Abstract
The genus Quercus (Fagaceae) is one of the most widely distributed and species-diverse trees in the Northern Hemisphere. The present study addresses the investigation of the phyto-chemical profile by ten assays, the antioxidant activity scavenging of DPPH radicals, total phenolic content, total flavonoids, and antimicrobial activity against three pathogenic bacteria with the foliage of two species of red oak (Quercus sartorii and Quercus rysophylla). Both species of oak showed a high phenolic content in the aqueous extract (22,342.10 ± 3076.5 mg GAE/kg of plant and 17,747.14 ± 1139.9 mg GAE/kg of plant, respectively). In the flavonoid content, Q. sartorii showed a higher amount in the ethanolic extract (24,587.42 ± 996.3 mg QE/kg of plant), while for Q. rysophylla, it was methanolic extract (19,875.66 ± 2754.01 QE/kg of plant). In the DPPH radical scavenging activity, Q. sartorii showed the highest percentage of inhibition in the methanolic extract (81.14 ± 1.7%), while in Q. rysophylla, it was the ethanolic extract (82.60 ± 2.7%). In the antimicrobial tests, inhibition halos were obtained in the strains Acinetobacter baumannii and Staphylococcus aureus of both species. All this gives a guideline to comprehensively elucidate the metabolites present in these two species for further study and application in the dispute against pathogenic bacteria or in diseases related to the imbalance of reactive oxygen species (ROS).
1. Introduction
The genus Quercus, belonging to the family Fagaceae, is among the most widely distributed and diverse tree genera in the Northern Hemisphere. The greatest diversity is exhibited in Mexico and East Asia [1]. In Mexico, there are about 170 species of oaks, among which 90 belong to the Quercus section (white oaks), 85 to the Lobatae section (red oaks), and only 4 to the Protobalanus section (intermediate oaks) [2]. The herbarium of the botanical garden of the “Benemérita Universidad Autónoma de Puebla” (HBUAP) has 182 species of oak [3]. A structural difference between the Quercus and Lobatae sections is the presence of tyloses in the cells of the white oak and their absence in the red oak [4]. These structures are formed within the xylem vessels, and develop from the natural process of heartwood formation or in response to external stimuli such as wounds or infections in the conductive part of the wood, to slow down or prevent the progression of pathogens [5]. For centuries, Quercus species have been used for their commercial value and their medicinal and nutritional uses. Oak galls have been used for tanning hides, dyeing fabrics, and as an ingredient in the manufacture of inks. In traditional medicine, they are used as hemostatic, astringent, antimicrobial, and healing agents, as well as for the treatment of acute diarrhea, eczema, scrofula, menorrhagia, hemorrhoids, tonsillitis, and inflammation of the oral, genital, and anal mucosa [6].
