Evaluating the Potential of Herbal Extracts as Treatment in Immune Thrombocytopenia: A Review of Evidence and Limitations
Abstract
1. Introduction
1.1. Current ITP Treatments
1.1.1. IVIG
1.1.2. Platelet Transfusions
1.1.3. Corticosteroids
1.1.4. Rituximab
1.1.5. Thrombopoietin Receptor Antagonists
1.1.6. Splenectomy
1.1.7. Plant Extract
2. Herbal Extract and Efficacy on Thrombocytopenia Treatment
2.1. Carica Papaya
2.1.1. Extraction
2.1.2. Mechanism of Action
2.2. Use of Papaya Leaf Extract for Thrombocytopenia in Humans
2.2.1. Carica papaya Leaf Extract in Chronic ITP
2.2.2. C. papaya for Drug-Induced ITP
2.2.3. C. papaya in Chemotherapy-Induced ITP
2.3. Limitations and Areas for Improvement
2.3.1. CPLE in Chemotherapy Induced Thrombocytopenia
2.3.2. Challenges in Evaluating CPLE Efficacy for Chronic ITP
2.4. Euphorbia Hirta and Equisetum Hyemale
2.4.1. Mechanism of Action on Thrombocytopenia
2.4.2. Current Research on Efficacy
2.4.3. Limitations and Areas for Improvement
2.5. Ipomoea batatas (L.) Lam (Sweet Potato)
2.5.1. Mechanism of Action on Thrombocytopenia
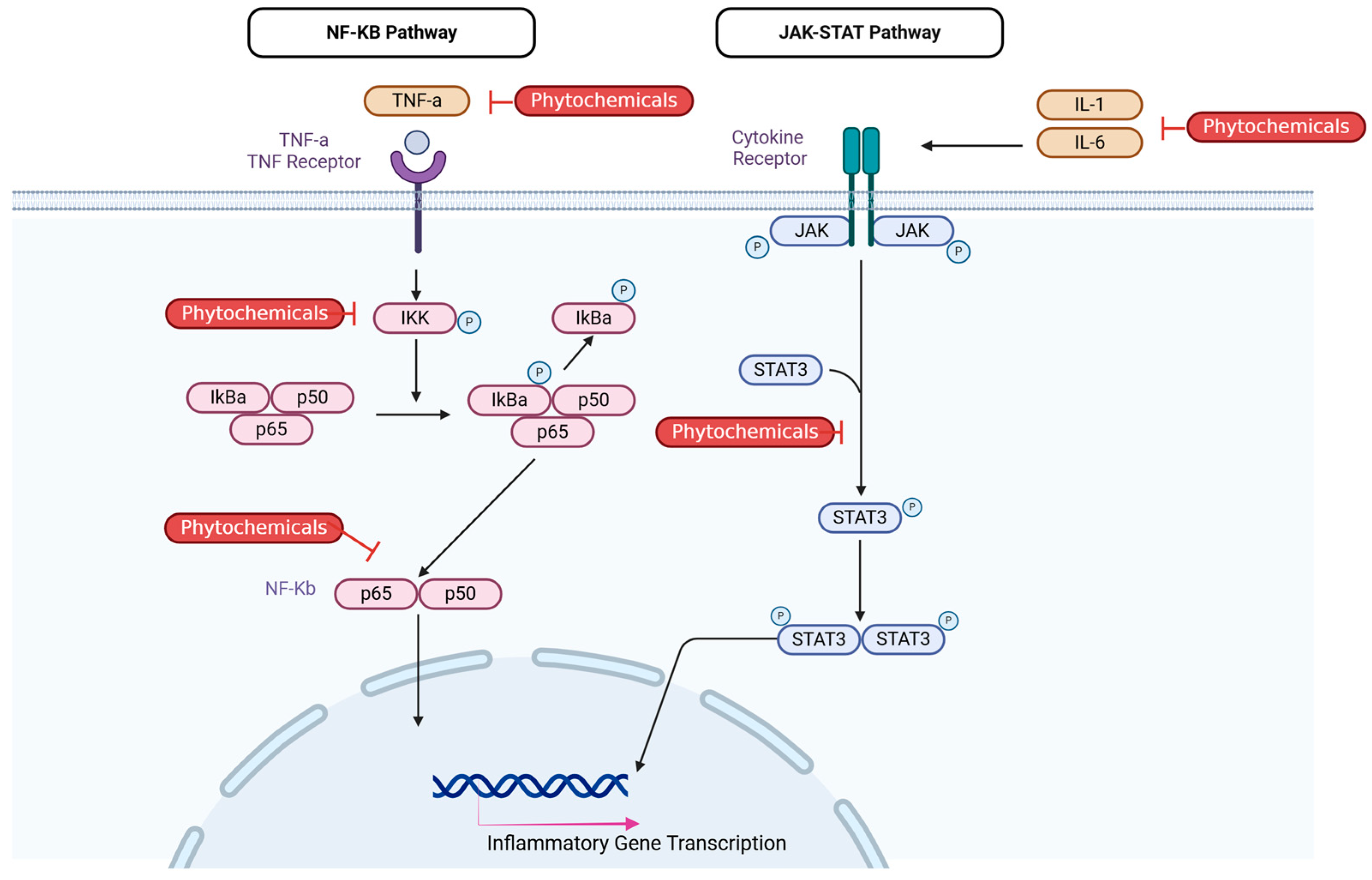
2.5.2. Current Research on Efficacy
2.5.3. Limitations and Areas for Improvement
2.6. Alternanthera sessilis
2.6.1. Mechanism of Action on Thrombocytopenia
2.6.2. Trials and Evidence of Efficacy
2.6.3. Weaknesses, Questionable Validity, and Areas for Improvement
2.7. Momordica charantia L. (Bitter Melon)
2.7.1. Mechanism of Action on Thrombocytopenia
2.7.2. Trials and Evidence of Efficacy
2.7.3. Limitations
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lambert, M.P.; Gernsheimer, T.B. Clinical updates in adult immune thrombocytopenia. Blood 2017, 129, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carballeira, D.; Bernardo, Á.; Caro, A.; Soto, I.; Gutiérrez, L. Pathophysiology, Clinical Manifestations and Diagnosis of Immune Thrombocytopenia: Contextualization from a Historical Perspective. Hematol. Rep. 2024, 16, 204–219. [Google Scholar] [CrossRef]
- Houwerzijl, E.J.; Blom, N.R.; van der Want, J.J.L.; Esselink, M.T.; Koornstra, J.J.; Smit, J.W.; Louwes, H.; Vellenga, E.; de Wolf, J.T.M. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood 2004, 103, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Bruce, S.; Freedman, J. Suppressed natural killer cell activity in patients with chronic autoimmune thrombocytopenic purpura. Am. J. Hematol. 1991, 37, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Dar, U.; Owais, F.; Ahmad, M.; Rizwani, G. Biochemical analysis of the crude extract of Momordica charantia (L.). Pak. J. Pharm. Sci. 2014, 27, 2237–2240. [Google Scholar] [PubMed]
- Madkhali, M.A. Recent advances in the management of immune thrombocytopenic purpura (ITP): A comprehensive review. Medicine 2024, 103, e36936. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Tărniceriu, C.C.; Hurjui, L.L.; Florea, I.D.; Hurjui, I.; Gradinaru, I.; Tanase, D.M.; Delianu, C.; Haisan, A.; Lozneanu, L. Immune Thrombocytopenic Purpura as a Hemorrhagic Versus Thrombotic Disease: An Updated Insight into Pathophysiological Mechanisms. Medicina 2022, 58, 211. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins & Kumar Basic Pathology, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Arnold, D.M.; Bussel, J.B.; Chong, B.H.; Cooper, N.; Gernsheimer, T.; Ghanima, W.; Godeau, B.; González-López, T.J.; Grainger, J.; et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019, 3, 3780–3817. [Google Scholar] [CrossRef] [PubMed]
