1. Introduction
Unlike upper extremity veins, where pathologies are relatively rare, lower extremity veins are quite often affected by abnormalities and/or health- or even life-threatening disease. These comprise chronic venous disease, which is seen in 25–40% of the adult population, and thromboembolic disease, which is one of the most common causes of mortality [
1,
2]. Therefore, knowledge of the anatomy of these veins is so important. Over the last two decades, there has been tremendous progress in the understanding of topographic anatomy and embryology-associated variability of the lower extremity veins. This progress has been primarily possible due to the fact that the majority of current invasive procedures for the treatment of lower extremity veins are performed under ultrasonographic control. Consequently, the detailed anatomy of these blood vessels that is easily seen during ultrasonographic examination, but cannot be revealed by traditional cadaver dissections, became accessible for research [
3].
Basing on this research, the revised description of the lower extremity venous anatomy was agreed upon at the pre-congress meeting of the 14th Congress of the International Union of Phlebology, which was held in Rome on 8–9 September 2001. This document was created under the auspices of the International Union of Phlebology, the International Federation of Associations of Anatomists, and the Federative International Committee on Anatomical Terminology and was published in 2002 [
4]. During the following years, there were some rather minor revisions to this terminology [
5,
6,
7,
8,
9,
10,
11], which followed new discoveries and better understanding of the anatomy of these veins, especially in the context of their topographic relation to the fascia and adjacent nerves.
Similarly, embryological studies enabled proper understanding of high variability in the anatomy of the lower extremity veins, while physiological studies provided very important information on the functional anatomy of these blood vessels. Of note, the complex interplay between the superficial, interfascial, and deep veins, as well as the proper function of muscular pumps of the lower extremity, is important in the setting of physiology and venous disease.
Unfortunately, of as yet, these relevant amendments have not been included in the anatomical textbooks and atlases. For example, in current editions of the “Gray’s Anatomy for Students” anatomy textbook (5th Edition; Drake RL, Vogl AW, Mitchell AWM, editors; Elsevier 2024), which is used by medical students worldwide, there is still unprecise and misleading information that the great and small saphenous veins are located in the subcutaneous connective tissue. Similarly, in the “Moore’s Clinically Oriented Anatomy” textbook (9th Edition; Agur AMR, Dalley II AF, editors; Wolters Kluwer 2023), which is also recommended for medical students, there is no information regarding the topographic relationship between saphenous veins and fascias, and there is incorrect information regarding the accessory saphenous vein. Both student textbooks describe saphenous veins in relation to the bones, instead of fascial compartments. The information about clinically relevant valves of the saphenofemoral junction is lacking as well. The specialist anatomical textbook, “Gray’s Anatomy. The Anatomical Basis of Clinical Practice (42nd Edition; Standring S, editor; Elsevier 2021), gives similarly scarce and rather obsolete information, although the document presenting the revised anatomical nomenclature [
4] is mentioned, still without revising these anatomical terms.
This review paper serves the purpose of summarizing the current anatomical knowledge of these veins. In addition, some proposals of the revised anatomical curriculum for undergraduate medical education will be presented. We hope that this paper can provide a useful framework for planning anatomical classes, especially considering the limitations of current textbooks and traditional cadaver dissections.
2. Topographic Anatomy of the Deep, Interfascial, and Epifascial Veins
According to the consensus documents of the vascular scientific societies, the veins of the lower extremities are categorized into three hierarchically ordered groups: the superficial (epifascial) veins, the interfascial veins, and the deep veins [
4,
5,
6,
7,
8,
9]. It differs from information given in anatomical textbooks, where only two groups of veins, namely, the superficial and deep, are distinguished. The traditionally defined superficial group comprises both the epifascial and interfascial categories. The separate category of the latter is claimed by vascular scientific societies. Of note, there is a good reason for categorizing the interfascial veins as a separate group. These veins are characterized by special topography (which will be further discussed); they are frequently affected by varicose vein disease; and their management, using current endovascular procedures, in many aspects significantly differs from the management of “true” superficial veins.
In addition to these three or two categories, the epifascial, interfascial, and deep veins are interconnected by the perforating veins. The perforating veins of the lower extremity are defined as veins penetrating the muscular fascia. There are about 150 such veins in the lower extremity, referred to as the perforators by clinicians, but only a few are of actual relevance. In the past, these perforators were described using eponyms, such as the Dodd’s or Cockett’s perforators. Nowadays, the use of eponyms in anatomy is discouraged. Instead, the anatomical terminology of perforating veins should designate their location, for example, the foot, leg, knee, or thigh perforators and the medial, posterior, or lateral ones [
7,
10]. Perforating veins are equipped with valves that promote the flow from the epifascial and interfascial veins toward the deep veins. The net flow through the perforator flow should be as above described. Still, some studies have revealed bidirectional flow in otherwise competent perforating veins [
10,
11]. There is an ongoing debate among doctors on the clinical meaning of perforating veins that exhibit reversed, i.e., from the deep toward the epifascial and interfascial veins, flow. Such a discussion is beyond the scope of this paper. Yet, it should be mentioned that there is a common agreement that perforating veins located above the knee level, which exhibit such a reversed flow direction, are always pathological. It is associated with the topography of the femoral vein, which is covered only by the sartorius muscle; consequently, the muscle pump in the thigh is not very efficient, resulting in obviously pathological flows if such an above-the-knee perforator becomes incompetent. On the contrary, perforators located below the knee, where deep veins are situated inside muscular compartments, should be assessed in the context of the entire venous hemodynamic anatomy of the lower extremity. A reversed flow in these veins should not be considered an isolated abnormality. Of note, it is generally accepted that such a reversed flow that exceeds 0.5 s is pathological. Shorter-duration outward flows still remain controversial. With the better ultrasonographic equipment, it appears that some of these refluxes actually represent vortical flow in tortuous perforators or develop after non-physiological provoking maneuvers, such as the leg compression/sudden release test [
12,
13,
14,
15].