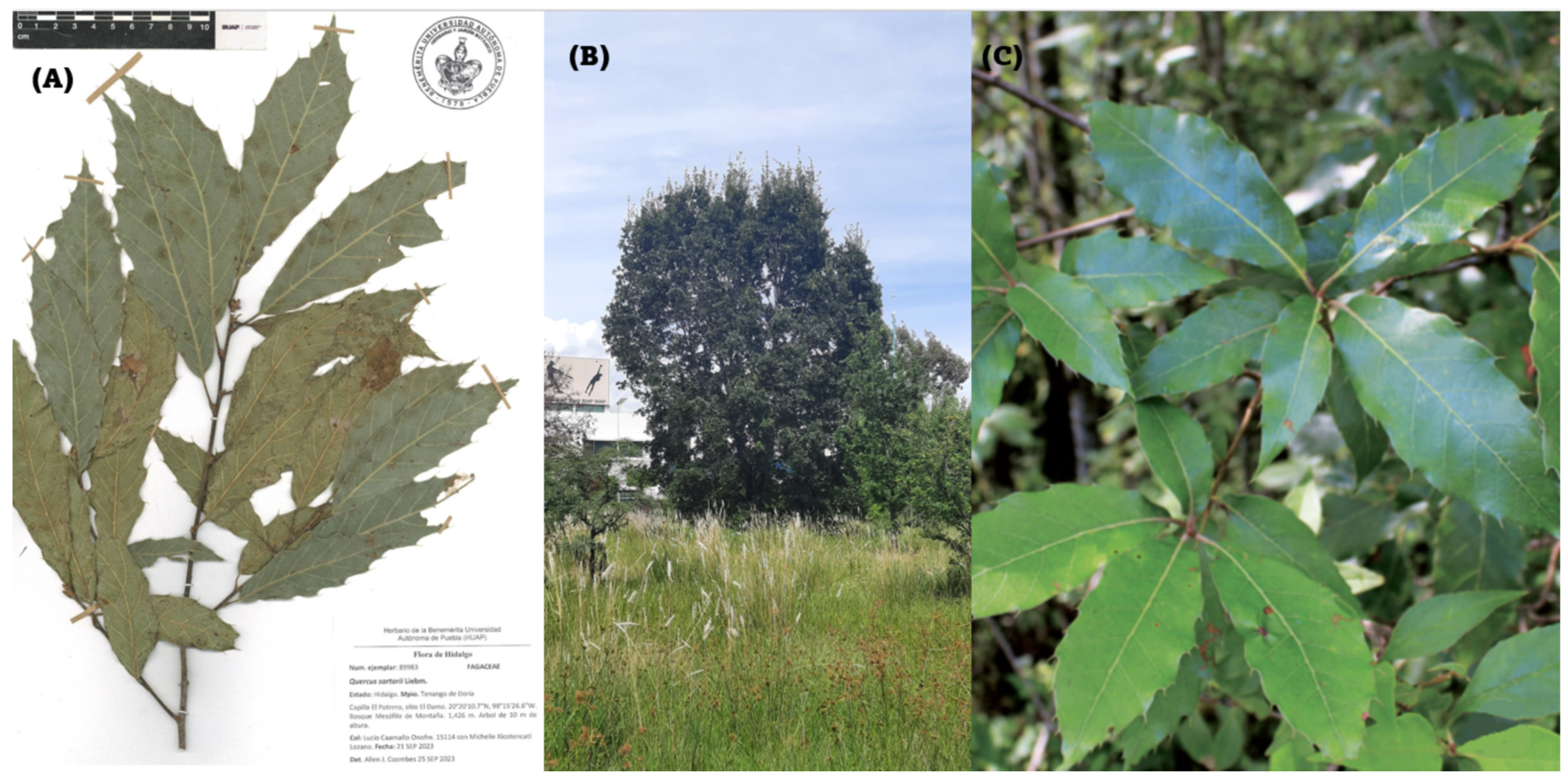
Quercus sartorii (Figure 1) belong to the Lobatae section [7]. They are trees that reach a height of between 10 and 25 m. The mature leaves feature petioles measuring 11.4–18 mm long by 1.2–2 mm wide. The laminae are leathery, elliptical, ovate, and lanceolate, with a round or truncated base. The apex of the leaf is sharp and ends in a ridged tip. Annual fruits, ovoid acorns, glabrous. Endemic to eastern Mexico, in the states of Hidalgo, Oaxaca, Puebla, San Luis Potosí, Tamaulipas, and Veracruz [8].
Figure 1.
Quercus sartorii. (A) Exemplary provided by the herbarium of the botanical garden of the “Benemérita Universidad Autónoma de Puebla”. (B) Photo of the tree. (C) Expansion of leaves (Photo E.C). In three pictures show both the height of the specimen, as well as the characteristic leaves feature petioles measuring 11.4–18 mm long by 1.2–2 mm wide.
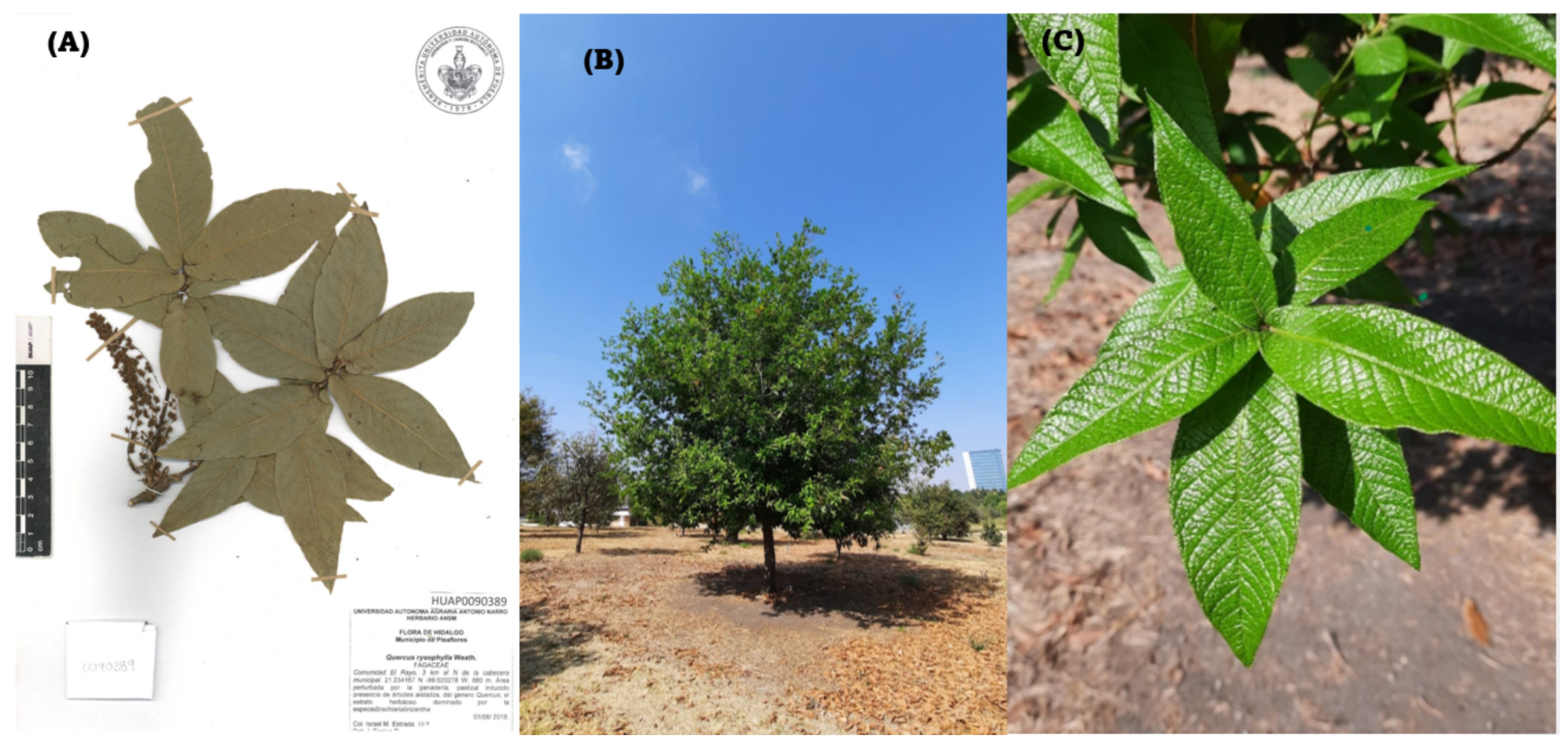
Quercus rysophylla (Figure 2) also belongs to the section Lobatae. They are trees between 12 and 20 m high. Mature leaves with petioles 3.3–6.2 mm long by 1.6–2.3 mm wide. The blades are subcoracious or cartaceous, elliptical, oblanceolate, or obovate and measure between 10.5 cm and 18.5 cm long and 3.5 cm to 7 cm wide. The base of the leaves is cordate or subcordate. The apex is acute to obtuse and sometimes acuminate. The biennial fruits are acorns, varying from ovoid to elliptical. The species is endemic to Mexico, in the states of Hidalgo, Querétaro, Nuevo León, San Luis Potosí, Tamaulipas, and Veracruz [8].
Figure 2.
Quercus rysophylla. (A) Exemplary provided by the herbarium of the botanical garden of the “Benemérita Universidad Autónoma de Puebla”. (B) Photo of the tree. (C) Expansion of leaves (Photo E. C). Three pictures show both the height of the specimen, as well as the characteristic leaves with petioles (3.3–6.2 mm long and 1.6–2.3 mm wide).
Quercus species contain several phytochemical components with significant differences between species due to their high variability. However, there are certain types of compounds that are found in all Quercus species. In the case of Q. rysophylla and Q. sartorii, there is little research on their phytochemical profile; however, other studies have already reflected the similarity that exists between numerous species such as Q. engleariana, Q. cerris, Q. mongolica, Q. canarienris, and Q. ilex, giving the possibility to compare our results with previous studies in this family [6]. The main phytochemicals with biological activity are phenolic compounds, which usually occur in the form of glycosides. These compounds constitute one of the most numerous groups of secondary metabolites in plants, they are of great relevance due to their pharmacological properties. They exhibit a wide variety of structures, from