- Sewell, W.A.C.; Jolles, S. Immunomodulatory action of intravenous immunoglobulin. Immunology 2002, 107, 387–393. [Google Scholar] [CrossRef]
- Barahona Afonso, A.F.; João, C.M.P. The Production Processes and Biological Effects of Intravenous Immunoglobulin. Biomolecules 2016, 6, 15. [Google Scholar] [CrossRef]
- Hsia, C.C.; Liu, Y.; Eckert, K.; Monga, N.; Elia-Pacitti, J.; Heddle, N.M. Intravenous Immunoglobulin (IVIg) Utilization in Immune Thrombocytopenia (ITP): A Multi-Center, Retrospective Review. Drugs Real World Outcomes 2015, 2, 35–42. [Google Scholar] [CrossRef]
- Imbach, P.; Barandun, S.; Baumgartner, C.; Hirt, A.; Hofer, F.; Wagner, H.P. High-dose intravenous gammaglobulin therapy of refractory, in particular idiopathic thrombocytopenia in childhood. Helv. Paediatr. Acta 1981, 36, 81–86. [Google Scholar] [PubMed]
- Goel, R.; Chopra, S.; Tobian, A.A.R.; Ness, P.M.; Frank, S.M.; Cushing, M.; Vasovic, L.; Kaicker, S.; Takemoto, C.; Josephson, C.D.; et al. Platelet transfusion practices in immune thrombocytopenia related hospitalizations. Transfusion 2019, 59, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Cirasino, L.; Robino, A.M.; Cattaneo, M.; Pioltelli, P.E.; Pogliani, E.M.; Terranova, L.; Morra, E.; Colombo, P.; Palmieri, G.A.; Piscitelli, P. Appropriate hospital management of adult immune thrombocytopenic purpura patients in major Italian institutions in 2000-2002: A retrospective analysis. Blood Coagul. Fibrinolysis 2010, 21, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Ness, P.M.; Takemoto, C.M.; Krishnamurti, L.; King, K.E.; Tobian, A.A.R. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Blood 2015, 125, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Handin, R.I.; Stossel, T.P. Effect of Corticosteroid Therapy on the Phagocytosis of Antibody-coated Platelets by Human Leukocytes. Blood 1978, 51, 771–779. [Google Scholar] [CrossRef]
- Atkinson, J.P.; Schreiber, A.D.; Frank, M.M. Effects of corticosteroids and splenectomy on the immune clearance and destruction of erythrocytes. J. Clin. Investig. 1973, 52, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- KAPLAN, M.E.; JANDL, J.H. Inhibition of red cell sequestration by cortisone. J. Exp. Med. 1961, 114, 921–937. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31, vii. [Google Scholar] [CrossRef] [PubMed]
- Hajime, M.; Takayasu, F.; Yumiko, l.; Hirokazu, K.; Shigenori, H.; Hironori, T.; Yoshiyuki, K.; Takeshi, Y.; Seiichiro, T.; Susumu, l. Mechanisms of Corticosteroid Action in Immune Thrombocytopenic Purpura (ITP): Experimental Studies Using ITP-Prone Mice, (NZW x BXSB) F1. Blood 1992, 79, 942–947. [Google Scholar] [CrossRef]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014, 14, 203–207. [Google Scholar] [PubMed]