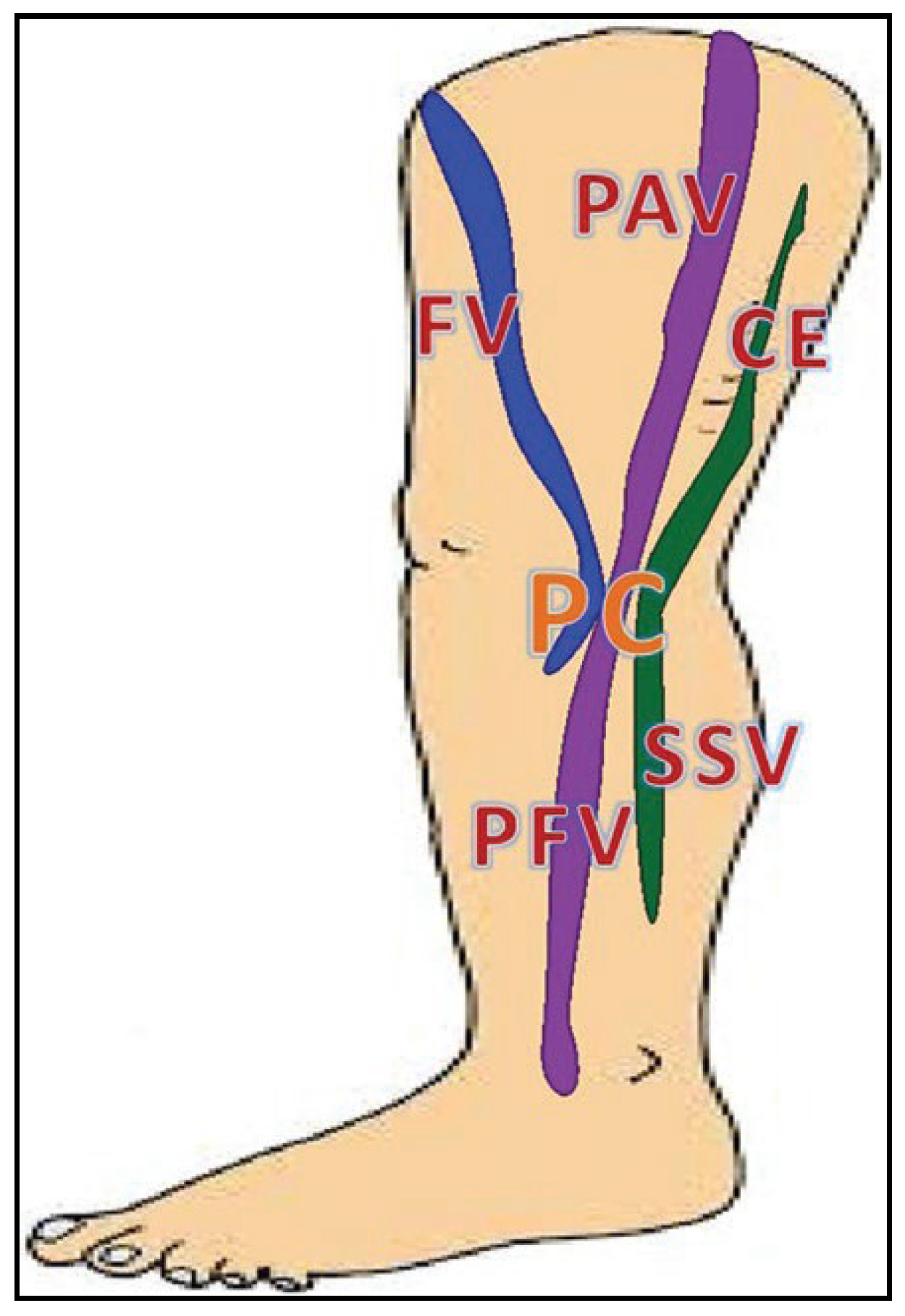
The deep vein group comprises veins located below the muscular fascia of the extremity (
Figure 1). Similarly, the superficial veins are the veins that are located above this fascia. However, actually, this fascial sheath consists of two fibrous layers. These layers usually are not seen during traditional cadaver dissection but can be easily recognized during ultrasonographic examination. These two sheaths, the superficial (the proper anatomical term of this fascia is the membranous layer of subcutaneous tissue) and muscular ones, fuse with each other, except for small areas surrounding the interfascial veins (
Figure 2). Here, these fascias are not fused and form a separate fascial compartment enclosing the vein and usually also a small artery, lymphatic vessels, adipose tissue, and sometimes an accompanying nerve. In an ultrasonographic transverse scan the interfascial compartment, with the vein located in its center, forms a picture called the “Egyptian eye” (
Figure 3) [
3,
5,
16].