simple molecules (e.g., phenolic acids) to polyphenols (e.g., stilbenes, flavonoids, and derived polymers). These compounds play a crucial role in defending against herbivores including positive effects in humans due to their antioxidant, antimicrobial, anti-inflammatory, and anticancer activity [9]. The main phenolic compounds identified in Quercus leaves are phenolic acids (e.g., gallic acid, ellagic acid, protocatechuic acid, gentisic acid, chlorogenic acid, vanillic acid, syringic acid, caffeic acid, p-coumaric acid, and ferulic acid), flavonoids (e.g., rutin, quercetin, epicatechin, naringenin, heseretin, formononetin, naringin, kaempferol), or tannins [10].
These identified metabolites play an important role in antioxidant activity, which is closely related to the oxidative stress that occurs when there is an imbalance between the antioxidant defenses of cells and an excessive number of oxidants. Antioxidants are essential for reducing oxidative stress, as they help slow down or prevent the spread of chain reactions that cause oxidation. They are crucial for neutralizing free radicals and preventing damage to tissues and cells, which consequently reduces the risk of chronic diseases [11]. However, these metabolites do not just help in protection against oxidative stress, as they also play a crucial role in antimicrobial activity. In Quercus extracts (especially bark extracts), gallic acid, ellagic acid, vescalagine, or castalagine are identified as antimicrobial compounds [10].
Bacteria are one of the main pathogens with drug resistance, posing a threat to humans and public health. This is why it is urgent to develop new antimicrobial drugs capable of inhibiting their growth, preventing the formation of biofilms and effectively eliminating bacteria, and this is where the Quercus genus can play a major role [11]. ESKAPE superbugs are a group of six bacteria responsible for most nosocomial infections that can escape antimicrobial effects (Table 1). This group includes Enterococcus spp., Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. [11].

Table 1.
ESKAPE principal infections associated.
Throughout the present work, the phytochemical profile, and antioxidant and antimicrobial capacity of two species of oak Quercus sartorii and Quercus rysophylla were determined. Recent studies on the phytochemical and antioxidant profile in this genus cover most of the species; however, Q. sartorii and Q. ryshopylla are not found, this is because they are endemic to Mexico, giving rise to a new source of antioxidants and antimicrobials with future applications.
2. Materials and Methods
2.1. Collection and Extraction
The study was carried out with the leaves of Quercus sartorii (20070441 A) and Quercus rysophylla (U20080202 B), the collection was carried out in the Botanical Garden of the “Benemérita Universidad Autónoma de Puebla” (BUAP).
