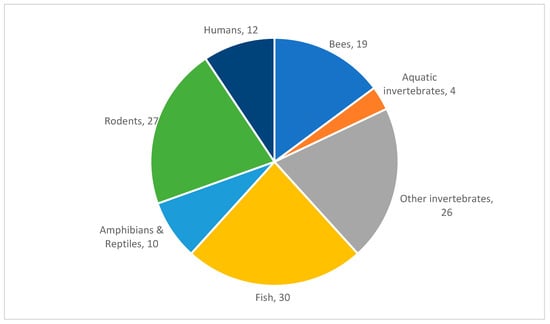
Figure 1.
Number of papers by taxa. This indicates the animal group studied and the number of papers about that group for all research articles which met inclusion criteria and are cited herein.
3. Results
3.1. Activity
Glyphosate affects the way many animals go about their day to day lives. Across studies about animal activity in a wide range of taxa, glyphosate affects activity levels, whether walking, crawling, swimming, or flying. When exposed to glyphosate, animals tend to be less active and do not move as far as unexposed animals (summarized in Table 5). In the honeybee, Apis mellifera, an experiment conducted using 7 μg glyphosate/bee, 14 μg/bee, and 28 μg/bee found that exposure to higher concentrations caused the bees to have trouble reaching their hive [98]. Bees returning to the hive in a straight line or with five right-angle turns, 2 h after being fed sucrose solution containing 5 mg/L or 10 mg/L glyphosate, took more time to reach the hive in both sets of mazes [60]. A similar method was used by Balbuena et al. [99] to examine flight paths of bees after food-based glyphosate exposure at 2.5 mg/L, 5 mg/L, or 10 mg/L. Although they performed this study outside and not under laboratory conditions, bees given herbicide took longer and took fewer direct flights. Similarly, 2.5 mg/L glyphosate exposure strongly impacted how long it took bees to return home [36]. A different method was used to record total activity level over a 24 h period. Bees were more active than controls when exposed to Roundup® at 1.2 mg/L or 6 mg/L, whereas exposure at 0.12 mg/L and 12 mg/L did not affect their activity levels, and exposure to 24 mg/L of Roundup® decreased their activity [100]. While this is an unusual response pattern, the mechanism leading to this change in behavior is not known. If the change in activity results from the endocrine-disruptive properties of GBHs, which are known to be non-monotonic, this may not be surprising (e.g., [101]). Conversely, feeding 50- or 100-ng doses of glyphosate per bee, caused bees to sleep more [102]. Other studies did not identify changes in activity level after Roundup® or glyphosate exposure. At concentrations of 0.5 mL/50 mL, 1 mL/50 mL, and 1.5 mL/50 mL, bees successfully returned to the hive [103]. However, these authors only considered whether the bees returned to the hive and not activity level or speed of return. Another study tested locomotion using a square box with four lights. One light was turned on at a time and when the bee was at the light it would turn off and the next light would turn on. This is an effective way to collect activity data because bees go toward lights. The data collected then were the duration of time it took for the bees to get to all the lights twice. When conducting this experiment with bees exposed to glyphosate at a concentration of 2.5 mg/L or 5 mg/L, they did not find a significant difference among the groups [104].
Walking animals also exhibit lower activity levels after exposure to glyphosate. In Swiss mice, the total distance traveled and velocity decrease when exposed to higher concentrations of GBHs given in oral gavages [105,106]. Exposure of mice to 50 mg/kg/day of a GBH had a large effect on their activity. The mice were less active and traveled a shorter distance than unexposed controls [107]. The Sprague Dawley rat also exhibited less locomotor activity when first injected with glyphosate at concentrations of 50, 100, or 150 mg glyphosate/kg. The rats traveled shorter distances, had less activity, and showed less stereotyped behavior in a dose-dependent pattern [108]. Another study showed less locomotor activity of Wistar rats injected with glyphosate at the lower concentration of 35 mg glyphosate/kg [109].
Similarly, arthropods also showed lower locomotor activity after GBH exposure. Arthropods tested include the small wolf spider, Pardosa milvina; the large wolf spider, Hogna helluo; and the ground beetle, Scarites quadriceps. All three arthropods showed a decrease in activity when they came in contact by touch with the herbicide [110]. The Madagascar hissing cockroach, Gromphadorhina portentosa, consumed up to 13.2 mg of glyphosate. Then, they were placed on a hamster wheel to see how long they could run for a maximum of 3 min. The results showed a significant decrease in time spent running on the wheel for cockroaches exposed to glyphosate compared to their unexposed conspecifics [111]. The fruit fly, Drosophila melanogaster, was also studied to see how different methods of feeding could affect their locomotor activity. The methods they used were putting the pesticide into agar-gelled feed (AM) and continuous liquid feeding (CLF), where flies ingested the pesticide in liquid, and how much they ingested could be quantified. Both methods decreased locomotor activity, though CLF yielded lower locomotor levels than when using the AM method [112].
The earthworm, Lumbricus terrestris, is also impacted when exposed to glyphosate. The GBH induced lower levels of activity when present throughout the soil [113]. In another earthworm, Octolasion cyaneum, there was very slight avoidance behavior towards the contaminated soil at the concentration of 249 μg, though this slight avoidance was not statistically significant [114].
Many aquatic organisms, including several types of fish, decrease activity and behave abnormally when exposed to GBH. Many studies using the zebrafish, Danio rerio, show that GBHs lower activity level at all stages, from larvae to adult fish. In a study that used both larvae and adults, glyphosate and Roundup® at 0.01, 0.065, or 0.5 mg/L reduced swimming distance [115]. Glyphosate concentrations of 0.01, 0.5, or 5 mg/L also increased swimming activity levels in exposed larval and adult zebrafish during the day [116]. When exposed to glyphosate at 1000 μg/L, zebrafish larvae decreased swimming distance, number of rotations, mean velocity, and body mobility [117].
One study of larval zebrafish showed an increase in activity when exposed to GBH at the concentrations of 0.1 and 10 uM for a 7-day exposure period [118]. However, another study done on larval zebrafish showed an increase in activity levels when exposed to Roundup® at 106 to 104 dilution but a decrease when exposed to GBH at 0.01 and 0.1 uM. Both exposure periods were 48 h at 5 days after fertilization [119]. The common carp, Cyprinus carpio, showed a decrease in activity level when exposed for 60 days versus an increase in activity level when exposed for only 12 h to GBH [120,121]. Collectively, these results show that in zebrafish and carp, the effect of GBH on activity depends on both concentration and duration of exposure. One clue to the variation might be that when glyphosate circulates in the water in the tanks, carp moved away from the part of the tank with contaminated water [121]. Under some conditions, changes in activity might reflect avoidance of contaminated water, while under other conditions might reflect an impairment in ability to behave normally. For example, when exposed to higher concentrations of glyphosate, the African catfish, Clarias gariepinus, showed loss of reflex, air gulping, and erratic swimming [122]. Two other studies that exposed catfish to glyphosate documented loss of equilibrium, increased startle responses, abnormal swimming, and restlessness [123,124]. When treated with glyphosate, the redbelly tilapia, Tilapia zilli, swam erratically and irregularly. After bursts of swimming, they became exhausted, more so at higher concentrations [125]. An additional study using the livebearer, Jenynsia multidentata, showed lower swimming activity levels when exposed to GBH [126]. The blue ridge two-lined salamander, Eurycea wildrae, exhibited lower burst distance swimming activity when exposed to GBH, and lower movement distance at higher temperatures [127]. Conversely, in a study using the hybrid fish surubim, a cross-breed of Pseudoplatystoma corruscans and Pseudoplatystoma reticulatum, fish showed higher swimming activity levels and increased ventilatory frequency when exposed to GBH [128]. Furthermore, the rainbow trout, Oncorhynchus mykiss, was moved from dark to light and back to dark conditions while exposed to different concentrations and formulations of GBH, including glyphosate alone. During the light period, fish have significantly lower activity levels compared to the dark periods, though during the dark periods, the fish swam a longer distance [129].
Other aquatic animals affected by glyphosate include the marsh frog, Pelophylax ridibundus. Marsh frog tadpoles exposed to water contaminated with 7.6 mg/L, 3.1 mg/L, and 0.7 mg/L glyphosate showed a decrease in activity level during the Gosner stage 25 [130]. In two different species of South American frogs, Boana faber and Leptodactylus latrans, tadpoles showed lethargy, convulsions, and rapid bursts of swimming when exposed to glyphosate at concentrations of 69, 161, 310, 550, and 1074.5 μg/L [131]. However, in the leopard treefrog, Boana pardalis, there was an insignificant decrease in activity levels when frogs were exposed to glyphosate [132]. The water flea, Daphnia magna, showed less swimming activity when exposed to GBH [133].


Table 5.
Effects of GBH on activity level.
Table 5.
Effects of GBH on activity level.
| Species | Herbicide/Ingredient Used | Exposure | Concentration | Results | Source |
|---|---|---|---|---|---|
| Honeybee (A. mellifera) | Herbazed 48% | Sucrose solutions | 0.5 mL/50 mL 1 mL/50 mL 1.5 mL/50 mL | No effect on navigation | [103] |
| Honeybee (A. mellifera) | Glyphosate | Distilled water solutions containing glyphosate | 2.5 mg/L 5 mg/L 10 mg/L | Longer to get to the hive | [99] |
| Honeybee (A. mellifera) | Glyphosate | Water and sucrose solutions containing glyphosate | 1.2 mg/L 6 mg/L 0.12 mg/L 12 mg/L 24 mg/L | Decrease activity | [100] |
| Honeybee (A. mellifera) | Glyphosate | Sucrose solutions containing glyphosate | 2.5 mg/L | Longer time to return to hive | [36] |
| Honeybee (A. mellifera) | Glyphosate | Water and sucrose solutions containing glyphosate | 2.5 mg/L, 5 mg/L | No significant difference in activity | [104] |
| Honeybee (A. mellifera) | Glyphosate isopropylamine salt (Monsanto Roundup® Original) | Sucrose solutions containing herbicide | 7 μg/bee, 14 μg/bee, 28 μg/bee. | Harder time reaching hive | [98] |
| Honeybee (A. mellifera) | Glyphosate | Sucrose solutions containing glyphosate | 50, 100 ng doses | Cause bees to sleep more | [102] |
| Honeybee (A. mellifera) | Glyphosate, in the Roundup® | Sucrose solutions containing glyphosate | 5 mg/L, 10 mg/L | Less time to reach the hive | [60] |
| Fruit fly (D. melanogaster) | Glyphosate | Continuous Liquid Feeding (CLF). | 10 mM, 30 mM, 50 mM | Decreased locomotor activity | [112] |
| Swiss mice | Roundup® | Oral gavages | 250 and 500 mg/kg/day | Total distance traveled and velocity decrease | [105,106] |
| CF-1 mice | Glyphosate isopropylamine salt | Saline solutions with glyphosate | 50 mg/kg/day | Less active and traveled a shorter distance | [107] |
| Wistar rat | Glyphosate | Water solutions containing glyphosate | 24 and 35 mg glyphosate/kg | Less locomotor activity | [109] |
| Sprague Dawley rat | Glyphosate | Saline solutions with glyphosate | 50, 100, or 150 mg glyphosate/kg | Lower locomotor activity | [108] |
| Wolf spider (P. milvina; H. helluo)Ground beetle (S. quadriceps) | Glyphosate | Saline solutions with glyphosate | 12 g/L | Lower locomotor activity | [110] |
| Madagascar hissing cockroach (G. portentosa) | Roundup® Ready-to-Use III | Food consumption | 13.2 mg of glyphosate | Decrease in activity level | [111] |
| Earthworm (O. cyaneum) | Glyphosate | Distilled water solutions containing glyphosate | 166, 332, 498, 664 and 830 g GLY/kg | Very slight avoidance of soil that contained glyphosate | [114] |
| Earthworm (L. terrestris) | Glyphosate based Herbicide | Food consumption | 243, 221, 218 mg | Lower levels of activity | [113] |
| Salamander (E. wildrae) | Commercially sold Roundup®. | In water | 0.0 mL/L, 0.5 mL/L, 1.0 mL/L, and 2.0 mL/L | Lower burst distance swimming activity and lower movement distance in higher temperatures | [127] |
| Rainbow trout larvae (O. mykiss) | Glyphosate based Herbicide | In water | 1.18 ± 0.036 and 1.95± 0.086 μg/L | Increased swimming activity and distance traveled during dark periods | [129] |
| Redbelly tilapia (T. zillii) | Glyphosate based Herbicide | In water | 108, 216, 324, 432 and 540 mg/L | Swam erratically and irregularly | [125] |
| Livebearer (J. multidentata) | Glyphosate based Herbicide | In water | 0.59 ± 0.07, 0.58 ± 0.14 and 0.56 ± 0.16 mg/L | Lower swimming activity levels | [126] |
| Surubim(P. corruscans and P. reticulatum cross-breed) | Roundup® Original | In water | 2.25, 4.5, 7.5, and 15 mg/L | Increase swimming activity levels and ventilatory frequency | [128] |
| Carp (C. carpio) | Glyphosate | In water | 5 and 15 mg/L | Decreased activity levels | [120] |
| Carp (C. carpio) | Glyphosate | In water | 0, 50, 100 and 150 mL/L | Increased activity levels | [121] |
| African catfish (C. gariepinus) | Dizensate (Glyphosate Herbicide) | In water | 9.6 mg/L, 14.4 mg/L, 19.2 mg/L, 21.6 mg/L and 24 mg/L | Loss of reflex, air gulping, and erratic swimming | [122] |
| African catfish (C. gariepinus) | Glyphosate | In water | 0.36, 0.48, 0.60, 0.72 and 0.84 mg/L | Loss of equilibrium, increased startle responses, abnormal swimming, and restlessness | [123] |
| African catfish (C. gariepinus) | Glyphosate, in the Roundup® | In water | 0.00 mg/L 0.30 mg/L 0.50 mg/L 0.70 mg/L 1.40 mg/L | Loss of equilibrium, increased startle responses, abnormal swimming, and restlessness | [124] |
| Zebrafish (D. rerio) | Glyphosate and Roundup® | In water | 0.01 mg/L, 0.065 mg/L, and 0.5 mg/L | Reduced swimming distance at both stages | [115] |
| Zebrafish larvae (D. rerio) | Glyphosate | In water | 0.05, 0.1, 0.5, 1, 10, 100, 1000, 10,000 mg/L | Decreased distance swam, number of rotations, mean velocity, and body mobility | [117] |
| Zebrafish larvae (D. rerio) | Glyphosate and Roundup® | In water | 0.1, 1, and 10 uM GLY 106- to 104-fold dilution Roundup® | Increase activity levels with Roundup® exposure and decrease with glyphosate exposure | [119] |
| Zebrafish larvae (D. rerio) | Glyphosate | In water | 0.01 and 10 uM | Increased in activity level | [118] |
| Zebrafish larvae (D. rerio) | Glyphosate | In water | 0.01, 0.1, 0.5, 1, 5, and 10 mg/L | Increased swimming activity levels | [116] |
| Marsh frog tadpoles (P. ridibundus) | Roundup® Power 2.0 | In water | 7.6 mg/L, 3.1 mg/L, and 0.7 mg/L | Decrease in activity level at Gosner stage 25 | [130] |
| Water flea (D. magna) | Glyphosate | In water | 0, 0.875, 1.75, 3.5, 7, 14, 28, and 56 mg/L); | Lower swimming activity levels | [133] |
| Treefrog larvae (B. pardalis) | Glyphosate based Herbicides | Food exposure | 2.40 mg/L, 4.00 mg/L, 1.21 mg/L,1.92 mg/L, 3.34 mg/L | Only ametryn had adverse side effects on the tadpole’s activity | [132] |
| South American frog tadpoles (B. faber and L. latrans) | Glyphosate (Roundup Original DI®) + 2,4–D(NORTOX®) | In water | 69, 161, 310, 550, and 1074.5 μg/L | lethargy, convulsions, and rapid bursts of swimming | [131] |
3.2. Foraging and Feeding Behavior
Exposure to glyphosate-based herbicides alters the feeding behavior of some organisms, while in other studies it has no effect. In many cases, GBH-laden food is avoided (summarized in Table 6), while organisms pre-exposed to varying concentrations of GBHs for several hours or a few days before experimental trials only sometimes alter feeding behavior (summarized in Table 7). Zebrafish larvae exposed throughout their first stage and tested 7 days after hatching exhibit altered feeding behavior. When zebrafish and their food, the rotifers, Brachionus calyciflorus and Lecane papuana, were exposed to GBH at concentrations of 0.8 mg/L, zebrafish decreased their food consumption. However, zebrafish pre-exposed to GBH consumed non-GBH food at normal rates. Since zebrafish rely on olfactory cues to find food, the authors suggest that changes in tastes or smells caused differential feeding on exposed rotifers [134]. Fruit flies consume less medium containing Roundup® in a dose-dependent pattern [135]. Similarly, flies preferred an organic sucrose solution to a solution that contained Roundup® Ready to Use, a GBH formulation with the active ingredients glyphosate and pelargonic acid. However, they did not show a preference for the solution with Roundup® Super Concentrate, another GBH formulation containing the surfactant POEA and the active ingredient glyphosate, despite exposure to equal concentrations of glyphosate from both formulations. In the same study, flies given organic corn medium containing either Roundup® Ready to Use or Roundup® Super Concentrate at various concentrations later consumed more sucrose than those pre-fed with non-GBH medium. The authors attribute this to the flies consuming less of the GBH-contaminated medium, which prompted them to later consume more sucrose solution [136]. The spider, Alpaida veniliae, showed lower consumption rates when prey were exposed to GBH [137]. Effects of GBH exposure on honeybees are mixed. Newly emerged adult honeybees given food infused with glyphosate showed a decrease in food intake compared to the control [138,139]. However, a study conducted in the winter found honeybees consumed more food when it contained GBH at concentrations of 0.1, 1, and 10 μg/L. This study also combined other reagents with glyphosate such as glyphosate + insecticide Imidacloprid, glyphosate + fungicide difenoconazole, and glyphosate + imidacloprid + difenoconazole. The mixtures and individual reagents caused the honeybees to consume more food than the control [140]. Honeybees required higher concentrations of sucrose solution to elicit proboscis extension after GBH exposure [35,96,103,113]. Similarly, honeybees feeding on sucrose solution displayed no difference in food intake regardless of exposure [104,141,142,143] and broiler hens showed no preference between feed containing or without GBH [144].
Species pre-exposed to glyphosate demonstrated varying effects. The pacu fish, Piaractus mesopotamicus, displayed decreased food consumption after they were exposed to glyphosate. Pacu were exposed chronically (10–15 days) to glyphosate at 0.2, 0.6 and 1.8 ppm. While the details differed among days, fish exposed to all concentrations exhibited decreased feeding on at least some days. Those exposed to 1.8 ppm also exhibited such a decrease [145]. Pre-exposed freshwater planarian, Girardia tigrina, exhibited decreased food consumption as the concentration of Roundup® increased [146]. Another study tested how glyphosate and Roundup® exposure influenced larvae of the damselfly, Coenagrion pulchellum. The larvae were exposed to 1 mg/L or 2 mg/L, both of which led to an increase in consumption of food compared to the control [147]. Predator cues did not affect food consumption when larvae of the damselfly, Enallagma cyathigerum, were pre-exposed to 2 mg/L of glyphosate for seven days [148]. Adult and spiderling Pardosa milvina environmentally exposed to GBH Buccaneer Plus ate more crickets than unexposed controls. The authors attribute this behavior to hyperactivity from exposure to Buccaneer Plus [149]. A similar study involving both P. milvina and another wolf spider, Tigrosa helluo, also showed that Buccaneer Plus altered predator efficiency. Environmental exposure via paper discs saturated with 12 mL/m2 GBH placed in random locations around the testing apparatus mimicked fields exposed to GBH. T. helluo were allowed to prey on crickets, Acheta domesticus, and on P. milvina; P. milvina were observed preying on crickets. In the presence of the herbicide, T. helluo were able to capture prey faster than the control for both prey types. While exposed and unexposed P. milvina did not differ in timing of predation, they required more lunges to capture their prey [150]. Another wolf spider, Hogna cf. bivittata, displayed pest-specific effects from GBH exposure, as they captured caterpillars and ants with lower efficiency than the control, but not when preying on crickets [151], while a different spider, Pardosa agricola, and the ground beetle, Poecilus cupreus, showed no significant difference in prey capture rates [152]. The water flea, Daphnia pulex, reduced grazing by 40% after pre-exposure at glyphosate concentrations of 50 mg/L [153]. Three-keeled pond turtle, Mauremys reevesii, eggs were exposed to glyphosate concentrations of 0, 2, 20, 200, and 2000 mg/L, which led to an increase in foraging time in the hatchlings at the two highest concentrations [154]. While some studies indicate that GBH exposure alters feeding behavior, others do not. For instance, in a study involving two predators, the southern hawker dragonfly, Aeshna cyanae and smooth newt, Lissotriton vulgaris, the predatory activity of organisms exposed to GBH, chronically or acutely, was no different from those unexposed, suggesting that GBH had no effect on the foraging of the predators [155,156].