Doctors take advantage of this topography of interfascial veins. Nowadays, thermal ablations (such as the laser, radiofrequency or steam procedures) of varicose veins, which constitute the most frequently used therapeutic modality for the treatment of varicosities, are performed under local anesthesia. A relatively low volume of the anesthetic fluid injected into the interfascial compartment, compresses the vein and separates it from adjacent skin and nerves. In the case of “true” superficial veins, a higher volume of anesthetic would be needed since there are no anatomical barriers built by these fibrous sheaths. Similarly, inflammatory reactions after chemical ablations (such as sclerotherapy or glue closure) are better constrained in the case of interfascial vein management than after the treatment of epifascial veins. Therefore, the topography of the target veins is very important during contemporary procedures for varicose veins. Good anatomical knowledge enables an adequate modification of the procedures, while minimizing the risk of associated adverse events.
3. Embryology of the Lower Extremity Veins
The anatomical pattern of the lower extremity veins, which is seen in the majority of adults, is very different from that present in embryos and fetuses. During embryological development of the lower extremities, the venous system undergoes several remodelings, although in some adult individuals a pattern of an embryonic or fetal venous anatomy is still present. Of note, some anatomical terms of the embryonic veins are used to describe blood vessels, which are quite different from those found in adults. To avoid confusion, a descriptor “primitive” is used. Therefore, it should be remembered that, for example, the primitive posterior tibial vein in an embryo is not the same blood vessel as the posterior tibial vein found in an adult, even if the location of these veins is similar. The latter does not develop from the primitive one but arises independently. It should also be noted that current knowledge on venous embryogenesis in humans is based on studies in animals, primarily rabbit embryos. Research on human embryos is very limited, and therefore, an extrapolation of findings in the animals to humans should be performed with caution. Nonetheless, comprehending the venous embryology of the lower extremities seems indispensable for understanding anatomical variability and venous outflows, particularly in the settings of venous pathology [
17].
In general, venous embryogenesis of the lower limb consists of three stages. During the first stage, the primitive fibular vein is the main vein of the lower extremity. During the second stage it is replaced by the axial vein and finally by the femoral vein. During the first step, which in humans takes place between the fifth and sixth week of the embryo’s life, the bud of the lower limb is vascularized by the primitive fibular vein. This blood vessel, similarly to the rest of caudal part of the embryo, receives inflow from the umbilical vein, through the posterior cardinal veins. The primitive fibular vein further develops its distal extension, the primitive lateral marginal vein, and its proximal extension, the primitive axial (sciatic) vein. Interestingly, at this stage of embryogenesis, the lower extremity receives blood inflow through the veins and is drained by the arteries. Therefore, all veins of the lower extremity bud at this step are valveless; otherwise, an inflow would not be possible. The persistent marginal vein, which is a pathological remnant of this stage of vasculogenesis, is valveless as well, which has severe clinical consequences for patients presenting with this abnormal blood vessel. The persistent marginal vein is actually a transformed primitive fibular vein and is seen in those patients (e.g., presenting with Klippel–Trenaunay syndrome) in whom the first step of venous vasculogenesis is not followed by the second one. Since the marginal vein is an embryonic vein, it is valveless, which results in high-volume venous reflux and associated severe venous symptoms.
The next step of the venous embryogenesis, which takes place between the seventh and eighth week of the embryo’s life, is regulated by so-called angio-guiding nerves. The nerves that develop at this stage of embryogenesis secrete several cytokines, such as vascular endothelial growth factor and ephrins. These signaling substances trigger the growth of embryonic veins. There are three main such angio-guiding nerves in the bud of the lower extremity. They are located in its central, anterior, and posterior part: the axial nerve (in adults it transforms into the sciatic nerve), the pre-axial nerve (it will transform into the femoral nerve), and the post-axial nerve (it later transforms into the posterior femoral cutaneous nerve). The axial nerve stimulates the development of the sciatic vein, the pre-axial nerve stimulates the development of the femoral vein, and the post-axial nerve stimulates the development of the cranial extension of the small saphenous vein [
18,
19].
At this step, in the lower leg, the primitive fibular vein develops two branches—the primitive anterior tibial vein and the connecting branch. The primitive anterior tibial vein becomes the main vein of the lower limb at this stage of vasculogenesis, while the primitive fibular vein becomes less important, does not grow so fast, and finally becomes the small saphenous vein. Cranially, the primitive anterior tibial vein and the primitive fibular vein merge to form the axial (sciatic) vein, which is the main venous channel draining the limb at this stage of embryogenesis. A tributary of the primitive fibular vein, the connecting branch, evolves into the primitive femoral vein (
Figure 4). This process is stimulated by the pre-axial nerve.
During the last step of venous embryogenesis, the aforementioned primitive femoral veins connect to the posterior cardinal veins, anteriorly from the axial veins (these connections will later become the iliac veins). The sciatic vein begins to involute, while the primitive femoral vein enlarges. As the primitive femoral vein grows distally, it forms the primitive posterior tibial vein, which later develops into the great saphenous vein. Since at this stage of embryogenesis the embryo limb rotates, the great saphenous vein is finally located at the medial aspect of the lower extremity [
18,
19].
At the end of the 12th week of human embryogenesis, the development of the lower extremity veins is almost completed, except for the femoral and sciatic veins. Initially, the axial (sciatic) vein is the dominant one. As it involutes, the femoral vein becomes the bigger one. Finally, the adult anatomical pattern of the lower extremity veins emerges, with the femoral vein being the main blood vessel providing blood outflow from the extremity. Small veins accompanying the sciatic vein seen in adults, which are remnants of the axial vein, are called the sciatic nerve veins. Rarely, a large vein is present alongside the sciatic nerve. Such a vein in adults is referred to as the persistent sciatic vein [
21]. Such an atypical, yet understandable considering the embryogenesis, anatomy can make the proper patient assessment challenging. For example, a hypoplastic femoral vein can be misdiagnosed as venous thrombosis. Also, patients presenting with unusual varicose veins associated with the sciatic nerve veins can be improperly managed if the doctor is unaware of such anatomical variant [
22].