- Vozoris, N.T.; Seemangal, J.; Batt, J. Prevalence, screening and treatment of latent tuberculosis among oral corticosteroid recipients. Eur. Respir. J. 2014, 44, 1373–1375. [Google Scholar] [CrossRef]
- Weiner, G.J. Rituximab: Mechanism of action. Semin. Hematol. 2010, 47, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Del Poeta, G.; Stipa, E.; Evangelista, M.L.; Trawinska, M.M.; Cooper, N.; Amadori, S. Response to B-cell–depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood 2007, 110, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.K.; Yang, Y.T.; Bennett, C.L. Why Biologics and Biosimilars Remain So Expensive: Despite Two Wins for Biosimilars, the Supreme Court’s Recent Rulings do not Solve Fundamental Barriers to Competition. Drugs 2018, 78, 1777–1781. [Google Scholar] [CrossRef]
- Goshua, G.; Gokhale, A.; Hendrickson, J.E.; Tormey, C.; Lee, A.I. Cost savings to hospital of rituximab use in severe autoimmune acquired thrombotic thrombocytopenic purpura. Blood Adv. 2020, 4, 539–545. [Google Scholar] [CrossRef]
- Kasi, P.M.; Tawbi, H.A.; Oddis, C.V.; Kulkarni, H.S. Clinical review: Serious adverse events associated with the use of rituximab—A critical care perspective. Crit. Care 2012, 16, 231. [Google Scholar] [CrossRef]
- Pulanić, D.; Bátorová, A.; Bodó, I.; Červinek, L.; Ionita, I.; Lissitchkov, T.; Melikyan, A.; Podolak-Dawidziak, M. Use of thrombopoietin receptor agonists in adults with immune thrombocytopenia: A systematic review and Central European expert consensus. Ann. Hematol. 2023, 102, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Birocchi, S.; Podda, G.M.; Manzoni, M.; Casazza, G.; Cattaneo, M. Thrombopoietin receptor agonists for the treatment of primary immune thrombocytopenia: A meta-analysis and systematic review. Platelets 2021, 32, 216–226. [Google Scholar] [CrossRef]
- Cheng, G.; Saleh, M.N.; Marcher, C.; Vasey, S.; Mayer, B.; Aivado, M.; Arning, M.; Stone, N.L.; Bussel, J.B. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): A 6-month, randomised, phase 3 study. Lancet 2011, 377, 393–402. [Google Scholar] [CrossRef]
- Tjepkema, M.; Amini, S.; Schipperus, M. Risk of thrombosis with thrombopoietin receptor agonists for ITP patients: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2022, 171, 103581. [Google Scholar] [CrossRef] [PubMed]
- Remiker, A.; Neunert, C. Splenectomy for immune thrombocytopenia: The evolution and preservation of treatment. Haematologica 2020, 105, 2507–2509. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Arnold, D.M.; McCrae, K.R. Splenectomy for immune thrombocytopenia: Down but not out. Blood 2018, 131, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Palandri, F.; Polverelli, N.; Sollazzo, D.; Romano, M.; Catani, L.; Cavo, M.; Vianelli, N. Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years. Am. J. Hematol. 2016, 91, 267. [Google Scholar] [CrossRef]
- WHO Headquarters (HQ). WHO Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Lim, X.Y.; Chan, J.S.W.; Japri, N.; Lee, J.C.; Tan, T.Y.C. Carica papaya L. Leaf: A Systematic Scoping Review on Biological Safety and Herb-Drug Interactions. