Evaluation of the Results of Pesticide Residue Analysis in Food Sampled between 2017 and 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Analyses of Pesticide Residues
2.3. Assessment of Compliance with Legal Limits (MRL)
- (a)
- the sampled lot is intended for the local market;
- (b)
- the lot is sampled before export.
3. Results
3.1. Summary of the Results of Pesticide Residue Monitoring during 2017–2021
3.2. Assessment of Compliance of Residues with MRLs
3.2.1. Commodities Marketed in Hungary
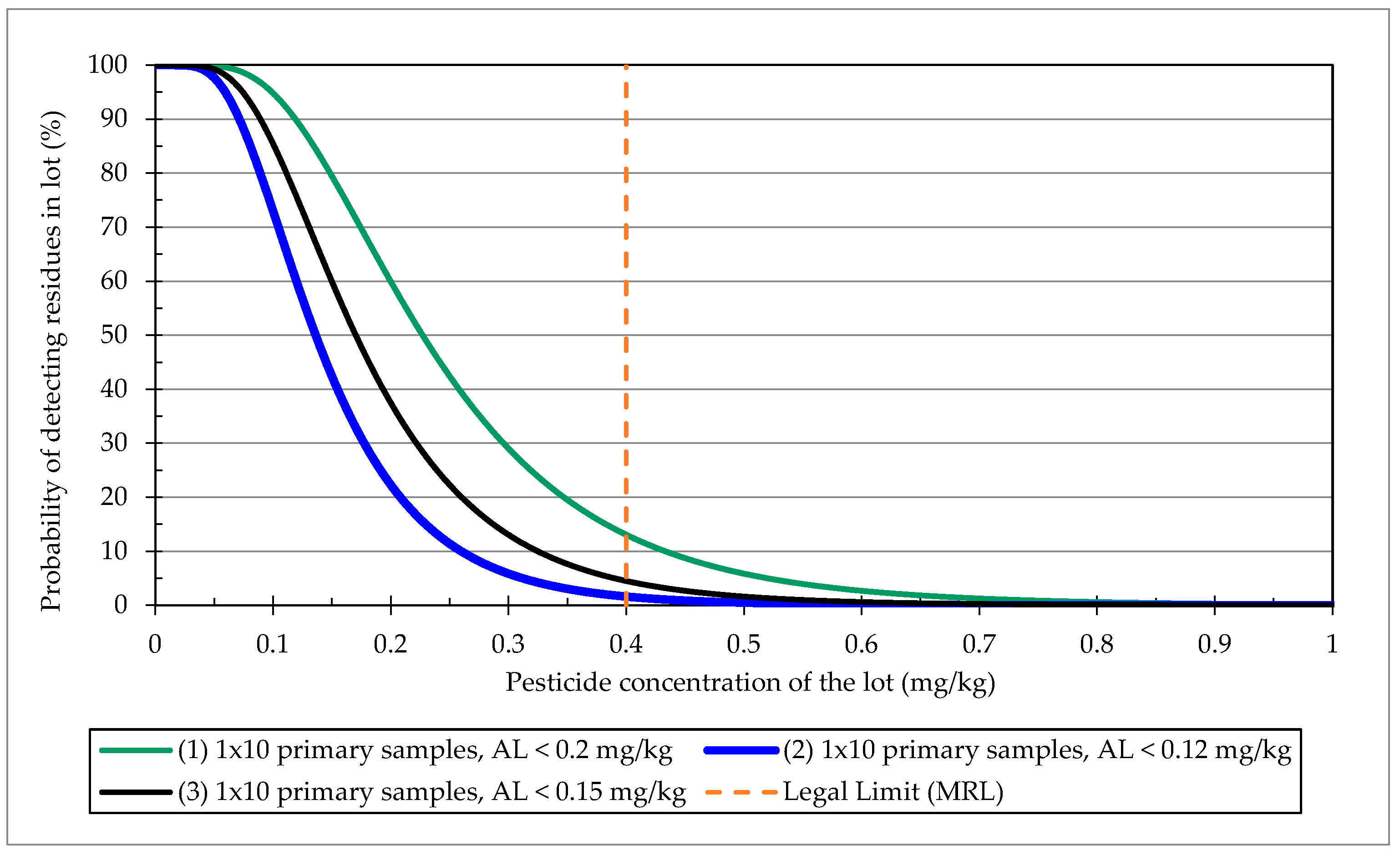
3.2.2. Prediction of Potential Compliance with MRLs if the Sampled Products Were Exported
- apple: chlorothalonil, chlorpyrifos, chlorpyrifos-methyl, fenhexamid, imidacloprid and methoxyfenozide;
- grape: chlorpyrifos, chlorpyrifos-methyl, diflubenzuron, dimethoate/omethoate, famoxadone, iprodione, pirimicarb and thiophanate-methyl;
- cherry: chlorpyrifos, dimethoate, omethoate, prochloraz;
- peach: chlorpyrifos, chlorpyrifos-methyl, diflubenzuron, fenbuconazole, imazalil, imidacloprid and propamocarb;
- peppers: buprofezin, chlorpyrifos-methyl, napropamid, triadimefon, triadimenol.
3.3. Evaluation of Plant Protection Practice
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AL | Action Limit |
| AS | Active Substance |
| EFSA | European Food Safety Authority |
| FAO | Food and Agriculture Organization |
| GAP | Good Agricultural Practices |
| JMPR | Joint Meeting on Pesticide Residues |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MRL | Maximum Residue Limit |
| NFCSO | Hungarian Food Chain Safety Office |
| US FDA | United States Food and Drug Administration |
| WHO | World Health Organization |
Appendix A
| Number of Tests | Apples | Cherries | Grapes | Green Peppers | Peaches and Nectarines | Active Substances 1 |
|---|---|---|---|---|---|---|
| No. of samples tested | 803 | 122 | 783 | 588 | 468 | 349 |
| No. of residues—matrix combinations tested | 227,571 | 32,962 | 113,132 | 165,388 | 90,851 | 60,458 |
| No. of ASs tested | 459 | 441 | 459 | 459 | 445 | 447 |
| Active Substances 1 | Apples | Cherries | Grapes, Table | Green Peppers | Peaches and Nectarines | Strawberries |
|---|---|---|---|---|---|---|
| 2,4-D | 202 | 52 | 212 | 148 | 80 | 99 |
| 2,4-DB | 48 | 12 | 42 | 19 | 13 | 32 |
| 2-Phenylphenol | 588 | 81 | 499 | 438 | 367 | 153 |
| 3,5-Dichloroaniline | 219 | 42 | 156 | 139 | 68 | 95 |
| 3-Chloroaniline | 158 | 40 | 125 | 90 | 53 | 70 |
| Abamectin (sum) | 449 | 41 | 166 | 346 | 190 | 92 |
| Acephate | 759 | 99 | 386 | 573 | 302 | 204 |
| Acetamiprid | 765 | 108 | 394 | 586 | 321 | 212 |
| Acetochlor | 694 | 102 | 316 | 489 | 278 | 178 |
| Aclonifen | 163 | 10 | 63 | 92 | 61 | 32 |
| Acrinathrin | 766 | 102 | 389 | 577 | 311 | 206 |
| Alachlor | 542 | 81 | 273 | 381 | 192 | 140 |
| Aldicarb (sum) | 572 | 92 | 343 | 471 | 248 | 165 |
| Aldrin and Dieldrin (sum) | 724 | 104 | 387 | 568 | 312 | 205 |
| Alphamethrin | 184 | 29 | 77 | 145 | 91 | 39 |
| Ametoctradin | 404 | 38 | 157 | 257 | 148 | 70 |
| Ametryn | 370 | 52 | 203 | 214 | 122 | 107 |
| Amidosulfuron | 140 | 18 | 36 | 97 | 75 | 35 |
| Aminopyralid | 14 | |||||
| Amitraz (sum) | 161 | 40 | 126 | 94 | 53 | 68 |
| AMPA | 14 | 1 | 11 | 15 | 4 | 2 |
| Atraton | 207 | 42 | 140 | 122 | 61 | 75 |
| Atrazine | 593 | 95 | 287 | 423 | 239 | 162 |
| Azamethiphos | 247 | 18 | 67 | 134 | 88 | 25 |
| Azinphos-ethyl | 700 | 103 | 320 | 494 | 287 | 178 |
| Azinphos-methyl | 769 | 103 | 393 | 581 | 318 | 206 |
| Aziprotryne | 370 | 52 | 203 | 214 | 122 | 107 |
| Azoxystrobin | 765 | 110 | 394 | 586 | 322 | 211 |
| Beflubutamid | 137 | 18 | 34 | 96 | 75 | 33 |
| Benalaxyl (sum of isomers) | 698 | 104 | 317 | 490 | 273 | 177 |
| Bendiocarb | 122 | 11 | 41 | 107 | 44 | 15 |
| Benfluralin | 379 | 71 | 210 | 289 | 131 | 108 |
| Bentazone (sum) | 218 | 41 | 104 | 207 | 103 | 48 |
| Benthiavalicarb (Benthiavalicarb-isopropyl) | 169 | 10 | 131 | 102 | 61 | 34 |
| Benzovindiflupyr | 151 | 13 | 320 | 83 | 31 | 8 |
| Bifenazate | 163 | 10 | 359 | 92 | 61 | 32 |
| Bifenox | 694 | 102 | 331 | 490 | 279 | 178 |
| Bifenthrin (sum of isomers) | 769 | 108 | 155 | 588 | 322 | 212 |
| Biphenyl | 565 | 79 | 316 | 417 | 210 | 151 |
| Bitertanol (sum of isomers) | 759 | 107 | 617 | 574 | 307 | 209 |
| Bixafen | 709 | 107 | 396 | 493 | 290 | 177 |
| Boscalid | 773 | 108 | 308 | 588 | 322 | 212 |
| Bromfenvinfos | 167 | 12 | 396 | 98 | 64 | 37 |
| Bromide ion | 14 | |||||
| Bromophos-methyl | 688 | 105 | 309 | 473 | 288 | 177 |
| Bromophos-ethyl | 691 | 105 | 170 | 477 | 287 | 177 |
| Bromopropylate | 771 | 108 | 395 | 587 | 321 | 212 |
| Bromoxynil and its salts | 382 | 65 | 356 | 246 | 132 | 106 |
| Bromuconazole (sum of diasteroisomers) | 696 | 108 | 63 | 499 | 290 | 184 |
| Bupirimate | 779 | 111 | 393 | 585 | 324 | 215 |
| Buprofezin | 759 | 108 | 203 | 583 | 314 | 211 |
| Butocarboxim | 146 | 18 | 70 | 103 | 77 | 35 |
| Butralin | 172 | 29 | 39 | 167 | 70 | 33 |
| Butylate | 370 | 52 | 39 | 214 | 122 | 107 |
| Cadusafos | 698 | 106 | 362 | 495 | 98 | 179 |
| Captafol | 221 | 32 | 291 | 200 | 298 | 55 |
| Captan (sum) | 692 | 99 | 386 | 540 | 304 | 211 |
| Carbaryl | 754 | 101 | 380 | 576 | 322 | 205 |
| Carbendazim and benomyl (sum) | 765 | 108 | 318 | 586 | 297 | 212 |