In the embryo, there are many connections between the aforementioned venous channels: pre-axial (primitive femoral), axial (sciatic), and post-axial. At the level of the popliteal fossa, all these three venous channels are connected (
Figure 4). It is referred to as the embryonic popliteal crossroad. The sapheno-popliteal junction is the remnant of this crossroad. Since connections between major embryonic venous channels involute differently, it explains the high variability of the sapheno-popliteal junction [
19].
4. Deep Veins of the Lower Extremities and the Veins of the Pelvis
Below the knee, deep veins of the lower extremities are usually doubled or tripled or form an irregular vascular network. Beginning from the popliteal vein proximally, there is usually a single vein, although in many individuals the paired popliteal or femoral veins can be present. In the lower leg, the deep veins accompany corresponding arteries. In this part of the lower extremity, the main veins comprise the posterior tibial veins that drain the posterior fascial compartment, the anterior tibial veins that drain the anterior fascial compartment, and the fibular veins that primarily drain the deep posterior fascial compartment [
23].
The popliteal vein, which is a continuation of the merged posterior and anterior tibial veins, is situated in the popliteal fossa. In addition to the tibial veins, the popliteal vein receives muscular tributaries, particularly the soleal and gastrocnemius veins, which are of high clinical relevance. The soleal and gastrocnemius veins drain the triceps surae muscle and constitute the most important part of the calf muscle pump. Although these veins drain the same muscle, there are significant morphological differences between them. The gastrocnemius veins are usually paired and exhibit typical venous morphology, with bicuspid valves that are located in the main venous trunks and branches. On the contrary, the morphology of the soleal veins is irregular; there are only a few, usually incomplete valves in their main trunks; and these veins build up rather a venous plexus instead of a typical vascular tree. Such a morphology of the soleal veins facilitates the proper function of the calf muscle pump but also promotes venous stasis inside this vascular plexus, which in immobilized individuals can result in thrombosis [
24].
In people presenting with typical anatomy, at the level of knee joint, the popliteal vein merges with the small saphenous vein that belongs to the interfascial veins. In other people this junction is situated above the knee level or even is missing [
19].
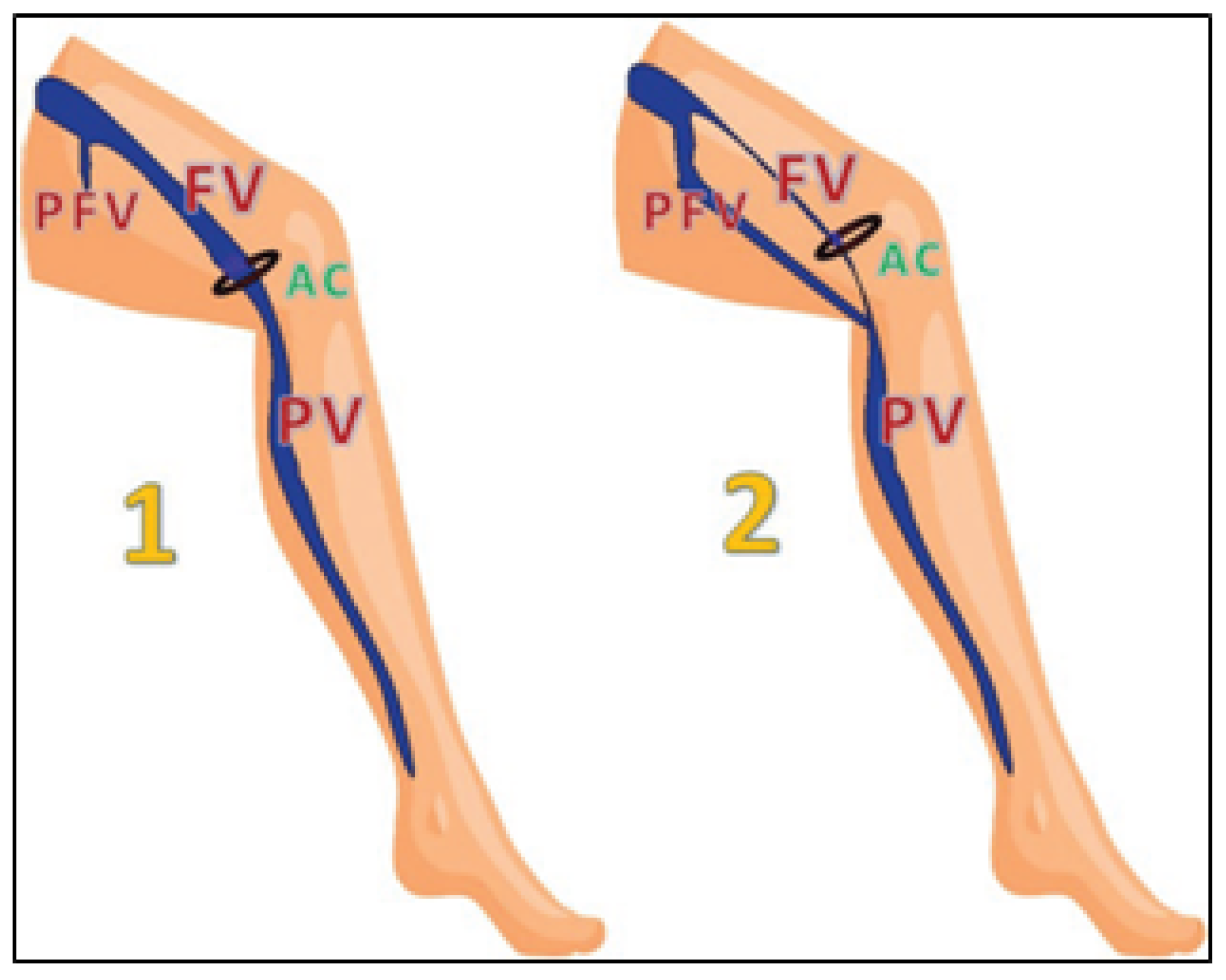
Above the knee, the popliteal vein continues proximally and at the hiatus adductorius (the opening in the adductor magnus muscle) becomes the femoral vein. In the thigh, the femoral vein is situates relatively superficially, just below the sartorius muscle. In the upper thigh, the femoral vein receives a big tributary: the profunda femoris vein, which is a short and wide vein that drains the posterior and medial fascial compartments of the thigh. There are also other, less clinically relevant tributaries of the femoral vein, particularly in the upper part of the thigh. Typically, the femoral vein is the main deep vein draining the lower extremity. Still, in about 3% of people, the main outflow from the lower leg continues within the posterior fascial compartment of the thigh. In these cases, the main continuation of the popliteal vein does not continue anteriorly, through the hiatus adductorius, but merges with the profunda femoris vein, either through the axiofemoral trunk (a wide vein developed from the merged axial and profunda femoris veins) or through the deep femoral trunk (a wide vein developed from the profunda femoris vein, which is elongated distally) (
Figure 5) [
22]. All these anatomical variants develop due to atypical embryological development of the lower limb veins but usually remain asymptomatic.
