Cyclic Organic Peroxides as New Fungicides against Phytopathogenic Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information and Materials
2.2. Synthesis of Starting Compounds
2.3. Procedure for the Synthesis Peroxide P1
4-Tert-butyl-1,1-dihydroperoxycyclohexane P1 [48]
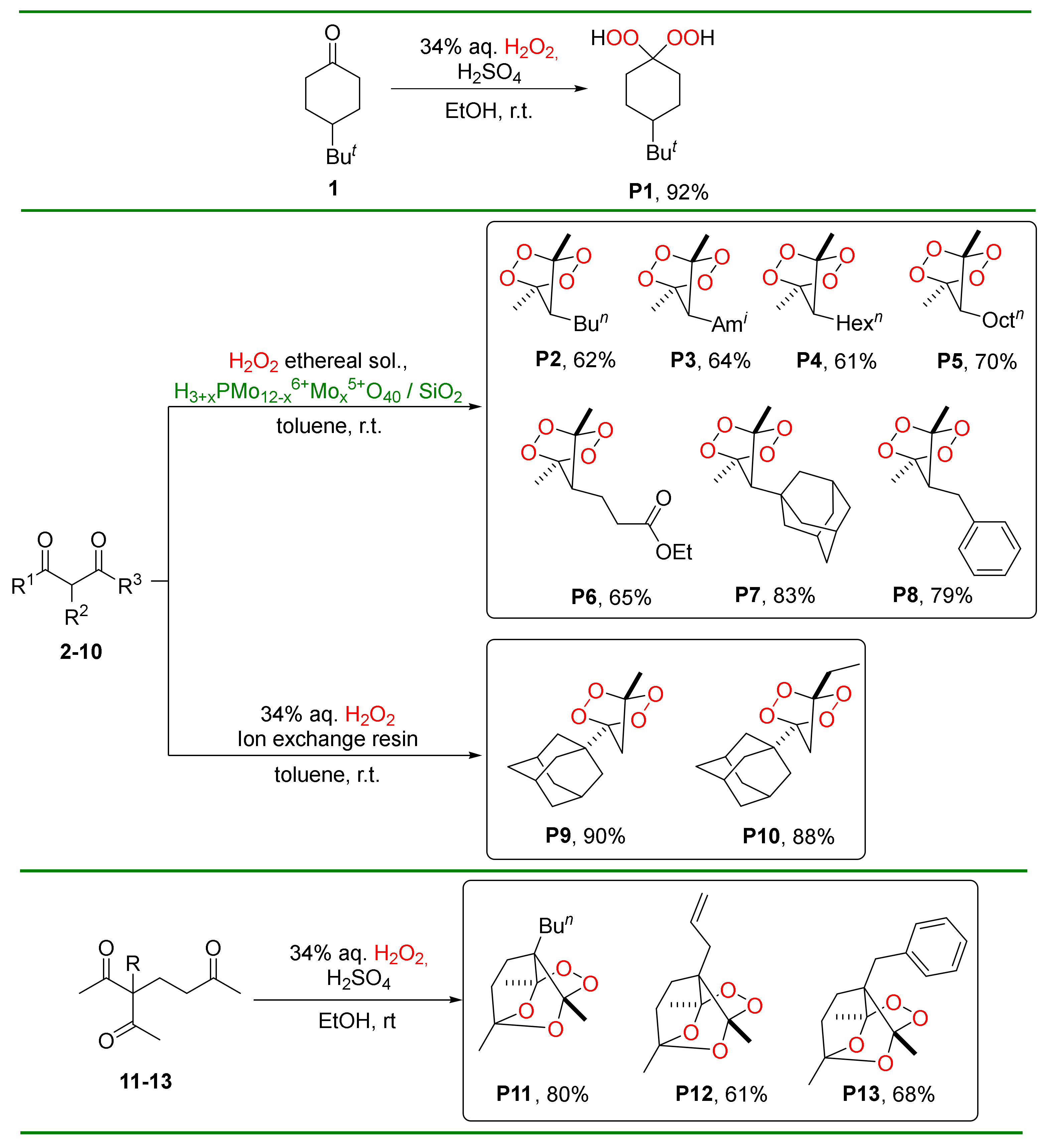
2.4. Synthesis of Bridged 1,2,4,5-Tetraoxanes P2–P10
2.4.1. 7-Butyl-1,4-dimethyl-2,3,5,6-tetraoxabicyclo[2.2.1]heptane, P2 [44]
2.4.2. 7-Isopentyl-1,4-dimethyl-2,3,5,6-tetraoxabicyclo[2.2.1]heptane, P3 [44]
2.4.3. 7-Hexyl-1,4-dimethyl-2,3,5,6-tetraoxabicyclo[2.2.1]heptane, P4 [44]
2.4.4. 1,4-Dimethyl-7-octyl-2,3,5,6-tetraoxabicyclo[2.2.1]heptane, P5 [44]
2.4.5. Ethyl 3-(1,4-dimethyl-2,3,5,6-tetraoxabicyclo[2.2.1]heptan-7-yl)propanoate, P6 [44]
2.4.6. 7-(Adamantan-1-yl)-1,4-dimethyl-2,3,5,6-tetraoxabicyclo[2.2.1]heptane, P7 [44]
2.4.7. 7-Benzyl-1,4-dimethyl-2,3,5,6-tetraoxabicyclo[2.2.1]heptane, P8 [49]
2.4.8. 4-(1-Adamantyl)-1-methyl-2,3,5,6-tetraoxabicyclo[2.2.1]heptane, P9 [21]
2.4.9. 4-(1-Adamantyl)-1-ethyl-2,3,5,6-tetraoxabicyclo[2.2.1]-heptane, P10 [21]
2.5. Synthesis of Tricyclic Peroxides P11–P13
2.5.1. 3a-Butyl-3,6,7a-trimethyltetrahydro-3H,4H-3,6-epoxy[1,2]-dioxolo[3,4-b]pyran, P11 [50]
2.5.2. 3a-Allyl-3,6,7a-trimethyltetrahydro-3H,4H-3,6-epoxy[1,2]-dioxolo[3,4-b]pyran, P12 [50]
2.5.3. 3a-Benzyl-3,6,7a-trimethyltetrahydro-3H,4H-3,6-epoxy[1,2]dioxolo[3,4-b]pyran, P13 [50]
2.6. Investigation of Fungicidal Activity In Vitro
2.7. Investigation of Fungicidal Activity to Control Leaf Blight on Detached Potato Leaves
2.7.1. Chemicals and Formulations
2.7.2. Plants
2.7.3. Pathogens and Inoculation
2.7.4. Study of Fungicidal Activity to Control Leaf Blight
3. Results and Discussion
3.1. The Synthesis of Acyclic and Cyclic Organic Peroxides
3.2. Study of the Fungicidal Activity of Synthesized Peroxides P1–P13 In Vitro
| No | Cmpd. | Mycelium Growth Inhibition ± SD, % C = 30 mg∙L−1 | ||||
| F.c. | R.s. | A.s. | P.i. | C.c. | ||
| 1 | P1 | 0 ± 0.0 | 1 ± 7.5 | 11 ± 2.1 | 37 ± 5.0 | 4 ± 8.5 |
| 2 | P2 | 87 ± 1.0 | 84 ± 2.3 | 62 ± 3.6 | 100 ± 0.0 | 44 ± 4.5 |
| 3 | P3 | 56 ± 2.2 | 50 ± 2.5 | 50 ± 2.5 | 100 ± 0.0 | 38 ± 2.5 |
| 4 | P4 | 22 ± 2.2 | 45 ± 2.2 | 50 ± 2.5 | 100 ± 0.0 | 57 ± 2.5 |
| 5 | P5 | 86 ± 1.1 | 88 ± 6.2 | 55 ± 4.2 | 100 ± 0.0 | 33 ± 4.5 |
| 6 | P6 | 54 ± 1.1 | 58 ± 2.2 | 35 ± 1.8 | 79 ± 4.8 | 18 ± 4.4 |
| 7 | P7 | 45 ±2.2 | 67 ± 1.1 | 63 ± 2.5 | 48 ± 3.7 | 25 ± 2.5 |