2.1.1. Collection of Leaves of the Species: Quercus sartorii and Quercus rysophylla
The oak leaves (six for Quercus) were collected manually with the help of previously disinfected gardening shears, considering taking complete leaves from the apex to the petiole, which presented good growing conditions and cutting from the node of the branches since these structures are usually areas where nutrients and phytochemical compounds essential for growth accumulate. The leaves were collected from different areas of the tree canopy during the spring season and were placed in a plastic box. The area of collection was at an altitude of 2000 m above sea level, in a region with a temperature sub-humid climate. They were then transferred to the laboratory, where they were placed separately in trays for drying the leaves using a drying oven (Rational Self Cooking Center) at a temperature of 35 °C for a period of 6 days and with a humidity percentage at 0% ensuring that the leaves were completely dehydrated without ventilation.
2.1.2. Differential Extraction by the Soxhlet Method
In total, 20.0 g of the dried oak leaves, previously crushed, were placed in a cloth sack which was placed in the siphon. For each extraction, six solvents (Hexane, Ethyl Acetate (AcOEt), Dichloromethane (DCM), Ethanol (EtOH), Methanol (MeOH), and Water) were used with a duration of 3 h for each. At the end of each extraction, the solvent was removed at reduced pressure and then dried in the muffle for half an hour. Finally, due to weight difference, a stock solution of 0.1 g/mL of each solvent was obtained, and placed in Falcon test tubes, this study was conducted in triplicate.
2.2. Phytochemical Testing
In total, 10.0 g of dried leaf of Quercus sartorii and Quercus rysophylla was colocated in a mortar. For each species, ten phytochemical tests for principal secondary metabolites [20,21,22]:
2.2.1. Lieberman–Burchard (Steroids and Terpenes)
The sample was placed in a test tube, adding chloroform a little less than half, and allowed to stand for five minutes. Subsequently, one milliliter of Lieberman–Burchard solution was added.
2.2.2. Shinoda Test (Flavonoid)
In total, 1 mg of the sample was placed in a test tube and coated with ethanol. It rested for two minutes and a piece of magnesium was added. Subsequently, 3 drops of concentrated HCl were added and no change in coloration was observed.
2.2.3. Salkowsky Test (Steroid and Flavone Identification)
The sample was added to the test tube and dissolved in chloroform by stirring in a circular motion, leaving it to stand for five minutes. Subsequently, 1 mL of sulfuric acid was added, and it was observed if there was any change in coloration.
2.2.4. Dragendorf (Alkaloid Detection)
The sample was placed in the test tube, and coated with ethanol. Subsequently, a part of this ethanol was placed (taking care not to add solids) in a well of the porcelain plate. Finally, one to two drops of the Dragendorf reagent were added to the ethanol observing some change in coloration.
2.2.5. Baljet Test (Sesquiterpenlactones)
The sample was added to the test tube, and coated with ethanol. Subsequently, 3 to 4 drops of Baljet’s solution were added, checking instantly and every 5 min for the next half hour for any change in coloration.
2.2.6. Phenolic Hydroxyls Test
The sample was placed in the test tube, and coated halfway with ethanol. Subsequently, part of this ethanol was placed in a well of the porcelain plate (avoiding solids). One to two drops of ferric chloride were added to the ethanol placed and observed if there was a change in coloration.
2.2.7. Saturations- KMnO4 Test
The sample with a volume of ethanol was placed in the test tube. Subsequently, part of this ethanol was placed in a well of the porcelain plate. Add a drop of the 2% KMnO4 solution over the placed ethanol and observe any change in color or precipitation.
2.2.8. Molish Test—For Sugars
The sample was placed in a test tube, adding 3 drops of Molish reagent. It stirred slightly. Subsequently, 1 mL of sulfuric acid was slowly added along the wall of the tube.
2.2.9. Coumarins (NaOH Test)
The sample was placed in the test tube, dissolving in 10% NaOH (if it could not be completely dissolved, the volume of solution coating the sample was added with 5 min of waiting time). Observe if there was a change in color to yellow. If there was no change, the test was negative. If there was a change in color, it was acidified by adding 3 drops of concentrated HCl; if the yellow coloration disappeared, the test was positive.
2.2.10. Saponin Test (Foam Test)
The sample was placed in the test tube, adding hot distilled water (at least 50 °C); it was shaken carefully so as not to water the contents of the tube, for fifteen seconds. Let it rest and observe whether there is foam formation.
2.3. Antioxidant Studies
2.3.1. Total Phenolic Content
The total phenolic content of the extracts was determined by the Folin–Ciocalteu assay, according to the general methodology reported by [23]. 150 μL of each extract was used in cells for spectrophotometers including a blank (methanol) and a positive control (gallic acid 0.1 mg/mL). Subsequently, 2250 μL of distilled water was added (to homogenize the sample); 150 μL of Folin’s reagent was added to each sample, homogenizing with the help of the micropipette. Then, 450 μL of 12% sodium carbonate was added, and it was left in darkness for a period of two hours at room temperature. Absorbance was measured at 760 nm in the UV-VIS spectrophotometer. Total phenolic compounds were determined using a gallic acid calibration curve. Data were expressed as milligrams of gallic acid equivalent (GAE) per kg of plant. Performing this procedure in triplicate for extraction.
2.3.2. Total Flavonoid Content
The methodology used is reported by [24] with some modifications. In test tubes, 4 mL of distilled water was added; then, 1 mL of sample to be worked with (solvent) was added, including a blank (methanol) and a positive control (quercetin 0.1 mg/mL). Subsequently, 300 μL of NaNO2 was added, and at the end of the last tube, we waited for 5 min. In total, 300 μL of AlCl3 at 10% was added; once the last tube was finished, we waited for 5 min. Then, 2 mL of 1 M NaOH was added and homogenized. Each tube was measured to 10 mL by adding 2.4 mL of distilled water. Once finished, it was left to incubate for a period of 40 min in the dark at room temperature. After this time, each of the samples was placed in cells to be read spectrophotometrically at a wavelength of 510 nm. The total flavonoid content was determined using a quercetin calibration curve. The data were expressed as milligrams of quercetin equivalents (QE) per kg of plant. Performing this procedure in triplicate for extraction.
2.3.3. DPPH (2,2-Diphenyl-1-picrylhydracil) Scavenging
The methodology used is the one reported by [25], 25 μL of each extract was used, including a blank (methanol) and a positive control (gallic acid 0.015 mg/mL). Subsequently, 200 μL of DPPH solution was added to each sample in a 96-well plate. The reaction was kept in darkness at room temperature for 40 min. Readings were taken on the ELISA equipment at 510 nm. This test was carried out in triplicate by extraction to ensure a reliable result. To determine the % inhibition of the DPPH free radical, the following formula was calculated:
where AbsR is the absorbance of the reference solution and AbsS is the absorbance of the sample, performing this procedure in triplicate for extraction.
2.4. Antimicrobial Activity
Three pathogenic ATCC bacteria were used: Staphylococcus aureus (BSA06), Acinetobacter baumannii (BAB09), and Enterococcus faecalis (BEF04) obtained from standards by the ISSSTE Hospital in the city of Puebla, Pue., Mexico.
2.4.1. Antibiogram
The methodology used is the one reported by [26]. The bacteria were cultured in the LB agar culture medium. Filter paper disks were impregnated with 8 μL of the extracts in 0.1 g/mL stock solution for 24 h at a temperature of 37 °C to later determine the inhibition halos around the sensidiscs. The antibiotics used to assess susceptibility were: aztreonam, ceftriaxone, and streptomycin. The result was expressed in mm. Performing this procedure in triplicate for extraction.
2.4.2. Minimal Inhibitory Concentration (MIC)
With each of the compounds with activity in the inhibition zone test, a medium solution was prepared with concentrations of 1000 µg/mL (original concentration) and 500 µg/mL in a 96-well plate in triplicate and it was left in incubation for 24 h at 37 °C; subsequently, the absorbance was measured using Accuris SmartReader 96 at 630 nm and it was considered as MIC since it presented an inhibition of 99% with respect to blank.
2.5. Statistical Analysis
Results were expressed as mean and standard deviation values (mean ± SD). The Anderson–Darling normality test was performed. Statistical differences between the groups were assessed using the Kruskal–Wallis test followed by the Mann–Whitney test. The statistical analysis was performed using the Minitab 18.0 program; p-values less than 0.05 were considered statistically significant.
3. Results and Discussion
3.1. Phytochemical Profile
After phytochemical tests, the colorimetric results obtained qualitatively indicate the presence or absence of secondary metabolites according to the intensity scale (the increase in the number of + is related to higher intensity) present in the leaves of both oak species (Table 2). For both Q. sartorii and Q. rysophylla, the tests that were positive were those corresponding to steroids, flavones, phenolic oxidyls, unsaturations, and carbohydrates. In the case of the metabolites with negative or low results, it could be related to the stage in which the plant was since it is well known that secondary metabolites are not vital and their synthesis is dependent on external factors such as photoperiod, temperature, water availability, among others [27]; such could be the case of flavonoids.

Table 2.
Phytochemical profile of Q. Sartorii and Q. rysophylla.
It is important to note that although flavones are a type of flavonoid, their presence was not expected in the results, as the presence of flavonoids in the leaves was not previously detected. Steroids have been reported to be products commonly found in timber tissues of various species of oak [7]. As an antioxidant, phenolic oxidyls are mainly related to this activity, in addition to being mediating molecules in the regulation of physiological processes.
A type of flavonoid found in oak leaves is quercetin and kaempferol, both compounds have antioxidant and anti-inflammatory properties. The antioxidant effect of quercetin may be due to its ability to inhibit enzymes such as xanthine oxidase, lipoxygenase, and NADPH oxidase, preventing cell death. Gallic acid, also known as 3,4,5-trihydroxybenzoic acid, is a phenolic acid with antioxidant properties, as it protects cells against oxidative stress that can be caused by environmental factors such as UV radiation and pollution. This acid is present in the leaves, bark, and fruit, and has been identified in oak species such as Q. acutissima and Q. brantii [10]. Vanillic acid, characterized by its antioxidant activity, is a compound of great relevance in the pharmaceutical industry due to its numerous bioactive properties; it also has a vasodilatory effect on the porcine endothelial coronary artery, which is identified in the leaves of Q. salicina [9]. Catechin has antioxidant properties, protecting cells from oxidative damage; these compounds can be found in the leaves of Q. resinosa [6,10]. Other compounds identified are fatty acids, including palmitic, oleic, and linoleic acids found in the fruit of Quercus. Although not all tests were positive, the presence of other metabolites such as flavonoids and sesquiterpenlactones cannot be ruled out, since in previous studies of the genus there are some constants in both groups of metabolites, such as custonolide, quercetin, and catechin [10].
