Effects of Congenital Adrenal Hyperplasia (CAH) and Biological Sex on Brain Size
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Image Acquisition and Processing
2.3. Statistical Analysis
3. Results
3.1. Intracranial Volume (ICV)
3.2. Total Brain Volume (TBV)
3.3. Supplementary Analyses (Effect of CAH Phenotype)
4. Discussion
4.1. No Significant CAH Effect
4.2. No Significant CAH-by-Sex Interaction
4.3. Significant Sex Effect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merke, D.P.; Fields, J.D.; Keil, M.F.; Vaituzis, A.C.; Chrousos, G.P.; Giedd, J.N. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: Potential prenatal and postnatal hormonal effects. J. Clin. Endocrinol. Metab. 2003, 88, 1760–1765. [Google Scholar] [CrossRef]
- Khalifeh, N.; Omary, A.; Cotter, D.L.; Kim, M.S.; Geffner, M.E.; Herting, M.M. Congenital Adrenal Hyperplasia and Brain Health: A Systematic Review of Structural, Functional, and Diffusion Magnetic Resonance Imaging (MRI) Investigations. J. Child. Neurol. 2022, 37, 758–783. [Google Scholar] [CrossRef]
- Beltz, A.M.; Demidenko, M.I.; Wilson, S.J.; Berenbaum, S.A. Prenatal androgen influences on the brain: A review, critique, and illustration of research on congenital adrenal hyperplasia. J. Neurosci. Res. 2023, 101, 563–574. [Google Scholar] [CrossRef]
- Herting, M.M.; Azad, A.; Kim, R.; Tyszka, J.M.; Geffner, M.E.; Kim, M.S. Brain Differences in the Prefrontal Cortex, Amygdala, and Hippocampus in Youth with Congenital Adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2020, 105, 1098–1111. [Google Scholar] [CrossRef]
- Van’t Westeinde, A.; Karlsson, L.; Thomsen Sandberg, M.; Nordenstrom, A.; Padilla, N.; Lajic, S. Altered Gray Matter Structure and White Matter Microstructure in Patients with Congenital Adrenal Hyperplasia: Relevance for Working Memory Performance. Cereb. Cortex 2020, 30, 2777–2788. [Google Scholar] [CrossRef]
- Webb, E.A.; Elliott, L.; Carlin, D.; Wilson, M.; Hall, K.; Netherton, J.; Reed, J.; Barrett, T.G.; Salwani, V.; Clayden, J.D.; et al. Quantitative Brain MRI in Congenital Adrenal Hyperplasia: In Vivo Assessment of the Cognitive and Structural Impact of Steroid Hormones. J. Clin. Endocrinol. Metab. 2018, 103, 1330–1341. [Google Scholar] [CrossRef]
