The Complexity of the Pancreatic Lymphatic System and the Key Role of Para-Aortic Lymph Node Metastasis in Pancreatic Cancer Prognosis Prediction: A Comprehensive Review
Abstract
1. Pancreatic Cancer
1.1. Epidemiology
1.2. Pancreas Anatomy
1.3. Pancreatic Cancer Subtypes
1.4. Diagnosis
2. Lymphatic System
2.1. Background
2.2. Lymphatic System in Metastasis
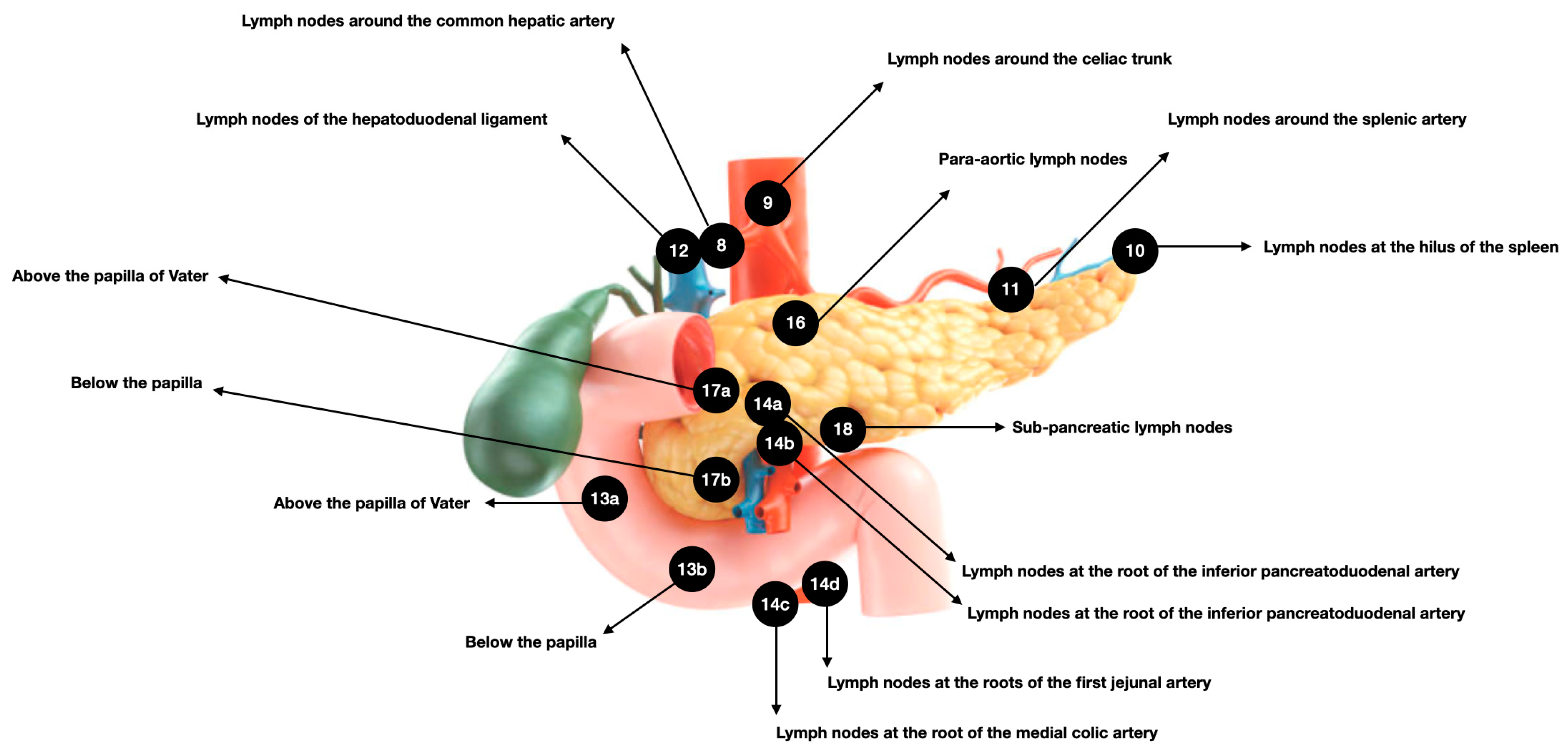
2.3. Pancreatic Lymphatic System
3. LNM in Pancreatic Cancer
3.1. Background
3.2. Clinical–Anatomical Classification
3.3. PALN
4. Imaging Diagnosis and Sentinel Lymph Node
5. Management of Para-Aortic Lymph Node Metastasis
6. Para-Aortic Lymph Node Metastasis in Patient Prognosis
7. Pancreatic Cancer from the Present to the Future
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Brand, R.E.; Goggins, M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology 2023, 164, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.X.; Lin, Y.Y.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- Ilic, I.; Ilic, M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J. Gastroenterol. 2022, 28, 4698–4715. [Google Scholar] [CrossRef]
- Covantsev, S.; Chicu, C.; Mazuruc, N.; Belic, O. Pancreatic ductal anatomy: More than meets the eye. Surg. Radiol. Anat. SRA 2022, 44, 1231–1238. [Google Scholar] [CrossRef]
- Mastracci, T.L.; Apte, M.; Amundadottir, L.T.; Alvarsson, A.; Artandi, S.; Bellin, M.D.; Bernal-Mizrachi, E.; Caicedo, A.; Campbell-Thompson, M.; Cruz-Monserrate, Z.; et al. Integrated Physiology of the Exocrine and Endocrine Compartments in Pancreatic Diseases: Workshop Proceedings. Pancreas 2022, 51, 1061–1073. [Google Scholar] [CrossRef]
- Torphy, R.J.; Fujiwara, Y.; Schulick, R.D. Pancreatic cancer treatment: Better, but a long way to go. Surg. Today 2020, 50, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Hezel, A.F.; Kimmelman, A.C.; Stanger, B.Z.; Bardeesy, N.; DePinho, R.A. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006, 20, 1218–1249. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, V.; Luchini, C. Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms of the Pancreas. Surg. Pathol. Clin. 2022, 15, 555–563. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Burns, W.R.; Frankel, T.L.; Cho, C.S.; Nathan, H. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann. Surg. Oncol. 2017, 24, 2023–2030. [Google Scholar] [CrossRef]
- Kang, H.; Kim, S.S.; Sung, M.J.; Jo, J.H.; Lee, H.S.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; Park, M.S.; et al. Evaluation of the 8th Edition AJCC Staging System for the Clinical Staging of Pancreatic Cancer. Cancers 2022, 14, 4672. [Google Scholar] [CrossRef]
- Shin, D.W.; Kim, J. The American Joint Committee on Cancer 8th edition staging system for the pancreatic ductal adenocarcinoma: Is it better than the 7th edition? Hepatobiliary Surg. Nutr. 2020, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.C.; Itkin, M. Lymphatic Anatomy. Tech. Vasc. Interv. Radiol. 2016, 19, 247–254. [Google Scholar] [CrossRef]