Table 6.
Effects of GBH in Food on Feeding Behavior.
Table 6.
Effects of GBH in Food on Feeding Behavior.
| Species | Herbicide/Ingredient Used | Concentration | Results | Source |
|---|---|---|---|---|
| Zebrafish (D. rerio) | Glyphosate | 0.8 mg/L | Decrease in food consumption | [134] |
| Fruit fly (D. melanogaster) | Roundup® | 1, 3.3, 10, 33 g/L | Decrease in food consumption | [135] |
| Fruit fly (D. melanogaster) | Roundup® Super Concentrate, Roundup® Ready to Use | 0, 0.5, 1,0, 2.0 g/L, 10 g/L | Flies consumed less of the exposed food, consumed more food after exposed to GBH | [136] |
| Spider (A. veniliae) | Glifoglex® 48 | 192 mg/L a.i. | Lower consumption rates for prey exposed to GBH | [137] |
| Honeybee (A. mellifera) | Glyphosate formulation not listed | 0, 2.5, 5 mg/L | No difference in food intake | [104] |
| Honeybee (A. mellifera) | Glyphosate | 10 ppb, 100 ppb, 1 ppm, 10 ppm | No difference in consumption ratios except for the 10-ppb solution showing they consumed more | [142] |
| Honeybee (A. mellifera) | Glyphosate | 2.5 mg/L | Honeybees showed decrease in food consumption | [138] |
| Honeybee (A. mellifera) | Glyphosate | 0.1, 1, 10 μg/L | Food consumption increased in the presence of glyphosate | [140] |
| Honeybee (A. mellifera) | Credit Extreme® 240 | 1.25, 2.5, 5 ng/bee | Food consumption decreased over a 10-day period for food exposed to differing concentrations of glyphosate | [139] |
| Honeybee (A. mellifera) | Glyphosate | 1.5, 7.5 mM | Food consumption did not vary for glyphosate in its isolated form or combined form with AMPA or Ncer | [143] |
| Honeybee (A. mellifera) | Glyphosate | 75, 150, 301 a.e. mg/L | Glyphosate exposure did not alter food consumption | [141] |
| Broiler hen | Gallup super 360 | 47 mg Gly equivalent/kg body weight/day | No difference between exposure and post exposure to GBH | [144] |

Table 7.
Effects of Pre-Exposure to GBH on Feeding Behavior.
Table 7.
Effects of Pre-Exposure to GBH on Feeding Behavior.
| Species | Herbicide/Ingredient Used | Exposure | Concentration | Results | Source |
|---|---|---|---|---|---|
| Damselfly larvae (C. pulchellum) | Glyphosate and Roundup® | Individuals | 1, 2 mg/L | Increase in food consumption | [147] |
| Damselfy larvae (E. cyathigerum) | Glyphosate | 7 days | 2 mg/L | Increase in food consumption | [148] |
| Southern hawker dragonfly (A. cyanea) Smooth newt (L. vulgari) | Glyphogan Classic | Mesocosm exposed | 6.5 mg/L | No visible effect | [155,156] |
| Wolf spider (P. milvina) | Buccaneer Plus | Testing apparatus exposed | 9 a.e. g/L | Captured more prey than control | [149] |
| Pacu (P. mesopotamicus) | Unspecified glyphosate formulation | Fish exposed | 0.2, 0.6, 1.8 ppm | Food consumption decreased exposure | [145] |
| Water flea (D. pulex) | Pure glyphosate | Organism exposed | 50 mg/L | Reduced grazing | [153] |
| Wolf spider (T. helluo and P. milvina) | Buccaneer Plus | Paper filter discs were exposed with herbicide | 12 mL/m2 | Tigrosa caught prey faster while P. milvina required more lunges to capture prey | [150] |
| Three-keeled pond turtle(M. reevesii) | Glyphosate—ammonium | Eggs were exposed | 0, 2, 20, 200, 2000 mg/L | Increase in the amount of time it took to forage | [154] |
| Wolf spider (H. cf. bivittata) | Unspecified glyphosate formulation | In a test tube 30 min | 280 mg/L a.i. | Lower consumption rates for specific prey | [151] |
| Planarian (G. tigrina) | Roundup ® | GBH 96 h | 1.87, 3.75, 7.5, 15 mg/L a.e. glyphosate | Decrease in consumption rates as concentration increased | [146] |
| Agrobiont spider (P. agricola) Ground beetle (P. cupreus) | Roundup ® Biaktiv | Freshly doused paper and paper left to dry for 1 day | No significant difference in predation rates | [152] |
3.3. Anti-Predator Behavior
Glyphosate-based herbicides like Roundup® have adverse effects on anti–predator capabilities of some organisms, but not others (Table 8). For some organisms, exposure to GBHs led to a decrease in predator awareness. Zebrafish exposed to GBH were found to be in areas that put them at a higher risk of predation indicating loss in predator awareness compared to unexposed fish [157,158,159,160,161]. In another fish, the common spiny loach, Lepidocephalichthys thermalis, exposure to Roundup® (3 h and 15 days at 0.8 mg/L) led to an increase in activity in the presence of conspecific alarm cues (CC) [162]. Wood frog, Lithobates sylvaticus, tadpoles exposed to injured conspecific cues and Roundup® did not change their activity, while tadpoles unexposed to Roundup® decreased activity when exposed to cues from injured conspecifics, which indicates that glyphosate impairs the tadpole’s ability to respond to the threat of predation [163]. Gulf coast toad, Incilius nebulifer, tadpoles pre-exposed to Roundup® and exogenous corticosterone (CORT) became more active in the presence of predator cues. Pre-exposure to the individual reagents and control all showed a decrease in activity [164]. Blue Ridge two-lined salamander showed synergistic effects of temperature and glyphosate on anti-predator behaviors. Use of refuge became less frequent in exposed salamanders at ambient temperatures (12 °C) and an interactive effect between elevated temperatures (23 °C) and glyphosate also had lower frequency of refuge use. Glyphosate led to a reduction in burst distance, speed and distance from a predator attack, unaffected by temperature [127]. When exposed to Roundup®, damselfly larvae exhibited more activity in the presence of predator cues than the controls, which reduced their activity in the presence of predator cues. Exposed larvae walked more, faced their food and fed more often than the controls. However, the predator, the emperor dragonfly, Anax imperator, was not more effective at eating exposed larvae than the controls, despite the change in anti-predator behavior, perhaps because exposed larvae’s increased swimming speeds may counteract the reduced anti-predator behavior [165]. In damselfly, exposure to either Roundup® or pure glyphosate at 1 or 2 mg/L induced slower escape speeds in the presence of predator cues. Roundup® induced significantly slower escape speeds than glyphosate, indicating that “inert” ingredients affected their anti-predator capabilities [147]. Exposure to GBH decreased the amount of time wolf spiders spend ambulatory compared to controls in response to predator cues from beetles but not giant wolf spiders’ predator cues [166].
In other organisms, exposure to GBH causes little to no effects on anti-predator behavior. For instance, threat of predation from newts and dragonflies did not affect the anti-predation behavior of tadpoles of the agile frog, Rana dalmatina. Tadpoles exposed to varying levels of herbicide exhibited different behaviors, as the concentration increased, tadpoles decreased their activity around the predators. It was also shown that more tadpoles hid more often at the higher concentrations from the predators, more tadpoles hid from the dragonfly larvae than newts. Overall, the authors suggest that exposure to the herbicide did not significantly alter the tadpole’s anti-predator response [167]. Likewise, the anti-predator capabilities of marsh frog tadpoles were not affected by exposure to Roundup® Power 2.0 [130].


Table 8.
Effects of GBH exposure on anti-predator behavior.
Table 8.
Effects of GBH exposure on anti-predator behavior.
| Species | Herbicide/Ingredient Used | Exposure | Concentration | Results | Source |
|---|---|---|---|---|---|
| Zebrafish (D. rerio) | Roundup® | Exposed for 30 min | 1.4 μL | After simulated bird attack, fish remained in the central zone compared to control and other tests. | [157] |
| Zebrafish (D. rerio) | Roundup® | Exposed from 3–120 h post fertilization | 4.8 μg/L | Exposed fish remained in the area which had a stimulus unlike control; exposed fish displayed hypermobility and more time spent in the central zone | [158] |
| Zebrafish (D. rerio) | Glyphosate | Exposed from 3–120 h post fertilization | 4.8 μg/L | After predatory stimulus, fish entered and spent more time in the central zone than the control | [159] |
| Zebrafish (D. rerio) | Roundup® | Pre-exposed for 96 h | 3, 5 mg/L | Exposed fish spent more time spent in the top zone of the tank compared to the bottom zone | [160] |
| Zebrafish (D. rerio) | Roundup® Ultramax | Embryos were pre-exposed for 72 h | 0, 1, 2, 5 µg a.i./mL | 5 µg a.i./mL led to less time spent at the bottom of the tank when a visual stimulus was encountered, indicating loss of fear | [161] |
| Common spiny loach (L. thermalis) | Roundup® | Pre-exposed for 3 h and 15 days; briefly exposed to Roundup® mixed with CC and other mixtures | 0.8 mg/L | Pre-exposure led to an increase in activity in the presence of conspecific alarm cues (cc); unexposed fish did not detect conspecific alarm cues when Roundup®+cc were mixed | [162] |
| Wood frog tadpoles (L. sylvaticus) | Roundup® weathermax | Tadpoles were pre-exposed for 1 h; unexposed tadpoles briefly exposed to CC mixed with Roundup® and other mixtures | 0.5 mg a.e./L | Pre-exposure led to no change in behavior in the presence of conspecific cues compared to control; unexposed tadpoles exposed to Roundup® mixed with CC led to no change in behavior compared to control suggesting Roundup® inactivated CC | [163] |
| Gulf coast toad tadpoles (I. nebulifer) | Roundup® ready to use | Tadpoles were pre exposed for 7 days | 0.736 mg a.e/L | Mixture of Roundup® and exogenous corticosterone led to more activity compared to individual reagents and control | [164] |
| Blue Ridge two-lined salamander (E. wilderae) | Roundup® ready to use | Exposed for 5 h. | 0.73, 1.46, 2.92 µg a.e./L. | Ambient temperatures + glyphosate led to a lower frequency of refuge as the concentration increased; reduction in burst speed (speed and distance away from a predator) occurred during exposure | [127] |
| Damselfly larvae (E. cyathigerum) | Roundup® | Pre-exposed for 24 h | 1.5 mg/L | Exposure led to more activity in the presence of predator cues compared to the control; survival rate from altered anti-predator behavior did not have a significant change on survival from predation | [165] |
| Damselfy (C. pulchellum) | Roundup® and glyphosate | Pre-exposed for 7 days | 1, 2 mg/L | Exposure to glyphosate and Roundup® led to a decrease in escape swimming speed with 2 mg /L of Roundup® inducing the slowest escape speed | [147] |
| Wolf spider (P. Milvina) | Buccaneer Plus | Semicircles were sprayed with herbicide and placed in testing apparatus. | 2.5% | Exposure led to less time moving when exposed to S quadriceps cues but not to H. Helluo | [166] |
| Agile frog (R. dalmanita) | Glyphogan classic | 21 days of exposure | 0, 2, 6.5 mg a.e./L | Increase in concentration led to a decline in activity in the presence of predators, except for newts which were similar to the control (no predator); hiding occurred more often at higher concentrations, except for newts and the control | [167] |
| Marsh frog tadpoles (P. ridibundus) | Roundup® power 2.0 | Embryos were exposed for 96 h | 0.7 mg a.e./L, 3.1 mg a.e./L and 7.6 mg a.e./L | Exposure had no effect on anti-predator behavior | [130] |
3.4. Reproductive and Maternal Behavior
As an endocrine disruptor, glyphosate and GBHs particularly affect animal reproduction and reproductive behavior. Exposure to glyphosate can lead to a variety of negative effects on the reproductive systems of animals, including courtship, mating, fertility, and maternal behavior (summarized in Table 9 and Table 10). Ait Bali et al. [106] found that the mice had difficulty conceiving and success rates rapidly declined when exposed to higher concentrations of glyphosate. The females who were not exposed to glyphosate had an 87% success rate for conceiving, females exposed to 250 mg/kg had a 60% success rate, and females exposed to 500 mg/kg had a 25% success rate. Similarly, fecundity rates and fertility rates of planaria decrease as the concentration of glyphosate increases [146]. In earthworms, L. terrestris and Aporrectodea caliginosa, and Japanese medaka, Oryzias latipes, it was found that fecundity and fertility rates were negatively impacted by GBH exposure [168,169]. The offspring of female Wistar rats exposed to GBH had lower rates of fertility as well [170].
Pinning behavior, considered crucial for the development of sexual competence in males, was diminished in both male and female offspring perinatally exposed to the highest dose of GBH. Both doses of GBH reduced female sexual behavior, as demonstrated by a decrease in the female’s receptiveness to the male’s sexual advances, measured by latency to the first lordosis, a postural change in females that indicates receptivity to mating, number of lordosis, and number of mounts without lordosis. Male sexual behavior, measured in latencies to the first mount, first intromission, first ejaculation, number of total mounts with or without intromissions, and number of ejaculations in 30 min, was unaffected. The study suggests that the prenatal and lactational exposure to GBH disrupted aromatase activity, leading to the impairment of sexual behavior in female offspring, including a precocious vaginal opening [171].
GBHs decrease masculinization of male mice exposed before puberty [172,173]. Both maternal exposure to glyphosate and exposure before puberty disturbed the masculinization process during the critical period of sexual hypothalamic differentiation. Sexual partner preference score, measured by (total time spent in estrous female area—total time spent in sexually active male area), increased and copulatory behavior was altered, with an increase in latency to first mount, first intromission, and mount after first ejaculation. In the same mice, exposure increased estradiol serum concentrations, but this did not lead to increased sexual arousal. However, the mice began puberty at a younger age, which may lead to an increase in sexual behaviors at a younger age [172].
Wolf spider males exhibit less courtship when exposed to glyphosate. Females were placed inside traps and 47.2% of the traps captured between one and four males. Traps with GBH on filter paper inside the trap captured fewer males than those treated with distilled water. Traps with GBH surrounding the opening also captured fewer males than those with only water on the filter paper ring. This suggests that the herbicide interferes with female pheromone production. In an olfactometer experiment, there was no significant difference in the choice of a corridor that the spider took regardless of the presence of a female or not, leading to belief that the spiders were not repulsed by GBH itself. The conclusion was made that the males had trouble in detecting and/or responding to the females pheromones [174]. Another study confirmed that exposure to glyphosate impaired sexual chemical communication between female and male wolf spiders, Pardosa agrestis, reducing the male spider’s ability to find their mate [175]. Male agrobiont spiders and beetles exhibit similar courtship behaviors and experience similar success rate and duration of mating regardless of whether the surface they were on contained GBH residues [152]. A similar study on wolf spiders and glyphosate showed no significant effect on courtship or sexual behavior in either sex of the spider [176]. This may be due to differences between species. While exposure levels from [176] are difficult to compare because of different experimental procedures (5.04 µg/cm2 at 30.34%), they appear to be comparable among the other three studies (12 g/L [174], 14.4 g/L [152] and 15 mL/L [175]).
Chronic sublethal exposure to glyphosate and another pesticide, thiacloprid, negatively affected colonies of the ant species, Cardiocondyla obscurior, decreasing the number of eggs and pupae when exposed to both pesticides simultaneously [177]. Specifically, queens’ reproductive performance decreased, possibly due to trade-offs between detoxification and reproduction. The density of endosymbionts in workers decreased, which could be responsible for the decrease in the queens’ reproductive performance. In addition, the pesticides had no effect on the sex ratio, but resulted in smaller colonies. The results highlight the importance of studying multiple stressors and the long-term effects of chronic exposure.
Exposure to glyphosate and its commercial formulations can interfere with the reproductive fitness of fish by affecting their neural and endocrine systems. Exposure to glyphosate (0.5 mg/L in Roundup®) decreases the sexual activity and sperm quality of male livebearers found in rice plantations in southern Brazil and northern Argentina [38,126]. Livebearers also experienced a reduction in copulation and mating success, thus decreasing sexual activity [178]. GBH exposure also negatively impacts mate attraction by changing territorial behavior, aggressiveness, and coloration of livebearers, zebrafish, and male Mozambique tilapia, Oreochromis mossambicus, all traits important in courtship behavior and mate attraction, which ultimately decreases reproduction. Territorial behavior is important because females lay their eggs within these territories, and those with more resources attract more females. Aggressiveness includes chasing and biting rival males to secure access to females. Finally, coloration indicates a male’s health and genetic quality, such that brighter and more colorful patterns attract females [178,179]. Adult zebrafish that are exposed to glyphosate in combination with warm temperatures showed significant malformities in offspring, which may ultimately negatively impact sexual development and behavior in later stages of life [180]. Conversely, GBH exposure did not significantly impact the fertility and reproductive potential of rainbow trout, since both the control and exposed fish had high fertility [129]. GBHs decreased ovary size and number of mature oocytes in fruit flies [181], which may account for GBH-induced reductions in fertility [61].
Several studies [106,171,182,183] show that female rats and mice who were exposed to glyphosate while pregnant exhibited less maternal behavior, including decreased nursing, grooming of offspring, brooding, and reduced time spent in the nest compared to unexposed pregnant females. This negatively affects the offspring, interfering with their development and interactions with their environment. For example, offspring of exposed mothers had reduced locomotor function and mental health impairments. It was also found that maternal exposure to GBH had negative effects on maternal care of offspring, resulting in decreased body weight of rats at 75 and 90 days of age, with male offspring being more susceptible than females [171]. Another study [184] found that perinatal exposure to GBH reduced maternal care and aggressive behavior in rats, which may impair their ability to protect their offspring from predators. This was due to hormonal deregulations that decreased maternal reflexes and motivation. The time and number of pups retrieved decreased with a high dose of GBH, and maternal grooming and nesting was also observed to decrease. Maternal grooming and nesting are important for the pups’ development of endocrine and emotional responses to stress, and the lack of such grooming or nesting by the mother can alter the pup’s endocrine development and its response to stress later in life.
Maternal behavior of Wistar rats exposed to two different concentrations of GBH during pregnancy and lactation was not affected, nor did it impact water and food intake of mothers or their body weight, gestational length, or litter size. There were also no visible external malformations in the pups or any effect on their body weight due to GBH intake by mothers. These findings suggest that exposure to GBH during pregnancy and lactation did not have any significant adverse effects on maternal behavior of rats [185]. No significant changes were observed in maternal behavior of Wistar rats between the experimental and control groups [183]. These conflicting results may be attributed to the lower doses of GBH in both Gallegos [185] and de Oliveira [183] and the different formulations that were used. Additionally, there were no observed behavioral changes in Japanese medaka fish despite induced altered expression in reproductive related genes [169].