| 8 | P8 | 78 ± 1.1 | 100 ± 0.0 | 89 ± 1.1 | 100 ± 0.0 | 47 ± 4.4 |
| 9 | P9 | 25 ± 5.0 | 56 ± 3.8 | 66 ± 2.1 | 67 ± 2.5 | 27 ± 6.4 |
| 10 | P10 | 12 ± 7.5 | 17 ± 6.3 | 66 ± 2.1 | 62 ± 2.5 | 30 ± 6.3 |
| 11 | P11 | 1 ± 7.5 | 0 ± 0.0 | 6 ± 2.1 | 5 ± 7.5 | 0 ± 0.0 |
| 12 | P12 | 0 ± 0.0 | 2 ± 7.5 | 15 ± 2.1 | 20 ± 7.5 | 0 ± 0.0 |
| 13 | P13 | 2 ± 7.5 | 1 ± 7.5 | 6 ± 2.1 | 0 ± 0.0 | 0 ± 0.0 |
| 14 | Azoxystrobin in Quadris® | 46 ± 2.7 | 85 ± 4.4 | 63 ± 3.6 | 95 ± 2.5 | 87 ± 2.5 |
| LSD0.95 | 12.8 | 12.3 | 12.9 | 16.1 | 12.6 | |
3.3. Study of Fungicidal Activity to Control Leaf Blight on Detached Potato Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Strange, R.N.; Scott, P.R. Plant Disease: A Threat to Global Food Security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. Progress of modern agricultural chemistry and future prospects. Pest. Manag. Sci. 2016, 72, 433–455. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. Chapter Two—The Evolution of Fungicide Resistance. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 90, pp. 29–92. [Google Scholar]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Y.; Wittlin, S.; Charman, S.A.; Chiu, F.C.K.; Chollet, J.; Katneni, K.; Mannila, J.; Morizzi, J.; Ryan, E.; et al. Comparative Antimalarial Activities and ADME Profiles of Ozonides (1,2,4-trioxolanes) OZ277, OZ439, and Their 1,2-Dioxolane, 1,2,4-Trioxane, and 1,2,4,5-Tetraoxane Isosteres. J. Med. Chem. 2013, 56, 2547–2555. [Google Scholar] [CrossRef]
- Giannangelo, C.; Fowkes, F.J.I.; Simpson, J.A.; Charman, S.A.; Creek, D.J. Ozonide Antimalarial Activity in the Context of Artemisinin-Resistant Malaria. Trends Parasitol. 2019, 35, 529–543. [Google Scholar] [CrossRef]
- Ingram, K.; Yaremenko, I.A.; Krylov, I.B.; Hofer, L.; Terent’ev, A.O.; Keiser, J. Identification of Antischistosomal Leads by Evaluating Bridged 1,2,4,5-Tetraoxanes, Alphaperoxides, and Tricyclic Monoperoxides. J. Med. Chem. 2012, 55, 8700–8711. [Google Scholar] [CrossRef]
- Keiser, J.; Ingram, K.; Vargas, M.; Chollet, J.; Wang, X.; Dong, Y.; Vennerstrom, J.L. In Vivo Activity of Aryl Ozonides against Schistosoma Species. Antimicrob. Agents Chemother. 2012, 56, 1090. [Google Scholar] [CrossRef]
- Vil’, V.; Yaremenko, I.; Ilovaisky, A.; Terent’ev, A. Peroxides with Anthelmintic, Antiprotozoal, Fungicidal and Antiviral Bioactivity: Properties, Synthesis and Reactions. Molecules 2017, 22, 1881. [Google Scholar] [CrossRef]
- Abrams, R.P.; Carroll, W.L.; Woerpel, K.A. Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells. ACS Chem. Biol. 2016, 11, 1305–1312. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Goswami, A.; Pratim Saikia, P.; Barua, N.C.; Rao, P.G. Artemisinin and its derivatives: A novel class of anti-malarial and anti-cancer agents. Chem. Soc. Rev. 2010, 39, 435–454. [Google Scholar] [CrossRef]
- Coghi, P.; Yaremenko, I.A.; Prommana, P.; Radulov, P.S.; Syroeshkin, M.A.; Wu, Y.J.; Gao, J.Y.; Gordillo, F.M.; Mok, S.; Wong, V.K.W.; et al. Novel Peroxides as Promising Anticancer Agents with Unexpected Depressed Antimalarial Activity. ChemMedChem 2018, 13, 902–908. [Google Scholar] [CrossRef]
- Dwivedi, A.; Mazumder, A.; du Plessis, L.; du Preez, J.L.; Haynes, R.K.; du Plessis, J. In vitro anti-cancer effects of artemisone nano-vesicular formulations on melanoma cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2041–2050. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Syroeshkin, M.A.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Cyclic peroxides as promising anticancer agents: In vitro cytotoxicity study of synthetic ozonides and tetraoxanes on human prostate cancer cell lines. Med. Chem. Res. 2017, 26, 170–179. [Google Scholar] [CrossRef]
- Alagbala, A.A.; McRiner, A.J.; Borstnik, K.; Labonte, T.; Chang, W.; D’Angelo, J.G.; Posner, G.H.; Foster, B.A. Biological mechanisms of action of novel C-10 non-acetal trioxane dimers in prostate cancer cell lines. J. Med. Chem. 2006, 49, 7836–7842. [Google Scholar] [CrossRef]
- Rubush, D.M.; Morges, M.A.; Rose, B.J.; Thamm, D.H.; Rovis, T. An Asymmetric Synthesis of 1,2,4-Trioxane Anticancer Agents via Desymmetrization of Peroxyquinols through a Brønsted Acid Catalysis Cascade. J. Am. Chem. Soc. 2012, 134, 13554–13557. [Google Scholar] [CrossRef]
- Chaudhari, M.B.; Moorthy, S.; Patil, S.; Bisht, G.S.; Mohamed, H.; Basu, S.; Gnanaprakasam, B. Iron-Catalyzed Batch/Continuous Flow C–H Functionalization Module for the Synthesis of Anticancer Peroxides. J. Org. Chem. 2018, 83, 1358–1368. [Google Scholar] [CrossRef]
- Gao, F.; Sun, Z.; Kong, F.; Xiao, J. Artemisinin-derived hybrids and their anticancer activity. Eur. J. Med. Chem. 2020, 188, 112044–112063. [Google Scholar] [CrossRef]
- Vil’, V.A.; Yaremenko, I.A.; Fomenkov, D.I.; Levitsky, D.O.; Fleury, F.; Terent’ev, A.O. Ion exchange resin-catalyzed synthesis of bridged tetraoxanes possessing in vitro cytotoxicity against HeLa cancer cells. Chem. Het. Compd. 2020, 56, 722–726. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Coghi, P.; Prommana, P.; Qiu, C.; Radulov, P.S.; Qu, Y.; Belyakova, Y.Y.; Zanforlin, E.; Kokorekin, V.A.; Wu, Y.Y.J.; et al. Synthetic Peroxides Promote Apoptosis of Cancer Cells by Inhibiting P-Glycoprotein ABCB5. ChemMedChem 2020, 15, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Sharma, V.; Jaiswal, P.K.; Gaikwad, A.N.; Sinha, S.K.; Puri, S.K.; Sharon, A.; Maulik, P.R.; Chaturvedi, V. Stable Tricyclic Antitubercular Ozonides Derived from Artemisinin. Org. Lett. 2015, 17, 4948–4951. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Walz, A.J.; Zhu, H.; Wu, C.; Moraski, G.; Möllmann, U.; Tristani, E.M.; Crumbliss, A.L.; Ferdig, M.T.; Checkley, L.; et al. Design, Synthesis, and Study of a Mycobactin−Artemisinin Conjugate That Has Selective and Potent Activity against Tuberculosis and Malaria. J. Am. Chem. Soc. 2011, 133, 2076–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.-W.; Lei, H.-S.; Fan, L.; Jiang, L.; Liu, J.; Peng, X.-M.; Xu, X.-R.; Chen, L.; Zhou, C.-H.; Zou, Y.-Y.; et al. Design, synthesis, and biological evaluation of dihydroartemisinin–fluoroquinolone conjugates as a novel type of potential antitubercular agents. Bioorg. Med. Chem. Lett. 2014, 24, 1912–1917. [Google Scholar] [CrossRef]
- Chou, S.; Marousek, G.; Auerochs, S.; Stamminger, T.; Milbradt, J.; Marschall, M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antiviral Res. 2011, 92, 364–368. [Google Scholar] [CrossRef]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.G.; Marschall, M. The Antiviral Activities of Artemisinin and Artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef]
- Efferth, T. Beyond malaria: The inhibition of viruses by artemisinin-type compounds. Biotechnol. Adv. 2018, 36, 1730–1737. [Google Scholar] [CrossRef]
- Reiter, C.; Fröhlich, T.; Gruber, L.; Hutterer, C.; Marschall, M.; Voigtlander, C.; Friedrich, O.; Kappes, B.; Efferth, T.; Tsogoeva, S.B. Highly potent artemisinin-derived dimers and trimers: Synthesis and evaluation of their antimalarial, antileukemia and antiviral activities. Bioorg. Med. Chem. 2015, 23, 5452–5458. [Google Scholar] [CrossRef]
- Miller, L.H.; Su, X.Z. Artemisinin: Discovery from the Chinese Herbal Garden. Cell 2011, 146, 855–858. [Google Scholar] [CrossRef]
- Huang, J.J.; Xia, Z.Q.; Wu, L.F. Study on the Constituents of Artemisia-Annua L. 1. The Isolation and Identification of 11r-(-)-Dihydroarteannuic Acid. Acta Chim. Sinica 1987, 45, 609–612. [Google Scholar]
- Copes, W.E.; Ojiambo, P.S. Efficacy of peroxygen disinfestants against fungal plant pathogens. A systemic review and meta-analysis. Crop Prot. 2023, 164, 106143. [Google Scholar] [CrossRef]
- Larsen, B.; White, S. Antifungal Effect of Hydrogen Peroxide on Catalase-Producing Strains of Candida spp. Infect. Dis. Obstet. Gynecol. 1995, 3, 73–78. [Google Scholar] [CrossRef]
- Cerioni, L.; Rapisarda, V.A.; Hilal, M.; Prado, F.E.; Rodríguez-Montelongo, L. Synergistic Antifungal Activity of Sodium Hypochlorite, Hydrogen Peroxide, and Cupric Sulfate against Penicillium digitatum. J. Food Prot. 2009, 72, 1660–1665. [Google Scholar] [CrossRef]
- Finnegan, M.; Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010, 65, 2108–2115. [Google Scholar] [CrossRef]
- Wessels, S.; Ingmer, H. Modes of action of three disinfectant active substances: A review. Regul. Toxicol. Pharmacol. 2013, 67, 456–467. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar] [CrossRef]
- Tenhaken, R.; Levine, A.; Brisson, L.F.; Dixon, R.A.; Lamb, C. Function of the oxidative burst in hypersensitive disease resistance. Proc. Natl. Acad. Sci. USA 1995, 92, 4158–4163. [Google Scholar] [CrossRef]
- Van Camp, W.; Van Montagu, M.; Inzé, D. H2O2 and NO: Redox signals in disease resistance. Trends Plant Sci. 1998, 3, 330–334. [Google Scholar] [CrossRef]
- Jamison, M.T.; Dalisay, D.S.; Molinski, T.F. Peroxide Natural Products from Plakortis zyggompha and the Sponge Association Plakortis halichondrioides–Xestospongia deweerdtae: Antifungal Activity against Cryptococcus gattii. J. Nat. Prod. 2016, 79, 555–563. [Google Scholar] [CrossRef]
- Phillipson, D.W.; Rinehart, K.L. Antifungal peroxide-containing acids from two Caribbean sponges. J. Am. Chem. Soc. 2002, 105, 7735–7736. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Syromyatnikov, M.Y.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Popov, V.N.; Terent’ev, A.O. Cyclic Synthetic Peroxides Inhibit Growth of Entomopathogenic Fungus Ascosphaera apis without Toxic Effect on Bumblebees. Molecules 2020, 25, 1954. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Demina, A.A.; Fomenkov, D.I.; Barsukov, D.V.; Subbotina, I.R.; Fleury, F.; Terent’ev, A.O. Catalyst Development for the Synthesis of Ozonides and Tetraoxanes Under Heterogeneous Conditions: Disclosure of an Unprecedented Class of Fungicides for Agricultural Application. Chem-Eur. J. 2020, 26, 4734–4751. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Nagata, R.; Yuba, K.; Matsuura, T. Synthesis of α-silyloxyhydroperoxides from the reaction of silyl enol ethers and hydrogen peroxide. Tetrahedron Lett. 1983, 24, 1737–1740. [Google Scholar] [CrossRef]
- Terent’ev, A.; Yaremenko, I.; Vil’, V.; Dembitsky, V.; Nikishin, G. Boron Trifluoride as an Efficient Catalyst for the Selective Synthesis of Tricyclic Monoperoxides from β,δ-Triketones and H2O2. Synthesis 2012, 45, 246–250. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Belyakova, Y.Y.; Radulov, P.S.; Novikov, R.A.; Medvedev, M.G.; Krivoshchapov, N.V.; Korlyukov, A.A.; Alabugin, I.V.; Terent′ev, A.O. Inverse α-Effect as the Ariadne’s Thread on the Way to Tricyclic Aminoperoxides: Avoiding Thermodynamic Traps in the Labyrinth of Possibilities. J. Am. Chem. Soc. 2022, 144, 7264–7282. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Platonov, M.M.; Ogibin, Y.N.; Nikishin, G.I. Convenient Synthesis of Geminal Bishydroperoxides by the Reaction of Ketones with Hydrogen Peroxide. Synth. Commun. 2007, 37, 1281–1287. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Borisov, D.A.; Chernyshev, V.V.; Nikishin, G.I. Facile and Selective Procedure for the Synthesis of Bridged 1,2,4,5-Tetraoxanes; Strong Acids As Cosolvents and Catalysts for Addition of Hydrogen Peroxide to β-Diketones. J. Org. Chem. 2009, 74, 3335–3340. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Yaremenko, I.A.; Chernyshev, V.V.; Dembitsky, V.M.; Nikishin, G.I. Selective Synthesis of Cyclic Peroxides from Triketones and H2O2. J. Org. Chem. 2012, 77, 1833–1842. [Google Scholar] [CrossRef]
- Andreeva, E.I. Metodicheskie Rekomendatsii po Opredeleniyu Fungitsidnoi Aktivnosti Novykh Soedinenii [Guidelines for Determination of Fungicidal Activity of New Compounds]; NIITEKhIM: Cherkassy, Ukraine, 1984; p. 32. (In Russian) [Google Scholar]
- Popkov, S.V.; Kovalenko, L.V.; Bobylev, M.M.; Molchanov, O.Y.; Krimer, M.Z.; Tashchi, V.P.; Putsykin, Y.G. The Synthesis and Fungicidal Activity of 2-Substituted 1-Azol-1-ylmethyl-6-arylidenecyclohexanols. Pestic. Sci. 1997, 49, 125–129. [Google Scholar] [CrossRef]
- Itoh, H.; Kajino, H.; Tsukiyama, T.; Tobitsuka, J.; Ohta, H.; Takahi, Y.; Tsuda, M.; Takeshiba, H. Synthesis of silicon-containing azole derivatives with magnesium bromide diethyl etherate, and an investigation of their fungicidal activities. Bioorg. Med. Chem. 2002, 10, 4029–4034. [Google Scholar] [CrossRef]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef]
- Pastuszak, J.; Szczerba, A.; Dziurka, M.; Hornyák, M.; Kopeć, P.; Szklarczyk, M.; Płażek, A. Physiological and Biochemical Response to Fusarium culmorum Infection in Three Durum Wheat Genotypes at Seedling and Full Anthesis Stage. Int. J. Mol. Sci. 2021, 22, 7433. [Google Scholar] [CrossRef]
- Johansson, P.M.; Johnsson, L.; Gerhardson, B. Suppression of wheat-seedling diseases caused by Fusarium culmorum and Microdochium nivale using bacterial seed treatment. Plant Pathology 2003, 52, 219–227. [Google Scholar] [CrossRef]
- Bains, P.S.; Bennypaul, H.S.; Lynch, D.R.; Kawchuk, L.M.; Schaupmeyer, C.A. Rhizoctonia disease of potatoes (Rhizoctonia solani): Fungicidal efficacy and cultivar susceptibility. Am. J. Pot. Res. 2002, 79, 99–106. [Google Scholar] [CrossRef]
- Niu, Z.; Zheng, L.; Yang, P.; Wang, J.; Tian, M.; Pan, Y.; Zhao, D.; Yang, Z.; Zhu, J. Detection of Alternaria solani with high accuracy and sensitivity during the latent period of potato early blight. Front. Microbiol. 2022, 13, 1016996. [Google Scholar] [CrossRef]
- Chaerani, R.; Voorrips, R.E. Tomato early blight (Alternaria solani): The pathogen, genetics, and breeding for resistance. J. Gen. Plant. Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Dong, S.-m.; Zhou, S.-q. Potato late blight caused by Phytophthora infestans: From molecular interactions to integrated management strategies. J. Integr. Agric. 2022, 21, 3456–3466. [Google Scholar] [CrossRef]
- Belov, G.L.; Belosokhov, A.F.; Kutuzova, I.A.; Statsyuk, N.V.; Chudinova, E.M.; Alexandrova, A.V.; Kokaeva, L.Y.; Elansky, S.N. Colletotrichum coccodes in potato and tomato leaves in Russia. J. Plant. Dis. Prot. 2017, 125, 311–317. [Google Scholar] [CrossRef]
- Lees, A.K.; Hilton, A.J. Black dot (Colletotrichum coccodes): An increasingly important disease of potato. Plant Pathology 2003, 52, 3–12. [Google Scholar] [CrossRef]
- Karadimos, D.A.; Karaoglanidis, G.S.; Tzavella–Klonari, K. Biological activity and physical modes of action of the Qo inhibitor fungicides trifloxystrobin and pyraclostrobin against Cercospora beticola. Crop Prot. 2005, 24, 23–29. [Google Scholar] [CrossRef]