- Luders, E.; Toga, A.W. Sex differences in brain anatomy. Prog. Brain Res. 2010, 186, 2–12. [Google Scholar]
- Luders, E.; Kurth, F. Structural differences between male and female brains. Handb. Clin. Neurol. 2020, 175, 3–11. [Google Scholar] [CrossRef]
- Salminen, L.E.; Tubi, M.A.; Bright, J.; Thomopoulos, S.I.; Wieand, A.; Thompson, P.M. Sex is a defining feature of neuroimaging phenotypes in major brain disorders. Hum. Brain Mapp. 2022, 43, 500–542. [Google Scholar] [CrossRef]
- Kaczkurkin, A.N.; Raznahan, A.; Satterthwaite, T.D. Sex differences in the developing brain: Insights from multimodal neuroimaging. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 71–85. [Google Scholar] [CrossRef]
- Merke, D.P.; Auchus, R.J. Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. N. Engl. J. Med. 2020, 383, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, R.B.; French, J.W.; Harman, H.H.; Dermen, D. Manual for Kit of Factor Referenced Cognitive Tests; Educational Testing Service: Princeton, NJ, USA, 1976. [Google Scholar]
- Gaser, C.; Dahnke, R.; Thompson, P.M.; Kurth, F.; Luders, E. CAT—A Computational Anatomy Toolbox for the Analysis of Structural MRI Data. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kurth, F.; Thompson, P.M.; Luders, E. Investigating the differential contributions of sex and brain size to gray matter asymmetry. Cortex 2018, 99, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Gaser, C.; Narr, K.L.; Toga, A.W. Why sex matters: Brain size independent differences in gray matter distributions between men and women. J. Neurosci. 2009, 29, 14265–14270. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Kurth, F.; Gingnell, M.; Engman, J.; Yong, E.L.; Poromaa, I.S.; Gaser, C. From baby brain to mommy brain: Widespread gray matter gain after giving birth. Cortex A J. Devoted Study Nerv. Syst. Behav. 