- Swartz, M.A. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 2001, 50, 3–20. [Google Scholar] [CrossRef]
- Hu, D.; Li, L.; Li, S.; Wu, M.; Ge, N.; Cui, Y.; Lian, Z.; Song, J.; Chen, H. Lymphatic system identification, pathophysiology and therapy in the cardiovascular diseases. J. Mol. Cell. Cardiol. 2019, 133, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.; Komnick, M.R.; Brigleb, P.H.; Dermody, T.S.; Esterházy, D. Lymph node sharing between pancreas, gut, and liver leads to immune crosstalk and regulation of pancreatic autoimmunity. Immunity 2023, 56, 2070–2085. [Google Scholar] [CrossRef]
- Ps, L. Overview of the pancreas. Adv. Exp. Med. Biol. 2010, 690, 3–12. [Google Scholar] [CrossRef]
- Renard, Y.; de Mestier, L.; Perez, M.; Avisse, C.; Lévy, P.; Kianmanesh, R. Unraveling Pancreatic Segmentation. World J. Surg. 2018, 42, 1147–1153. [Google Scholar] [CrossRef]
- Fink, D.M.; Steele, M.M.; Hollingsworth, M.A. The lymphatic system and pancreatic cancer. Cancer Lett. 2016, 381, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Cesmebasi, A.; Malefant, J.; Patel, S.D.; Plessis, M.D.; Renna, S.; Tubbs, R.S.; Loukas, M. The surgical anatomy of the lymphatic system of the pancreas. Clin. Anat. 2015, 28, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Cui, M.; Yang, G.; Wang, H.; Feng, M.; You, L.; Zhao, Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol. Cancer 2018, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Luo, G.; Liu, C.; Wu, C.; Liu, L.; Liu, Z.; Ni, Q.; Long, J.; Yu, X. Molecular mechanism underlying lymphatic metastasis in pancreatic cancer. BioMed Res. Int. 2014, 2014, 925845. [Google Scholar] [CrossRef] [PubMed]
- Keleg, S.; Büchler, P.; Ludwig, R.; Büchler, M.W.; Friess, H. Invasion and metastasis in pancreatic cancer. Mol. Cancer 2003, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Nagakawa, T.; Kobayashi, H.; Ueno, K.; Ohta, T.; Kayahara, M.; Mori, K.; Nakano, T.; Takeda, T.; Konishi, I.; Miyazaki, I. The pattern of lymph node involvement in carcinoma of the head of the pancreas—A histologic study of the surgical findings in patients undergoing extensive nodal dissections. Int. J. Pancreatol. 1993, 13, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Fodor, M.; Stättner, S. The Lymphatic System and Lymph Nodes of the Pancreas. In Textbook of Pancreatic Cancer; Søreid, K., Stättner, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 173–185. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, S.H.; Choi, J.J.; Kang, C.M.; Hwang, H.K.; Lee, W.J. Clinical necessity of the immunohistochemical reassessment of para-aortic lymph nodes in resected pancreatic ductal adenocarcinoma. Oncol. Lett. 2013, 6, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, H.; Olsson, H.; Green, H.; Björnsson, B.; Elander, N.O. Impact of resection margins and para-aortic lymph node metastases on recurrence patterns and prognosis in resectable pancreatic cancer—A long-term population-based cohort study. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2023, 25, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Hwang, H.K.; Lee, W.J.; Kang, C.M. Unexpected Para-aortic Lymph Node Metastasis in Pancreatic Ductal Adenocarcinoma: A Contraindication to Resection? J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2020, 24, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Yoon, Y.S.; Han, H.S.; Lee, J.S.; Na, H.Y.; Ahn, S.; Park, J.; Jung, K.; Jung, J.H.; Kim, J.; et al. Clinical Impact of Unexpected Para-Aortic Lymph Node Metastasis in Surgery for Resectable Pancreatic Cancer. Cancers 2021, 13, 4454. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Zhang, X.M.; Kou, J.T.; Fa, H.; Zhang, X.X.; Dai, Y.; He, Q. Analysis of prognostic factors for pancreatic head cancer according to para-aortic lymph node. Cancer Med. 2016, 5, 2701–2707. [Google Scholar] [CrossRef]
- Paiella, S.; Sandini, M.; Gianotti, L.; Butturini, G.; Salvia, R.; Bassi, C. The prognostic impact of para-aortic lymph node metastasis in pancreatic cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2016, 42, 616–624. [Google Scholar] [CrossRef]
- Burk, K.S.; Lo, G.C.; Gee, M.S.; Sahani, D.V. Imaging and Screening of Pancreatic Cancer. Radiol. Clin. N. Am. 2017, 55, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Kobayashi, S.; Takahashi, H.; Yoshioka, T.; Iwagami, Y.; Tomimaru, Y.; Shigekawa, M.; Akita, H.; Noda, T.; Asaoka, T.; et al. Pancreatic CT density is an optimal imaging biomarker for earlier detection of malignancy in the pancreas with intraductal papillary mucinous neoplasm. Pancreatology 2022, 22, 488–496. [Google Scholar] [CrossRef]
- O’Neill, E.; Hammond, N.; Miller, F.H. MR imaging of the pancreas. Radiol. Clin. N. Am. 2014, 52, 757–777. [Google Scholar] [CrossRef]
- Kobi, M.; Veillette, G.; Narurkar, R.; Sadowsky, D.; Paroder, V.; Shilagani, C.; Gilet, A.; Flusberg, M. Imaging and Management of Pancreatic Cancer. Semin. Ultrasound CT MR 2020, 41, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xia, Y.; Yao, J.; Han, X.; Lambert, L.; Zhang, T.; Tang, W.; Jin, G.; Jiang, H.; Fang, X.; et al. Large-scale pancreatic cancer detection via non-contrast CT and deep learning. Nat. Med. 2023, 29, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.N.; Goh, K.S.; Huang, C.R.; Chiang, T.C.; Lee, C.Y.; Jeng, Y.M.; Peng, S.J.; Chien, H.J.; Chung, M.H.; Chou, Y.H.; et al. Lymphatic vessel remodeling and invasion in pancreatic cancer progression. eBioMedicine 2019, 47, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Ettrich, T.J.; Seufferlein, T. Systemic Therapy for Metastatic Pancreatic Cancer. Curr. Treat. Options Oncol. 2021, 22, 106. [Google Scholar] [CrossRef] [PubMed]