3.2. Antioxidant Profile
3.2.1. DPPH (2,2-Diphenyl-1-picrylhydracil) Scavenging Results
According to the antioxidant assays, most of the extracts exerted remarkable DDPH removal activities as shown in Table 3. The highest DPPH radical scavenging activity in Q. sartorii was observed in methanolic extract (81.14 ± 1.7%), followed by ethanolic extract (79.99 ± 2.2%). However, for Q. rysophylla, it was the ethanolic extract (82.60 ± 2.7%) followed by the methanolic extract (80.82 ± 3.2%). It is important to note the trend that is marked according to the increase in polarity, this can be associated with the fact that many of the structures, to which antioxidant activity is conferred, are generally endowed with hydroxyl groups or heteroatoms which increase their affinity to polar solvents. This effect is preserved by means of dilutions, since in a 1:5 dilution the levels in all extracts decrease proportionally, while in 1:10 its antioxidant capacity is reduced below the detection limit except for ethanol and methanol, where the antioxidant potential was higher. This is indicative of the type of species related to these solvents; although there are both antioxidant and oxidizing species in a natural extract, this allows us to generalize that for these extracts the former predominate.

Table 3.
Percentage inhibition of Q. sartorii and Q. rysophylla extracts.
3.2.2. Total Flavonoid and Total Phenolic Content
Total flavonoids and total phenolic content were determined spectrophotometrically. Flavonoid content was reported as milligrams of quercetin equivalents (QE) per kg of plant, while phenolic content as milligrams of gallic acid equivalent (GAE) per kg of plant, as shown in Table 4 and Table 5 for both species.

Table 4.
Total flavonoid content of Q. sartorii and Q. rysophylla extracts (mg QE/kg of plant).

Table 5.
Total phenolic content of Q. sartorii and Q. rysophylla extracts.
In Q. sartorii and Q. rysophylla, the phenolic content of the aqueous extract was higher than that of the other extracts, presenting 22,342.1 ± 3076.5 mg EAG/kg of plant and 17,747.14 ± 1139.9 mg GAE/kg of plant, respectively. For flavonoids, Q. sartorii showed the highest number of total flavonoids in the ethanolic extract (24,587.42 ± 996.3 mg QE/kg of plant), followed by the aqueous extract (22,947.13 ± 2326.1 mg QE/kg of plant). While for Q. rysophylla, the methanolic extract showed the highest number of total flavonoids (19,875.66 ± 2754 mg QE/kg of plant). In both cases, the metabolites contain hydroxyl groups in their structure, which confer significant polarity. This property explains their tendency to exhibit greater affinity for alcoholic or aqueous solvents, as these solvents possess higher dielectric constant. Similarly, it is possible to correlate the content of these metabolites with the antioxidant activity exhibited by inhibiting a radical such as DPPH, since in different studies they have been given an important facility to donate hydrogen atoms.
3.3. Antimicrobial Activity of Extracts
Extracts of AcOEt and DCM from both oak species show halos of inhibition against Acinetobacter baumanni. The extracts of Q. sartorii are the ones with slightly larger halos, 1.02 mm, and 1.20 mm, respectively. In Q. rysophylla, hexane extract has some antibacterial activity (0.6 mm), while ethanol extract is the most effective (1.5 mm). Against Staphylococcus aureus, the hexane extract in Q. sartorii does not show antibacterial activity; however, in Q. rysophylla, it shows an inhibition halo of 1.3 mm; this extract is the most effective against this bacterium. AcOEt and DCM have a similar inhibition for both species, with close values (about 0.5–0.7 mm), having a comparable effectiveness against S. aureus. Only ethanol and methanol extracts in Q. sartorii show inhibition halos of 0.60 mm and 0.55 mm, respectively. None of the species has inhibition halos with any of the extracts against Enterococcus faecalis (Table 6).

Table 6.
Inhibition halos of extracts in mm of Q. sartorii and Q. rysophylla.
Quercus sartorii shows greater antibacterial activity against Acinetobacter baumanni and Staphylococcus aureus, especially with the extracts in AcOEt, DCM, and Ethanol. Quercus rysophylla, on the other hand, exhibits some antibacterial activity with hexane extract against both bacteria, which is remarkable since Q. sartorii shows no inhibition in this extract.