2020, 126, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Manjon, J.V.; Coupe, P.; Marti-Bonmati, L.; Collins, D.L.; Robles, M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J.Magn. Reson. Imaging 2010, 31, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Unified segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, J.C.; Giedd, J.N.; Rapoport, J.L. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans. Med. Imaging 1997, 16, 176–186. [Google Scholar] [CrossRef]
- Tohka, J.; Zijdenbos, A.; Evans, A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage 2004, 23, 84–97. [Google Scholar] [CrossRef]
- Arnold, A.P. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009, 55, 570–578. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Arnold, A.P. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011, 14, 677–683. [Google Scholar] [CrossRef]
- Arnold, A.P. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 2004, 5, 701–708. [Google Scholar] [CrossRef]
- Phoenix, C.H.; Goy, R.W.; Gerall, A.A.; Young, W.C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 1959, 65, 369–382. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Seidman, L.J.; Horton, N.J.; Makris, N.; Kennedy, D.N.; Caviness, V.S., Jr.; Faraone, S.V.; Tsuang, M.T. Normal Sexual Dimorphism of the Adult Human Brain Assessed by In Vivo Magnetic Resonance Imaging. Cereb. Cortex 2001, 11, 490–497. [Google Scholar] [CrossRef]
- Hines, M. Neuroscience and Sex/Gender: Looking Back and Forward. J. Neurosci. 2020, 40, 37–43. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Lin, W.; Prastawa, M.W.; Looney, C.B.; Vetsa, Y.S.; Knickmeyer, R.C.; Evans, D.D.; Smith, J.K.; Hamer, R.M.; Lieberman, J.A.; et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J. Neurosci. 2007, 27, 1255–1260. [Google Scholar] [CrossRef]
- Benavides, A.; Metzger, A.; Tereshchenko, A.; Conrad, A.; Bell, E.F.; Spencer, J.; Ross-Sheehy, S.; Georgieff, M.; Magnotta, V.; Nopoulos, P. Sex-specific alterations in preterm brain. Pediatr. Res. 2019, 85, 55–62. [Google Scholar] [CrossRef]
- Carruth, L.L.; Reisert, I.; Arnold, A.P. Sex chromosome genes directly affect brain sexual differentiation. Nat. Neurosci. 2002, 5, 933–934. [Google Scholar] [CrossRef]