| Carbofuran (sum) | 697 | 103 | 336 | 556 | 231 | 349 |
| Carboxin | 696 | 108 | 39 | 499 | 16 | 184 |
| Carfentrazone-ethyl (sum) | 293 | 40 | 210 | 243 | 288 | 54 |
| Chinomethionat | 379 | 71 | 73 | 289 | 156 | 108 |
| Chlorantraniliprole | 766 | 108 | 7 | 578 | 228 | 202 |
| Chlorbromuron | 163 | 10 | 361 | 92 | 280 | 32 |
| Chlordane (sum of cis- and trans-chlordane) | 653 | 104 | 40 | 468 | 280 | 171 |
| Chlorfenapyr | 692 | 99 | 140 | 541 | 271 | 205 |
| Chlorfenson | 207 | 42 | 19 | 122 | 39 | 75 |
| Chlorfenvinphos | 690 | 102 | 58 | 487 | 61 | 177 |
| Chlorfluazuron | 134 | 15 | 36 | 73 | 75 | 11 |
| Chloridazon | 506 | 98 | 380 | 389 | 16 | 151 |
| Chlorobenzilate | 374 | 54 | 307 | 220 | 61 | 108 |
| Chlorothalonil | 772 | 108 | 390 | 587 | 283 | 212 |
| Chlorotoluron | 706 | 107 | 285 | 494 | 307 | 182 |
| Chloroxuron | 140 | 18 | 397 | 100 | 321 | 35 |
| Chlorpropham | 578 | 81 | 396 | 435 | 18 | 145 |
| Chlorpyrifos | 803 | 108 | 311 | 588 | 321 | 212 |
| Chlorpyrifos-methyl | 803 | 108 | 1 | 588 | 215 | 212 |
| Chlorsulfuron | 1 | 4 | 318 | 14 | 321 | 10 |
| Chlozolinate | 208 | 42 | 63 | 123 | 123 | 71 |
| Cinidon-ethyl | 370 | 52 | 398 | 214 | 122 | 107 |
| Clethodim (sum) | 184 | 30 | 318 | 167 | 314 | 33 |
| Clofentezine | 759 | 108 | 1 | 583 | 283 | 211 |
| Clomazone | 706 | 107 | 393 | 494 | 321 | 182 |
| Clopyralid | 1 | 248 | ||||
| Clothianidin | 722 | 99 | 206 | 535 | 60 | 189 |
| Coumaphos | 653 | 93 | 321 | 443 | 322 | 173 |
| Cyanazine | 113 | 10 | 140 | 58 | 32 | 10 |
| Cyanofenphos | 207 | 42 | 3 | 122 | 61 | 75 |
| Cyantraniliprole | 47 | 2 | 328 | 7 | 8 | |
| Cyazofamid | 656 | 105 | 53 | 491 | 299 | 186 |
| Cycloate | 516 | 70 | 71 | 316 | 199 | 142 |
| Cycloxydim (sum) | 169 | 10 | 394 | 102 | 61 | 34 |
| Cyflufenamid | 273 | 45 | 186 | 254 | 102 | 37 |
| Cyfluthrin (sum of isomers) | 741 | 102 | 553 | 569 | 299 | 187 |
| Cymoxanil | 765 | 108 | 203 | 586 | 322 | 212 |
| Cypermethrin (sum of isomers) | 802 | 108 | 394 | 588 | 321 | 212 |
| Cyproconazole | 754 | 106 | 15 | 576 | 305 | 203 |
| Cyprodinil | 780 | 107 | 388 | 587 | 315 | 211 |
| Cyprosulfamide | 5 | 2 | ||||
| Cyromazine | 21 | 307 | 70 | 8 | 14 | |
| Dazomet | 58 | 17 | 322 | 17 | 14 | 40 |
| DDT | 701 | 108 | 322 | 501 | 290 | 184 |
| Deltamethrin | 803 | 108 | 204 | 588 | 322 | 212 |
| Demeton-S-Methyl | 367 | 54 | 82 | 219 | 124 | 108 |
| Desethyl-Atrazine | 552 | 86 | 275 | 385 | 196 | 144 |
| Desisopropyl-Atrazine | 552 | 86 | 214 | 385 | 196 | 144 |
| Desmedipham | 402 | 63 | 189 | 257 | 168 | 129 |
| Dialifos | 207 | 42 | 395 | 122 | 61 | 75 |
| Diazinon | 773 | 108 | 387 | 588 | 322 | 212 |
| Dicamba | 146 | 48 | 252 | 97 | 62 | 72 |
| Dichlobenil | 525 | 94 | 63 | 396 | 212 | 145 |
| Dichlofenthion | 535 | 96 | 320 | 401 | 227 | 145 |
| Dichlofluanid | 696 | 106 | 336 | 493 | 287 | 183 |
| Dichlormid | 338 | 59 | 146 | 276 | 121 | 94 |
| Dichlorprop | 210 | 52 | 74 | 139 | 74 | 82 |
| Dichlorvos | 771 | 108 | 176 | 586 | 322 | 212 |
| Diclobutrazol | 163 | 10 | 259 | 91 | 32 | 10 |
| Dicloran | 595 | 81 | 394 | 457 | 215 | 155 |
| Dicofol (sum of p, p’ and o, p’ isomers) | 712 | 99 | 615 | 519 | 340 | 213 |
| Dicrotophos | 372 | 62 | 1 | 232 | 135 | 114 |
| Diethofencarb | 765 | 108 | 318 | 586 | 322 | 212 |
| Difenoconazole | 766 | 108 | 141 | 586 | 322 | 212 |
| Diflovidazin (Flufenzin) | 204 | 44 | 394 | 127 | 63 | 76 |
| Diflubenzuron | 765 | 108 | 321 | 586 | 322 | 212 |
| Diflufenican | 696 | 108 | 120 | 499 | 290 | 184 |
| Dimethachlor | 700 | 107 | 203 | 491 | 275 | 181 |
| Dimethenamid (sum of isomers) | 370 | 52 | 286 | 214 | 122 | 107 |
| Dimethipin | 379 | 71 | 394 | 289 | 131 | 108 |
| Dimethoate | 765 | 108 | 101 | 586 | 322 | 212 |
| Dimethomorph (sum of isomers) | 767 | 108 | 318 | 586 | 322 | 212 |
| Dimoxystrobin | 707 | 107 | 393 | 494 | 283 | 182 |
| Diniconazole (sum of isomers) | 759 | 108 | 140 | 583 | 314 | 211 |
| Dioxacarb | 1 | |||||
| Dioxathion | 207 | 42 | 273 | 122 | 61 | 75 |
| Diphenylamine | 591 | 81 | 394 | 439 | 215 | 153 |
| Diquat | 317 | 1 | ||||
| Disulfoton (sum) | 488 | 79 | 255 | 348 | 184 | 133 |
| Ditalimfos | 698 | 106 | 142 | 493 | 288 | 177 |
| Dithianon | 243 | 30 | 324 | 172 | 105 | 35 |
| Dithiocarbamates | 605 | 77 | 320 | 399 | 247 | 176 |
| Diuron | 690 | 108 | 202 | 496 | 282 | 183 |
| Dodine | 423 | 37 | 157 | 335 | 169 | 90 |
| Emamectin B1a (free base) | 421 | 52 | 535 | 337 | 171 | 84 |
| Endosulfan (sum) | 772 | 108 | 395 | 588 | 322 | 212 |
| Endrin | 701 | 108 | 322 | 501 | 290 | 184 |
| Endrin Aldehyde | 692 | 108 | 109 | 495 | 290 | 178 |
| Endrin, Keto- | 312 | 37 | 394 | 205 | 160 | 74 |
| EPN | 769 | 106 | 362 | 535 | 319 | 207 |
| Epoxiconazole | 766 | 108 | 29 | 586 | 322 | 212 |
| epsilon-HCH | 48 | 8 | 35 | 48 | 25 | 13 |
| EPTC (ethyl dipropylthiocarbamate) | 207 | 42 | 140 | 122 | 61 | 75 |
| Ethephon | 60 | 46 | 44 | 30 | ||
| Ethiofencarb | 119 | 7 | 46 | 68 | 32 | 12 |
| Ethiofencarb-Sulfone | 119 | 7 | 46 | 68 | 32 | 12 |
| Ethiofencarb-Sulfoxide | 119 | 7 | 392 | 68 | 32 | 12 |
| Ethion | 767 | 103 | 394 | 579 | 318 | 206 |
| Ethirimol | 765 | 108 | 388 | 586 | 322 | 210 |
| Ethofumesate | 367 | 54 | 126 | 219 | 124 | 108 |
| Ethoprophos | 699 | 106 | 157 | 495 | 288 | 179 |