The activity detected by the extracts in both species may be due to the fact that some compounds may be acting synergistically, enhancing the antimicrobial action. Phenolic compounds are important for the inhibition of bacterial growth, they contribute to the break-down of lipids present in cell and mitochondrial membranes which ends in bacterial cell death. This can be closely correlated with the number of hydroxyl groups, as well as the toxicity it generates on the microorganism. This may explain the results in E. faecalis because it has a thicker wall compared to S. aureus, and with a higher peptidoglycan content, it has a higher barrier and lower permeability; the MIC was calculated for the bacteria with the greatest effect, finding only for A. Baumani a value of 500 μg/mL with the AcOEt and DCM extracts of Q. sartorii; however, for Q. rysophylla, it was in the ethanol extract, in the rest of the extracts where activity was presented it was up to the maximum concentration evaluated where this effect was observed; however, for the MIB, in the case of sowing after this dose, there was growth inferring that a higher dose would be needed to achieve the bactericidal effect or only the inhibitory effect predominates.
According to our results, the aqueous, ethanolic, and methanolic extracts presented better results in phenolic and flavonoid content. Most of these compounds exhibit a higher affinity for more polar solvents due to the presence of functional groups in their structures that allow strong interactions through hydrogen bonds. Therefore, this type of extract, due to its phenol and flavonoid content, is an important source for future research focused on emerging treatments such as those proposed by nanotechnology, specifically in the improvement of treatments for diseases of worldwide incidence, such as different types of cancer [28,29]. This also results in high antioxidant activity, as a correlation with DDPH removal activities is observed, as shown with ethanolic and methanolic extracts.
4. Conclusions
The biological activities of Q. sartorii and Q. rysophylla were successfully determined. Both species of oak showed the highest phenolic content in the water extracts; however, a significant difference is shown between them. Water, being more polar than the other extracts, can extract these phenolic compounds, which results in a higher content. In the flavonoid content, the ethanolic extract was higher in Q. sartorii and the methanolic extract in Q. rysopylla. In contrast to DPPH free radical scavenging, the methanolic extract had a higher percentage of inhibition for Q. sartorii; however, for Q. rysophylla, it was ethanolic extract. The dichloromethane extract of Q. sartorii showed the highest antimicrobial activity against Acinetobacter baumanii, while, for Q. rysophylla, it was the ethanolic extract. Against Staphylococcus aureus were acetate extract and hexane extract in Q. sartorii and Q. rysophylla, respectively. These results indicate that the study carried out with the leaves of both species of oak constitutes a great source of bioactive compounds, they have the potential to be applied in industry as an important source of antioxidants, adding to the antimicrobial effect, which can be used in the preservation and improvement of certain types of food, for which future research should evaluate the stability in time and temperature changes. In this way, the quality of food preservation is improved.
Author Contributions
E.C.-M.: research, methodology, statistical analysis, data curation, writing—original draft. J.A.H.-R.: research, data curation, statistical analysis, writing—revision and editing. J.L.A.P.-S.: research, contribution, and characterization of the species. S.R.R.-C.: writing, revision and editing, research. A.C.-C.: conceptualization, supervision, project management, writing, revision, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are within the manuscript.
Acknowledgments
J.A.H.R. for scholarship CVU 1176708, Allen Coombes for language revision, ACC for IMX-CONHCYT-698207.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal Uses, Phytochemistry, and Pharmacological Activities of Quercus Species. Evid.-Based Complement. Altern. Med. eCAM 2020, 2020, 1920683. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gutiérrez, J.O.; Alvarado-Segura, A.A.; Machuca-Velazco, R.; Borja-de-la-Rosa, A. Caracterización anatómica y propiedades físicas de la madera de monte bajo de dos especies de Quercus del volcán Popocatépetl. Madera Bosques 2023, 29, e2911580. [Google Scholar] [CrossRef]
- Red de Herbarios Mexicanos Herbario del Jardín Botánico BUAP, Puebla, Mexico. Available online: https://www.herbanwmex.net/portal/collections/misc/collprofiles.php?collid=484 (accessed on 29 November 2024).
- Rocha, N.; González-Laredo, R.; Vázquez-Cabral, B.; Moreno-Jiménez, M.; Gallegos-Infante, J.; Gamboa-Gómez, C.; Flores-Rueda, A. Oak Leaves as a New Potential Source for Functional Beverages: Their Antioxidant Capacity and Monomer Flavonoid Composition. In Functional and Medicinal Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 381–411. ISBN 978-0-12-816397-9. [Google Scholar]