Nikbakhtzadeh and Fuentes [186] found that exposure to glyphosate was lethal to eggs, larvae and pupae, prolonged larval development, and delayed pupation of the mosquito, Culex quinquefasciatus. Female mosquitoes avoided ovipositing in glyphosate-contaminated water [186], but glyphosate at 5 mg/L from Roundup® Super Concentrate had no effect on where female field crickets, Gryllus lineaticeps, chose to lay their eggs [187].



Table 9.
Effects of GBH exposure on courtship and mating behavior.
Table 9.
Effects of GBH exposure on courtship and mating behavior.
| Animal | Behavior | Exposure | Test Used | Outcome | Source |
|---|---|---|---|---|---|
| Swiss mice, male and female | Courtship | In food: glyphosate 250 mg/kg and 500 mg/kg | Copulation, fertility, and fecundity rates |
| [106] |
| Mozambique tilapia (O. mossambicus), male and female | Courtship | 5 ppm, 8 ppm and 10 ppm of glyphosate | Color pattern, chasing distance of males, chasing occurrences, size of territory |
| [179] |
| Planarian (G. tigrina) male and female | Fertility | Borosilicate glass beakers: Roundup® Original 1.87, 3.75, 7.5 and 15 mg a.e./L | Fertility and Fecundity rates |
| [146] |
| Rainbow trout (O. mykiss) | Fertility and fecundity | 360 and 420 g/L Glyphosate | Fertility and Fecundity rates |
| [129] |
| Earthworm (L. terrestris and A. caliginosa) | Fertility and fecundity | Unspecified concentration of GBH | Fertility and fecundity rates |
| [168] |
| Male wolf spider (P. milvina) | Courtship behavior | Roundup® II Original diluted to 12 g/L | Olfactometer experiment and pitfall experiment |
| [174] |
| Livebearer (J. multidentata), male and female | Courtship behavior | Roundup®: 5, 10, 20, 35, 60, and 100 mg/L | Number of persecutions, copulation attempts, number of copulations, and mating success |
| [178] |
| BALB/c mouse, male and female | Courtship behavior | Roundup® Transorb: 50 mg/kg of glyphosate | Open-field test, elevated plus-maze test, and forced swim test |
| [173] |
| Wolf spider (P. agrestis) | Courtship behavior | 15 mL/L of Roundup® and 3 mL/L of Nurelle D | Two-choice olfactometer and Y-maze set-up |
| [175] |
| Ant (C. obscurior) | Fertility | fed with 75% honey-water mix- ture containing 3 μg/g thiacloprid, 100 μg/g glyphosate or 3 μg/g thiacloprid+ 100 μg/g glyphosate | Egg production, pupae production |
| [177] |
| Many fish species from embryo to adult | Courtship | Many different concentrations of glyphosate | Many different tests done from each paper |
| [38] |
| Agrobiont spider (P. agricola), ground beetle (P. cupreus) | Courtship | Roundup® Biaktiv Diluted to 1 part GBH and 25 parts water (1:25) = 14.4 g/L | Predation, locomotion, Avoidance, defence, and mating |
| [152] |
| Wistar rat, male and female | Fertility | MAGNUM SUPER II, 2 mg/kg/day or 200 mg/kg/day | Fertility rates |
| [170] |
| Fruit fly (D. melanogaster) | Fertility | Roundup® Super Concentrate: 0.5, 1.0, and 2.0 g/L and Roundup® Ready to Use: 1.0, 2.0, and 4.0 g/L | Ovary size, number of mature oocytes, body weight of females |
| [181] |
| Mosquito (C. quinquefasciatus) | Fertility | Roundup® super concentrate: 0.5 and 1 g/liter | Oviposition experiment, egg viability experiment, and triple-choice oviposition experiment |
| [186] |
| Wistar rat, male and female | Courtship | Roundup® Transorb: 50 and 150 mg/kg | Observations on male and female sexual behavior |
| [171] |
| Wistar rat PND 90 and adult | Courtship | Roundup® Transorb: 0.25 mL/ 100 g of body weight between 7 and 8 am from GD18 to PND5 | Sexual partner preference score, sexual behavior |
| [172] |
| Japanese medaka (O. latipes) | Courtship | Embryos exposed to 0.5 mg/L glyphosate, 0.5 mg/L and 5 mg/L Roundup® for 15 days | Fecundity and fertilization efficiency |
| [169] |
| Zebrafish (D. rerio) | Fertility | 1 ppm and 5 ppm glyphosate for 96 h temperatures: 28.5 °C, 29 °C, 29.5 °C, and 30 °C |
| [180] | |
| Fruit fly (D. melanogaster) | Courtship | Roundup® sprayed on GMO corn and then fed to fruit fly | Portion mated (females) and courtship rate (males) |
| [61] |
| Wolf spider (P. milvina) | Courtship | Hi-Yield® Kilzall 5.040 μL/cm2 | Body shakes and leg raises |
| [176] |

Table 10.
Effects of GBH exposure on maternal behavior.
Table 10.
Effects of GBH exposure on maternal behavior.
| Species | Exposure | Behavior | Outcome | Source |
|---|---|---|---|---|
| Swiss mice, male and female | In food: glyphosate 250 mg/kg and 500 mg/kg | Nest building |
| [106] |
| Sprague Dawley Rat, male and female | Vanilla wafer cookie: Glyphosate: 5 mg kg−1 d−1 and Roundup® Plus: 5 mgkg−1d−1 | Maternal behavior |
| [182] |
| Wistar rat, male and female | Glifloglex®: 0.65 g/L and 1.30 g/L | Maternal Behavior |
| [185] |
| Wistar rat, male and female | 50 mg/kg per day of GBH | Maternal behavior |
| [183] |
| Wistar rats, male and female | Roundup® Transorb: 50 and 150 mg/kg of GLY-BH | Pup retrieval, percentage of dams that retrieved all pups, total number of pups retrieved for each dam, grooming of the pups, fullmaternal behavior, nest building, maternal aggressive behavior |
| [184] |
| Cricket (G. lineaticeps) | Roundup®: 5 mg GLY/L of water and glyphosate: 5 mg/L of water | Choice oviposition experiment and no-choice oviposition Experiment |
| [187] |
3.5. Learning, Memory, and Cognition
Learning involves the acquisition of new information, while memory is the ability to retain that information and apply it in future situations. Studies that focus on visual and olfactory learning tasks indicate that some sensory learning systems are extremely susceptible to GBH exposure, while in other situations they may not be affected at all (Table 11). In the mosquito, Aedes aegypti, for example, habituated less to a visual stimulus after exposure to a dose only 5% of the lethal dose, and almost completely lost habituation at higher but still field-relevant concentrations [188]. The effect of GBH on honeybee sensory learning is more complicated. In two-color discrimination associative learning, whether the association was between neutral stimuli and electric shock [100] or between a sucrose reward and an aversive solution [189], GBH exposure did not affect visual learning. However, in a 10-color discrimination scenario, which is a realistic foraging situation for honeybees, GBH-exposed bees failed to learn during the second half of training, resulting in significantly worse performance than unexposed control bumblebees, Bombus terrestris [189]. The same authors found no effect on 10-odor discrimination. Glyphosate exposure did impair olfactory learning in 9-day-old young adult honeybees (but not at 5 or 14 days, [138]) and adult honeybees [98,104] in some two-choice associative learning situations, but not in another [190]. Apparently, difficult sensory learning tasks are more likely to be damaged by GBH exposure than simple ones.
Even in paradigms in which sensory learning is not impaired by GBH exposure, memory often is (Table 12). Though Hernandez et al. [190] found no effect on learning, or on memory overall, exposure did shorten memory retention from long-term to medium-term sensory memory. Helander et al. [189] found that sensory memory was significantly and strongly impaired in the same situations as learning and 10-color discrimination but not 2-color or 10-odor situations. Importantly, this was true whether bees were exposed to GBH before or after learning acquisition. Similarly, Herbert et al. [104] and Luo et al. [98] identified deficiencies in short-term and medium-term olfactory memory in exposed honeybees. A study of farmers in Uganda identified that visual memory is also impaired by pesticide exposure in humans, as are language memory, perceptual motor function, complex attention, and processing speed. Specifically, glyphosate exposure is associated with impaired visual memory, as measured by the Benton visual retention test [191].
Spatial learning ability has been assessed based on maze completion in rats and turtles, and homeward flight paths in honeybees. In an open field experiment, honeybees exposed to GBH took longer to accomplish homeward flight, and were less likely to transition from an indirect flight path on their first trial to a direct path on the second [99]. Similarly, turtles exposed to GBH took longer to complete a cross maze, and those exposed to high concentration took longer than low concentration [154]. Rat spatial learning was examined using the Morris Water Maze test, where they use visual cues outside of the water to find a submerged platform in opaque water. Those rats exposed to GBH took longer to find the correct quadrant and the platform during the second half of the learning phase, regardless of the exposure concentration [192].


Table 11.
Effects of GBH exposure on learning.
Table 11.
Effects of GBH exposure on learning.
| Animal | Exposure | Specific Behavior | Behavior Test Used | Behavioral Outcomes | Source |
|---|---|---|---|---|---|
| Rat pups PND 28–35 | Glyphosate 35 mg/kg 70 mg/kg every 2 days PND 7–27 | Spatial learning | Morris water maze test | Learning less at days 3 and 4 | [192] |
| Honeybee (A. mellifera) | Glyphosate PESTANAL 2.5 mg/L 5 mg/L 10 mg/L | Spatial learning | Homeward flight path | Proportion direct second trial > first trial in controls (3/15 vs. 12/15) but not exposed bees (8/16 vs. 11/16) Proportion with indirect on the first trial →direct on second decreased with concentration but not significant | [99] |
| Honeybee (A. mellifera) | Roundup® or glyphosate 0.12 mg/L (1.2 ng/bee) 0.24 mg/L (2.4 ng/bee) 2 weeks of sucrose solution | Aversive stimulus learning, visual learning | Associative learning task: 2-color choice paired with shock | No effect | [100] |
| Honeybee (A. mellifera) young bees | Glyphosate | Associative olfactory learning | Training: olfactory stimulus paired with reward, vs. unpaired | 5 days old: no effect 9 days old: impaired 14 days old: no effect | [138] |
| Bumblebee (B. terrestris) | Roundup® Gold 0.1 uL once before training | Associative visual learning | 10-color choice paired with sucrose reward or aversive solution | Untreated bees increased performance during each of the five bouts. Treated bees failed to learn between 3 and 4, or 4 and 5 and performed significantly worse than controls during 4 and 5 | [189] |
| Associative visual learning | 2-color choice | No effect | |||
| Associative olfactory learning | 10-odor choice | No effect | |||
| Honeybee (A. mellifera) | Glyphosate 2.5 mg/L 5 mg/L Daily, 15 days | Associative olfactory learning | Proboscis extension response (PER) | Sucrose sensitivity, elemental and non-elemental learning impaired | [104] |
| Honeybee (A. mellifera) adult foragers | Glyphosate 375 ng 1500 ng Single dose or divided over three days | Associative olfactory learning | PER | No effect | [190] |
| Honeybee (A. mellifera) | Roundup®, unspecified formulation 0.72 g/L 3.6 g/L = recommended dose7.2 g/L 3 h/day, 11 days | Associative olfactory learning | PER | %PER lower in bees exposed to paired sucrose and odor during 2nd and 3rd conditioning sessions for ½ RC and 2 RC but not 1 RC | [98] |
| Honeybee (A. mellifera) | ED50 = 10 mg/L, ED25 = 5 mg/L dissolved Roundup® granules in saturated sucrose solution administered 2 h before testing | Spatial learning | Simple maze completion time | >10× longer for ED50 and >6× longer for ED25 bees to complete | [60] |
| Complex maze | Even greater differences in completion time and course corrections (though control bees did make course corrections, exposure increased >10× | ||||
| Mosquito (A. aegypti) fourth-instar larvae (5–8 days from hatching) | Glyphosate from hatching | Non-associative visual learning | Habituation to shadow | Decreased by doses <5% of lethal dose 50 μg/L—no effect, normal habituation 100 μg/L—intermediate 210 μg and 2 mg/L—almost no habituation | [188] |
GBH exposure impaired spatial memory even more strongly than spatial learning. In honeybees, those exposed to GBH at 25% or 50% of the ED50 (ED50 = 10 mg/L in sucrose) concentration took 6 or 10 times longer to complete a simple maze 2 h after training than unexposed controls. After 24 h, the results were only slightly less pronounced. In addition, while control bees required no course corrections, exposed bees did in a dose-dependent manner. Differences between exposed and unexposed bees were even greater in a complex maze, both in terms of completion time and course corrections [60], again indicating that more complex types of learning and memory decrease more than simple ones. Rodents’ spatial memory was also impaired, including rats tested in the water maze test mentioned above [192] (but not [193]). In addition, chronic GBH exposure reduces short term spatial memory in a y-maze among young mice exposed through maternal dosing, prenatally, and through lactation [106] (but not those exposed only during gestation [194]) and chronically exposed adult mice [195].
Mice and rats explore and spend more time with an unfamiliar (novel) object than one they have spent time interacting with in the past. However, mice exposed to GBH fail to discriminate between novel and familiar objects. In adults, chronic and subchronic exposure significantly reduce discrimination. This effect most prominently impacts short-term (6-h) memory [107]; and is dose-dependent when young mice are exposed through maternal dosing [106]. These results are similar in rats, both in terms of an increase in variance among exposed females and overall novel object recognition impairment in males [196] or both sexes [192]. In contrast, Del Castilo et al. [193] did not observe a decrease in novel object recognition in 3-month-old mice exposed to GBH since pregnancy; the difference may be attributed to lower doses.
Consistent GBH exposure is detrimental to aversive stimulus-avoidance memory in mice and in fish. In both taxa, electric shocks are applied when the animal enters a dark area of their arena during training. Short-term memory is measured as the latency to enter the dark area 2 h later; long-term memory is tested 24 h after training. Mice exposed to 500 mg/kg, whether as adults [195] or through maternal dosing [106] exhibited shorter latency to enter the dark area after 24 h, whether dosing was chronic, subchronic or acute. Maternally dosed and chronically dosed adults’ short-term avoidance memory was also impaired at this dose. A lower dose of 250 mg/kg impaired avoidance memory after acute and subchronic dosing in adults (short-term memory), subchronic and chronic dosing in adults (long-term memory), and maternal dosing (long-term only). In zebrafish [115] and a livebearer fish [126], long-term aversive stimulus memory is impaired by GBH exposure. These are also among the few learning and memory papers that directly compare exposure to different formulations. Consistent with results from a wide variety of taxa comparing the effects of glyphosate to those of formulated GBHs on many different behavioral, physiological, morphological, and genetic endpoints (reviewed in [28,56]), Bridi [115] found that Roundup® exposure affected memory more than exposure to glyphosate alone, and Sanchez et al. [126] compared two different Roundup® formulations with somewhat different results.
Most information about how GBH exposure affects humans is based on case studies resulting from accidental acute exposure or intentional exposure during suicide attempts. Most of these case studies indicate that short-term and/or verbal memory loss occurs. The exception is Wang et al. [75], who report a case in which the patient exhibited parkinsonian syndrome, but without short-term memory loss. Other cases do report short-term memory loss, often beginning quickly (hours to days after exposure) [95,197,198]. In some cases, memory loss lasted for many months [95] or years [199] through the end of the study. In other cases, dramatic improvements were observed [197,198]. Types of memory affected include word recall [197], confusion, verbal memory, general memory and delayed memory [95], and both retrograde and anteriograde amnesia [95,198]. While there is some variation among these case studies, both in how patients were assessed and the memory impairments reported, the overall pattern indicates that short-term and language memory are most often affected.


Table 12.
Effects of GBH exposure on memory.
Table 12.
Effects of GBH exposure on memory.
| Animal | Exposure | Specific Learning/Memory Behavior | Behavior Test Used | Behavioral Outcomes | Source |
|---|---|---|---|---|---|
| Swiss mice male 1 month | GBH, unspecified formulation 250 or 500 mg/kg/day: acute (once); subchronic (daily for 6 weeks); chronic (daily for 12 weeks) | Recognition memory | Novel object recognition | Chronic and subchronic—reduced discrimination Acute—similar average discrimination ability (NS), more variation | [195] |
| Spatial working memory | Y-maze | Chronic—reduced spontaneous alternation Subchronic—no effectAcute—no effect | |||
| Aversive stimulus memory | Passive avoidance task | Short-term memory (2 h): chronic—500 mg/kg reduced latency subchronic and acute—250 mg/kg reduced latency Long-term memory (24 h): chronic and subchronic—250 and 500 mg/kg reduced latency acute—500 mg/kg reduced latency | |||
| Swiss mice male and female offspring 60+ days | GBH, unspecified formulation 250 or 500 mg/kg/day: maternal gestation and lactation | Working memory | Y-maze | Lower alternation in a dose dependent manner | [106] |
| Recognition memory | Novel object recognition test | Reduced ratio of time with novel object in a dose-dependent manner Lower discrimination index | |||
| Aversion avoidance memory | Passive avoidance test | Short-term memory (2 h): decreased latency at 500 mg/kg Long-term memory (24 h): decreased latency at 250 and 500 mg/kg | |||
| Mice male 4 weeks | Glifloglex® 4 mg/day 3X/wk, 50 mg/kg/day: intranasal | Recognition memory | Novel object recognition test | Short-term (6 h): impaired Long-term (24 h): not impaired (recovered) | [107] |
| Swiss mice male and female 3 months | Roundup®, unspecified formulation 0.075% w/v: Drinking water | Recognition memory | Novel object recognition test | No differences for males or females | [193] |
| Spatial memory | Water maze | No difference for males or females | |||
| Rats male and female adults | Glifloglex® 0.65 g/L (NOAEL; 100 mg/kg/day) 1.3 g/L (200 mg/kg/day) gestation and lactation | Recognition memory | Novel object recognition test | Females—no significant effect; high variation among exposed females during familiarization phase Males—impaired | [196] |
| Rat pups PND 28–35 | Glyphosate 35 mg/kg 70 mg/kg every 2 days PND 7–27 | Recognition memory | Novel object recognition test | Decrease in time spent with novel object at both concentrations | [192] |
| Spatial memory | Morris water maze test | Lower in rats exposed to either concentration | |||
| Bumblebee (B. terrestris) | Roundup® Gold 0.1 uL once before training | Associative Visual memory | 10-color choice paired with sucrose or aversive solution | Whether treated before learning bout 1 or after learning bout 5, exposed bees performed significantly and much worse than control bees | [189] |
| Associative Visual memory | 2-color choice | No effect | |||
| Associative Olfactory memory | 10-odor choice | No effect | |||
| Honeybee (A. mellifera) | Glyphosate 2.5 mg/L 5 mg/L Daily, 15 days | Associative olfactory memory | Proboscis extension response (PER) | Short-term memory decreased | [104] |
| Honeybee (A. mellifera) adult foragers | Glyphosate 375 ng 1500 ng Single dose or divided over three days | Associative Olfactory learning | PER | memory retrieval at 14 min or 24 h after conditioning did impair memory retention patterns—unexposed bees were more likely to have successful long term than medium term memory, while exposed bees were more likely to have successful medium term memory. | [190] |
| Honeybee (A. mellifera) | Roundup®, unspecified formulation 0.72 g/L 3.6 g/L = recommended dose 7.2 g/L 3 h/day, 11 days | Associative Olfactory learning | PER | % PER lower for all exposed bees in memory trials 1–5, but only significant for 1/2RC in all trials and in T1, T4, and T5 for 1RC | [98] |
| Honeybee (A. mellifera) | ED50 = 10 mg/L, ED25= 5 mg/L dissolved Roundup® granules in saturated sucrose solution administered 2 h before testing | Spatial memory | Simple maze completion time- | 24 h after exposure, times were lower than at 2 h, but still 5–8.5X longer than controls Simple maze course corrections—control bees 0, ED25 1.3, ED50 2.35 | [60] |
| Complex maze | Substantially and significantly after 24 h | ||||
| Zebrafish (D. rerio) 3-day larvae adults | Glyphosate Roundup® 96 h 0.01 mg/L 0.065 mg/L and 0.5 mg/L | Aversion avoidance memory | Dark/light association | Impaired by 0.5 mg/L Roundup®; other concentrations not significant Glyphosate alone not significant | [115] |
| Livebearer (J. multidentata) | Roundup® Original Roundup® Transorb Roundup® WG 96 h 0.5 mg/L | Long-term memory | Avoidance inhibition test | All fish spent more time in the light area in testing than training RWG: less time in light area in testing | [126] |
| Three–keeled pond turtle (M. reevesii) | Glyphosate ammonium eggs 2, 20, 200 or 2000 mg/L | Spatial learning | Maze | Longer time to cross maze; dose-dependent | [154] |
| Human | Accidental exposure | Parkinsonism | Mental exam | Short-term memory loss | [199] |
| Human | Occupational exposure | Benton visual retention test | Impaired visual memory | [191] | |
| Human | Unknown exposure amount | Encephalopathy | Neuropsychological test | Day 2—memory problems Day 12—overall test score 22/30, 0/3 word recall, impaired memory and executive function 3 years—neuropsychological tests 28/30, ⅔ word recall, improvements in memory and executive functioning | [197] |
| Human | Commercial formulation Unknown amount | Hippocampal infarction | IQ and memory tests | At admission—memory normal Several hours—memory deficit, short-term memory loss, including of suicide attempt Day 9—short-term recall deficits 3 weeks—partial improvement; IQ = 70, verbal memory 52, general memory 64, delayed memory; 65 indicates retrograde and anterograde amnesia. 2 months—verbal memory 74, general memory 84, delayed memory 86 6 months—memory impairments remain. | [95] |
| Human | Chronic occupational exposure | Parkinsonism | No short-term memory loss | [75] | |
| Human | Unknown amount | Encephalopathy | Short-term memory impairment, retrograde and anterograde amnesia | [198] |
3.6. Social Behaviors
Studies on a variety of animal species have assessed the effects of pesticides and herbicides on social behavior. The scope of these investigations includes effects on anxiety-related and depressive-like behavior, aggression, autism spectrum disorders (ASD), and more (summarized in Table 13). In critical developing stages of the brain such as the prenatal, postnatal, and adolescent periods, exposure may be even more detrimental, having possible impacts not just on higher cognitive functioning like in learning and memory, but also on social and emotional behavior as well as the development of ASD [105,200]. Generally, herbicides like glyphosate have been reported to affect motor and emotional functioning in addition to sociability in several non-target animals. Though neurotoxicity of glyphosate on humans is less frequently studied, studies have made associations between glyphosate and neuropathology like ASD and Parkinson’s disease.