| Ethoxyquin | 222 | 55 | 143 | 164 | 116 | 93 |
| Etofenprox | 781 | 99 | 204 | 575 | 301 | 203 |
| Etoxazole | 404 | 38 | 148 | 257 | 148 | 74 |
| Etridiazole | 208 | 42 | 322 | 123 | 60 | 71 |
| Etrimfos | 698 | 106 | 327 | 495 | 288 | 179 |
| Famoxadone | 592 | 90 | 393 | 458 | 229 | 171 |
| Fenamidone | 759 | 108 | 396 | 583 | 314 | 211 |
| Fenamiphos (sum) | 566 | 88 | 324 | 450 | 228 | 155 |
| Fenarimol | 760 | 102 | 393 | 574 | 303 | 205 |
| Fenazaquin | 759 | 108 | 394 | 583 | 314 | 211 |
| Fenbuconazole | 763 | 108 | 18 | 584 | 317 | 211 |
| Fenbutatin oxide | 47 | 394 | 27 | 19 | 9 | |
| Fenchlorphos (sum) | 487 | 92 | 245 | 372 | 217 | 138 |
| Fenhexamid | 765 | 108 | 391 | 586 | 322 | 212 |
| Fenitrothion | 765 | 106 | 140 | 571 | 317 | 193 |
| Fenoxycarb | 765 | 108 | 1 | 586 | 322 | 212 |
| Fenpicoxamid | 15 | 151 | 3 | |||
| Fenpropathrin | 771 | 106 | 392 | 582 | 319 | 207 |
| Fenpropidin | 763 | 108 | 392 | 585 | 320 | 212 |
| Fenpropimorph (sum of isomers) | 706 | 90 | 308 | 524 | 256 | 171 |
| Fenpyrazamine | 276 | 40 | 394 | 260 | 96 | 34 |
| Fenpyroximate | 765 | 108 | 396 | 586 | 322 | 212 |
| Fenson (Fenison) | 207 | 42 | 388 | 122 | 61 | 75 |
| Fensulfothion | 649 | 100 | 39 | 458 | 278 | 168 |
| Fensulfothion-Oxon | 113 | 7 | 39 | 58 | 32 | 10 |
| Fensulfothion-Sulfone | 113 | 7 | 388 | 58 | 32 | 10 |
| Fenthion (sum) | 515 | 83 | 242 | 399 | 221 | 145 |
| Fenuron | 1 | 4 | 398 | 14 | 18 | 10 |
| Fenvalerate (sum) | 801 | 108 | 140 | 588 | 322 | 212 |
| Fipronil (sum) | 733 | 103 | 390 | 570 | 313 | 204 |
| Flazasulfuron | 137 | 18 | 316 | 96 | 75 | 33 |
| Flonicamid (sum) | 447 | 78 | 255 | 353 | 176 | 110 |
| Florasulam | 385 | 80 | 138 | 302 | 151 | 116 |
| Fluazifop-P | 180 | 52 | 333 | 152 | 73 | 88 |
| Fluazifop-P-butyl | 22 | 270 | 46 | 9 | 18 | |
| Fluazinam | 690 | 105 | 211 | 495 | 281 | 182 |
| Flubendiamide | 580 | 74 | 394 | 468 | 261 | 126 |
| Flucythrinate (sum of isomers) | 376 | 73 | 297 | 294 | 133 | 109 |
| Fludioxonil | 766 | 108 | 392 | 586 | 322 | 212 |
| Flufenacet | 646 | 105 | 3 | 465 | 261 | 162 |
| Flufenoxuron | 741 | 108 | 39 | 575 | 305 | 188 |
| Flumethrin | 113 | 7 | 243 | 58 | 32 | 10 |
| Flumetralin | 6 | 141 | 5 | |||
| Flumioxazine | 341 | 39 | 377 | 269 | 131 | 67 |
| Fluometuron | 513 | 72 | 351 | 322 | 201 | 143 |
| Fluopicolide | 764 | 105 | 268 | 573 | 321 | 210 |
| Fluopyram | 615 | 92 | 175 | 475 | 230 | 163 |
| Fluoxastrobin | 500 | 58 | 388 | 359 | 210 | 101 |
| Flupyradifurone | 29 | 3 | 3 | |||
| Fluquinconazole | 755 | 104 | 15 | 578 | 305 | 206 |
| Flurochloridone | 597 | 88 | 1 | 376 | 230 | 153 |
| Fluroxypyr (sum) | 16 | 11 | 246 | 25 | 38 | 17 |
| Flusilazole | 761 | 104 | 354 | 580 | 313 | 207 |
| Flutolanil | 503 | 78 | 47 | 332 | 171 | 131 |
| Flutriafol | 771 | 104 | 182 | 578 | 313 | 206 |
| Fluvalinate (sum of isomers) | 768 | 106 | 783 | 581 | 410 | 249 |
| Fluxapyroxad | 421 | 49 | 361 | 296 | 179 | 88 |
| Folpet (sum) | 684 | 92 | 252 | 522 | 270 | 192 |
| Fomesafen | 163 | 10 | 39 | 92 | 61 | 32 |
| Fonofos | 521 | 77 | 297 | 327 | 210 | 143 |
| Foramsulfuron | 146 | 18 | 204 | 103 | 77 | 35 |
| Forchlorfenuron | 140 | 18 | 292 | 100 | 75 | 35 |
| Formetanate | 429 | 77 | 322 | 359 | 166 | 128 |
| Formothion | 624 | 92 | 8 | 428 | 243 | 161 |
| Fosetyl-Al (efozit-Al) | 330 | 24 | ||||
| Fosthiazate | 763 | 108 | 354 | 586 | 201 | 203 |
| Fuberidazole | 308 | 28 | 140 | 195 | 130 | 67 |
| Furilazole | 207 | 42 | 7 | 122 | 32 | 75 |
| Glufosinate | 27 | 11 | ||||
| Glyphosate | 82 | 2 | 36 | 27 | 75 | 17 |
| Halosulfuron methyl | 140 | 18 | 138 | 100 | 8 | 35 |
| Haloxyfop | 201 | 52 | 592 | 208 | 353 | 104 |
| Heptachlor (sum) | 653 | 104 | 307 | 471 | 280 | 177 |
| Heptenophos | 698 | 106 | 322 | 495 | 143 | 179 |
| Hexachlorobenzene | 701 | 108 | 205 | 501 | 315 | 184 |
| Hexachlorocyclohexane, alpha-isomer | 700 | 108 | 322 | 501 | 290 | 183 |
| Hexachlorocyclohexane, beta-isomer | 700 | 108 | 495 | 501 | 290 | 183 |
| Hexachlorocyclohexane, delta-isomer | 679 | 100 | 398 | 482 | 276 | 176 |
| Hexaconazole | 775 | 107 | 394 | 581 | 124 | 210 |
| Hexaflumuron | 394 | 60 | 391 | 245 | 290 | 110 |
| Hexazinone | 382 | 54 | 394 | 221 | 322 | 108 |
| Hexythiazox | 765 | 108 | 207 | 586 | 322 | 212 |
| Imazalil | 765 | 108 | 128 | 586 | 143 | 212 |
| Imazamox | 353 | 78 | 7 | 278 | 53 | 109 |
| Imazapyr | 164 | 40 | 394 | 100 | 321 | 69 |
| Imazethapyr | 6 | 391 | 10 | 2 | ||
| Imidacloprid | 765 | 108 | 141 | 586 | 315 | 212 |
| Indoxacarb | 775 | 107 | 141 | 581 | 63 | 210 |
| Iodosulfuron-methyl | 184 | 27 | 94 | 164 | 288 | 33 |
| Ioxynil | 204 | 44 | 389 | 127 | 199 | 76 |
| Ipconazole | 516 | 70 | 394 | 317 | 313 | 142 |
| Iprodione | 765 | 104 | 38 | 578 | 322 | 206 |
| Iprovalicarb | 765 | 108 | 322 | 586 | 35 | 212 |
| Isocarbophos | 768 | 107 | 204 | 576 | 68 | 208 |
| Isodrin | 116 | 12 | 312 | 64 | 287 | 15 |
| Isofenphos | 698 | 106 | 390 | 495 | 270 | 178 |
| Isofenphos-methyl | 688 | 99 | 90 | 484 | 305 | 176 |
| Isoprocarb | 367 | 54 | 249 | 219 | 284 | 108 |
| Isoprothiolane | 693 | 108 | 34 | 508 | 178 | 197 |
| Isoproturon | 493 | 79 | 210 | 337 | 75 | 133 |
| Isopyrazam | 205 | 18 | 335 | 120 | 124 | 35 |
| Isoxaben | 137 | 18 | 56 | 96 | 143 | 33 |
| Isoxadifen-ethyl | 431 | 53 | 140 | 301 | 42 | 106 |
| Isoxaflutole | 376 | 60 | 393 | 239 | 175 | 109 |
| Kresoxim-methyl | 770 | 107 | 398 | 578 | 251 | 209 |
| Lambda-cyhalothrin | 802 | 108 | 322 | 588 | 290 | 213 |
| Lenacil | 696 | 108 | 394 | 499 | 290 | 184 |
| Lindane | 701 | 108 | 380 | 501 | 322 | 184 |
| Linuron | 765 | 108 | 371 | 586 | 276 | 212 |
| Lufenuron | 719 | 99 | 383 | 535 | 287 | 180 |
| Malathion (sum) | 721 | 100 | 383 | 564 | 322 | 199 |
| Mandipropamid | 765 | 108 | 319 | 586 | 70 | 212 |
| MCPA and MCPB | 180 | 52 | 138 | 121 | 73 | 81 |
| Mecarbam | 688 | 108 | 389 | 493 | 73 | 183 |
| Mecoprop (sum) | 180 | 52 | 169 | 121 | 305 | 81 |
| Mefenpyr-diethyl | 172 | 29 | 138 | 167 | 282 | 33 |
| Mepanipyrim | 764 | 104 | 204 | 575 | 124 | 205 |
| Mepiquat | 141 | |||||
| Mepronil | 367 | 54 | 36 | 219 | 63 | 108 |
| Meptyldinocap | 204 | 44 | 281 | 127 | 75 | 76 |
| Mesosulfuron-methyl | 146 | 18 | 3 | 103 | 62 | 35 |
| Mesotrione | 194 | 44 | 391 | 113 | 315 | 76 |
| Metaflumizone (sum of E- and Z- isomers) | 548 | 90 | 2 | 395 | 265 | 148 |
| Metalaxyl and metalaxyl-M (sum of isomers) | 771 | 104 | 455 | 578 | 373 | 206 |
| Metaldehyde | 163 | 10 | 321 | 92 | 318 | 32 |
| Metamitron | 696 | 108 | 390 | 499 | 275 | 184 |
| Metazachlor | 700 | 107 | 395 | 491 | 302 | 181 |
| Metconazole (sum of isomers) | 688 | 105 | 345 | 498 | 287 | 177 |
| Methabenzthiazuron | 140 | 18 | 305 | 100 | 212 | 35 |
| Methacrifos | 616 | 100 | 388 | 461 | 1 | 167 |
| Methamidophos | 767 | 103 | 317 | 579 | 290 | 206 |
| Methidathion | 760 | 102 | 395 | 575 | 321 | 201 |
| Methiocarb (sum) | 598 | 90 | 327 | 455 | 316 | 162 |
| Methomyl | 766 | 108 | 394 | 586 | 322 | 212 |
| Methoxychlor | 701 | 108 | 356 | 501 | 32 | 184 |
| Methoxyfenozide | 765 | 108 | 39 | 586 | 290 | 212 |
| Metobromuron | 696 | 108 | 32 | 499 | 92 | 184 |
| Metolachlor and S-metolachlor (sum of isomers) | 705 | 98 | 678 | 496 | 468 | 171 |
| Metoxuron | 113 | 10 | 316 | 58 | 322 | 10 |
| Metrafenone | 764 | 108 | 113 | 547 | 273 | 211 |
| Metribuzin | 695 | 104 | 321 | 487 | 157 | 177 |
| Metsulfuron-methyl | 332 | 48 | 39 | 270 | 288 | 70 |
| Mevinphos | 698 | 106 | 133 | 493 | 77 | 179 |
| Molinate | 516 | 70 | 63 | 317 | 291 | 142 |
| Monocrotophos | 686 | 98 | 203 | 534 | 61 | 195 |
| Monolinuron | 163 | 10 | 39 | 92 | 122 | 32 |
| Myclobutanil | 775 | 107 | 362 | 581 | 199 | 210 |
| N,N-Diethyl-m-toluamid (DEET) | 652 | 105 | 302 | 462 | 266 | 174 |
| Napropamide (sum of isomers) | 370 | 52 | 243 | 214 | 77 | 107 |
| Nicosulfuron | 146 | 18 | 167 | 103 | 201 | 35 |
| Nitenpyram | 513 | 72 | 204 | 322 | 90 | 143 |
| Nitrofen | 300 | 49 | 203 | 165 | 124 | 84 |
| Novaluron | 367 | 54 | 294 | 219 | 122 | 108 |
| Nuarimol | 370 | 52 | 112 | 214 | 249 | 107 |
| o.p’-DDD | 631 | 92 | 322 | 434 | 251 | 164 |
| o.p’-DDE | 631 | 92 | 203 | 434 | 290 | 164 |
| Ofurace | 370 | 52 | 73 | 214 | 309 | 107 |
| Omethoate | 728 | 108 | 393 | 575 | 105 | 205 |
| Oxadiazon | 198 | 19 | 394 | 130 | 314 | 53 |
| Oxadixyl | 759 | 108 | 39 | 583 | 322 | 211 |
| Oxamyl | 765 | 108 | 1 | 586 | 77 | 212 |
| Oxasulfuron | 146 | 18 | 247 | 103 | 168 | 35 |
| Oxathiapiprolin | 15 | 284 | 3 | 118 | ||
| Oxycarboxin | 163 | 10 | 322 | 92 | 249 | 172 |
| Oxydemeton-methyl (sum) | 551 | 83 | 374 | 442 | 216 | 161 |
| Oxyfluorfen | 492 | 78 | 294 | 347 | 61 | 32 |
| Paclobutrazol | 760 | 104 | 389 | 580 | 218 | 140 |
| Paraoxon | 526 | 77 | 362 | 324 | 318 | 206 |
| Parathion | 773 | 108 | 391 | 541 | 322 | 212 |
| Parathion-methyl (sum) | 768 | 106 | 391 | 580 | 318 | 211 |
| Penconazole | 772 | 104 | 110 | 578 | 269 | 175 |
| Pencycuron | 765 | 108 | 87 | 586 | 315 | 210 |
| Pendimethalin | 775 | 107 | 390 | 581 | 68 | 33 |
| Penflufen (sum of isomers) | 205 | 18 | 303 | 115 | 313 | 206 |
| Penthiopyrad | 282 | 42 | 203 | 202 | 321 | 212 |
| perchlorate | 1 | 107 | ||||
| Permethrin (sum of isomers) | 772 | 108 | 242 | 587 | 122 | 142 |
| Pethoxamid | 370 | 52 | 320 | 214 | 199 | 182 |
| Phenkapton | 207 | 42 | 245 | 122 | 61 | 75 |
| Phenmedipham | 402 | 63 | 394 | 257 | 168 | 129 |
| Phenthoate | 516 | 70 | 5 | 317 | 200 | 143 |
| Phorate (sum) | 342 | 78 | 41 | 282 | 158 | 115 |
| Phorate (sum) | 700 | 106 | 8 | 495 | 288 | 179 |
| Phosmet (sum) | 595 | 81 | 394 | 438 | 216 | 151 |
| Phosphamidon | 700 | 106 | 358 | 495 | 288 | 179 |
| Phosphane and phosphide salts | 1 | 320 | 12 | |||
| Phoxim | 513 | 72 | 102 | 323 | 270 | 143 |
| Picolinafen | 516 | 70 | 229 | 317 | 282 | 139 |
| Picoxystrobin | 690 | 108 | 63 | 496 | 244 | 32 |
| Piperonyl butoxide | 163 | 10 | 6 | 92 | 53 | 9 |
| Pirimicarb | 773 | 103 | 394 | 579 | 102 | 212 |
| Pirimicarb, desmethyl- | 333 | 63 | 140 | 219 | 107 | 110 |
| Pirimiphos-ethyl | 654 | 94 | 389 | 476 | 319 | 207 |
| Pirimiphos-methyl | 769 | 106 | 17 | 582 | 313 | 19 |
| Prochloraz (sum) | 507 | 87 | 54 | 412 | 42 | 18 |
| Procymidone | 750 | 99 | 388 | 569 | 199 | 205 |
| Profenofos | 759 | 102 | 348 | 574 | 131 | 178 |
| Profluralin | 379 | 71 | 255 | 289 | 273 | 133 |
| Promecarb | 326 | 48 | 245 | 267 | 215 | 138 |
| Prometryn | 571 | 86 | 394 | 398 | 201 | 212 |
| Propachlor | 516 | 73 | 337 | 316 | 298 | 184 |
| Propamocarb | 715 | 108 | 359 | 586 | 252 | 178 |
| Propaquizafop | 467 | 68 | 203 | 290 | 279 | 107 |
| Propargite | 650 | 96 | 102 | 518 | 122 | 67 |
| Propazine | 370 | 52 | 390 | 214 | 138 | 209 |
| Propetamphos | 309 | 28 | 394 | 195 | 307 | 212 |
| Propham | 516 | 70 | 210 | 317 | 303 | 108 |
| Propiconazole (sum of isomers) | 768 | 107 | 276 | 578 | 322 | 141 |
| Propisochlor | 547 | 81 | 321 | 387 | 282 | 162 |
| Propoxur | 690 | 108 | 271 | 496 | 242 | 137 |
| Propyzamide | 765 | 108 | 320 | 586 | 192 | 183 |
| Proquinazid | 618 | 89 | 63 | 474 | 274 | 32 |
| Prosulfocarb | 605 | 74 | 309 | 483 | 61 | 180 |
| Prosulfuron | 163 | 10 | 178 | 92 | 291 | 80 |
| Prothioconazole: prothioconazole-desthio (sum of isomers) | 542 | 90 | 333 | 450 | 296 | 184 |
| Prothiofos | 693 | 107 | 102 | 481 | 136 | 67 |
| Pymetrozine | 540 | 74 | 43 | 482 | 61 | 20 |
| Pyraclostrobin | 765 | 108 | 164 | 586 | 288 | 78 |
| Pyraflufen-ethyl | 135 | 19 | 394 | 111 | 322 | 211 |
| Pyrazophos | 698 | 106 | 393 | 495 | 85 | 211 |
| Pyrethrins | 271 | 47 | 320 | 152 | 313 | 178 |
| Pyridaben | 759 | 108 | 100 | 583 | 286 | 6 |
| Pyridalyl | 151 | 13 | 204 | 134 | 143 | 108 |
| Pyridaphenthion | 696 | 102 | 207 | 486 | 31 | 109 |
| Pyridate | 353 | 78 | 391 | 278 | 124 | 210 |
| Pyrifenox | 367 | 54 | 285 | 219 | 315 | 164 |
| Pyrimethanil | 776 | 107 | 395 | 581 | 277 | 207 |
| Pyriofenone | 273 | 36 | 70 | 201 | 322 | 33 |
| Pyriproxyfen | 765 | 108 | 383 | 586 | 78 | 204 |
| Pyroxsulam | 184 | 27 | 242 | 161 | 301 | 142 |
| Quinalphos | 700 | 106 | 117 | 495 | 131 | 179 |
| Quinmerac | 503 | 98 | 393 | 388 | 68 | 211 |
| Quinoclamine | 190 | 18 | 300 | 112 | 314 | 170 |
| Quinoxyfen | 759 | 108 | 304 | 583 | 262 | 175 |
| Quintozene (sum) | 658 | 102 | 204 | 466 | 123 | 25 |
| Resmethrin (sum of isomers) | 366 | 54 | 243 | 216 | 63 | 143 |
| Rimsulfuron | 69 | 15 | 203 | 75 | 201 | 107 |
| Rotenone | 513 | 72 | 63 | 322 | 122 | 32 |
| Secbumeton | 309 | 28 | 102 | 196 | 138 | 177 |
| Sedaxane | 26 | 4 | 3 | 67 | ||
| Silthiofam | 309 | 65 | 196 | 168 | ||
| Simazine | 370 | 52 | 104 | 214 | 32 | 10 |
| Simetryn | 113 | 10 | 394 | 91 | 31 | 212 |
| Spinetoram (XDE-175) | 151 | 13 | 394 | 139 | 322 | 212 |
| Spinosad (sum) | 765 | 108 | 394 | 586 | 322 | 212 |
| Spirodiclofen | 765 | 108 | 187 | 586 | 317 | 78 |
| Spiromesifen | 770 | 106 | 38 | 582 | 175 | 39 |
| Spirotetramat (sum) | 540 | 67 | 344 | 406 | 324 | 142 |
| Spiroxamine (sum of isomers) | 775 | 107 | 102 | 581 | 138 | 2 |
| Sulfotep | 690 | 102 | 346 | 487 | 31 | 211 |
| Sulfoxaflor (sum of isomers) | 151 | 13 | 393 | 83 | 224 | 212 |
| Tau-Fluvalinate | 613 | 81 | 394 | 468 | 314 | 206 |
| Tebuconazole | 774 | 104 | 393 | 580 | 226 | 205 |
| Tebufenozide | 759 | 108 | 391 | 583 | 322 | 147 |
| Tebufenpyrad | 766 | 108 | 260 | 586 | 313 | 211 |
| Tecnazene | 535 | 96 | 388 | 403 | 314 | 33 |
| Teflubenzuron | 759 | 108 | 34 | 583 | 303 | 33 |
| Tefluthrin | 760 | 102 | 34 | 574 | 75 | 144 |
| Tepraloxydim | 137 | 18 | 322 | 96 | 201 | 32 |
| Terbacil | 516 | 73 | 63 | 320 | 288 | 10 |
| Terbufos | 698 | 106 | 39 | 494 | 61 | 10 |
| Terbufos-sulfone | 163 | 10 | 39 | 92 | 32 | 182 |
| Terbufos-sulfoxide | 113 | 7 | 361 | 1 | 61 | 162 |
| Terbumeton | 113 | 10 | 285 | 58 | 273 | 151 |
| Terbuthylazine | 651 | 98 | 396 | 516 | 237 | 146 |
| Terbutryn | 583 | 93 | 291 | 422 | 321 | 210 |
| Tetrachlorvinphos | 526 | 77 | 316 | 328 | 315 | 212 |
| Tetraconazole | 775 | 107 | 392 | 581 | 279 | 212 |
| Tetradifon | 772 | 108 | 252 | 587 | 201 | 178 |
| Tetramethrin | 695 | 102 | 394 | 490 | 321 | 32 |
| Thiabendazole | 765 | 105 | 394 | 585 | 321 | 70 |
| Thiacloprid | 766 | 108 | 40 | 586 | 321 | 212 |
| Thiamethoxam | 766 | 108 | 113 | 586 | 32 | 180 |
| Thiencarbazone-methyl | 128 | 10 | 394 | 61 | 157 | 69 |
| Thifensulfuron-methyl | 332 | 48 | 361 | 270 | 322 | 117 |
| Thiodicarb | 765 | 108 | 112 | 586 | 271 | 184 |
| Thiofanox | 326 | 48 | 340 | 267 | 180 | 205 |
| Thiometon | 470 | 74 | 254 | 287 | 270 | 35 |
| Thiophanate-methyl | 684 | 97 | 227 | 501 | 149 | 112 |
| Tolclofos-methyl | 759 | 102 | 307 | 574 | 75 | 48 |
| Tolylfluanid (sum) | 445 | 76 | 210 | 345 | 303 | 210 |
| Tralkoxydim | 140 | 18 | 391 | 100 | 280 | 210 |
| Triadimefon | 775 | 107 | 391 | 581 | 85 | 36 |
| Triadimenol | 775 | 107 | 39 | 581 | 124 | 35 |
| Tri-allate | 367 | 54 | 395 | 219 | 78 | 171 |
| Triasulfuron | 146 | 18 | 39 | 103 | 319 | 212 |
| Triazophos | 770 | 106 | 306 | 582 | 77 | 182 |
| Tribenuron-methyl | 146 | 18 | 394 | 103 | 267 | 108 |
| Trichlorfon | 518 | 70 | 313 | 319 | 149 | 12 |
| Triclopyr | 7 | 112 | 10 | 25 | ||
| Tricyclazole | 600 | 105 | 318 | 457 | 322 | 211 |
| Trifloxystrobin | 765 | 108 | 200 | 586 | 281 | 178 |
| Triflumizole | 690 | 105 | 394 | 495 | 132 | 101 |
| Triflumuron | 765 | 108 | 191 | 586 | 279 | 143 |
| Trifluralin | 694 | 102 | 7 | 506 | 114 | 69 |
| Triflusulfuron | 15 | 46 | 3 | 42 | ||
| Triforine | 327 | 50 | 243 | 192 | 200 | 177 |
| Trimethacarb | 326 | 48 | 1 | 267 | 272 | 151 |
| Triticonazole | 685 | 101 | 38 | 490 | 76 | 206 |
| Uniconazole | 144 | 18 | 279 | 95 | 32 | 182 |
| Valifenalate | 119 | 7 | 389 | 68 | 269 | |
| Vamidothion | 643 | 96 | 33 | 477 | 311 | |
| Vinclozolin | 765 | 102 | 319 | 577 | 13 | |
| Zoxamide | 690 | 122 | 495 |
References
- Research Institute of Organic Agriculture (FIBL). European and Global Organic Farming Statistics. 2023. Available online: https://www.organic-world.net/statistics.html (accessed on 25 May 2023).
- European Council Regulation No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32009R1107 (accessed on 25 May 2023).
- European Council. Commission Regulation No 2022/1438, Amending Annex II to Regulation (EC) No 1107/2009 as Regards Specific Criteria for the Approval of Active Substances that are Micro-Organisms. Available online: http://data.europa.eu/eli/reg/2022/1441/oj (accessed on 25 May 2023).
- European Commission Regulation No 283/2013 of 1 March 2013 Setting Out the Data Requirements for Active Substances, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:093:0001:0084:EN:PDF (accessed on 25 May 2023).
- European Commission Regulation No 546/2011 of 10 June 2011 Implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as Regards Uniform Principles for Evaluation and Authorisation of Plant Protection Products. Available online: http://data.europa.eu/eli/reg/2009/1107/2022-11-21 (accessed on 25 May 2023).
- European Council Regulation No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC. Available online: http://data.europa.eu/eli/reg/2005/396/2023-02-28 (accessed on 25 May 2023).
- Austrian Agency for Health and Food Safety. National Pesticide Residue Control Programmes. Available online: https://www.ages.at/en/plant/pesticides/pesticide-residues#c5252 (accessed on 25 May 2023).
- Australian Government Department of Agriculture, Fisheries and Forestry. National Residue Survey. Available online: https://www.agriculture.gov.au/agriculture-land/farm-food-drought/food/nrs (accessed on 25 May 2023).
- German Federal Office of Consumer Protection. Tables for the National Reporting of Pesticide Residues in food 2020. Available online: www.bvl.bund.de/DE/Arbeitsbereiche/01_Lebensmittel/01_Aufgaben/02_AmtlicheLebensmittelueberwachung/07_PSMRueckstaende/01_nb_psm_2020_tabellen/nbpsm_2020_tabellen_node.html (accessed on 25 May 2023).
- Japanese Ministry of Health, Labour and Welfare. Agricultural Chemical Residues in Foods. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/shokuhin/zanryu/index_00016.htm (accessed on 25 May 2023).
- USDA. PDP Databases and Annual Summaries (1992–2020). Available online: www.ams.usda.gov/datasets/pdp/pdpdata (accessed on 25 May 2023).
- Zhang, M.; Zeiss, M.R.; Geng, S. Agricultural pesticide use and food safety: California’s model. J. Integr. Agric. 2015, 14, 2340–2357. [Google Scholar] [CrossRef]
- Jardim, A.N.O.; Caldas, E.D. Brazilian monitoring programs for pesticide residues in food-results from 2001 to 2010. Food Control 2012, 25, 607–616. [Google Scholar] [CrossRef]
- Lei, Y.; Luo, Y.; Fang, N.; Li, Y.; Wang, X.; He, H.; Jiang, J.; Yu, J.; Zhang, C.; Zhao, X. Residues and dietary risk assessment of imidacloprid in bamboo shoot (Phyllostachys praecox), winter jujube (Ziziphus jujuba Mill. cv. Dongzao), Dendrobium officinale Kimura et Migo, and Fritillaria. Agronomy 2023, 13, 1076. [Google Scholar] [CrossRef]
- Wang, X.; Fang, N.; Wang, X.; Zhao, X.; Zhang, C.; Wang, Q. Residue analysis and dietary risk assessment of metalaxyl in Chinese bayberry and Dendrobium officinale. Agronomy 2023, 13, 186. [Google Scholar] [CrossRef]
- Meftaul, M.; Venkateswarlu, K.; Parven, A.; Annamalai, P.; Megharaj, M. Human health risk assessment of pesticides in lettuce and spinach grown in urban backyard garden soils. J. Food Compos. 2023, 115, 104977. [Google Scholar] [CrossRef]
- Mandal, K.; Singh, R.; Sharma, S.; Kataria, D. Dissipation and kinetic studies of fluopyram and trifloxystrobin in chilli. J. Food Compos. 2023, 115, 105008. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Lin, T.; Fan, C.; Li, Y.; Zhang, Z.; Li, J. Migration behavior and dietary exposure risk assessment of pesticides residues in honeysuckle (Lonicera japonica Thunb.) based on modified QuEChERS method coupled with tandem mass spectrometry. Food Res. Int. 2023, 166, 112572. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Sulyok, M.; Faas, J.; Krska, R.; Khiaosa-ard, R.; Zebeli, Q. Residues of pesticides and veterinary drugs in diets of dairy cattle from conventional and organic farms in Austria. Environ. Pollut. 2023, 316, 120626. [Google Scholar] [CrossRef]
- Lajmanovich, R.; Repetti, M.R.; Cuzziol Boccioni, A.P.; Michlig, M.P.; Demonte, L.; Attademo, A.M.; Peltzer, P.M. Cocktails of pesticide residues in Prochilodus lineatus fish of the Salado River (South America): First record of high concentrations of polar herbicides. Sci. Total Environ. 2023, 870, 162019. [Google Scholar] [CrossRef]
- De Cock, A.; Forio, M.A.E.; Croubels, S.; Dominguez-Granda, L.; Jacxsens, L.; Lachat, C.; Roa-López, H.; Ruales, J.; Scheyvaerts, V.; Hidalgo, M.C.S.; et al. Health risk-benefit assessment of the commercial red mangrove crab: Implications for a cultural delicacy. Sci. Total Environ. 2023, 862, 160737. [Google Scholar] [CrossRef]
- Environmental Working Group. Dirty Dozen Clean Fifteen. Available online: https://www.ewg.org/https://www.ewg.org/foodnews/clean-fifteen.php (accessed on 25 May 2023).