- Sho, M.; Murakami, Y.; Motoi, F.; Satoi, S.; Matsumoto, I.; Kawai, M.; Honda, G.; Uemura, K.; Yanagimoto, H.; Kurata, M.; et al. Postoperative prognosis of pancreatic cancer with para-aortic lymph node metastasis: A multicenter study on 822 patients. J. Gastroenterol. 2015, 50, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Lupinacci, R.M.; Svrcek, M.; Lesurtel, M.; Bubenheim, M.; Vuarnesson, H.; Balladur, P.; Paye, F. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br. J. Surg. 2014, 101, 530–538. [Google Scholar] [CrossRef]
- Doi, R.; Kami, K.; Ito, D.; Fujimoto, K.; Kawaguchi, Y.; Wada, M.; Kogire, M.; Hosotani, R.; Imamura, M.; Uemoto, S. Prognostic implication of para-aortic lymph node metastasis in resectable pancreatic cancer. World J. Surg. 2007, 31, 147–154. [Google Scholar] [CrossRef]
- Sperti, C.; Gruppo, M.; Valmasoni, M.; Pozza, G.; Passuello, N.; Beltrame, V.; Moletta, L.; Blandamura, S. Para-aortic node involvement is not an independent predictor of survival after resection for pancreatic cancer. World J. Gastroenterol. 2017, 23, 4399–4406. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Plodeck, V.; Mierke, F.; Distler, M.; Aust, D.E.; Saeger, H.D.; Weitz, J.; Welsch, T. Para-aortic lymph node metastases in pancreatic cancer should not be considered a watershed for curative resection. Sci. Rep. 2017, 7, 7688. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, Y.; Luo, G.; Cheng, H.; Guo, M.; Liu, Z.; Xu, J.; Long, J.; Liu, L.; Fu, D.; et al. Which patients with para-aortic lymph node (LN16) metastasis will truly benefit from curative pancreaticoduodenectomy for pancreatic head cancer? Oncotarget 2016, 7, 29177–29186. [Google Scholar] [CrossRef]
- Sillesen, M.; Hansen, C.P.; Burgdorf, S.K.; Dencker, E.E.; Krohn, P.S.; Gisela Kollbeck, S.L.; Stender, M.T.; Storkholm, J.H. Impact of para aortic lymph node removal on survival following resection for pancreatic adenocarcinoma. BMC Surg. 2023, 23, 214. [Google Scholar] [CrossRef]
- Van Wijk, L.; De Klein, G.W.; Kanters, M.A.; Patijn, G.A.; Klaase, J.M. The ultimate preoperative C-reactive protein-to-albumin ratio is a prognostic factor for survival after pancreatic cancer resection. Eur. J. Med. Res. 2020, 25, 46. [Google Scholar] [CrossRef]
- Eskander, M.F.; de Geus, S.W.L.; Kasumova, G.G.; Ng, S.C.; Al-Refaie, W.; Ayata, G.; Tseng, J.F. Evolution and impact of lymph node dissection during pancreaticoduodenectomy for pancreatic cancer. Surgery 2017, 161, 968–976. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Kitago, M.; Kitagawa, Y. Evidence and Future Perspectives for Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Scoping Review. Cancers 2024, 16, 1632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, T.; Lu, D.; Zhen, J.; Zhang, L. CD44 overexpression related to lymph node metastasis and poor prognosis of pancreatic cancer. Int. J. Biol. Markers 2018, 33, 308–313. [Google Scholar] [CrossRef]