Herbicides like glyphosate have been shown to cause neurotoxic effects linked to changes in mood such as anxiety-related and depressive-like behavior. In rodents, the open field (OF) test is commonly used to assess locomotor activity and emotional reactivity to new environments, where a tendency to stay in the peripheral areas in the OF arena, termed thigmotaxis, and thus, less time spent in the center, indicates anxiety-like behavior [107]. Elevated plus maze (EPM) tests have also similarly been used to assess anxiety in rodents. Employing these methods, studies on GBH exposure in mice have reported decreased locomotor activity [107,201], indicating effects on nervous system function, and increased anxiogenic behavior [105,106,107,202,203]. Ait Bali et al. [105,202,203] have shown that these effects are dose-dependent and occurred after subchronic (6 weeks) and chronic (12 weeks) GBH exposure, but not acute. Another study by Bicca et al. [204] assessed subchronic exposure of Zamba® GBH at 50 mg/kg on rodents and explored the potential therapeutic effects of the flavonoid, quercetin. The results showed increased anxiety in the EPM test with fewer open arm entries and less time spent in the open arm. These effects were largely recovered by quercetin. Conversely, some studies did not find anxiolytic behavioral effects of glyphosate in the OF test [193,201,205], and Joaquim et al. [201] noted reduced exploratory behavior of only male mice during the EPM test. Exposure to GBH during critical development stages of the brain may have important effects on emotional behavior later in life. De Castro Vieira Carneiro et al. [194] reported that mice between postnatal day (PND) 25–28 that were exposed to 0.3 mg/kg/day of GBH during gestation crossed lines more frequently in OF tests, suggesting hyperactivity, and exhibited increased marble burying behavior which may be indicative of anxiety. In another study, rats were exposed to either 0.65 or 1.3 g/L of GBH during gestation and lactation. Females at PND 45 that were exposed to the highest concentration of GBH crossed fewer squares in an OF test, indicating decreased locomotor activity. These effects were also observed in 90-day-old male and female rats exposed to either concentration of GBH. The authors state their findings are positively correlated to an increase in emotional response in adulthood [185].
Glyphosate also affects locomotor activity and anxiety in other non-target organisms such as zebrafish. A study assessing the potential effects of global warming on glyphosate toxicity found that zebrafish exposed to glyphosate at increasing temperatures spent more time at the bottom of the tank and had more erratic movements suggesting increased anxiety [206]. They also found that glyphosate exposure at increasing temperatures caused disruptions in the zebrafish’s circadian rhythm, where they spent less time swimming during the light portion of the cycle and more in the dark portion. Ivantsova et al. [118] compared the effects of glyphosate and AMPA (glyphosate’s main metabolite) as well as a mixture of the two on zebrafish larvae. While glyphosate, but not AMPA or the mixture, induced hyperactivity in zebrafish, there were no observed effects on anxiety with any of the treatments.
In livebearers, unlike what has generally been seen in rodents, unexposed fish spend more time in the peripheral areas in an OF test than the central area where there is increased predatory susceptibility, indicating natural anxiety behavior [126]. Two formulations of GBH, Roundup® Original and Roundup® Transorb, increased the amount of time fish spent in the central area, while a third formulation, Roundup® WG, did not. The authors suggest that this could be due to a depressive-like state coinciding with reduced alertness. Similarly, Lanzarin et al. [161] found that zebrafish embryos exposed to the highest tested concentration of GBH did not exhibit evasion behavior when introduced to an aversive stimulus compared to the unexposed embryos. This decreased perception of fear could be due to adverse effects on CNS development, specifically in the habenula region of the brain which plays a role in aversive response control.
While it is unclear whether the effects of GBH on evasion behavior are caused by a depressive-like state, other studies have also linked glyphosate exposure to depression-like behavior. Ait Bali et al. [105,202] reported that subchronic and chronic, but not acute, exposure to GBH not only induced anxiety in mice but also depressive behavior, where mice subjected to a tail suspension test and splash test showed a dose-dependent increase in immobility time and decrease in grooming time, respectively. These results were in agreement with Joaquim et al. [201], who also reported increased immobility in the tail suspension test with both male and female mice acutely exposed to GBH. Mice exposed to GBH also spent more time immobile in a forced swim test and this, like the anxiety effects previously discussed, also improved with quercetin therapy [204]. Rats exposed to 0.36% glyphosate in water from gestational day 5 until PND 60 demonstrated prolonged immobility time and decreased time climbing in a forced swim test, indicative of depressive-like behavior, though no effects on anhedonia-like behavior were seen [207].
Glyphosate affects honeybees’ ability to carry out social activities that rely on functions such as directional flight [99], appetite [104], associative learning, and circadian rhythms [100]. Decreased social interaction was also observed in rodents exposed to GBH during gestation [183,208] and also throughout the lifespan from pregnancy until adulthood [193]. Impaired social behavior was also found in livebearers exposed to Roundup® for 96 h [126], as demonstrated by a preference for the side of the aquarium with fewer fish. De Oliviera et al. [183] found that maternal exposure to GBH reduced the number of ultrasonic vocalizations emitted by pups, which is an early social communicative deficit. Additionally, the pups exhibited increased latency to reach the GBH-treated dam’s shavings, signifying a defect in olfactory discrimination which is important for social behavior development. In a three-chamber sociability test, GBH-treated mice spent less time and made less visits to another conspecific and spent more time with an inanimate object, indicating adverse effects on adult mice social skills [106]. While many studies have reported negative effects of GBH on social behavior, a few studies [161,194] did not identify such effects.
Changes in aggression also occur in some animals exposed to glyphosate. In Sanchez et al.’s [126] study, livebearers were tested for aggressive behavior by observing their proximity to their own reflection in a mirror, which indicated preference for an “opponent”. All GBH-treated fish spent more time in proximity to the mirror and thus demonstrated more aggressiveness than non-treated fish. In contrast, Bridi et al. [115] reported that glyphosate impaired aggressive behavior in zebrafish, utilizing a similar methodology. Pinning behavior is an important assessment of play fighting behavior in rodents where the goal of the rat is to wrestle the opponent onto its back and stand over it [171]. Pinning behavior was impaired in both male and female offspring of dams exposed to the highest GBH dose of 150 mg/kg/day from day 15 of gestation to PND 7. Since pinning behavior is critical to the development of male rat sexual competence, this could affect sexual behavior in adulthood.
Over the past few decades, there has been a rapid increase in the prevalence of autism spectrum disorder (ASD), a neurodevelopmental disorder characterized by difficulties in social communication and unusually limited and repetitive behaviors and interests [209]. With the simultaneous increase in global herbicide and pesticide use, many studies assessing the association between pesticides and neurodevelopmental disorders like ASD have shown a strong relationship [200,210,211,212]. Del Castilo et al. [193] found that both male and female 3-month-old mice exposed to GBH since pregnancy exhibit more repetitive marble burying, a behavior used to assess stereotyped behavior in mouse models of autism. Other studies also support these findings [183,194], reporting an increase in repetitive/stereotyped behavior in rodents exposed to GBH during gestation and throughout the lifespan [193]. In addition, in a novel object recognition test, mice demonstrated cognitive deficits after maternal glyphosate exposure, suggesting ASD-like cognitive impairment [208].
Some case studies have assessed the effects of pesticides and herbicides on neurological disorders like ASD and Parkinson’s disease in humans. For example, a study by von Ehrenstein et al. [79] examined birth data between 1998–2010 from the Central Valley of California, a major agricultural location. The risk of developing ASD correlates with exposure to glyphosate and other herbicides such as chlorpyrifos, diazinon, malathion, avermectin, and permethrin. This risk increases following prenatal exposure to ambient pesticides within 2000 m of their mother’s residence during pregnancy. Exposure during the first year of life can further increase the risk of ASD with intellectual disability comorbidity. Other studies have linked glyphosate exposure, through occupational exposure or accidental ingestion, to Parkinsonian syndrome [75,199,213]. A case study by Zheng et al. [214] detailed the events of a previously healthy 58-year-old woman following acute glyphosate exposure, where the patient developed Parkinsonian syndrome that completely resolved after treatment with ATP, pralidoxime iodide, and scopolamine hydrobromide.
Social behavior is important to the procreation, survivability, and adaptability of species throughout the animal kingdom. In honeybees, for instance, social interaction and cooperation is vital to the survivability of the entire colony, as each member has specific tasks to carry out [215]. Social behavior in rodents also plays a role in the development of other cognitive and emotional processes which later form part of the adult behavioral repertoire [183]. Thus, disruptions in social behavior can have damaging effects to not just the individual organism but to whole populations, species, or ecosystems.


Table 13.
Effects of GBHs on Social Behaviors.
Table 13.
Effects of GBHs on Social Behaviors.
| Animal | Exposure | Specific Behavior | Behavior Test Used | Behavioral Outcomes | Source |
|---|---|---|---|---|---|
| Zebrafish (D. rerio) | Glyphosate and AMPA (0.1, 1, or 10 µM) or mix of both (1 µM) for 7 days | Locomotor activity and anxiety | Distance moved during alternating light and dark periods, Dark/light preference | Hyperactivity of zebrafish exposed to glyphosate but not AMPA or the mixture No effect on anxiety-like responses | [118] |
| Mice | Roundup® Transorb 25, 50, or 100 mg/kg; | Generalized behavior and anxiety | Open field, elevated plus maze, tail suspension | Decreased locomotion in female mice No effect on anxiety but reduced exploration in male mice Increased immobility time both males and females | [201] |
| Zebrafish(D. rerio) | Roundup® UltraMax 0, 1, 2 and 5 µg a.i./mL; 72 h | Escape-like response, anxiety/stress, social behavior | Visual stimulus response, nearest neighbor distance and inter-individual | Exposed larvae did not exhibit evasion behavior | [161] |
| Rat | ZappQI620 Syngenta; 50 mg/kg/day | Social behavior, ASD | Open field, social play behavior test, homing behavior test, hole board test | Reduced number of ultrasonic vocalizations in pups Decreased social interaction time in pups and deficit in olfactory discrimination Increased stereotyped behavior | [183] |
| Rat | Roundup® Transorb; 50 and 150 mg/kg | Play fighting behavior | Intruder play fighting test | Pinning behavior impaired in both male and females offspring | [171] |
| Zebrafish (D. rerio) | Glyphosate 1 ppm and 5 ppm for 96 h at 28.5, 29, 29.5, or 30 °C | Circadian rhythm and anxiety | Locomotor test, novel diving tank test | Disruptions in circadian rhythm Fish spent more time at the bottom of the tank with more erratic movements | [206] |
| Mice | GBH, unspecified formulation (0.039% w/v) | Social behavior, ASD | Three-chamber test, novel object recognition | Deficits in social interaction in offspring ASD-like cognitive impairment | [208] |
| Mice | Roundup®; lifelong exposure to low doses (0.075% w/v) | Social behavior | Open field, social approach test | Reduced time spent exploring the stranger mouse and increased repetitive behavior No effect on anxiety or locomotion | [193] |
| Mice | Glifloglex®; 2 mg/nostrils/day | Anxiety | Plus maze | Increased thigmotaxis and higher anxiety | [107] |
| Mice | Roundup® Original | depression | Forced swim test | Increased immobility time and decreased climbing activity | [207] |
| Livebearer (J. multidentata) | Roundup® Original, Roundup® Transorb, and Roundup® WG | Aggression, anxiety, social behavior | Open field, proximity to own reflection in a mirror | More time spent in proximity to the mirror More time spent in central area of open field | [126] |
| Mice | Glyphosate 0.3 mg/kg daily per oral | Anxiety, locomotor activity | Open field, social interaction, and marble-burying | More frequent crossing of lines in open field Increased marble burying behavior No effect on social behavior | [194] |
| Mice | Zamba® 50 mg/kg | Anxiety, depression | Elevated plus maze Forced swim test | Fewer open arm entries and less time spent in open arm Increased immobility time | [204] |
| Mice | Roundup® 500 mg/kg/day | Anxiety, depression | Open field, elevated plus maze, tail suspension, splash test | Increased anxiogenic and depressive behavior after subchronic and chronic exposure Dose-dependent increase in immobility time and decrease in grooming time | [105] |
| Mice | Roundup® 250 or 500 mg/kg/day | Anxiety, depression | Open field, elevated plus maze, tail suspension, splash test | Decreased time spent in center of open field after subchronic and chronic exposure Increased immobility time after chronic exposure Decreased grooming time after both subchronic and chronic exposure | [202] |
| Mice | Roundup® 250 or 500 mg/kg/day | Anxiety, social behavior | Three-chamber sociability test | More time spent with inanimate object Fewer visits made to conspecific | [106] |
| Mice | Roundup® 250 or 500 mg/kg/day | Anxiety | Open field, elevated plus maze | Increased anxiogenic and depressive behavior after subchronic and chronic exposure | [203] |
| Zebrafish (D. rerio) | Roundup® or glyphosate (0.01, 0.065, and 0.5 mg/L | Aggression | Proximity to own reflection in a mirror | Reduced time spent in proximity to the mirror Decreased number of entries into the mirror contact zone | [115] |
| Human | Acute glyphosate exposure | Parkinsonism | Parkinsonism syndrome that resolved after treatment | [214] | |
| Human | Ambient pesticides including glyphosate | ASD | Increased risk for ASD after prenatal exposure Increased risk for ASD with comorbid intellectual disability after exposure during infancy | [79] |
4. Discussion
Glyphosate-based herbicides impact a wide variety of behaviors among animals, including activity, foraging and feeding, anti-predator behavior, courtship, mating, maternal behavior, learning, memory, anxiety-like behavior, depression-like behavior, aggression, and other social behaviors. When given the opportunity, many types of animals are able to detect GBH contamination. They avoid areas contaminated with GBH when possible (e.g., fish [121,129]). In addition, animals sometimes eat less when the food itself is contaminated with GBH. Food containing GBH led to some species decreasing food consumption [134,135,136], while in others, it had no effect [144]. For example, bees showed different results in different studies, even though they had similar modes of exposure. Bees increased consumption of GBH-contaminated food [140], decreased food consumption [138,139], or their food consumption was not affected by glyphosate contamination [104,141,142,143]. It appears that the variation could be caused by season, which may affect honeybees’ willingness to feed [140,216]. It could also be due to the differences in the herbicide formulation used, and dosage, as each formulation contains different in ingredients in addition to glyphosate. The decrease in food consumption sometimes seen may be due to GBHs affecting the taste or smell of the food, thereby either disrupting food recognition or overcoming the recognition of food with a stronger aversive cue. Taken together, these provide evidence of the ability of at least some animals in some situations to detect and avoid GBH contamination. However, there is some concern that co-exposure to different pollutants may confuse animals’ abilities to perceive, identify, and/or avoid individual toxins, and specifically, that glyphosate can mask identification of other agrochemicals [217].
GBH exposure affects many different animal species by changing their level of activity. Many studies indicate that glyphosate reduces activity, including overall activity levels, speed, distance traveled, coordination, and navigation efficiency. GBH-induced activity reduction affects terrestrial animals across taxa (see Table 5), regardless of the mode of activity (walking, crawling, flying), age or stage of animals (e.g., [115,116]), or method of GBH administration [112]. However, in aquatic animals like fish, GBH exposure can increase or decrease activity depending on concentration, duration of exposure, and distribution of the contaminant within the body of water. Increases in activity might allow animals to swim away from contaminated water, while decreases in activity might reflect a more general or long-term response to GBH exposure. Decreased activity would likely affect many other aspects of animals’ lives, including foraging and feeding, anti-predatory behavior, courtship, mating, and other social behaviors, ultimately impacting both the survival and reproductive aspects of fitness for exposed animals. When GBH contamination is widespread at high enough concentrations, species and ecosystems become threatened.
Pre-exposure to GBH for different durations affects foraging and feeding behavior. In most studies, animals decreased the amount of uncontaminated food consumed or increased the time to forage or feed on uncontaminated food after pre-exposure [145,146,153,154]. However, increases in food consumption after GBH exposure were also documented [147,148,149], while other studies showed that pre-exposure had no effect on feeding behavior [152,155,156]. Some predators (primarily wolf spiders) became hyperactive after exposure to GBH but less coordinated, improving their catches per minute but increasing the number of lunges required for each catch [149,150]. Impacts of GBH on foraging and feeding behavior will result in negative consequences for exposed organisms if they expend too much energy foraging or acquire too little food, particularly if the result is that they are unable to maintain sufficient energy and nutrients to maintain normal functions, including reproduction.
Exposure to GBH affected many prey species’ anti-predator capabilities. Zebrafish pre-exposed to GBH return to and spend more time in areas which had a predatory stimulus, indicating loss of predator awareness and putting them more at risk of predation. Zebrafish naturally spend time in peripheral areas, suggesting that they are risk-aversive, which can make them less susceptible to predation, but GBH exposure impaired their ability to detect danger and apparently decreased their baseline level of fear [157,158,159,160,161]. Other species displayed an increase in activity after pre-exposure to GBH and brief exposure to predator cues [162,165], making them more prone to predation. Meanwhile, in other species a decrease in activity was recorded in the presence of predators and predator cues after exposure to GBH [166,167]. GBH exposure impaired some species physically, decreasing escape speed after exposure [127,147]. Furthermore, in a few studies, no effect from pre-exposure to GBH on anti-predator behavior were recorded [130,167]. Deactivation or masking of predator cues by GBHs was documented [162,163], similar to GBHs masking the presence of other agrotoxins, as mentioned above [217]. Since some organisms rely on olfactory detection of predator cues in their environment; cues deactivated or masked by GBHs may increase vulnerability. In essence, the evidence suggests that exposure to GBH disrupts some animals’ anti-predator capabilities, making them vulnerable to predation with potential repercussions for food webs and ecosystems.