- De Vries, G.J.; Rissman, E.F.; Simerly, R.B.; Yang, L.Y.; Scordalakes, E.M.; Auger, C.J.; Swain, A.; Lovell-Badge, R.; Burgoyne, P.S.; Arnold, A.P. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 2002, 22, 9005–9014. [Google Scholar] [CrossRef]
- Giedd, J.N.; Raznahan, A.; Mills, K.L.; Lenroot, R.K. Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol. Sex Differ. 2012, 3, 19. [Google Scholar] [CrossRef]
- Gur, R.E.; Gur, R.C. Sex differences in brain and behavior in adolescence: Findings from the Philadelphia Neurodevelopmental Cohort. Neurosci. Biobehav. Rev. 2016, 70, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Herting, M.M.; Sowell, E.R. Puberty and structural brain development in humans. Front. Neuroendocrinol. 2017, 44, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, N.; Op de Macks, Z.; Shirtcliff, E.A.; Pfeifer, J.H. Puberty and the human brain: Insights into adolescent development. Neurosci. Biobehav. Rev. 2018, 92, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Kurth, F.; Gaser, C.; Luders, E. Development of sex differences in the human brain. Cogn. Neurosci. 2021, 12, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Chavarria, M.C.; Sanchez, F.J.; Chou, Y.Y.; Thompson, P.M.; Luders, E. Puberty in the corpus callosum. Neuroscience 2014, 265, 1–8. [Google Scholar] [CrossRef]
- Lenroot, R.K.; Gogtay, N.; Greenstein, D.K.; Wells, E.M.; Wallace, G.L.; Clasen, L.S.; Blumenthal, J.D.; Lerch, J.; Zijdenbos, A.P.; Evans, A.C.; et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007, 36, 1065–1073. [Google Scholar] [CrossRef]
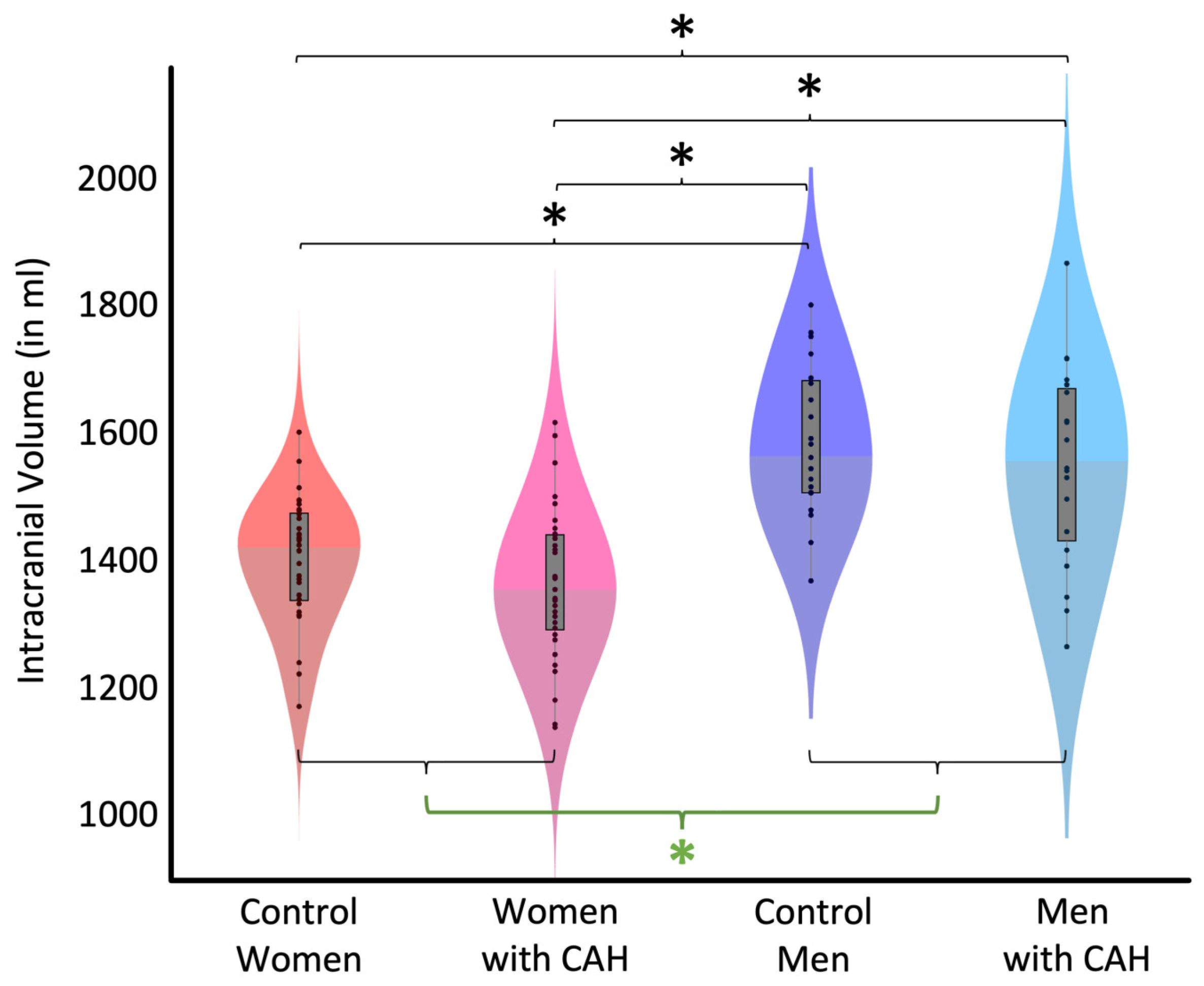
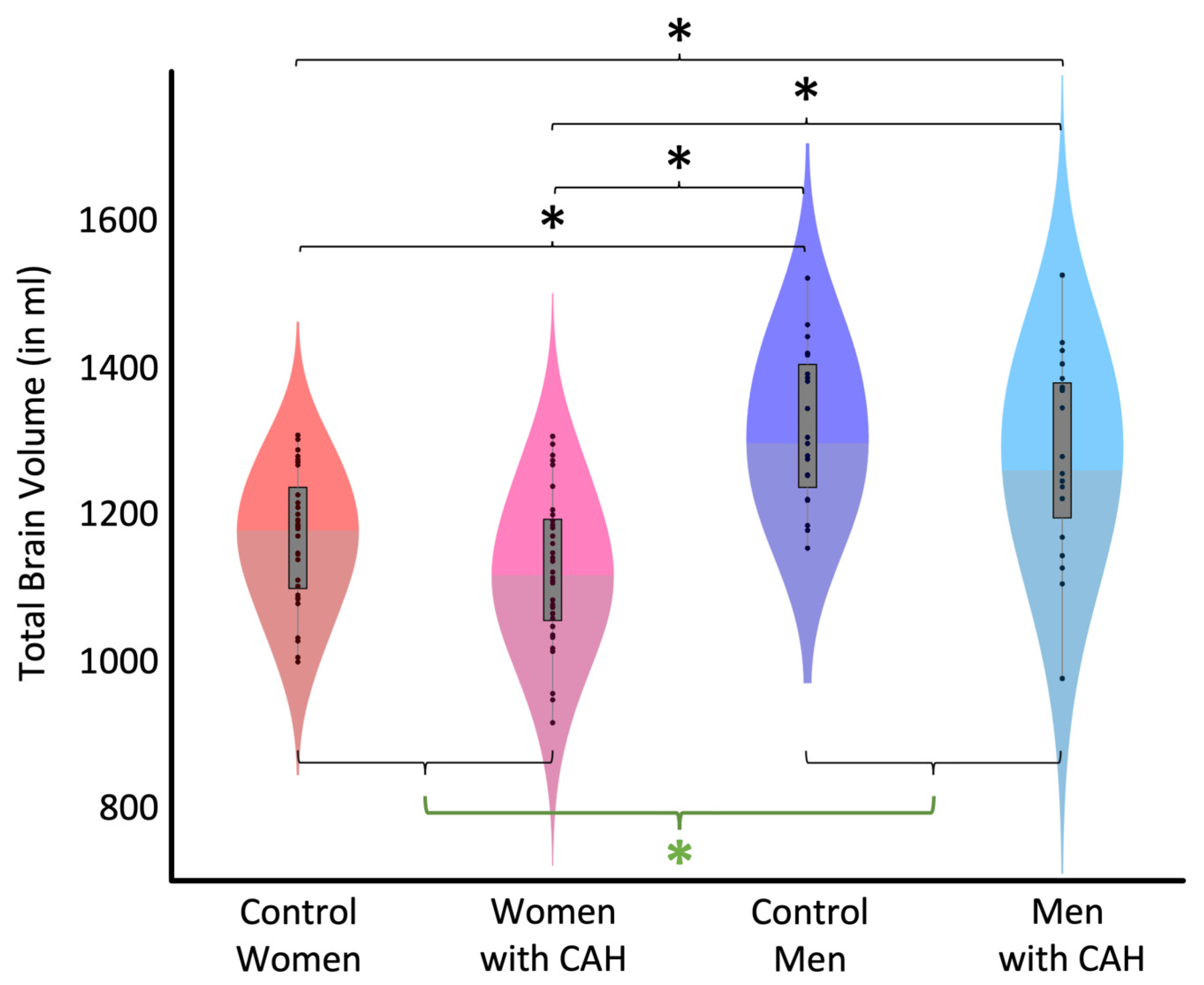
| Control Women | Women with CAH | Control Men | Men with CAH | All Women | All Men |
|---|---|---|---|---|---|
| 1399.06 | 1361.53 | 1585.52 | 1549.07 | 1380.29 | 1567.30 |
| ±99.05 | ±117.84 | ±118.74 | ±153.96 | ±109.65 | ±136.96 |
| Effect Size (Cohen’s d) | t (df) | Significance (p) | |
|---|---|---|---|
| Control Women vs. Control Men | −1.08 | −5.47 (102) | <0.001 |
| Control Women vs. Women with CAH | 0.25 | 1.27 (102) | 0.166 |