Just below the inguinal ligament, the femoral vein merges with the main vein of the interfascial system, the great saphenous vein. In some individuals, in the groin the femoral vein receives another tributary belonging to the interfascial system: the anterior accessory saphenous vein. In this area, there can also be situated connections with the venous system of the pelvis and the superficial epigastric vein draining the abdominal wall (the latter vein is the important landmark during endovascular procedures on the great saphenous vein). Above the inguinal ligament, the femoral vein continues proximally as the external iliac vein. Usually, the external iliac vein has no valves. A valve of this vein is seen in about 25–35% of people but often is not functional. The external iliac vein receives several tributaries, including the deep circumflex ilac vein and the inferior epigastric vein, which provide important outflow routes in a case of thrombosis at the iliofemoral level. The external iliac vein continues proximally and, after joining the internal iliac vein, forms the common iliac vein. The common iliac veins are not symmetric, which is associated with the embryological development of these veins. The right common iliac vein is shorter and usually runs more axially than the left one; therefore, the right femoral access for endovascular procedures (e.g., for the treatment of pulmonary embolism) is preferred. Both common iliac veins are typically valveless. The left common iliac vein is crossed by the right common iliac artery. In about 50% of people, there is a narrowing of the left iliac vein in this area, which is typically asymptomatic and without clinical relevance. Still, this anatomical feature represents a permissive lesion; it can result in impaired venous drainage from the left lower extremity and/or venous thrombosis. In such a case, this clinical entity is referred to as May–Thurner syndrome. Out of the clinically relevant tributaries of the common iliac veins, the ascending lumbar veins are of particular importance. These veins, which connect with the azygous vein system in the chest, provide an alternative outflow route from the lower part of the body in a case of thrombotic or neoplastic occlusions of the interior vena cava and/or iliac veins. The ascending lumbar veins are usually better developed on the left side, which is associated with embryological development of these blood vessels; still, it should be remembered that the anatomy of these veins is highly variable [
25].
The internal iliac veins provide the main outflow route from the pelvic walls and organs. Relatively often, the internal iliac vein is not a single vessel, but there are two or three such veins or even a complex venous plexus. Irrespective of this variable morphology, the tributaries of the internal iliac veins are usually categorized into three groups: the first one comprises veins draining the pelvic walls, and the second one groups the veins providing outflow from the sacrum and adjacent anatomical structures, while the third one comprises veins draining the pelvic viscera. Veins from the latter group do not form typical blood vessels but rather venous plexuses. These comprise the external and internal rectal venous plexuses, the vaginal venous plexus, the uterine venous plexus, the vesical venous plexus, and the pudendal venous plexus [
25]. Of note, the uterine venous plexus is drained both toward the internal iliac veins and toward the ovarian veins. These vascular connections with the gonadal veins are of importance in several clinical conditions, particularly in the case of compromised outflow from the left renal vein (the so-called nutcracker syndrome) resulting in overload of the left ovarian vein and subsequent dilatation of the pelvic veins (pelvic congestion syndrome) of even the lower extremity veins (varicose veins of pelvic origin). The aforementioned bidirectional outflow from the uterine venous plexus is also important during pregnancy.
5. Interfascial Veins
The interfascial veins of the lower extremity are superficially covered by the membranous layer of subcutaneous tissue (the so-called saphenous fascia) and deeply by the muscular fascia [
5,
16]. It distinguishes them from the “true” superficial, i.e., epifascial, veins that are located superficially from the saphenous fascia. The group of interfascial veins comprises four veins: the great saphenous vein, the small saphenous vein, the anterior accessory saphenous vein, and the Giacomini’s vein. Out of them, the great and small saphenous veins are seen in a majority of people, while the remaining two are present only in some individuals [
7,
8].