Glyphosate and GBHs can act as endocrine disruptors and negatively impact the reproductive systems of animals. Exposure to glyphosate has been linked to reduced courtship, mating, and fertility rates, as well as impaired maternal behavior in animals. Studies have found that exposure to glyphosate can lead to difficulty conceiving in mice and decreased fecundity and fertility rates in different species of fish, invertebrates, and rodents [38,61,146,168,170,178,179,180,181,182,184,186,195]. GBH exposure has also been linked to diminished pinning behavior in rodents, decreased female sexual behavior, and disrupted aromatase activity, leading to the impairment of sexual behavior in female offspring [171]. Exposure to glyphosate has been found to decrease masculinization of male rodents exposed before puberty and impaired sexual chemical communication between female and male wolf spiders, reducing the male spider’s ability to find their mate [169,172,173,174,175]. Chronic sublethal exposure to glyphosate and another pesticide, thiacloprid, negatively affected colonies of ants, decreasing the number of eggs and pupae and resulting in smaller colonies [177]. While some studies have found no adverse effects in terms of the impact of GBH on courtship, fertility rates, and maternal behavior [129,152,176,183,185,187], it is important to note that the effects of glyphosate and GBHs on reproductive behavior can vary depending on the species, the level of exposure, and the stage of reproduction. Overall, these studies suggest that exposure to glyphosate can lead to disruptions in maternal behavior, courtship, and fertility which can have negative impacts on the development and survival of offspring.
Researchers working with bees, rats and mice, and other animals have extensively explored the effects of GBH on a variety of types of learning and memory. While there are some mixed results, taken together, these studies suggest that GBH exposure reduces spatial and some types of sensory learning. Memory of all types discussed are impaired by GBH exposure, whether exposure occurs before learning or between learning and recall ([189]; human studies). Spatial learning [60,99,192] and memory [60,154,192,195] are both impaired by GBH exposure, potentially interfering with the ability of animals to navigate effectively in their environment. For example, exposure impairs cognitive abilities needed to integrate spatial information to successfully return to the hive [99]. GBH exposure interferes with some types of sensory learning [98,104,138,188,189,190] and all types of sensory memory studied [98,104,189,190], which could impact foraging, recognition of food, recognition of mates, recognition of offspring, and other social interactions. Other types of memory harmed by GBH exposure include recognition memory and aversive stimulus memory [106,107,115,126,192,195,196]. In addition, case studies and population assessments indicate that GBH exposure, both incidental and through ingestion, impairs various types of memory in humans, especially visual memory [191], word recall [95,197], and short-term memory [95,198,199], while in some cases, it induces both anterograde and retrograde amnesia [95,198]. In terms of both learning and memory, more difficult tasks seem to be more affected by exposure than simple ones [60,189].
The effects of glyphosate and GBH on anxiety-like behavior and social behavior, in addition to the development of neurological disorders, have been demonstrated throughout the literature. Most commonly, GBH exposure causes increased anxiety [105,106,107,202,203,204] and changes in motor activity, whether animals exhibit decreased locomotion [107,201] or hyperactivity [118,194,206]. GBHs also cause depressive-like behavior [105,201,202,204,207], changes in aggressiveness [115,126], and ASD-like cognitive impairment [183,193,208]. Social behavior is also affected by GBH exposure, as demonstrated by deficits in early communication and olfactory discrimination [183], impairment of functions needed to carry out social activities [37,99,100,104], and decreased social interaction with other animals of the same species [106,126,183,193,208]. Interestingly, impaired evasion behavior was seen in multiple aquatic systems, demonstrated by an increased time spent in the central areas of the tank [126,161]. Of the studies assessed here, however, rodents generally exhibit the opposite behavior, spending more time in the periphery of an open field arena. This suggests that responses to GBH may be species and/or habitat-specific. In addition, epidemiological studies report adolescent-related psychiatric illness and sensorimotor deficits resulting from GBH exposure [105]. Other studies in humans have demonstrated a relationship between glyphosate and neurological disorders such as ASD [79,200,210,211] and Parkinsonism [75,199,213,214].
While there is variation among the reported results, some of these could be attributed to differences in methodology, especially age of exposure, duration of exposure, dose or concentration, and GBH formulations. For example, prolonged exposures cause more toxic effects and more severe effects than acute exposure [105,201,202]. The dose of GBH fed to honeybees may have led to variation in amount of feeding behavior [104,138,139,140,141,142,143]. Similarly, Gallegos [185] and de Oliveira [183] observed no adverse effects of GBH exposure on maternal behavior, which may be attributed to lower doses and different formulations of GBH than in other similar studies. Griesinger [174] and Ward [176] used different concentrations and different formulations of GBH, which may be the cause of their conflicting results about courtship behavior. No effect of glyphosate on associative learning was found when a very low dose was administered [190], while other studies used higher concentrations and identified learning deficits [98,104]. A study that did not observe effects on either anxiety or novel object recognition used lower doses of glyphosate than in other studies [193].
While it is important for researchers to use ecologically relevant doses, any concentration up to the recommended dose for application is ecologically relevant in some situations, such as brief exposures when an animal is in an agricultural field or other area while it is being sprayed, or eating nectar, pollen, or other parts from a plant that was recently sprayed. A great deal of variability in experimental approaches, including concentration, duration, method and medium of exposure, etc., make generalizations difficult. However, the methods used in most lab and field studies seem to use exposures that are between concentrations as applied and those measured in different environments at times mostly unspecified with regard to local or nearby spraying (compare Table 1 to Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13).
Commercial formulations of GBH include a variety of adjuvants and other ingredients that are often listed as proprietary information and therefore are not disclosed on packaging. These additives could have synergistic effects, making it difficult to attribute toxicity to any one specific ingredient, and this could contribute to the differences between formulations. Bridi [115] found that Roundup® exposure affected memory more than exposure to glyphosate alone, and Sanchez et al. [126] compared two different Roundup® formulations with somewhat different results. Another study found that Roundup® had more of a negative impact on anti-predator capabilities and an increase in food consumption compared to glyphosate [147]. Zebrafish embryos exposed to Roundup® and glyphosate had contrasting effects—Roundup® induced more swimming and glyphosate had the opposite effect [119].
4.1. Mechanisms of GBH-Induced Behavioral Impairments
Glyphosate can act via a variety of mechanisms, both in general and as it relates to behavioral toxicity. Two of these mechanisms, oxidative stress and disruption of the gut microbiome, are general, disrupt the function of a variety of organs throughout the body, and have widespread effects on many aspects of physiology and behavior. Other mechanisms that specifically cause behavioral responses to GBH exposure involve endocrine and neurological control of these behaviors.
Exposure to glyphosate increases reactive oxygen species and causes inflammation, both of which affect learning and memory. Increases in anti-oxidant enzymes SOD and PO correlate with learning and memory impairments in mice [195], and is associated with synaptic plasticity for short-term and long-term memory storage [106]. Recognition memory impairment is related to other markers of antioxidant stress, including MDA content, CAT activity, and GPx activity in rats [196]. GBH-induced memory loss in humans has also been attributed to oxidative stress [198]. Oxidative stress can also lead to inflammation, which can cause morphological changes. Several proteins associated with inflammation increase in concentration in the hippocampus and prefrontal cortex after exposure to GBHs, specifically GFAP and Iba-1, and of TNF-alpha in the hippocampus only [106]. Oxidative stress has also been proposed as a mechanism of glyphosate-induced toxicity with regard to avoidance behavior, [114], reproductive behaviors [114,183], and anxiety and depression [204,207].
Several studies have reported that glyphosate disrupts the normal gut microbiota, promoting overgrowth of pathogenic bacteria [55,105,202,218,219], which could have dangerous impacts on the organisms’ health in general. Because mammal gut microbiota utilize the shikimate pathway to synthesize precursors to neurotransmitters like serotonin and dopamine [219,220,221], disruption of this pathway by glyphosate could also have important neurologic health implications, including causing anxiety and depression-like behavior in mice [202].
Glyphosate as an endocrine modulator has been suggested as a potential mechanism of toxicity, particularly regarding reproductive outcomes, including courtship, mating behavior and maternal behaviors. Glyphosate inhibition of aromatase, the enzyme responsible for the irreversible conversion of androgens to estrogen, causes an imbalance between androgens and estrogens. Particularly during the neonatal period in rats, this disruption in steroid sex hormones could interfere with brain development of the sexual organization and thus impair sexual behavior in adulthood [106,171]. Male rats who were exposed to glyphosate showed higher levels of testosterone and estradiol compared to the control group. The higher levels of testosterone levels could explain why the male’s sexual partner preference increased dramatically towards the females [172]. Exposure to the herbicide GLY-BH decreased specific binding to D1-DA receptors in the nucleus accumbens, basal extracellular dopamine levels, and high-potassium-induced dopamine release in the striatum. This resulted in hypoactivity and impaired maternal behavior, possibly due to blockage of the Ait-Balistriatal and mesolimbic systems. The decrease in maternal grooming behavior may be a result of perinatal exposure to the herbicide, as it has been shown that decreased grooming behavior is associated with decreased D1 receptor activation [184].
Neurological mechanisms of glyphosate toxicity that contribute to impairments in learning, memory, anxiety, and other social behaviors include structural localization, neurotransmitter activity, and oxidative stress and inflammation specifically within the brain. The two most important structures related to learning and memory and implicated in GBH toxicity are the hippocampus and the prefrontal cortex. GBH toxicity to the prefrontal cortex may be morphogenic, related to the expression of brain-derived neurotrophic factor and tyrosine-related kinase receptor [106,195]. In rats, hippocampus involvement is related to decreased dendritic complexity, synaptic spine formation and maturation, and therefore decreased formation of synaptic terminals [192]. Hippocampal lesions were also associated with learning and memory loss in mice [106]. In humans, hippocampal lesions after GBH poisoning [95,197,198] resolved after several months [95,197].
One cause of GBH induced neurotoxicity to the hippocampus, PFC, and striatum is that glyphosate mimics glycine, a required cofactor for glutamate activation of NMDA. According to this mechanism, glyphosate hyper-excites NMDA glutamate receptors, causing cell death [196,197,198,199]. In bees, glyphosate binds to NMDA receptors more efficiently than endogenous glycine, making GBHs more toxic to bees than to humans [60]. Another neurotransmitter involved in glyphosate toxicity is acetylcholine. Specifically, GBHs interfere with acetylcholine esterase activity, reducing its concentration in the hippocampus, prefrontal cortex and striatum [106,195,196].
Potential mechanisms of glyphosate neurotoxicity related to depression and anxiety have also been extensively discussed. Sulukan et al. [206] suggested a possible mechanism involving an important receptor for serotonin, 5-HT4R, which modulates depression and anxiety responses. Some studies have discussed changes to the dopaminergic, seritonergic, and glutaminergic systems as possible explanations for anxiety and depressive-like behavior [105,161,183,185]. Influences on brain development, sexual and play behavior in rodents could be associated with glyphosate as an endocrine modulator, since glyphosate inhibits aromatase, the enzyme responsible for the irreversible conversion of androgens to estrogen [171]. Other mechanisms have been explored, such as inflammatory cellular disorganization [106], disruption of manganese homeostasis [219], changes in gene expression [118,203], and oxidative stress [204]. In conclusion, more research is warranted to elucidate the mechanism by which glyphosate causes neurotoxicity in non-target animals.
4.2. Further Research
Many behavioral areas have been researched in only a few taxa (Figure 1), often common model systems. None of the articles we were able to identify studied behavioral effects in mammals other than mice, rats, humans, and birds, and only a few used amphibian or reptile systems. While human behavior was examined in terms of effects of GBH on memory, there were no studies of how other human behaviors are affected by GBHs. Even among invertebrate models, honeybees were disproportionately the focus of the studies reviewed here. While honeybees serve a critically important role in pollination, so that their behavior might be disproportionately important, and their exposure disproportionately likely, for example in agriculture, other insects and non-insect invertebrates should also be studied in terms of the effects of GBH on behavior. Overall, future studies are needed to address the effect of glyphosate and GBH on behavior in a wider range of taxa, including mammals other than mice and rats.
Additionally, studies have been conducted on animals in a laboratory setting. More research is needed to understand the full extent of glyphosate’s impact on activity, foraging, anti-predatory behavior, and reproductive behaviors in organisms that are exposed to GBH in natural settings. Field-based studies should focus on exposure at environmentally relevant doses, ranging from those applied in agricultural settings to smaller concentrations that would persist in areas affected by agricultural overspray, drift, or runoff. These field-based studies are critical to better understand the impact of GBHs on the behavior of animals in their environments, and by extension the overall impacts of GBH exposure on ecosystems and ecosystem services.
More research is also needed on mechanisms of behavioral changes, tying together physiological results with consequential behaviors. While research on physiological toxicity is extensive, the relationship between these physiological effects and behaviors are only partially understood. Given that experimental research on humans is nearly impossible, fully delineating the mechanisms of behavioral GBH toxicity in animals can help us understand which specific behavioral results from animal experiments are likely to also apply to human behaviors, and ultimately the potential impacts on human populations and human wellbeing.
5. Conclusions
Glyphosate, the active ingredient in many herbicides, has been the subject of much debate due to its potential toxic effects on non-target organisms. In this review, we examined the effects of glyphosate and GBHs on animal behavior. While there are some discrepancies between the methodologies and results, studies demonstrate that glyphosate impairs activity, foraging and feeding, anti-predator behavior, reproductive and maternal behaviors, learning and memory, and social behaviors in animals. Although glyphosate toxicity in non-target organisms has been studied, its mechanisms and effects on human health are still unclear, justifying further research and investigation. Due to the possible dangers and unknown impact of glyphosate in humans and on the environment, we advise caution when using glyphosate and GBHs and suggest implementation of alternative agricultural and municipal practices.
Overall, animal behavior endpoints should be considered in regulatory decisions about chemical environmental contaminants. Appropriate performance of many types of behavior impact reproduction and survival, food webs, and therefore, ecosystem health [95]. Behavior, as a sub-lethal endpoint, provides a particularly sensitive test for biotic disruptions that result from environmental contamination with chemical pollutants, and importantly, provides more accurate assessment of the effect of contaminants on real-world ecosystem health than mortality-based endpoints alone [222]. For example, the sensitivity of behavioral endpoints are 10 to 1000 times greater than those for mortality at accurately providing an early warning about chemical contaminants. [223]. Other benefits of examining animal behavior in ecotoxicological assessment include that it is often inexpensive, non-invasive, and increasingly can be automated [96,223]. In fact, a variety of new tools and technologies, reviewed in [222], allow for behavioral investigation of the effects of contaminants with higher resolution and at larger scales, further increasing the usefulness of behavioral outcomes for making regulatory decisions. Including animal behavior in regulatory decisions would therefore allow a more complete and accurate understanding of the impacts of new and emerging contaminants, low concentrations of contaminants, and allow identification of contaminants of concern much more quickly than waiting for more widespread indications of ecosystem collapse, perhaps after it is too late.
Author Contributions
Conceptualization, B.T.; methodology, B.T.; validation, B.T.; investigation, B.T, C.M., B.G. and K.B.; data curation, B.T., K.M., C.M., B.G. and K.B.; writing—original draft preparation, B.T., K.M., C.M., B.G. and K.B.; writing—review and editing, B.T., K.M., C.M., B.G. and K.B.; visualization, B.T., K.M., C.M., B.G. and K.B.; supervision, B.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were not applicable because no original research was conducted, nor original data collected, in preparation of this manuscript.
Informed Consent Statement
While some articles cited involved research on humans, we did not engage in any original human-subject research. Therefore, informed consent is neither required nor appropriate.
Data Availability Statement
Since this is a review article, it is not based on our original data. Therefore, we do not make data available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef]
- Data from Two Sets of Testing Conducted by Arnesco Labs and Discussed in Murphy, D & Rowlands, H. 16 November 2016. Glyphosate: Unsafe on Any Plate. Available online: https://detoxproject.org/wp-content/uploads/2022/08/Final-Report.pdf (accessed on 12 May 2023).
- Based on Data Sets from The Detox Project. Available online: https://detoxproject.org/wp-content/uploads/2016/10/anresco_reports_food_testing_2016.pdf (accessed on 12 May 2023).
- Bøhn, T.; Millstone, E. The introduction of thousands of tonnes of glyphosate in the food chain—An evaluation of glyphosate tolerant soybeans. Foods 2019, 8, 669. [Google Scholar] [CrossRef]
- Torretta, V.; Katsoyiannis, I.A.; Viotti, P.; Rada, E.C. Critical review of the effects of glyphosate exposure to the environment and humans through the food supply chain. Sustainability 2018, 10, 950. [Google Scholar] [CrossRef]
- Lozano-Kasten, F.; Sierra-Diaz, E.; Chavez, H.G.; Lucano, A.A.P.; Cremades, R.; Pinto, E.S. Seasonal urinary levels of glyphosate in children from agricultural communities. Dose-Response 2021, 19, 15593258211053184. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Martínez-Larrañaga, M.R.; Martínez, M.A.; Castellano, V.J.; Martínez, M.; Martin, M.T.; Nozal, M.J.; Bernal, J.L. Toxicokinetics of glyphosate and its metabolite aminomethyl phosphonic acid in rats. Toxicol. Lett. 2009, 190, 91–95. [Google Scholar] [CrossRef]
- Contardo-Jara, V.; Klingelmann, E.; Wiegand, C. Bioaccumulation of glyphosate and its formulation Roundup Ultra in Lumbriculus variegatus and its effects on biotransformation and antioxidant enzymes. Environ. Pollut. 2009, 157, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Schrödl, W.; Pedersen, I.B.; Shehata, A.A. Detection of glyphosate in malformed piglets. J. Environ. Anal. Toxicol. 2014, 4, 230. [Google Scholar] [CrossRef]
- Kongtip, P.; Nankongnab, N.; Phupancharoensuk, R.; Palarach, C.; Sujirarat, D.; Sangprasert, S.; Sermsuk, M.; Sawattrakool, N.; Woskie, S.R. Glyphosate and paraquat in maternal and fetal serums in Thai women. J. Agromedicine 2017, 22, 282–289. [Google Scholar] [CrossRef]
- Gillezeau, C.; van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Environ. Health 2019, 18, 2. [Google Scholar] [CrossRef]
- Niemann, L.; Sieke, C.; Pfeil, R.; Solecki, R. A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. J. Verbrauch. Lebensm. 2015, 10, 3–12. [Google Scholar] [CrossRef]
- Fagan, J.; Bohlen, L.; Patton, S.; Klein, K. Organic diet intervention significantly reduces urinary glyphosate levels in US children and adults. Environ. Res. 2020, 189, 109898. [Google Scholar] [CrossRef] [PubMed]
- Vicini, J.L.; Jensen, P.K.; Young, B.M.; Swarthout, J.T. Residues of glyphosate in food and dietary exposure. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5226–5257. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015, 13, 107. [Google Scholar] [CrossRef]
- CCME (Canadian Council of Ministers of the Environment). Available online: https://ccme.ca/en/res/glyphosate-en-canadian-water-quality-guidelines-for-the-protection-of-aquatic-life.pdf (accessed on 12 May 2023).