| Women with CAH vs. Men with CAH | −1.09 | −5.50 (102) | <0.001 |
| Control Men vs. Men with CAH | 0.19 | 0.96 (102) | 0.407 |
| Women with CAH vs. Control Men | −1.30 | −6.57 (102) | <0.001 |
| Control Women vs. Men with CAH | −0.87 | −4.40 (102) | <0.001 |
| Control Women | Women with CAH | Control Men | Men with CAH | All Women | All Men |
|---|---|---|---|---|---|
| 1167.09 | 1123.43 | 1316.61 | 1279.37 | 1145.26 | 1297.99 |
| ±88.08 | ±102.30 | ±104.44 | ±134.87 | ±97.24 | ±120.55 |
| Effect Size (Cohen’s d) | t (df) | Significance (p) | |
|---|---|---|---|
| Control Women vs. Control Men | −0.99 | −5.00 (102) | <0.001 |
| Control Women vs. Women with CAH | 0.33 | 1.68 (102) | 0.166 |
| Women with CAH vs. Men with CAH | −1.03 | −5.21 (102) | <0.001 |
| Control Men vs. Men with CAH | 0.22 | 1.12 (102) | 0.407 |
| Women with CAH vs. Control Men | −1.28 | −6.46 (102) | <0.001 |
| Control Women vs. Men with CAH | −0.74 | −3.75 (102) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luders, E.; Gaser, C.; Spencer, D.; Thankamony, A.; Hughes, I.; Srirangalingam, U.; Gleeson, H.; Hines, M.; Kurth, F. Effects of Congenital Adrenal Hyperplasia (CAH) and Biological Sex on Brain Size. Anatomia 2024, 3, 155-162. https://doi.org/10.3390/anatomia3030012
Luders E, Gaser C, Spencer D, Thankamony A, Hughes I, Srirangalingam U, Gleeson H, Hines M, Kurth F. Effects of Congenital Adrenal Hyperplasia (CAH) and Biological Sex on Brain Size. Anatomia. 2024; 3(3):155-162. https://doi.org/10.3390/anatomia3030012
Chicago/Turabian StyleLuders, Eileen, Christian Gaser, Debra Spencer, Ajay Thankamony, Ieuan Hughes, Umasuthan Srirangalingam, Helena Gleeson, Melissa Hines, and Florian Kurth. 2024. "Effects of Congenital Adrenal Hyperplasia (CAH) and Biological Sex on Brain Size" Anatomia 3, no. 3: 155-162. https://doi.org/10.3390/anatomia3030012
APA StyleLuders, E., Gaser, C., Spencer, D., Thankamony, A., Hughes, I., Srirangalingam, U., Gleeson, H., Hines, M., & Kurth, F. (2024). Effects of Congenital Adrenal Hyperplasia (CAH) and Biological Sex on Brain Size. Anatomia, 3(3), 155-162. https://doi.org/10.3390/anatomia3030012