The great saphenous vein begins in the area of the medial malleolus and runs proximally at the medial aspect of the lower leg and thigh. Importantly, only the vein that is located between two previously described fascial sheaths can be called the great saphenous vein. In many individuals, there are many parallel veins at the medial aspect of the extremity, and not all of them can be called saphenous ones. It is of particular importance in the lower leg. In this part of the extremity, the great saphenous vein is accompanied by the saphenous nerve [
26,
27,
28,
29]. Other superficial veins in this area are not situated in the proximity of major cutaneous nerves. Therefore, invasive procedures for the treatment of associated varicose veins, like surgical or thermal ablations, can be complicated by symptomatic nerve injury if the great saphenous vein is managed, while the procedures on adjacent superficial veins are usually free from such adverse events. Of note, the saphenous compartment surrounding the great saphenous vein in the lower leg is usually quite narrow, especially in slim individuals. Therefore, ultrasonographic assessment of topographic anatomy in this area can be challenging (for this reason, invasive procedures associated with a risk of nerve injury of the below-the-knee part of the great saphenous vein are discouraged, if alternative treatment modalities are available).
Femoral segment of the great saphenous vein is not accompanied by the saphenous nerve, except for its most distal portion, at the level of knee joint. But the upper segment of the great saphenous vein in the thigh is accompanied by the anterior cutaneous branch of the femoral nerve, which sensorially innervates the medial aspect of the thigh. Injuries of this nerve are usually low-symptomatic or asymptomatic, thus not as significant as those of the saphenous nerve. However, the saphenous compartment in the thigh is also occupied by lymphatic vessels carrying lymph from the superficial structures of the foot and lower leg and also providing one of the outflow routes from the popliteal lymph nodes [
30,
31]. This proximity of lymphatic vessels should be taken into account during invasive procedures of the great saphenous vein, especially during traditional surgical stripping.
In the groin, the great saphenous vein joins the femoral vein. The most proximal part of the great saphenous vein, in the proximity of its connection with the femoral vein, is referred to as the sapheno-femoral junction. In this area, the great saphenous vein receives several tributaries that can be of clinical relevance during the treatment for varicose veins, especially if venous reflux originates in the perineum or pelvis [
32,
33]. These tributaries can also contribute to the recurrencies of varicose veins. These rather tiny veins comprise the superficial epigastric vein, the superficial circumflex iliac vein, the superficial external pudendal vein, and the anterior circumflex femoral vein. In some individuals, any of these tributaries can empty directly into the femoral vein, instead of the great saphenous vein.
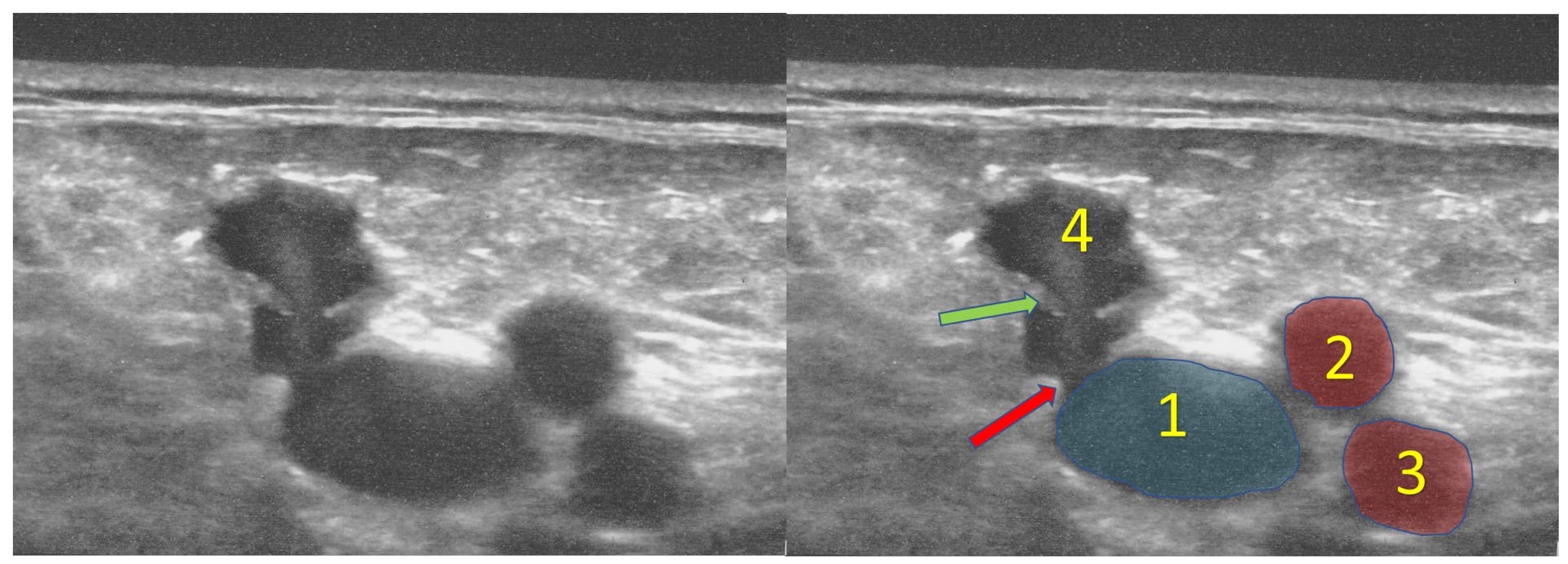
In the sapheno-femoral junction, there are typically two valves: the terminal valve that is located close (1–2 mm) to the estuary of the great saphenous vein and the pre-terminal valve that is usually located 3–5 cm distally (
Figure 6). These two valves are crucial for outflow from the great saphenous vein. They also play an important role in the pathogenesis of varicose veins and are of particular interest during endovascular procedures for incompetent great saphenous veins [
4,
7].