- Maeda, S.; Shinchi, H.; Kurahara, H.; Mataki, Y.; Maemura, K.; Sato, M.; Natsugoe, S.; Aikou, T.; Takao, S. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br. J. Cancer 2008, 98, 1389–1397. [Google Scholar] [CrossRef]
- Meng, F.; Hua, S.; Chen, X.; Meng, N.; Lan, T. Lymph node metastasis related gene BICC1 promotes tumor progression by promoting EMT and immune infiltration in pancreatic cancer. BMC Med. Genom. 2023, 16, 263. [Google Scholar] [CrossRef] [PubMed]
- Izumi, S.; Nakamura, S.; Mano, S.; Akaki, S. Well differentiation and intact Smad4 expression are specific features of groove pancreatic ductal adenocarcinomas. Pancreas 2015, 44, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, M.; Korc, M. Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin. Cancer Res. 2004, 10, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Kurahara, H.; Takao, S.; Maemura, K.; Mataki, Y.; Kuwahata, T.; Maeda, K.; Sakoda, M.; Iino, S.; Ishigami, S.; Ueno, S.; et al. M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas 2013, 42, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, T.; Miyamoto, A.; Maeda, S.; Hama, N.; Tsujie, M.; Ikeda, M.; Sekimoto, M.; Nakamori, S. CA19-9 level determines therapeutic modality in pancreatic cancer patients with para-aortic lymph node metastasis. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 75–80. [Google Scholar] [CrossRef]
- Søreide, K.; Ismail, W.; Roalsø, M.; Ghotbi, J.; Zaharia, C. Early Diagnosis of Pancreatic Cancer: Clinical Premonitions, Timely Precursor Detection and Increased Curative-Intent Surgery. Cancer Control J. Moffitt Cancer Cent. 2023, 30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.F.V.e.; Ballini, A.; Di Domenico, M.; Padín-Iruegas, M.E. The Complexity of the Pancreatic Lymphatic System and the Key Role of Para-Aortic Lymph Node Metastasis in Pancreatic Cancer Prognosis Prediction: A Comprehensive Review. Anatomia 2024, 3, 124-135. https://doi.org/10.3390/anatomia3020010
Silva FFVe, Ballini A, Di Domenico M, Padín-Iruegas ME. The Complexity of the Pancreatic Lymphatic System and the Key Role of Para-Aortic Lymph Node Metastasis in Pancreatic Cancer Prognosis Prediction: A Comprehensive Review. Anatomia. 2024; 3(2):124-135. https://doi.org/10.3390/anatomia3020010
Chicago/Turabian StyleSilva, Fábio França Vieira e, Andrea Ballini, Marina Di Domenico, and María Elena Padín-Iruegas. 2024. "The Complexity of the Pancreatic Lymphatic System and the Key Role of Para-Aortic Lymph Node Metastasis in Pancreatic Cancer Prognosis Prediction: A Comprehensive Review" Anatomia 3, no. 2: 124-135. https://doi.org/10.3390/anatomia3020010
APA StyleSilva, F. F. V. e., Ballini, A., Di Domenico, M., & Padín-Iruegas, M. E. (2024). The Complexity of the Pancreatic Lymphatic System and the Key Role of Para-Aortic Lymph Node Metastasis in Pancreatic Cancer Prognosis Prediction: A Comprehensive Review. Anatomia, 3(2), 124-135. https://doi.org/10.3390/anatomia3020010