- Silva, V.; Mol, H.G.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Coupe, R.H.; Kalkhoff, S.J.; Capel, P.D.; Gregoire, C. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag. Sci. 2012, 68, 16–30. [Google Scholar] [CrossRef]
- Hébert, M.P.; Fugère, V.; Gonzalez, A. The overlooked impact of rising glyphosate use on phosphorus loading in agricultural watersheds. Front. Ecol. Environ. 2019, 17, 48–56. [Google Scholar] [CrossRef]
- Thompson, D.G.; Wojtaszek, B.F.; Staznik, B.; Chartrand, D.T.; Stephenson, G.R. Chemical and biomonitoring to assess potential acute effects of Vision herbicide on native amphibian larvae in forest wetlands. Environ. Toxicol. Chem. 2004, 23, 843–849. [Google Scholar] [CrossRef]
- Rendón-von Osten, J.; Dzul-Caamal, R. Glyphosate residues in groundwater, drinking water and urine of subsistence farmers from intensive agriculture localities: A survey in Hopelchén, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef]
- Zhao, J.; Pacenka, S.; Wu, J.; Richards, B.K.; Steenhuis, T.; Simpson, K.; Hay, A.G. Detection of glyphosate residues in companion animal feeds. Environ. Pollut. 2018, 243, 1113–1118. [Google Scholar] [CrossRef]
- El Agrebi, N.; Tosi, S.; Wilmart, O.; Scippo, M.L.; de Graaf, D.C.; Saegerman, C. Honeybee and consumer’s exposure and risk characterisation to glyphosate-based herbicide (GBH) and its degradation product (AMPA): Residues in beebread, wax, and honey. Sci. Total Environ. 2020, 704, 135312. [Google Scholar] [CrossRef]
- Poulsen, M.E.; Andersen, J.H.; Petersen, A.; Jensen, B.H. Results from the Danish monitoring programme for pesticide residues from the period 2004–2011. Food Control 2017, 74, 25–33. [Google Scholar] [CrossRef]
- Richard, S.; Moslemi, S.; Sipahutar, H.; Benachour, N.; Seralini, G.E. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ. Health Perspect. 2005, 113, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Bernay, B.; Séralini, G.E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 313, 122–128. [Google Scholar] [CrossRef]
- Perego, M.C.; Caloni, F.; Cortinovis, C.; Schutz, L.F.; Albonico, M.; Tsuzukibashi, D.; Spicer, L.J. Influence of a Roundup formulation on glyphosate effects on steroidogenesis and proliferation of bovine granulosa cells in vitro. Chemosphere 2017, 188, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Silva, T.L.; Andreani, T.; Silva, A.M. Glyphosate vs. glyphosate-based herbicides exposure: A review on their toxicity. J. Xenobiot. 2022, 12, 21–40. [Google Scholar] [CrossRef]
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate toxicity for animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef]
- Richmond, M.E. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J. Environ. Stud. Sci. 2018, 8, 416–434. [Google Scholar] [CrossRef]
- Agostini, L.P.; Dettogni, R.S.; Dos Reis, R.S.; Stur, E.; Dos Santos, E.V.; Ventorim, D.P.; Garcia, F.M.; Cardoso, R.C.; Graceli, J.B.; Louro, I.D. Effects of glyphosate exposure on human health: Insights from epidemiological and in vitro studies. Sci. Total Environ. 2020, 705, 135808. [Google Scholar] [CrossRef]
- de Brito Rodrigues, L.; de Oliveira, R.; Abe, F.R.; Brito, L.B.; Moura, D.S.; Valadares, M.C.; Grisolia, C.K.; de Oliveira, D.P.; de Oliveira, G.A.R. Ecotoxicological assessment of glyphosate-based herbicides: Effects on different organisms. Environ. Toxicol. Chem. 2017, 36, 1755–1763. [Google Scholar] [CrossRef]
- Lares, B.A.; Vignatti, A.M.; Echaniz, S.A.; Gutiérrez, M.F. Effects of glyphosate on cladocera: A synthetic review. Aquat. Toxicol. 2022, 249, 106232. [Google Scholar] [CrossRef] [PubMed]
- Battisti, L.; Potrich, M.; Sampaio, A.R.; de Castilhos Ghisi, N.; Costa-Maia, F.M.; Abati, R.; dos Reis Martinez, C.B.; Sofia, S.H. Is glyphosate toxic to bees? A meta-analytical review. Sci. Total Environ. 2021, 767, 145397. [Google Scholar] [CrossRef]
- Farina, W.M.; Balbuena, M.S.; Herbert, L.T.; Goñalons, C.M.; Vázquez, D.E. Effects of the herbicide glyphosate on honey bee sensory and cognitive abilities: Individual impairments with implications for the hive. Insects 2019, 10, 354. [Google Scholar] [CrossRef]
- Tan, S.; Li, G.; Liu, Z.; Wang, H.; Guo, X.; Xu, B. Effects of glyphosate exposure on honeybees. Environ. Toxicol. Pharmacol. 2022, 90, 103792. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.R.; Moraes, J.S.; Martins, C.M.G. Effects of the herbicide glyphosate on fish from embryos to adults: A review addressing behavior patterns and mechanisms behind them. Aquat. Toxicol. 2022, 251, 106281. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Reichenbecher, W.; Teichmann, H.; Tappeser, B.; Lötters, S. Questions concerning the potential impact of glyphosate-based herbicides on amphibians. Environ. Toxicol. Chem. 2013, 32, 1688–1700. [Google Scholar] [CrossRef]
- López, S.L.; Aiassa, D.; Benítez-Leite, S.; Lajmanovich, R.; Manas, F.; Poletta, G.; Sánchez, N.; Simoniello, M.F.; Carrasco, A.E. Pesticides used in South American GMO-based agriculture: A review of their effects on humans and animal models. Adv. Mol. Toxicol. 2012, 6, 41–75. [Google Scholar] [CrossRef]
- Disner, G.R.; Falcão, M.A.P.; Andrade-Barros, A.I.; Leite dos Santos, N.V.; Soares, A.B.S.; Marcolino-Souza, M.; Gomes, K.S.; Lima, C.; Lopes-Ferreira, M. The toxic effects of glyphosate, chlorpyrifos, abamectin, and 2,4-D on animal models: A systematic review of Brazilian studies. Integr. Environ. Assess. Manag. 2021, 17, 507–520. [Google Scholar] [CrossRef]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef]
- Tsui, M.T.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- Milesi, M.M.; Lorenz, V.; Durando, M.; Rossetti, M.F.; Varayoud, J. Glyphosate herbicide: Reproductive outcomes and multigenerational effects. Front. Endocrinol. 2021, 12, 672532. [Google Scholar] [CrossRef]
- Mink, P.J.; Mandel, J.S.; Sceurman, B.K.; Lundin, J.I. Epidemiologic studies of glyphosate and cancer: A review. Regul. Toxicol. Pharmacol. 2012, 63, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Davoren, M.J.; Schiestl, R.H. Glyphosate-based herbicides and cancer risk: A post-IARC decision review of potential mechanisms, policy and avenues of research. Carcinogenesis 2018, 39, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rana, I.; Shaffer, R.M.; Taioli, E.; Sheppard, L. Exposure to glyphosate-based herbicides and risk for non-Hodgkin lymphoma: A meta-analysis and supporting evidence. Mutat. Res. Rev. Mutat. Res. 2019, 781, 186–206. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; Watson, R.E.; DeSesso, J.M. Developmental and reproductive outcomes in humans and animals after glyphosate exposure: A critical analysis. J. Toxicol. Environ. Health Part B Crit. Rev. 2012, 15, 39–96. [Google Scholar] [CrossRef]
- Belle, R.; Marc, J.; Morales, J.; Cormier, P.; Mulner-Lorillon, O. Letter to the editor: Toxicity of Roundup and glyphosate. J. Toxicol. Environ. Health Part B Crit. Rev. 2012, 15, 233–237. [Google Scholar] [CrossRef]
- de Araújo-Ramos, A.T.; Passoni, M.T.; Romano, M.A.; Romano, R.M.; Martino-Andrade, A.J. Controversies on endocrine and reproductive effects of glyphosate and glyphosate-based herbicides: A mini-review. Front. Endocrinol. 2021, 12, 627210. [Google Scholar] [CrossRef]
- Madani, N.A.; Carpenter, D.O. Effects of glyphosate and glyphosate-based herbicides like Roundup™ on the mammalian nervous system: A review. Environ. Res. 2022, 214, 113933. [Google Scholar] [CrossRef]
- Roberts, J.R.; Dawley, E.H.; Reigart, J.R. Children’s low-level pesticide exposure and associations with autism and ADHD: A review. Pediatr. Res. 2019, 85, 234–241. [Google Scholar] [CrossRef]
- Biosca-Brull, J.; Pérez-Fernández, C.; Mora, S.; Carrillo, B.; Pinos, H.; Conejo, N.M.; Collado, P.; Arias, J.L.; Martín-Sánchez, F.; Sánchez-Santed, F.; et al. Relationship between autism spectrum disorder and pesticides: A systematic review of human and preclinical models. Int. J. Environ. Res. Public Health 2021, 18, 5190. [Google Scholar] [CrossRef]
- Miani, A.; Imbriani, G.; De Filippis, G.; De Giorgi, D.; Peccarisi, L.; Colangelo, M.; Pulimeno, M.; Castellone, M.D.; Nicolardi, G.; Logroscino, G.; et al. Autism spectrum disorder and prenatal or early life exposure to pesticides: A short review. Int. J. Environ. Res. Public Health 2021, 18, 10991. [Google Scholar] [CrossRef]
- He, X.; Tu, Y.; Song, Y.; Yang, G.; You, M. The relationship between pesticide exposure during critical neurodevelopment and autism spectrum disorder: A narrative review. Environ. Res. 2022, 203, 111902. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Khan, S.; Patrikar, M.; Markad, A.; Kumar, N.; Choudhari, A.; Sagar, P.; Indurkar, S. Exposure risk and environmental impacts of glyphosate: Highlights on the toxicity of herbicide co-formulants. Environ. Chall. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Turkmen, R.; Dogan, I. Determination of acute oral toxicity of glyphosate isopropylamine salt in rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 19298–19303. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Moore, L.J.; Rodgers, J.H., Jr.; Bowerman, W.W.; Yarrow, G.K.; Chao, W.Y. Comparative toxicity of two glyphosate formulations (original formulation of Roundup® and Roundup WeatherMAX®) to six North American larval anurans. Environ. Toxicol. Chem. 2011, 30, 2756–2761. [Google Scholar] [CrossRef]
- Folmar, L.C.; Sanders, H.O.; Julin, A.M. Toxicity of the herbicide glyphosate and several of its formulations to fish and aquatic invertebrates. Arch. Environ. Contam. Toxicol. 1979, 8, 269–278. [Google Scholar] [CrossRef]
- Zgurzynski, M.I.; Lushington, G.H. Glyphosate impact on Apis mellifera navigation: A combined behavioral and chemoinformatics study. EC Pharmacol. Toxicol. 2019, 7, 806–824. [Google Scholar]
- Talyn, B.; Lemon, R.; Badoella, M.; Melchiorre, D.; Villalobos, M.; Elias, R.; Muller, K.; Santos, M.; Melchiorre, E. Roundup®, but Not Roundup-Ready® Corn, Increases Mortality of Drosophila melanogaster. Toxics 2019, 7, 38. [Google Scholar] [CrossRef]
- Parlapiano, I.; Biandolino, F.; Grattagliano, A.; Ruscito, A.; Libralato, G.; Prato, E. Effects of commercial formulations of glyphosate on marine crustaceans and implications for risk assessment under temperature changes. Ecotoxicol. Environ. Saf. 2021, 213, 112068. [Google Scholar] [CrossRef]
- Perry, M.J.; Mandrioli, D.; Belpoggi, F.; Manservisi, F.; Panzacchi, S.; Irwin, C. Historical evidence of glyphosate exposure from a US agricultural cohort. Environ. Health 2019, 18, 42. [Google Scholar] [CrossRef]
- Jayasumana, C.; Gunatilake, S.; Siribaddana, S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 2015, 16, 103. [Google Scholar] [CrossRef]
- Santos, R.; Piccoli, C.; Cremonese, C.; Freire, C. Thyroid and reproductive hormones in relation to pesticide use in an agricultural population in Southern Brazil. Environ. Res. 2019, 173, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Abell, A.; Ernst, E.; Bonde, J.P. Semen quality and sexual hormones in greenhouse workers. Scand. J. Work Environ. Health 2000, 26, 492–500. [Google Scholar] [CrossRef] [PubMed]
- de Cock, J.; Westveer, K.; Heederik, D.; te Velde, E.; van Kooij, R. Time to pregnancy and occupational exposure to pesticides in fruit growers in The Netherlands. Occup. Environ. Med. 1994, 51, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Sanin, L.H.; Carrasquilla, G.; Solomon, K.R.; Cole, D.C.; Marshall, E.J.P. Regional differences in time to pregnancy among fertile women from five Colombian regions with different use of glyphosate. J. Toxicol. Environ. Health Part A 2009, 72, 949–960. [Google Scholar] [CrossRef]
- Parvez, S.; Gerona, R.R.; Proctor, C.; Friesen, M.; Ashby, J.L.; Reiter, J.L.; Lui, Z.; Winchester, P.D. Glyphosate exposure in pregnancy and shortened gestational length: A prospective Indiana birth cohort study. Environ. Health 2018, 17, 23. [Google Scholar] [CrossRef]
- Winchester, P.; Proctor, C.; Ying, J. County-level pesticide use and risk of shortened gestation and preterm birth. Acta paediatr. 2016, 105, e107–e115. [Google Scholar] [CrossRef]
- Garry, V.F.; Harkins, M.E.; Erickson, L.L.; Long-Simpson, L.K.; Holland, S.E.; Burroughs, B.L. Birth defects, season of conception, and sex of children born to pesticide applicators living in the Red River Valley of Minnesota, USA. Environ. Health Perspect. 2002, 110 (Suppl. S3), 441–449. [Google Scholar] [CrossRef]
- Martinez, A.; Al-Ahmad, A.J. Effects of glyphosate and aminomethylphosphonic acid on an isogeneic model of the human blood-brain barrier. Toxicol. Lett. 2019, 304, 39–49. [Google Scholar] [CrossRef]
- Pan, L.; Xu, M.; Yang, D.; Wang, B.; Zhao, Q.; Ding, E.M.; Zhu, B. The association between coronary artery disease and glyphosate exposure found in pesticide factory workers. J. Public Health Emerg. 2017, 1, 4. [Google Scholar] [CrossRef]
- Mills, P.J.; Caussy, C.; Loomba, R. Glyphosate excretion is associated with steatohepatitis and advanced liver fibrosis in patients with fatty liver disease. Clin. Gastroenterol. Hepatol. 2020, 18, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fan, X.N.; Tan, Y.Y.; Cheng, Q.; Chen, S.D. Parkinsonism after chronic occupational exposure to glyphosate. Park. Relat. Disord. 2011, 17, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.; Huss, A.; van der Mark, M.; Nijssen, P.C.G.; Mulleners, W.M.; Sas, A.M.G.; van Laar, T.; de Snoo, G.R.; Kromhout, H.; Vermeulen, R.C. Environmental exposure to pesticides and the risk of Parkinson’s disease in the Netherlands. Environ. Int. 2017, 107, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.; Amiri, S.; Denney, J.T.; Monsivais, P.; Hystad, P.; Amram, O. Estimated residential exposure to agricultural chemicals and premature mortality by Parkinson’s disease in Washington state. Int. J. Environ. Res. Public Health 2018, 15, 2885. [Google Scholar] [CrossRef]
- Shaw, W. Elevated urinary glyphosate and clostridia metabolites with altered dopamine metabolism in triplets with autistic spectrum disorder or suspected seizure disorder: A case study. Integr. Med. 2017, 16, 50–57. [Google Scholar]
- Von Ehrenstein, O.S.; Ling, C.; Cui, X.; Cockburn, M.; Park, A.S.; Yu, F.; Wu, J.; Ritz, B. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: Population based case-control study. BMJ 2019, 364, l962. [Google Scholar] [CrossRef]
- Anifandis, G.; Amiridis, G.; Dafopoulos, K.; Daponte, A.; Dovolou, E.; Gavriil, E.; Gorgogietas, V.; Kachpani, E.; Mamuris, Z.; Messini, C.I.; et al. The in vitro impact of the herbicide roundup on human sperm motility and sperm mitochondria. Toxics 2017, 6, 2. [Google Scholar] [CrossRef]
- Bolognesi, C.; Carrasquilla, G.; Volpi, S.; Solomon, K.R.; Marshall, E.J.P. Biomonitoring of genotoxic risk in agricultural workers from five Colombian regions: Association to occupational exposure to glyphosate. J. Toxicol. Environ. Health Part A 2009, 72, 986–997. [Google Scholar] [CrossRef]
- Woźniak, E.; Sicińska, P.; Michałowicz, J.; Woźniak, K.; Reszka, E.; Huras, B.; Zakrzewski, J.; Bukowska, B. The mechanism of DNA damage induced by Roundup 360 PLUS, glyphosate and AMPA in human peripheral blood mononuclear cells-genotoxic risk assessement. Food Chem. Toxicol. 2018, 120, 510–522. [Google Scholar] [CrossRef]
- Nagy, K.; Argaw Tessema, R.; Szász, I.; Smeirat, T.; Al Rajo, A.; Ádám, B. Micronucleus formation induced by glyphosate and glyphosate-based herbicides in human peripheral white blood cells. Front. Public Health 2021, 9, 639143. [Google Scholar] [CrossRef]
- Sritana, N.; Suriyo, T.; Kanitwithayanun, J.; Songvasin, B.H.; Thiantanawat, A.; Satayavivad, J. Glyphosate induces growth of estrogen receptor alpha positive cholangiocarcinoma cells via non-genomic estrogen receptor/ERK1/2 signaling pathway. Food Chem. Toxicol. 2018, 118, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Santovito, A.; Ruberto, S.; Gendusa, C.; Cervella, P. In vitro evaluation of genomic damage induced by glyphosate on human lymphocytes. Environ. Sci. Pollut. Res. Int. 2018, 25, 34693–34700. [Google Scholar] [CrossRef] [PubMed]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Stur, E.; Aristizabal-Pachon, A.F.; Peronni, K.C.; Agostini, L.P.; Waigel, S.; Chariker, J.; Miller, D.M.; Thomas, S.D.; Rezzoug, F.; Detogni, R.S.; et al. Glyphosate-based herbicides at low doses affect canonical pathways in estrogen positive and negative breast cancer cell lines. PLoS ONE 2019, 14, e0219610. [Google Scholar] [CrossRef]
- De Roos, A.; Zahm, S.H.; Cantor, K.P.; Weisenburger, D.D.; Holmes, F.F.; Burmeister, L.F.; Blair, A. Integrative assessment of multiple pesticides as risk factors for non-Hodgkin’s lymphoma among men. Occup. Environ. Med. 2003, 60, e11. [Google Scholar] [CrossRef]
- Eriksson, M.; Hardell, L.; Carlberg, M.; Akerman, M. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int. J. Cancer 2008, 123, 1657–1663. [Google Scholar] [CrossRef]