In some individuals, in the groin the femoral vein communicates with another interfascial vein, the anterior accessory saphenous vein. This vein, if present, runs parallelly to the great saphenous vein, slightly laterally from it. Usually, the anterior accessory saphenous vein is a short blood vessel, with a length of 10 cm or less, and rarely continues to the distal part of the thigh. In the groin, the anterior accessory saphenous vein is situated anteriorly from the femoral artery and vein, while the great saphenous vein is located medially from the femoral vein. This topography is helpful in distinguishing these two veins, especially in patients after surgical excisions of varicose veins in this area (
Figure 7). The anterior accessory saphenous vein either connects with the femoral vein either directly or empties to the most proximal part of the great saphenous vein [
4,
7].
The small saphenous vein is located in the posterior aspect of the lower leg. This vein, similarly to the great saphenous vein, is situated in the saphenous compartment, between two layers of fascia. It should be remembered that here the saphenous compartment is much smaller in comparison with that of the great saphenous vein. Therefore, ultrasonographic examination should be done carefully, in order to distinguish this vein from an epifascial one. In addition, it should be remembered that the small saphenous vein is characterized by a high anatomical variability. In some individuals, it is doubled or tripled; there are some people presenting with a junction between the small saphenous vein and the popliteal vein, which is located above the level of the knee joint. In many individuals the connection between the small saphenous vein and the popliteal vein at the knee level (the sapheno-popliteal junction) is missing. In others, there is a proximal continuation of the small saphenous vein, which is located at the posterior aspect of the thigh in the interfascial compartment. Such a vein is called the cranial extension of the small saphenous vein. Another possibility is an interfascial continuation of the small saphenous vein toward the great saphenous vein. This anatomic variant is referred to as the Giacomini’s vein (of note, the use of eponyms in anatomy is discouraged; it has been suggested to call this vein the intersaphenous vein) [
34]. This high variability is associated with the embryological history of the small saphenous vein since it develops from the primitive fibular vein, while the sapheno-popliteal junction, the most proximal part of the small saphenous vein, where it merges with the popliteal vein, is the remnant of the popliteal crossroad [
24]. Of note, in some individuals, at the level of knee joint, there is present a connection between the superficially located vein and the popliteal vein. Since this epifascial vein is not situated in the interfascial compartment, as the small saphenous vein is, such a vein is referred to as the perforator of the popliteal fossa. Detailed ultrasonographic evaluation of the fascias surrounding the veins in the popliteal region can distinguish this perforator from the small saphenous vein. This distinction is important because varicosities associated with this perforator should be managed differently from those resulting from small saphenous vein incompetence.
6. Epifascially Located Superficial Veins
There are numerous epifascial (i.e., located superficially from the membranous layer of the subcutaneous tissue) veins in the lower extremities. A term “epifascial veins” would clearly distinguish these veins from the interfascial ones, e.g., the great saphenous vein. Yet, such a descriptor is not widely used. Epifascial veins are characterized by very high anatomical variability. Since there are no such veins constantly present, they are devoid of anatomical terms. This does not mean that they are not clinically relevant. Quite the contrary: usually varicose vein disease primarily affects these veins, while pathological dilatations and refluxes in the interfascial veins are seen later [
35].
Out of the clinically relevant epifascial veins, the lateral subdermic venous plexus (described also as the vein of Albanese) should be mentioned. These veins are situated at the lateral aspect of the lower thigh and upper lower leg (
Figure 8) and probably represent a remnant of the primitive lateral marginal vein. The lateral subdermic venous plexus is characterized by unique pattern of the venous drainage—blood outflow from these veins is directed toward the perforators located at the lateral aspect of the knee. Of note, while the flow in the calf component of this plexus is directed upward, flow in the femoral part is directed downward. The lateral subdermic venous plexus is often the first site where varicose veins develop, especially in women. The above-described unique anatomy and outflow routes should be considered while treating varicose veins in this area [
36,
37].
7. Veins of the Foot
Veins of the foot, in spite of their rather small size, play an important role in the lower extremity venous return, particularly through the so-called foot venous pump. This pump, together with calf and femoral pumps, is indispensable for physiological venous outflow from the lower limb.
Although the anatomic organization of the foot veins may seem similar to that of the lower leg and thigh, actually, these veins differ significantly, especially regarding their functional anatomy. Foot perforators typically promote blood flow from the deep toward the superficial veins, which is unique in the lower extremities. Such a flow is possible because their valves are oriented inversely to other lower extremity perforators. Functionally, veins of the foot should not be seen in the context of the superficial and deep systems but rather as the medial and the lateral units. The medial unit, comprising both superficial and deep (particularly, the medial plantar vein) veins, empties either to the posterior tibial vein system or toward the great saphenous vein. The flow from the lateral unit (the lateral plantar vein is here the main blood vessel) is directed either toward the anterior tibial vein system or to the small saphenous vein. The medial and lateral units are connected by the deep venous plantar arch.