- European Commission Implementing Regulation (EU) 2021/601 of 13 April 2021 Concerning a Coordinated Multiannual Control Programme of the Union for 2022, 2023 and 2024 to Ensure Compliance with Maximum Residue Levels of Pesticides and to Assess the Consumer Exposure to Pesticide Residues in and on Food of Plant and Animal Origin. Available online: http://data.europa.eu/eli/reg_impl/2021/601/oj (accessed on 25 May 2023).
- EFSA. National summary reports on pesticide residue analysis performed in 2020. EFSA Support. Publ. 2022, 19, 7216E. [Google Scholar] [CrossRef]
- EFSA; Carrasco Cabrera, L.; Medina Pastor, P. The 2019 European Union report on pesticide residues in food. EFSA J. 2021, 19, e06491. [Google Scholar] [CrossRef]
- EFSA. Eurobarometer 2022. Available online: www.efsa.europa.eu/sites/default/files/2022-09/EB97.2-food-safety-in-the-EU_report.pdf (accessed on 25 May 2023).
- FAO. Submission and Evaluation of Pesticide Residues Data for the Estimation of Maximum Residue Levels in Food and Feed, 3rd ed.; FAO Plant Production and Protection Paper 225; FAO: Rome, Italy, 1996; Available online: https://www.fao.org/3/i5452e/i5452e.pdf (accessed on 25 May 2023).
- Szenczi-Cseh, J.; Ambrus, Á. Uncertainty of exposure assessment of consumers to pesticide residues derived from food consumed. J. Environ Sci. Health B 2017, 52, 658–670. [Google Scholar] [CrossRef]
- FAO. Portion of Commodities to Which Codex MRLs Apply and Which Is Analyzed (CAC/GL 41-1993). Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B41-1993%252FCXG_041e.pdf (accessed on 25 May 2023).
- FAO. Recommended Methods of Sampling for the Determination of Pesticide Residues for Compliance with MRLs CXG33-1999. Available online: www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B33-1999%252FCXG_033e.pdf (accessed on 25 May 2023).
- European Commission Directive 2002/63/EK Establishing Community Methods of Sampling for the Official Control of Pesticide Residues in and on Products of Plant and Animal Origin and Repealing Directive 79/700/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002L0063 (accessed on 25 May 2023).
- ISO. ISO/IEC 17025:2017 General Requirements for the Competence of Testing and Calibration Laboratories. Available online: www.iso.org/ISO-IEC-17025-testing-and-calibration-laboratories.html (accessed on 25 May 2023).
- FAO. CAC/GL 70-2009 Guidelines for Settling Disputes on Analytical (Test) Results. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B70-2009%252Fcxg_070e.pdf (accessed on 25 June 2023).
- Ambrus, Á.; Doan, V.V.N.; Szenczi-Cseh, J.; Szemánné-Dobrik, H.; Vásárhelyi, A. Quality control of pesticide residue measurements and evaluation of their results. Molecules 2023, 28, 954. [Google Scholar] [CrossRef]
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed SANTE 11312/2021. Available online: https://food.ec.europa.eu/system/files/2022-02/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 25 May 2023).
- Ambrus, Á.; Szenczi-Cseh, J.; Doan, V.V.N.; Vásárhelyi, A. Evaluation of monitoring data in foods. Agrochemicals 2023, 2, 69–95. [Google Scholar] [CrossRef]
- Farkas, Z.; Slate, A.; Whitaker, T.; Kötelesné Suszter, G.; Ambrus, Á. Use of combined uncertainty information for testing compliance with MRLs. J. Agric. Food Chem. 2015, 63, 4418–4428. [Google Scholar] [CrossRef]
- Farkas, Z.; Cook, J.M.; Ambrus, Á. Estimation of uncertainty of measured residues and testing compliance with MRLs. In Food Safety Assessment of Pesticide Residues; Ambrus, Á., Hamilton, D., Eds.; World Scientific: Hackensack, NJ, USA, 2017; pp. 404–466. [Google Scholar]
- Sharma, G.M.; Pereira, M.; Wang, S.S.; Chirtel, S.J.; Whitaker, T.B.; Wehling, P.; Arlinghaus, M.; Canida, T.; Jackson, L.S.; Williams, K.M. Evaluation of sampling plans for measurement of gluten in oat groats. Food Control 2020, 114, 107241. [Google Scholar] [CrossRef]
- Farkas, Z.; Horváth, Z.; Szabó, I.J.; Ambrus, Á. Estimation of sampling uncertainty of pesticide residues based on supervised residue trial data. J. Agric. Food Chem. 2015, 63, 4409–4417. [Google Scholar] [CrossRef]
- Horváth, Z.; Ambrus, Á. Principles of control of small-scale production of fruits and vegetables and planning risk-based monitoring programmes. In Food Safety Assessment of Pesticide Residues, 1st ed.; Ambrus, Á., Hamilton, D., Eds.; World Scientific: Hackensack, NJ, USA, 2017; pp. 467–506. [Google Scholar]
- European Commission Implementing Regulation (EU) 2019/533 of 28 March 2019 Concerning a Coordinated Multiannual Control Programme of the Union for 2020, 2021 and 2022 to Ensure Compliance with Maximum Residue Levels of Pesticides and to Assess the Consumer Exposure to Pesticide Residues in and on Food of Plant and Animal Origin. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R0533 (accessed on 25 May 2023).
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and ‘dispersive solid-phase extraction’ for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Anastassiades, M.; Scherbaum, E.; Tasdelen, B.; Stajnbaher, D. Recent developments in QuEChERS methodology for pesticide multiresidue analysis. In Pesticide Chemistry. Crop Protection, Public Health, Environmental Safety; Ohkawa, H., Miyagawa, H., Lee, P.W., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2007; pp. 439–458. [Google Scholar] [CrossRef]
- Anastassiades, M.; Wachtler, A.K.; Kolberg, D.I.; Eichhorn, E.; Marks, H.; Benkenstein, A.; Zechmann, S.; Mack, D.; Wildgrube, C.; Barth, A.; et al. Quick Method for the Analysis of Highly Polar Pesticides in Food Involving Extraction with Acidified Methanol and LC-or IC-MS/MS Measurement I. Food of Plant Origin (QuPPe-PO-Method). Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlSRM/EurlSrm_meth_QuPPe_PO_V11_1.pdf (accessed on 27 June 2023).