- Vazquez, M.A.; Maturano, E.; Etchegoyen, A.; Difilippo, F.S.; Maclean, B. Association between cancer and environmental exposure to glyphosate. Int. J. Clin. Med. 2017, 8, 73–85. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, J.H.; Hong, C.K.; Cho, K.W.; Park, Y.H.; Kim, Y.W.; Hwang, S.Y. Heart rate–corrected QT interval predicts mortality in glyphosate-surfactant herbicide–poisoned patients. Am. J. Emerg. Med. 2014, 32, 203–207. [Google Scholar] [CrossRef]
- Lee, H.L.; Chen, K.W.; Chi, C.H.; Huang, J.J.; Tsai, L.M. Clinical presentations and prognostic factors of a glyphosate—Surfactant herbicide intoxication a review of 131 cases. Acad. Emerg. Med. 2000, 7, 906–910. [Google Scholar] [CrossRef]
- Roberts, D.M.; Buckley, N.A.; Mohamed, F.; Eddleston, M.; Goldstein, D.A.; Mehrsheikh, A.; Bleeke, M.S.; Dawson, A.H. A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin. Toxicol. 2010, 48, 129–136. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, Y.J.; Park, S.; Gil, H.W.; Song, H.Y.; Hong, S.Y. Serum S100 protein could predict altered consciousness in glyphosate or glufosinate poisoning patients. Clin. Toxicol. 2017, 55, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Nishiyori, Y.; Nishida, M.; Shioda, K.; Suda, S.; Kato, S. Unilateral hippocampal infarction associated with an attempted suicide: A case report. J. Med. Case Rep. 2014, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Clotfelter, E.D.; Bell, A.M.; Levering, K.R. The role of animal behaviour in the study of endocrine-disrupting chemicals. Anim. Behav. 2004, 68, 665–676. [Google Scholar] [CrossRef]
- Zala, S.M.; Penn, D.J. Abnormal behaviours induced by chemical pollution: A review of the evidence and new challenges. Anim. Behav. 2004, 68, 649–664. [Google Scholar] [CrossRef]
- Luo, Q.H.; Gao, J.; Guo, Y.; Liu, C.; Ma, Y.Z.; Zhou, Z.Y.; Dai, P.L.; Hou, C.S.; Wu, Y.Y.; Diao, Q.Y. Effects of a commercially formulated glyphosate solutions at recommended concentrations on honeybee (Apis mellifera L.) behaviours. Sci. Rep. 2021, 11, 2115. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, M.S.; Tison, L.; Hahn, M.L.; Greggers, U.; Menzel, R.; Farina, W.M. Effects of sublethal doses of glyphosate on honeybee navigation. J. Exp. Biol. 2015, 218, 2799–2805. [Google Scholar] [CrossRef]
- Delkash-Roudsari, S.; Chicas-Mosier, A.M.; Goldansaz, S.H.; Talebi-Jahromi, K.; Ashouri, A.; Abramson, C.I. Assessment of lethal and sublethal effects of imidacloprid, ethion, and glyphosate on aversive conditioning, motility, and lifespan in honey bees (Apis mellifera L.). Ecotoxicol. Environ. Saf. 2020, 204, 111108. [Google Scholar] [CrossRef]
- Séralini, G.E.; Clair, E.; Mesnage, R.; Gress, S.; Defarge, N.; Malatesta, M.; Hennequin, D.; de Vendômois, J.S. Republished study: Long-term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Environ. Sci. Eur. 2014, 26, 14. [Google Scholar] [CrossRef]
- Vázquez, D.E.; Balbuena, M.S.; Chaves, F.; Gora, J.; Menzel, R.; Farina, W.M. Sleep in honey bees is affected by the herbicide glyphosate. Sci. Rep. 2020, 10, 10516. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F.; Abuzeid, M.A. Effects of two herbicides on healthy and Nosema infected honey bee workers. Arthropods 2018, 7, 31–41. [Google Scholar]
- Herbert, L.T.; Vázquez, D.E.; Arenas, A.; Farina, W.M. Effects of field-realistic doses of glyphosate on honeybee appetitive behaviour. J. Exp. Biol. 2014, 217, 3457–3464. [Google Scholar] [CrossRef]
- Ait Bali, Y.; Ba-Mhamed, S.; Bennis, M. Behavioral and Immunohistochemical Study of the Effects of Subchronic and Chronic Exposure to Glyphosate in Mice. Front. Behav. Neurosci. 2017, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Ait Bali, Y.; Ba-M’hamed, S.; Gambarotta, G.; Sassoè-Pognetto, M.; Giustetto, M.; Bennis, M. Pre- and postnatal exposure to glyphosate-based herbicide causes behavioral and cognitive impairments in adult mice: Evidence of cortical and hippocampal dysfunction. Arch. Toxicol. 2020, 94, 1703–1723. [Google Scholar] [CrossRef] [PubMed]
- Baier, C.J.; Gallegos, C.E.; Raisman-Vozari, R.; Minetti, A. Behavioral impairments following repeated intranasal glyphosate-based herbicide administration in mice. Neurotoxicol. Teratol. 2017, 64, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Plata, I.; Giordano, M.; Díaz-Muñoz, M.; Rodríguez, V.M. The herbicide glyphosate causes behavioral changes and alterations in dopaminergic markers in male Sprague-Dawley rat. Neurotoxicology 2015, 46, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Coullery, R.; Pacchioni, A.M.; Rosso, S.B. Exposure to glyphosate during pregnancy induces neurobehavioral alterations and downregulation of Wnt5a-CaMKII pathway. Reprod. Toxicol. 2020, 96, 390–398. [Google Scholar] [CrossRef]
- Evans, S.C.; Shaw, E.M.; Rypstra, A.L. Exposure to a glyphosate-based herbicide affects agrobiont predatory arthropod behaviour and long-term survival. Ecotoxicology 2010, 19, 1249–1257. [Google Scholar] [CrossRef]
- Kanabar, M.; Bauer, S.; Ezedum, Z.M.; Dwyer, I.P.; Moore, W.S.; Rodriguez, G.; Mall, A.; Littleton, A.T.; Yudell, M.; Kanabar, J.; et al. Roundup negatively impacts the behavior and nerve function of the Madagascar hissing cockroach (Gromphadorhina portentosa). Environ. Sci. Pollut. Res. Int. 2021, 28, 32933–32944. [Google Scholar] [CrossRef]
- Soares, J.J.; Gonçalves, M.B.; Gayer, M.C.; Bianchini, M.C.; Caurio, A.C.; Soares, S.J.; Puntel, R.L.; Roehrs, R.; Denardin, E.L.G. Continuous liquid feeding: New method to study pesticides toxicity in Drosophila melanogaster. Anal. Biochem. 2017, 537, 60–62. [Google Scholar] [CrossRef]
- Zaller, J.G.; Weber, M.; Maderthaner, M.; Gruber, E.; Takács, E.; Mörtl, M.; Klátyik, S.; Győri, J.; Römbke, J.; Leisch, F.; et al. Effects of glyphosate-based herbicides and their active ingredients on earthworms, water infiltration and glyphosate leaching are influenced by soil properties. Environ. Sci. Eur. 2021, 33, 51. [Google Scholar] [CrossRef]
- Salvio, C.; Menone, M.L.; Rafael, S.; Iturburu, F.G.; Manetti, P.L. Survival, reproduction, avoidance behavior and oxidative stress biomarkers in the Earthworm Octolasion cyaneum exposed to glyphosate. Bull. Environ. Contam. Toxicol. 2016, 96, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Bridi, D.; Altenhofen, S.; Gonzalez, J.B.; Reolon, G.K.; Bonan, C.D. Glyphosate and Roundup® alter morphology and behavior in zebrafish. Toxicology 2017, 392, 32–39. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, J.; Kuang, X.; Li, S.; Li, X.; Chen, D.; Zhao, X.; Feng, X. Biological impacts of glyphosate on morphology, embryo biomechanics and larval behavior in zebrafish (Danio rerio). Chemosphere 2017, 181, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Forner-Piquer, I.; Faucherre, A.; Byram, J.; Blaquiere, M.; de Bock, F.; Gamet-Payrastre, L.; Ellero-Simatos, S.; Audinat, E.; Jopling, C.; Marchi, N. Differential impact of dose-range glyphosate on locomotor behavior, neuronal activity, glio-cerebrovascular structures, and transcript regulations in zebrafish larvae. Chemosphere 2021, 267, 128986. [Google Scholar] [CrossRef] [PubMed]
- Ivantsova, E.; Wengrovitz, A.S.; Souders, C.L.; Martyniuk, C.J. Developmental and behavioral toxicity assessment of glyphosate and its main metabolite aminomethylphosphonic acid (AMPA) in zebrafish embryos/larvae. Environ. Toxicol. Pharmacol. 2022, 93, 103873. [Google Scholar] [CrossRef]
- Lacroix, R.; Ibhazehibo, K.; Kaushik, G.; Kurrasch, D. Exposure to glyphosate or roundup during zebrafish embryogenesis differentially affects metabolism and swimming behaviours. bioRxiv 2022. [Google Scholar] [CrossRef]
- Chen, J.; Rao, C.; Yuan, R.; Sun, D.; Guo, S.; Li, L.; Yang, S.; Qian, D.; Lu, R.; Cao, X. Long-term exposure to polyethylene microplastics and glyphosate interferes with the behavior, intestinal microbial homeostasis, and metabolites of the common carp (Cyprinus carpio L.). Sci. Total. Environ. 2022, 814, 152681. [Google Scholar] [CrossRef]
- Yalsuyi, A.M.; Vajargah, M.F.; Hajimoradloo, A.; Galangash, M.M.; Prokić, M.D.; Faggio, C. Evaluation of behavioral changes and tissue damages in common carp (Cyprinus carpio) after exposure to the herbicide glyphosate. Vet. Sci. 2021, 8, 218. [Google Scholar] [CrossRef]
- Akinsorotan, A.M.; Zelibe, S.A.A.; Olele, N.F. Acute toxicity and behavioural changes on African catfish (Clarias gariepinus) exposed to dizensate (Glyphosate herbicide). Int. J. Sci. Eng. Res. 2013, 4, 1189–1193. [Google Scholar]
- Isaac, A.O.; Joshua, O.S.; Jehu, A. Behavioural and some physiological assessment of glyphosate and paraquat toxicity to juveniles of African catfish, Clarias gariepinus. Pak. J. Zool. 2017, 49, 175–181. [Google Scholar] [CrossRef]
- Uchenna, U.B.; Uka, A.; Obiahu, O.H. The impact of sub-lethal concentrations of glyphosate on growth and haematology of African catfish under aquatic ecological micro-climate. Environ. Chem. Ecotoxicol. 2022, 4, 164–170. [Google Scholar] [CrossRef]
- Nwani, C.D.; Ibiam, U.A.; Ibiam, O.U.; Nworie, O.; Onyishi, G.; Atama, C. Investigation on acute toxicity and behavioral changes in Tilapia zillii due to glyphosate-based herbicide, forceup. J. Anim. Plant Sci. 2013, 23, 888–892. [Google Scholar]
- Sánchez, J.A.A.; Barros, D.M.; de Los Angeles Bistoni, M.; Ballesteros, M.L.; Roggio, M.A.; Martins, C.G.M. Glyphosate-based herbicides affect behavioural patterns of the livebearer Jenynsia multidentata. Environ. Sci. Pollut. Res. Int. 2021, 28, 29958–29970. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.S.; Cecala, K.K. Interactive effects of temperature and glyphosate on the behavior of blue ridge two-lined salamanders (Eurycea wilderae). Environ. Toxicol. Chem. 2016, 35, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Sinhorin, V.D.G.; Sinhorin, A.P.; Teixeira, J.M.S.; Miléski, K.M.L.; Hansen, P.C.; Moeller, P.R.; Moreira, P.S.A.; Baviera, A.M.; Loro, V.L. Metabolic and behavior changes in surubim acutely exposed to a glyphosate-based herbicide. Arch. Environ. Contam. Toxicol. 2014, 67, 659–667. [Google Scholar] [CrossRef]
- Le Du-Carrée, J.; Saliou, F.; Cachot, J.; Morin, T.; Danion, M. Developmental effect of parental or direct chronic exposure to environmental concentration of glyphosate on the larvae of rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 2021, 237, 105894. [Google Scholar] [CrossRef]
- Bolis, A.; Gazzola, A.; Pellitteri-Rosa, D.; Colombo, A.; Bonfanti, P.; Bellati, A. Exposure during embryonic development to Roundup® Power 2.0 affects lateralization, level of activity and growth, but not defensive behaviour of marsh frog tadpoles. Environ. Pollut. 2020, 263, 114395. [Google Scholar] [CrossRef]
- Pavan, F.A.; Samojeden, C.G.; Rutkoski, C.F.; Folador, A.; Da Fré, S.P.; Müller, C.; Hartmann, P.A.; Hartmann, M.T. Morphological, behavioral and genotoxic effects of glyphosate and 2,4-D mixture in tadpoles of two native species of South American amphibians. Environ. Toxicol. Pharmacol. 2021, 85, 103637. [Google Scholar] [CrossRef]
- Moutinho, M.F.; de Almeida, E.A.; Espíndola, E.L.G.; Daam, M.A.; Schiesari, L. Herbicides employed in sugarcane plantations have lethal and sublethal effects to larval Boana pardalis (Amphibia, Hylidae). Ecotoxicology 2020, 29, 1043–1051. [Google Scholar] [CrossRef]
- Hansen, L.R.; Roslev, P. Behavioral responses of juvenile Daphnia magna after exposure to glyphosate and glyphosate-copper complexes. Aquat. Toxicol. 2016, 179, 36–43. [Google Scholar] [CrossRef]
- Alvarado-Suárez, G.B.; Silva-Briano, M.; Arzate-Cárdenas, M.A.; Carbajal-Hernández, A.L.; Yáñez-Rivera, B.; Rico-Mártinez, R. Feeding behavior of early life stages of the zebrafish Danio rerio is altered by exposure to glyphosate. Environ. Sci. Pollut. Res. 2022, 29, 85172–85184. [Google Scholar] [CrossRef] [PubMed]
- Strilbytska, O.M.; Semaniuk, U.V.; Strutynska, T.R.; Burdyliuk, N.I.; Tsiumpala, S.; Bubalo, V.; Lushchak, O. Herbicide Roundup shows toxic effects in nontarget organism Drosophila. Arch. Insect Biochem. Physiol. 2022, 110, e21893. [Google Scholar] [CrossRef]
- Elias, R.; Talyn, B.; Melchiorre, E. Dietary behavior of Drosophila melanogaster fed with genetically-modified corn or Roundup®. J. Xenobiot. 2021, 11, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Benamú, M.A.; Schneider, M.I.; Sánchez, N.E. Effects of the herbicide glyphosate on biological attributes of Alpaida veniliae (Araneae, Araneidae), in laboratory. Chemosphere 2010, 78, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Mengoni Goñalons, C.; Farina, W.M. Impaired associative learning after chronic exposure to pesticides in young adult honey bees. J. Exp. Biol. 2018, 221, jeb176644. [Google Scholar] [CrossRef]
- Helmer, S.H.; Kerbaol, A.; Aras, P.; Jumarie, C.; Boily, M. Effects of realistic doses of atrazine, metolachlor, and glyphosate on lipid peroxidation and diet-derived antioxidants in caged honey bees (Apis mellifera). Environ. Sci. Pollut. Res. 2015, 22, 8010–8021. [Google Scholar] [CrossRef]
- Almasri, H.; Tavares, D.A.; Pioz, M.; Sené, D.; Tchamitchian, S.; Cousin, M.; Brunet, J.L.; Belzunces, L.P. Mixtures of an insecticide, a fungicide and a herbicide induce high toxicities and systemic physiological disturbances in winter Apis mellifera honey bees. Ecotoxicol. Environ. Saf. 2020, 203, 111013. [Google Scholar] [CrossRef]
- Thompson, H.M.; Levine, S.L.; Doering, J.; Norman, S.; Manson, P.; Sutton, P.; von Mérey, G. Evaluating exposure and potential effects on honeybee brood (Apis mellifera) development using glyphosate as an example. Integr. Environ. Assess. Manag. 2014, 10, 463–470. [Google Scholar] [CrossRef]
- Liao, L.H.; Wu, W.Y.; Berenbaum, M.R. Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food. Sci. Rep. 2017, 7, 15924. [Google Scholar] [CrossRef]
- Blot, N.; Veillat, L.; Rouzé, R.; Delatte, H. Glyphosate, but not its metabolite AMPA, alters the honeybee gut microbiota. PLoS ONE 2019, 14, e0215466. [Google Scholar] [CrossRef]
- Fréville, M.; Estienne, A.; Ramé, C.; Lefort, G.; Chahnamian, M.; Staub, C.; Venturi, E.; Lemarchand, J.; Maximin, E.; Hondelatte, A.; et al. Chronic dietary exposure to a glyphosate-based herbicide results in total or partial reversibility of plasma oxidative stress, cecal microbiota abundance and short-chain fatty acid composition in broiler hens. Front. Physiol. 2022, 13, 974688. [Google Scholar] [CrossRef]
- Giaquinto, P.C.; de Sá, M.B.; Sugihara, V.S.; Gonçalves, B.B.; Delício, H.C.; Barki, A. Effects of glyphosate-based herbicide sub-lethal concentrations on fish feeding behavior. Bull. Environ. Contam. Toxicol. 2017, 98, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Córdova López, A.M.; Sarmento, R.A.; de Souza Saraiva, A.; Pereira, R.R.; Soares, A.M.V.M.; Pestana, J.L.T. Exposure to Roundup® affects behaviour, head regeneration and reproduction of the freshwater planarian Girardia tigrina. Sci. Total Environ. 2019, 675, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Janssens, L.; Stoks, R. Stronger effects of Roundup than its active ingredient glyphosate in damselfly larvae. Aquat. Toxicol. 2017, 193, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Janssens, L.; Stoks, R. Synergistic effects between pesticide stress and predator cues: Conflicting results from life history and physiology in the damselfly Enallagma cyathigerum. Aquat. Toxicol. 2013, 132–133, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Behrend, J.E.; Rypstra, A.L. Contact with a glyphosate-based herbicide has long-term effects on the activity and foraging of an agrobiont wolf spider. Chemosphere 2018, 194, 714–721. [Google Scholar] [CrossRef]
- Rittman, S.; Wrinn, K.M.; Evans, S.C.; Webb, A.W.; Rypstra, A.L. Glyphosate-based herbicide has contrasting effects on prey capture by two co-occurring wolf spider species. J. Chem. Ecol. 2013, 39, 1247–1253. [Google Scholar] [CrossRef]
- Lacava, M.; García, L.F.; Viera, C.; Michalko, R. The pest-specific effects of glyphosate on functional response of a wolf spider. Chemosphere 2021, 262, 127785. [Google Scholar] [CrossRef]
- Michalková, V.; Pekár, S. How glyphosate altered the behaviour of agrobiont spiders (Araneae: Lycosidae) and beetles (Coleoptera: Carabidae). Biol. Control 2009, 51, 444–449. [Google Scholar] [CrossRef]
- Bengtsson, G.; Hansson, L.A.; Montenegro, K. Reduced grazing rates in Daphnia pulex caused by contaminants: Implications for trophic cascades. Environ. Toxicol. Chem. 2004, 23, 2641–2648. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Ye, M.; Dang, W.; Lu, H. Effects of glyphosate-ammonium exposure on embryonic development, hatchling locomotor performance and learning ability in the three-keeled pond turtle, Asian J. Ecotoxicol. 2019, 6, 128–135. [Google Scholar] [CrossRef]
- Ujszegi, J.; Gál, Z.; Mikó, Z.; Hettyey, A. No observable effect of a glyphosate-based herbicide on two top predators of temporal water bodies. Environ. Toxicol. Chem. 2015, 34, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Ujszegi, J.; Gál, Z.; Mikó, Z.; Hettyey, A. No effect of a glyphosate-based herbicide on larval dragonflies (Aeshna cyanea) and adult newts (Lissotriton vulgaris) in a laboratory-based experiment. Acta Zool. Acad. Sci. Hung. 2016, 62, 355–367. [Google Scholar] [CrossRef]