- Mitani, S.; Araki, S.; Yamaguchi, T.; Takii, Y.; Ohshima, T.; Matsuo, N. Biological properties of the novel fungicide cyazofamid againstPhytophthora infestans on tomato andPseudoperonospora cubensis on cucumber. Pest. Manag. Sci. 2002, 58, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Gisi, U. Differential Activity of Carboxylic Acid Amide Fungicides Against Various Developmental Stages of Phytophthora infestans. Phytopathology 2007, 97, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Sudisha, J.; Amruthesh, K.N.; Deepak, S.A.; Shetty, N.P.; Sarosh, B.R.; Shetty, H.S. Comparative efficacy of strobilurin fungicides against downy mildew disease of pearl millet. Pestic. Biochem. Physiol. 2005, 81, 188–197. [Google Scholar] [CrossRef]
- Horsfall, J.G.; Barrat, R.W. An improved grating system for measuring plant diseases. Phytopathology 1945, 35, 655. [Google Scholar]

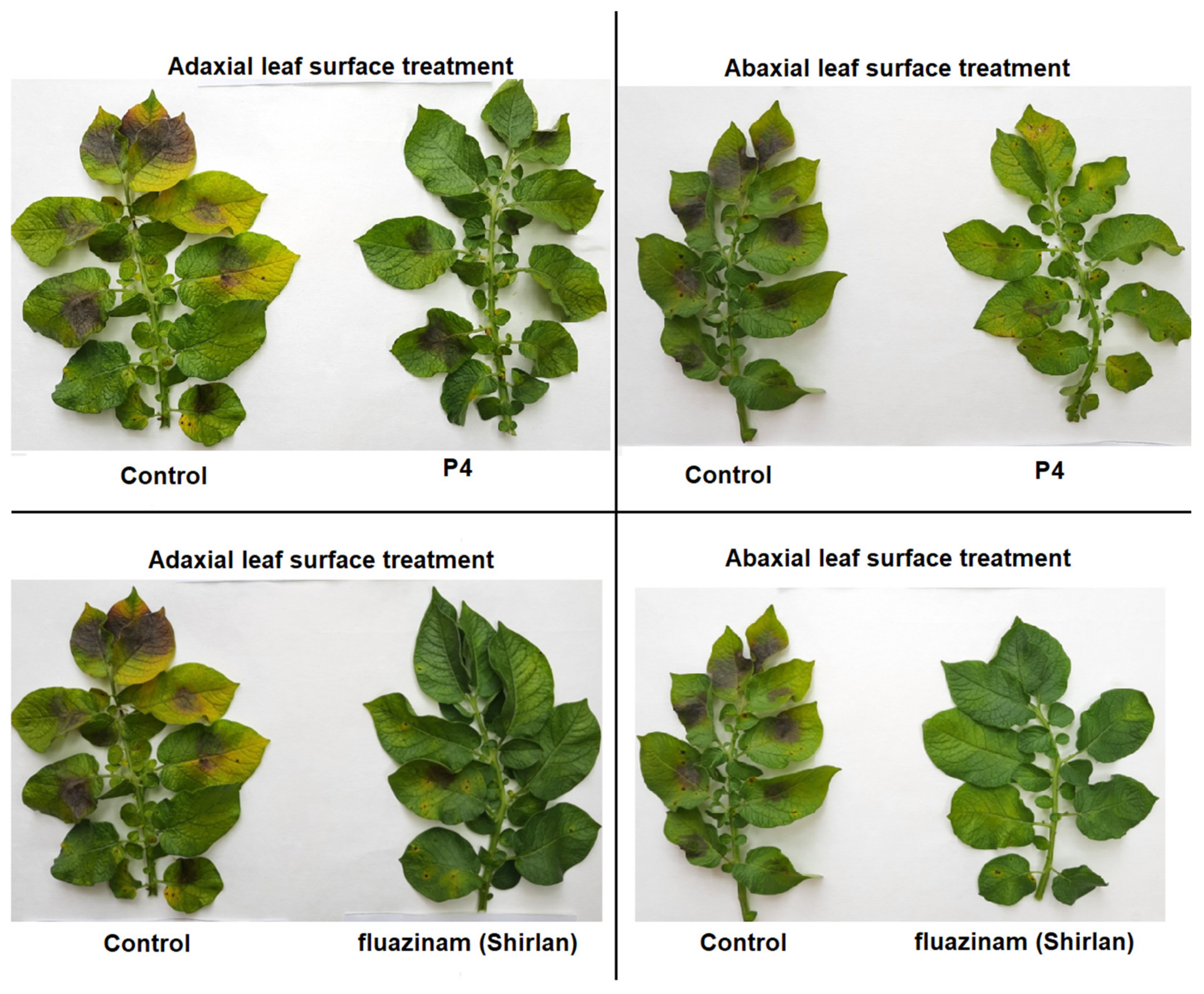
| No. | Fungicide Treatment | Application Dose (mg/mL) | Abaxial Leaf Surface Treatment 1 | Adaxial Leaf Surface Treatment 2 | ||
|---|---|---|---|---|---|---|
| Average Spot Growth Diameter, mm | Points | Average Spot Growth Diameter, mm | Points | |||
| 1 | P4 | 0.17 | 9.0 | 4.2 | 20.4 | 9.6 |
| 2 | fluazinam in Shirlan® | 2.0 | 0.5 | 0 | 8.6 | 4.8 |
| 3 | Control (H2O) | 22.8 | 11.4 | 23.8 | 11.4 | |
| LSD0.95 | 2.8 | 1.3 | 2.5 | 1.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaremenko, I.A.; Radulov, P.S.; Belyakova, Y.Y.; Fomenkov, D.I.; Vil’, V.A.; Kuznetsova, M.A.; Demidova, V.N.; Glinushkin, A.P.; Terent’ev, A.O. Cyclic Organic Peroxides as New Fungicides against Phytopathogenic Fungi. Agrochemicals 2023, 2, 355-366. https://doi.org/10.3390/agrochemicals2030021
Yaremenko IA, Radulov PS, Belyakova YY, Fomenkov DI, Vil’ VA, Kuznetsova MA, Demidova VN, Glinushkin AP, Terent’ev AO. Cyclic Organic Peroxides as New Fungicides against Phytopathogenic Fungi. Agrochemicals. 2023; 2(3):355-366. https://doi.org/10.3390/agrochemicals2030021
Chicago/Turabian StyleYaremenko, Ivan A., Peter S. Radulov, Yulia Yu. Belyakova, Dmitrii I. Fomenkov, Vera A. Vil’, Maria A. Kuznetsova, Valentina N. Demidova, Alexei P. Glinushkin, and Alexander O. Terent’ev. 2023. "Cyclic Organic Peroxides as New Fungicides against Phytopathogenic Fungi" Agrochemicals 2, no. 3: 355-366. https://doi.org/10.3390/agrochemicals2030021
APA StyleYaremenko, I. A., Radulov, P. S., Belyakova, Y. Y., Fomenkov, D. I., Vil’, V. A., Kuznetsova, M. A., Demidova, V. N., Glinushkin, A. P., & Terent’ev, A. O. (2023). Cyclic Organic Peroxides as New Fungicides against Phytopathogenic Fungi. Agrochemicals, 2(3), 355-366. https://doi.org/10.3390/agrochemicals2030021