- Ambrus, Á.; Buczkó, J.; Hamow, K.Á.; Juhász, V.; Solymosné Majzik, E.; Szemánné Dobrik, H.; Szitás, R. Contribution of sample processing to variability and accuracy of the results of pesticide residue analysis in plant commodities. J. Agric. Food Chem. 2016, 64, 6071–6081. [Google Scholar] [CrossRef]
- Ambrus, Á.; Vásárhelyi, A. Pesticide residue analysis in Hungary between 1967 and 2015. J. Food Investig. 2016, 62, 919–942. Available online: http://real-j.mtak.hu/18410/1/EVIK2016-1.pdf (accessed on 20 June 2023).
- Ambrus, Á.; Szenczi-Cseh, J.; Griff, T.; Kerekes, K.; Miklós, G.; Szigeti, T.; Vásárhelyi, A. Food safety assessment of the mycotoxin and pesticide residue contamination of our foods, Part 1. Pesticide residues. J. Food Investig. 2020, 66, 2773–2801. Available online: https://eviko.hu/Portals/0/ujsagok/Arcivum/2020/1_szam/EVIK2020-1.pdf (accessed on 20 June 2023).
- Ambrus, Á.; Szenczi-Cseh, J.; Griff, T.; Kerekes, K.; Miklós, G.; Szigeti, T.J.; Vásárhelyi, A. Food safety assessment of the mycotoxin and pesticide residue contamination of our foods, Part 2. Mycotoxins. J. Food Investig. 2020, 66, 2923–2949. [Google Scholar]
- Ambrus, Á. Variability of pesticide residues in crop units. Pest Manag. Sci. 2006, 62, 693–714. [Google Scholar] [CrossRef]
- Horváth, Z.; Ambrus, Á.; Mészáros, L.; Braun, S. Characterization of distribution of pesticide residues in crop units. J. Environ. Sci. Health—B Pestic. Food Contam. Agric. Wastes 2013, 48, 615–625. [Google Scholar] [CrossRef]
- Commission Regulation No 149/2008 of 29 January 2008 Amending Regulation No 396/2005 of the European Parliament and of the Council by Establishing Annexes II, III and IV Setting Maximum Residue Levels for Products Covered by Annex I Thereto. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32008R0149 (accessed on 20 June 2023).
| Year | PT Code | Test Material | No. of Components/ No. of Residues | No. of Participating Labs | AZ2 * Range |
|---|---|---|---|---|---|
| 2019 | EUPT-CF13 | Rye | 192/19 | 157 | 0.1 |
| EUPT-FV21 | Red cabbage | 237/21 | 188 | 0.1–0.4 | |
| EUPT-SM11 | Red cabbage | not identified no./16 | 67 | 50–93% ** | |
| 2020 | EUPT-CF14 | Rice | 202/20 | 158 | 0.1–1.3 |
| EUPT-FV22 | Onion | 244/19 | 176 | 0.2–1.1 | |
| EUPT-SRM15 | Rice | 30/16 | 60 | 0.5–1.1 | |
| EUPT-SM12 | Onion | not identified no./17 | 62 | 76% ** | |
| 2021 | EUPT-CF15 | Rapeseed cake | 213/22 | 137 | 0.3–2.0 |
| EUPT-FV23 | Aubergine | 256/20 | 182 | 0.2–1.3 | |
| EUPT-SRM16 | Sesame | 21/13 | 132 | 0.3–0.6 | |
| EUPT-SM13 | Aubergine | not identified no./18 | 60 | 78–89% ** |
| Commodity | No. of | |||||
|---|---|---|---|---|---|---|
| Samples 2 | Analytes 3 | Tests 4 | R > MRL 5 | MRL ≥ R ≥ LOQ 6 | R < LOQ 7 | |
| All commodities 1 | 9924 | 622 | 2,652,560 | 102 | 5261 | 4560 |
| Apples | 803 | 459 | 227,571 | 1 | 587 | 215 |
| Cherries, sour | 122 | 441 | 32,962 | 1 | 95 | 26 |
| Grapes, table | 783 | 459 | 113,132 | 1 | 703 | 79 |
| Peaches/nectarines | 468 | 445 | 90,851 | 1 | 350 | 117 |
| Peppers, green, red | 616 | 459 | 165,388 | 4 | 298 | 314 |
| Strawberries | 349 | 447 | 60,458 | 3 | 291 | 55 |
| Commodity | No. of Tested | No. of Lots Complied 1 | No. of Lots and Proportion of Their Compliance due to Residues Detected 1 | |||
|---|---|---|---|---|---|---|
| Lots | As-S | |||||
| Apple | 1944 | 50 | 1545 > 92% | 9 tau-fluvalinate (89%) | 8 folpet (88%) | 21 lambda-cyhalothrin (81%) |
| Cherries | 195 | 21 | 162 > 96% | 23 (dithiocarbamates 87%) | 6 thiamethoxam (83%) | 4 deltamethrin (50%) |
| Grape | 986 | 62 | 869 > 90% | 8 buprofezin 2 (0%) | 4 pyraclostrobin (78%) | 36 acetamiprid (86%) |
| Peach | 521 | 34 | 465 > 95% | acetamiprid (87.5%) | prochloraz (83.3%) | carbendazim (70%) |
| Peppers, sweet | 631 | 48 | 460 > 90% | # 3 | ||
| Strawberry | 588 | 40 | 444 > 90% | # 4 | ||
| Year | No. of Samples Analyzed | Samples w. Multiple Residues | Max. no. of AS | No. of Samples Containing Multiple Residues 1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Apples | Cherries, Sour | Grapes, Table | Peaches and Nectarines | Peppers Sweet | Strawberries | ||||
| 2017 | 1902 | 761 | 23 | 75 | 16 | 57 | 45 | 35 | 33 |
| 2018 | 1995 | 820 | 13 | 101 | 16 | 53 | 51 | 44 | 35 |
| 2019 | 1842 | 916 | 15 | 107 | 10 | 49 | 59 | 45 | 36 |
| 2020 | 1750 | 625 | 16 | 89 | 8 | 42 | 39 | 45 | 3 |
| 2021 | 1666 | 719 | 11 | 103 | 9 | 32 | 37 | 43 | 20 |
| Commodity | Max (Average) No. of AS Found in One Sample | Relevant Groups of | |||||
|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | Diseases | Arthropod Pests | |
| Apples | 23 (3.9) | 13 (3.9) | 8 (3.8) | 9 (3.7) | 11 (3.5) | 3 | 5 |
| Cherries, sour | 8 (3.4) | 7 (3.7) | 6 (3.6) | 6 (3.5) | 6 (3.8) | 3 | 5 |
| Grapes, table | 12 (4) | 11 (4.1) | 11 (3.8) | 11 (4.1) | 7 (3.3) | 3 | 3 |
| Peaches and nectarines | 6 (2.7) | 7 (3.4) | 9 (3.1) | 9 (4.1) | 5 (2.9) | 4 | 4 |
| Peppers, sweet | 10 (3.7) | 11 (3.2) | 15 (3.8) | 15 (3.6) | 10 (3.1) | 3–4 | 4 |
| Strawberries | 7 (3.4) | 9 (4.3) | 11 (4.8) | 7 (4.3) | 9 (5.0) | 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrus, Á.; Vásárhelyi, A.; Ripka, G.; Szemánné-Dobrik, H.; Szenczi-Cseh, J. Evaluation of the Results of Pesticide Residue Analysis in Food Sampled between 2017 and 2021. Agrochemicals 2023, 2, 409-435. https://doi.org/10.3390/agrochemicals2030023
Ambrus Á, Vásárhelyi A, Ripka G, Szemánné-Dobrik H, Szenczi-Cseh J. Evaluation of the Results of Pesticide Residue Analysis in Food Sampled between 2017 and 2021. Agrochemicals. 2023; 2(3):409-435. https://doi.org/10.3390/agrochemicals2030023
Chicago/Turabian StyleAmbrus, Árpád, Adrienn Vásárhelyi, Géza Ripka, Henriett Szemánné-Dobrik, and Júlia Szenczi-Cseh. 2023. "Evaluation of the Results of Pesticide Residue Analysis in Food Sampled between 2017 and 2021" Agrochemicals 2, no. 3: 409-435. https://doi.org/10.3390/agrochemicals2030023
APA StyleAmbrus, Á., Vásárhelyi, A., Ripka, G., Szemánné-Dobrik, H., & Szenczi-Cseh, J. (2023). Evaluation of the Results of Pesticide Residue Analysis in Food Sampled between 2017 and 2021. Agrochemicals, 2(3), 409-435. https://doi.org/10.3390/agrochemicals2030023