- Pompermaier, A.; Kirsten, K.; Soares, S.M.; Fortuna, M.; Kalichak, F.; Idalencio, R.; Koakoski, G.; Barreto, R.E.; Barcellos, L.J.G. Waterborne agrichemicals compromise the anti-predatory behavior of zebrafish. Environ. Sci. Pollut. Res. 2020, 27, 38559–38567. [Google Scholar] [CrossRef] [PubMed]
- Pompermaier, A.; Varela, A.C.C.; Mozzato, M.T.; Soares, S.M.; Fortuna, M.; Alves, C.; Tamagno, W.A.; Barcellos, L.J.G. Impaired initial development and behavior in zebrafish exposed to environmentally relevant concentrations of widely used pesticides. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 257, 109328. [Google Scholar] [CrossRef]
- Pompermaier, A.; Tamagno, W.A.; Alves, C.; Barcellos, L.J.G. Persistent and transgenerational effects of pesticide residues in zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 262, 109461. [Google Scholar] [CrossRef]
- da Costa Chaulet, F.; de Alcantara Barcellos, H.H.; Fior, D.; Pompermaier, A.; Koakoski, G.; da Rosa, J.G.S.; Fagundes, M.; Barcellos, L.J.G. Glyphosate- and Fipronil-Based Agrochemicals and Their Mixtures Change Zebrafish Behavior. Arch. Environ. Contam. Toxicol. 2019, 77, 443–451. [Google Scholar] [CrossRef]
- Lanzarin, G.A.B.; Venâncio, C.A.S.; Monteiro, S.M.; Félix, L.M. Behavioural toxicity of environmental relevant concentrations of a glyphosate commercial formulation—RoundUp® UltraMax—During zebrafish embryogenesis. Chemosphere 2020, 253, 126636. [Google Scholar] [CrossRef]
- Tapkir, S.D.; Kharat, S.S.; Kumkar, P.; Gosavi, S.M. Impact, recovery and carryover effect of Roundup® on predator recognition in common spiny loach, Lepidocephalichthys thermalis. Ecotoxicology 2019, 28, 189–200. [Google Scholar] [CrossRef]
- Moore, H.; Chivers, D.P.; Ferrari, M.C.O. Sub-lethal effects of RoundupTM on tadpole anti-predator responses. Ecotoxicol. Environ. Saf. 2015, 111, 281–285. [Google Scholar] [CrossRef]
- Gabor, C.R.; Perkins, H.R.; Heitmann, A.T.; Forsburg, Z.R.; Aspbury, A.S. RoundupTM With Corticosterone Functions as an Infodisruptor to Antipredator Response in Tadpoles. Front. Ecol. Evol. 2019, 7, 114. [Google Scholar] [CrossRef]
- Janssens, L.; Stoks, R. How does a pesticide pulse increase vulnerability to predation? Combined effects on behavioral antipredator traits and escape swimming. Aquat. Toxicol. 2012, 110–111, 91–98. [Google Scholar] [CrossRef]
- Wrinn, K.M.; Evans, S.C.; Rypstra, A.L. Predator cues and an herbicide affect activity and emigration in an agrobiont wolf spider. Chemosphere 2012, 87, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Mikó, Z.; Ujszegi, J.; Gál, Z.; Hettyey, A. Effects of a glyphosate-based herbicide and predation threat on the behaviour of agile frog tadpoles. Ecotoxicol. Environ. Saf. 2017, 140, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Gaupp-Berghausen, M.; Hofer, M.; Rewald, B.; Zaller, J.G. Glyphosate-based herbicides reduce the activity and reproduction of earthworms and lead to increased soil nutrient concentrations. Sci. Rep. 2015, 5, 12886. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Vera, M.K.M.; Bhandari, R.K. Developmental and epigenetic effects of Roundup and glyphosate exposure on Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2019, 210, 215–226. [Google Scholar] [CrossRef]
- Milesi, M.M.; Lorenz, V.; Pacini, G.; Repetti, M.R.; Demonte, L.D.; Varayoud, J.; Luque, E.H. Perinatal exposure to a glyphosate-based herbicide impairs female reproductive outcomes and induces second-generation adverse effects in Wistar rats. Arch. Toxicol. 2018, 92, 2629–2643. [Google Scholar] [CrossRef]
- Ricci, E.L.; Ribeiro, M.O.; Fukushima, A.R.; Sandini, T.M.; Rocha, P.R.D.; Waziry, P.A.F.W.; Bernardi, M.M.; Spinosa, H.d.S. Perinatal exposure to an aromatase inhibitor glyphosate-base herbicide reduced male and female social behavior in juvenile age and the sexual behavior at adult female rat. Braz. J. Vet. Res. Anim. Sci. 2022, 59, e186467. [Google Scholar] [CrossRef]
- Romano, M.A.; Romano, R.M.; Santos, L.D.; Wisniewski, P.; Campos, D.A.; de Souza, P.B.; Viau, P.; Bernardi, M.M.; Nunes, M.T.; de Oliveira, C.A. Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Arch. Toxicol. 2011, 86, 663–673. [Google Scholar] [CrossRef]
- Joaquim, A.D.O.; Macrini, D.J.; Ricci, E.L.; Rodrigues, P.A.; Spinosa, H.D.S.; Suffredini, I.B.; Bernarndi, M.M. Effects of exposure to glyphosate in male and female mice behavior in pubertal period. Braz. J. Vet. Res. Anim. Sci. 2014, 51, 194–203. [Google Scholar] [CrossRef]
- Griesinger, L.M.; Evans, S.C.; Rypstra, A.L. Effects of a glyphosate-based herbicide on mate location in a wolf spider that inhabits agroecosystems. Chemosphere 2011, 84, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Leccia, F.; Kysilková, K.; Kolářová, M.; Hamouzová, K.; Líznarová, E.; Korenko, S. Disruption of the chemical communication of the European agrobiont ground-dwelling spider Pardosa agrestis by pesticides. J. Appl. Entomol. 2015, 140, 609–616. [Google Scholar] [CrossRef]
- Ward, W.; Heinly, B.; Preston, J.; Johnson, C.; Sweger, A.; Persons, M. Lethal and sublethal effects of five common herbicides on the wolf spider, Pardosa milvina (Araneae: Lycosidae). Ecotoxicology 2022, 31, 1565–1582. [Google Scholar] [CrossRef] [PubMed]
- Leponiemi, M.; Schultner, E.; Dickel, F.; Freitak, D. Chronic sublethal pesticide exposure affects brood production, morphology and endosymbionts, but not immunity in the ant, Cardiocondyla obscurior. Ecol. Entomol. 2021, 47, 273–283. [Google Scholar] [CrossRef]
- Hued, A.C.; Oberhofer, S.; de los Ángeles Bistoni, M. Exposure to a Commercial Glyphosate Formulation (Roundup®) Alters Normal Gill and Liver Histology and Affects Male Sexual Activity of Jenynsia multidentata (Anablepidae, Cyprinodontiformes). Arch. Environ. Contam. Toxicol. 2011, 62, 107–117. [Google Scholar] [CrossRef]
- Sandun, K.; Bandara, N.; Amarasinghe, U.S. Effect of glyphosate-based herbicide, Roundup™ on territory deference of male Oreochromis mossambicus (Osteichthyes, Cichlidae) associated with mating behaviour. Sri Lanka J. Aquat. Sci. 2015, 20, 1–10. [Google Scholar] [CrossRef]
- Sulukan, E.; Baran, A.; Kankaynar, M.; Kızıltan, T.; Bolat, İ.; Yıldırım, S.; Ceyhun, H.A.; Ceyhun, S.B. Global warming and glyphosate toxicity (II): Offspring zebrafish modelling with behavioral, morphological and immunohistochemical approaches. Sci. Total Environ. 2023, 856, 158903. [Google Scholar] [CrossRef]
- Muller, K.; Herrera, K.; Talyn, B.; Melchiorre, E. Toxicological Effects of Roundup® on Drosophila melanogaster Reproduction. Toxics 2021, 9, 161. [Google Scholar] [CrossRef]
- Dechartres, J.; Pawluski, J.L.; Gueguen, M.; Jablaoui, A.; Maguin, E.; Rhimi, M.; Charlier, T.D. Glyphosate and glyphosate-based herbicide exposure during the peripartum period affects maternal brain plasticity, maternal behaviour and microbiome. J. Neuroendocrinol. 2019, 31, e12731. [Google Scholar] [CrossRef]
- de Oliveira, M.A.L.; Rojas, V.C.T.; de Sá, J.C.; de Novais, C.O.; Silva, M.S.; de Almeida Paula, H.A.; Kirsten, T.B.; Bernardi, M.M.; Pinheiro, L.C.; Giusti-Paiva, A.; et al. Perinatal exposure to glyphosate-based herbicides induced neurodevelopmental behaviors impairments and increased oxidative stress in the prefrontal cortex and hippocampus in offspring. Int. J. Dev. Neurosci. 2022, 82, 528–538. [Google Scholar] [CrossRef]
- Rocha, P.R.D.A.; Ribeiro, M.O.; Sandini, T.M.; Camargo, E.L.R.A.; Bernardi, M.M.; Spinosa, H.D.S. Perinatal glyphosate-based herbicide impaired maternal behavior by reducing the striatal dopaminergic activity and delayed the offspring reflex development. Atas Saúde Ambient. 2019, 7, 130–156. [Google Scholar]
- Gallegos, C.E.; Bartos, M.; Bras, C.; Gumilar, F.; Antonelli, M.C.; Minetti, A. Exposure to a glyphosate-based herbicide during pregnancy and lactation induces neurobehavioral alterations in rat offspring. Neurotoxicology 2016, 53, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Nikbakhtzadeh, M.R.; Fuentes, Y. Deterrent Effects of Glyphosate on Oviposition and Larval Development of Culex quinquefasciatus. J. Am. Mosq. Control Assoc. 2022, 38, 198–207. [Google Scholar] [CrossRef]
- Stahlschmidt, Z.R.; Vo, C. Spatial bet hedging, thermal trade-offs and glyphosate: Crickets integrate multivariate information during oviposition. Anim. Behav. 2022, 185, 105–112. [Google Scholar] [CrossRef]
- Baglan, H.; Lazzari, C.R.; Guerrieri, F.J. Glyphosate impairs learning in Aedes aegypti mosquito larvae at field-realistic doses. J. Exp. Biol. 2018, 221, jeb187518. [Google Scholar] [CrossRef]
- Helander, M.; Lehtonen, T.K.; Saikkonen, K.; Despains, L.; Nyckees, D.; Antinoja, A.; Solvi, C.; Loukola, O.J. Field-realistic acute exposure to glyphosate-based herbicide impairs fine-color discrimination in bumblebees. Sci. Total Environ. 2023, 857, 159298. [Google Scholar] [CrossRef]
- Hernández, J.; Riveros, A.J.; Amaya-Márquez, M. Sublethal doses of glyphosate impair olfactory memory retention, but not learning in the honey bee (Apis mellifera scutellata). J. Insect Conserv. 2021, 25, 683–694. [Google Scholar] [CrossRef]
- Fuhrimann, S.; Farnham, A.; Staudacher, P.; Atuhaire, A.; Manfioletti, T.; Niwagaba, C.B.; Namirembe, S.; Mugweri, J.; Winkler, M.S.; Portengen, L.; et al. Exposure to multiple pesticides and neurobehavioral outcomes among smallholder farmers in Uganda. Environ. Int. 2021, 152, 106477. [Google Scholar] [CrossRef]
- Luna, S.; Neila, L.P.; Vena, R.; Borgatello, C.; Rosso, S.B. Glyphosate exposure induces synaptic impairment in hippocampal neurons and cognitive deficits in developing rats. Arch. Toxicol. 2021, 95, 2137–2150. [Google Scholar] [CrossRef]
- Del Castilo, I.; Neumann, A.S.; Lemos, F.S.; De Bastiani, M.A.; Oliveira, F.L.; Zimmer, E.R.; Rêgo, A.M.; Hardoim, C.C.P.; Antunes, L.C.M.; Lara, F.A.; et al. Lifelong Exposure to a Low-Dose of the Glyphosate-Based Herbicide RoundUp® Causes Intestinal Damage, Gut Dysbiosis, and Behavioral Changes in Mice. Int. J. Mol. Sci. 2022, 23, 5583. [Google Scholar] [CrossRef]
- de Castro Vieira Carneiro, C.L.; Chaves, E.M.C.; Neves, K.R.T.; Braga, M.D.M.; Assreuy, A.M.S.; de Moraes, M.E.A.; Aragão, G.F. Behavioral and neuroinflammatory changes caused by glyphosate: Base herbicide in mice offspring. Birth Defects Res. 2023, 115, 488–497. [Google Scholar] [CrossRef]
- Ait Bali, Y.A.; Kaikai, N.E.; Ba-M’hamed, S.; Bennis, M. Learning and memory impairments associated to acetylcholinesterase inhibition and oxidative stress following glyphosate based-herbicide exposure in mice. Toxicology 2019, 415, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, C.E.; Baier, C.J.; Bartos, M.; Bras, C.; Domínguez, S.; Mónaco, N.; Gumilar, F.; Giménez, M.S.; Minetti, A. Perinatal glyphosate-based herbicide exposure in rats alters brain antioxidant status, glutamate and acetylcholine metabolism and affects recognition memory. Neurotox. Res. 2018, 34, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Park, H.S.; Oh, J.H.; Lee, J.S. Glyphosate-induced encephalopathy: A case report. J. Clin. Neurol. 2019, 15, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Sugisaki, T.; Ryota, Y.; Yoshiteru, S.; Okayasu, H.; Shioda, M.; Suzuki, K.; Yasui-Furukori, N.; Shimoda, K. Transient glyphosate encephalopathy due to a suicide attempt. Neuropsychopharmacol. Rep. 2021, 41, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.R.; Leiros da Costa, M.D.; Bacheschi, L.A.; Scaff, M.; Leite, C.C. Parkinsonism after glycine-derivate exposure. Mov. Disord. Off. J. Mov. Disor. Soc. 2001, 16, 565–568. [Google Scholar] [CrossRef]
- Wang, L.; Tang, S.; Wu, S.; Yao, L.; Su, D.; Wang, Y. Maternal Exposure to Pesticides and Risk of Autism Spectrum Disorders in Offspring: A Meta-analysis. J. Autism Dev. Disord. 2022, 52, 1640–1651. [Google Scholar] [CrossRef]
- Joaquim, A.O.; Spinosa, H.S.; Macrini, D.J.; Rodrigues, P.A.; Ricci, E.L.; Artiolli, T.S.; Moreira, N.; Suffredini, I.B.; Bernardi, M.M. Behavioral effects of acute glyphosate exposure in male and female Balb/c mice. Braz. J. Vet. Res. Anim. Sci. 2012, 49, 367–376. [Google Scholar] [CrossRef]
- Ait Bali, Y.; Ba-M’hamed, S.; Elhidar, N.; Nafis, A.; Soraa, N.; Bennis, M. Glyphosate based- herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol. Teratol. 2018, 67, 44–49. [Google Scholar] [CrossRef]
- Ait Bali, Y.; Kaikai, N.E.; Ba-M’hamed, S.; Sassoè-Pognetto, M.; Giustetto, M.; Bennis, M. Anxiety and Gene Expression Enhancement in Mice Exposed to Glyphosate-Based Herbicide. Toxics 2022, 10, 226. [Google Scholar] [CrossRef]
- Bicca, D.F.; Spiazzi, C.C.; Ramalho, J.B.; Soares, M.B.; Cibin, F.W.S. A subchronic low-dose exposure of a glyphosate-based herbicide induces depressive and anxious-like behavior in mice: Quercetin therapeutic approach. Environ. Sci. Pollut. Res. Int. 2021, 28, 67394–67403. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sepúlveda, C.J.; Cáceres-Chacón, M.; Alvelo-Fernández, P.; Haddock-Martínez, H.; Ramos-Sánchez, R.; Martínez-Guzmán, O.; Rivera-López, M.; Sierra-Mercado, D. Effects of glyphosate on locomotion and anxiety-like behavior in rats. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Sulukan, E.; Baran, A.; Şenol, O.; Kankaynar, M.; Yıldırım, S.; Bolat, İ.; Ceyhun, H.A.; Toraman, E.; Ceyhun, S.B. Global warming and glyphosate toxicity (I): Adult zebrafish modelling with behavioural, immunohistochemical and metabolomic approaches. Sci. Total Environ. 2023, 858, 160086. [Google Scholar] [CrossRef] [PubMed]
- Cattani, D.; Cesconetto, P.A.; Tavares, M.K.; Parisotto, E.B.; De Oliveira, P.A.; Rieg, C.E.H.; Leite, M.C.; Prediger, R.D.S.; Wendt, N.C.; Razzera, G.; et al. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 2017, 387, 67–80. [Google Scholar] [CrossRef]
- Pu, Y.; Yang, J.; Chang, L.; Qu, Y.; Wang, S.; Zhang, K.; Xiong, Z.; Zhang, J.; Tan, Y.; Wang, X.; et al. Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA 2020, 117, 11753–11759. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Becerra, T.A.; von Ehrenstein, O.S.; Heck, J.E.; Olsen, J.; Arah, O.A.; Jeste, S.S.; Rodriguez, M.; Ritz, B. Autism spectrum disorders and race, ethnicity, and nativity: A population-based study. Pediatrics 2014, 134, e63–e71. [Google Scholar] [CrossRef]
- Carter, C.J.; Blizard, R.A. Autism genes are selectively targeted by environmental pollutants including pesticides, heavy metals, bisphenol A, phthalates and many others in food, cosmetics or household products. Neurochem. Int. 2016, 101, 83–109. [Google Scholar] [CrossRef]
- Silva, M.H. Effects of low-dose chlorpyrifos on neurobehavior and potential mechanisms: A review of studies in rodents, zebrafish, and Caenorhabditis elegans. Birth Defects Res. 2020, 112, 445–479. [Google Scholar] [CrossRef]
- Malhotra, R.C.; Ghia, D.K.; Cordato, D.J.; Beran, R.G. Glyphosate-surfactant herbicide-induced reversible encephalopathy. J. Clin. Neurosci. 2010, 17, 1472–1473. [Google Scholar] [CrossRef]
- Zheng, Q.; Yin, J.; Zhu, L.; Jiao, L.; Xu, Z. Reversible Parkinsonism induced by acute exposure glyphosate. Park. Relat. Disord. 2018, 50, 121. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Fruciano, C.; Marchant, J.; Hildebrand, F.; Forslund, S.; Bork, P.; Engel, P.; Hughes, W.O.H. The gut microbiome is associated with behavioural task in honey bees. Insectes Sociaux 2018, 65, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Mena, F.; Romero, A.; Blasco, J.; Araújo, C.V. Can a mixture of agrochemicals (glyphosate, chlorpyrifos and chlorothalonil) mask the perception of an individual chemical? A hidden trap underlying ecological risk. Ecotoxicol. Environ. Saf. 2022, 230, 113172. [Google Scholar] [CrossRef]
- Motta, E.V.S.; Raymann, K.; Moran, N.A. Glyphosate perturbs the gut microbiota of honey bees. Proc. Natl. Acad. Sci. USA 2018, 115, 10305–10310. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate, pathways to modern diseases III: Manganese, neurological diseases, and associated pathologies. Surg. Neurol. Int. 2015, 6, 45. [Google Scholar] [CrossRef]
- Mesnage, R.; Teixeira, M.; Mandrioli, D.; Falcioni, L.; Ducarmon, Q.R.; Zwittink, R.D.; Mazzacuva, F.; Caldwell, A.; Halket, J.; Amiel, C.; et al. Use of shotgun metagenomics and metabolomics to evaluate the impact of glyphosate or roundup mon 52276 on the gut microbiota and serum metabolome of sprague-dawley rats. Environ. Health Perspect. 2021, 129, 17005. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate, pathways to modern diseases II: Celiac sprue and gluten intolerance. Interdiscip. Toxicol. 2013, 6, 159–184. [Google Scholar] [CrossRef]
- Bertram, M.G.; Martin, J.M.; McCallum, E.S.; Alton, L.A.; Brand, J.A.; Brooks, B.W.; Cerveny, D.; Fick, J.; Ford, A.T.; Hellström, G.; et al. Frontiers in quantifying wildlife behavioural responses to chemical pollution. Biol. Rev. 2022, 97, 1346–1364. [Google Scholar] [CrossRef]
- Thoré, E.S.; Philippe, C.; Brendonck, L.; Pinceel, T. Towards improved fish tests in ecotoxicology-efficient chronic and multi-generational testing with the killifish Nothobranchius furzeri. Chemosphere 2021, 273, 129697. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).