During contraction of the lower leg muscles, the outflow route to the deep veins of the lower leg is blocked; consequently, the venous outflow from the foot is directed to the saphenous veins. On the contrary, during lower leg muscle relaxation, blood flows out of the foot through deep veins of the lower leg. During walking, there is a dynamic interplay between the foot and lower leg veins and between the foot and calf muscle pumps, which is facilitated by the above-mentioned unique valve orientation in the foot perforators. This interplay can be compromised if the foot is deformed or improper footwear is used [
38,
39,
40,
41,
42].
8. Educational Challenges
Doctors are expected to be familiar with human anatomy, especially regarding clinical entities that are quite common and in which clinical anatomy plays a significant role. A substantial percentage of medical errors and subsequent legal litigations result from inadequate anatomical knowledge. Since the lower extremity vein pathologies are very common, this issue is of particular relevance.
Over the last three decades, there has been big progress in understanding the anatomy of the lower extremity veins. Consensus documents by the vascular scientific societies, as well as review papers, properly describing the updated anatomy and classification of these veins, were published more than 10 years ago [
4,
6,
7,
8]. At the same time, there was a substantial shift in the treatment of pathologies affecting lower extremity veins, from the traditional surgical methods to endovascular procedures. The latter modality is usually performed under ultrasonographic control. Nowadays, varicose veins are rarely managed with ligation and stripping, as has been done in the 20th century. Instead, they are preferentially treated using thermal or chemical ablations, with ultrasonography being the indispensable tool during such procedures.
With the advance of ultrasonography as the research tool for anatomical studies, it became apparent that previous information, based on cadaver dissections, was incomplete or even inaccurate. It is because of the fact that tiny fascial sheaths, which are of paramount importance in terms of anatomy of these veins, easily disintegrate during embalming of the cadaver bodies. This disintegration occurs both after traditional embalming with formaldehyde and with novel chemical preservatives such as Thiel fluid (
Figure 9,
Figure 10,
Figure 11 and
Figure 12). Theoretically, this problem could be overcome using fresh frozen cadavers for anatomical studies (such specimens are used in studies on the anatomy of the face). Still, ultrasonographic examinations are far more reliable and easy to perform. Therefore, ultrasonographic anatomy is currently a backbone of postgraduate medical education in such medical specialties as vascular surgery, phlebology, and angiology. Since postgraduate medical education primarily relies on practical courses, assisted by specialist manuals, scientific papers, and lectures given by experts, the problem of an obsolete anatomy is not so important. On the contrary, the undergraduate medical education still struggles with this obstacle. Unfortunately, over the last two decades, nothing has changed in the anatomical manuals and textbooks. The current anatomical textbooks provide no information about fascias covering these veins. Important anatomical variants, such as the anterior accessory saphenous vein and Giacomini’s vein, or detailed anatomy of the sapheno-femoral junction, are not mentioned. They still describe the topography of lower extremity veins in relation to the bones, tendons, and ligaments, which is understandable considering how these veins appear during cadaver dissections, yet largely ignoring the vast body of evidence coming from current studies. Actually, such topography is without any clinical application, while the topography based on fascial compartments is the key to contemporary treatments. Of note, the same obsolete information is given by students’ textbooks and specialist anatomical books. Consequently, this problem affects not only students but medical university educators as well, unless one is a vascular specialist.
Traditional teaching of anatomy is based on cadaver dissections, augmented by studying and memorizing anatomical textbooks and atlases. Yet, in recent times, there has been a shift to other educational methods. Supervised ultrasonography of living bodies is one of such methods. It is practiced at many medical universities and has been found to be efficient and attractive, although it requires some practical skills from the educators [
43,
44,
45,
46]. At our university, we also use this educational tool. Since students cannot find adequate information on the lower extremity veins in their textbooks, while they will need such knowledge a few years later during undergraduate clinical classes, as well as after graduation, “living anatomy” classes, with the use of ultrasonography (
Figure 13 and
Figure 14), seem to be a reliable option, especially regarding the anatomy of the lower extremities. During such classes, students can comprehend the topographic anatomy of the lower extremity veins, the relation of these blood vessels to the adjacent anatomical structures, functioning valves (
Supplementary File S1), and normal and abnormal blood flow direction, as well as many anatomical variants. Importantly, a “living anatomy” class enables the presentation of a high variability of interfascial veins.
The other potentially valuable option could be the use of specially prepared plastinated specimens. Of note, during a typical plastination process, the veins and fascia sheath are removed; for the purpose of teaching anatomy of the lower extremity veins, such specimens should be adequately prepared. The so-called sheet plastinates, which are thin (1–5 mm thick) slices of the human bodies, where the liquids have been replaced by polymers, provide such an educational tool. In the sheet plastinates of the thigh or the lower leg, the veins are clearly visible, and their topography is fully revealed [
47,
48]. Alternatively, high-resolution glass prints, which are less expensive copies of the sheet plastinates, can be used.
Also, traditional cadaver dissections, which are still a backbone of the undergraduate anatomical education, may remain in a prominent role. Still, anatomical educators should be aware of the topographic features of the lower extremity veins, especially with regard to their relationship to the fascias. Such information should also be included in anatomical dissection manuals. Considering the shortage of cadaver bodies at many medical universities, the new possibility of three-dimensional scanning (nowadays it can be done with a standard smartphone) of cadaver specimens, and preserving each step of the dissection digitally, provides an additional possible educational tool.