- Decombeix, A.-L.; Harper, C.J.; Galtier, J.; Meyer-Berthaud, B.; Krings, M. Tyloses in Fossil Plants: New Data from a Mississippian Tree, with a Review of Previous Records. Bot. Lett. 2022, 169, 510–526. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. The Polyphenolic Profile of Oak (Quercus) Species: A Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Moctezuma Vega, B.X. Interacción Entre Adultos de Macrodactylus mexicanus (Coleoptera: Melolonthidae) con Quercus grahamii y Quercus sartorii (Fagaceae), Mediada por Compuestos Volátiles; Benemérita Universidad Autónoma de Puebla: Puebla, Mexico, 2022. [Google Scholar]
- Valencia-A, S.; Flores-Franco, G.; Jiménez-Ramírez, J.; Mora-Jarvio, M. Distribution and Diversity of Fagaceae in Hidalgo, Mexico. Bot. Sci. 2017, 95, 660–721. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Burlacu, E.; Nisca, A.; Tanase, C. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, S.; Wang, G.; Shao, X.; Wang, Y.; Li, Y.; Ding, Y. The Genus Quercus: Metabolites, Biological Activity and Mechanisms of Action. Phytochem. Rev. 2024, 24, 259–302. [Google Scholar] [CrossRef]
- Boeder, A.; Spiller, F.; Carlström, M.; Izídio, G. Enterococcus Faecalis: Implications for Host Health. World J. Microbiol. Biotechnol. 2024, 40, 190. [Google Scholar] [CrossRef]
- Elashiry, M.M.; Bergeron, B.E.; Tay, F.R. Enterococcus faecalis in Secondary Apical Periodontitis: Mechanisms of Bacterial Survival and Disease Persistence. Microb. Pathog. 2023, 183, 106337. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Pasachova Garzón, J.; Ramirez Martinez, S.; Muñoz Molina, L. Staphylococcus aureus: Generalidades, mecanismos de patogenicidad y colonización celular. Nova 2019, 17, 25–38. [Google Scholar] [CrossRef]
- Chávez-Jacobo, V.M. La batalla contra las superbacterias: No más antimicrobianos, no hay ESKAPE. TIP Rev. Espec. Cienc. Químico-Biológicas 2020, 23. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- da Fonseca, A.d.S.; Mencalha, A.L.; de Paoli, F. Antimicrobial Photodynamic Therapy against Acinetobacter baumannii. Photodiagnosis Photodyn. Ther. 2021, 35, 102430. [Google Scholar] [CrossRef]
- Wu, H.-J.; Xiao, Z.-G.; Lv, X.-J.; Huang, H.-T.; Liao, C.; Hui, C.-Y.; Xu, Y.; Li, H.-F. Drug-resistant Acinetobacter baumannii: From Molecular Mechanisms to Potential Therapeutics (Review). Exp. Ther. Med. 2023, 25, 209. [Google Scholar] [CrossRef]
- Alqethami, A.; Aldhebiani, A.Y. Medicinal Plants Used in Jeddah, Saudi Arabia: Phytochemical Screening. Saudi J. Biol. Sci. 2021, 28, 805–812. [Google Scholar] [CrossRef]
- Cortes-Torres, A.G.; López-Castillo, G.N.; Marín-Torres, J.L.; Portillo-Reyes, R.; Luna, F.; Baca, B.E.; Sandoval-Ramírez, J.; Carrasco-Carballo, A. Cymbopogon Citratus Essential Oil: Extraction, GC–MS, Phytochemical Analysis, Antioxidant Activity, and In Silico Molecular Docking for Protein Targets Related to CNS. Curr. Issues Mol. Biol. 2023, 45, 5164–5179. [Google Scholar] [CrossRef]
- Marami, L.M.; Dilba, G.M.; Babele, D.A.; Sarba, E.J.; Gizaw, A.; Bune, W.M.; Bayu, M.D.; Admasu, P.; Mekbeb, A.; Tadesse, M.; et al. Phytochemical Screening and In-Vitro Evaluation of Antibacterial Activities of Echinops Amplexicaulis, Ruta Chalepensis and Salix Subserrata Against Selected Pathogenic Bacterial Strains in West Shewa Zone, Ethiopia. J. Exp. Pharmacol. 2021, 13, 511–520. [Google Scholar] [CrossRef]
- Zulfisa, Z.; Fika, R.; Agusfina, M.; Yonrizon, Y.; Muhsanah, A. Determination of Total Phenolic Content of Ethanol Extract of Broken Bone Twigs (Euphorbia tirucalli Linn.) by Folin-Ciocalteu Method Spectrophotometrically. J. EduHealth 2023, 14, 1326–1331. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Carrasco-Sandoval, J.; Falcó, I.; Sánchez, G.; Fabra, M.J.; López-Rubio, A.; Rodriguez, A.; Henríquez-Aedo, K.; Aranda, M. Multivariable Optimization of Ultrasound-Assisted Extraction for the Determination of Phenolic and Antioxidants Compounds from Arrayan (Luma apiculata (DC.) Burret) Leaves by Microplate-Based Methods and Mass Spectrometry. J. Appl. Res. Med. Aromat. Plants 2022, 28, 100356. [Google Scholar] [CrossRef]
- Saraluz, S.-O.; Alatriste, V.; Lucina, M.-T.J.; Paulina, M.-S.I.; Alan, C.-C. Loeselia mexicana: Antioxidant and Antimicrobial Properties by Soxhlet Differential Extraction. GSC Biol. Pharm. Sci. 2023, 23, 115–120. [Google Scholar] [CrossRef]
- Alami, M.M.; Guo, S.; Mei, Z.; Yang, G.; Wang, X.; Alami, M.M.; Guo, S.; Mei, Z.; Yang, G.; Wang, X. Environmental Factors on Secondary Metabolism in Medicinal Plants: Exploring Accelerating Factors. Med. Plant Biol. 2024, 3, e016. [Google Scholar] [CrossRef]
- Wahnou, H.; Liagre, B.; Sol, V.; El Attar, H.; Attar, R.; Oudghiri, M.; Duval, R.E.; Limami, Y. Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment. Cancers 2023, 15, 3826. [Google Scholar] [CrossRef]
- Wahnou, H.; Limami, Y.; Oudghiri, M. Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management. BioChem 2024, 4, 38–61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).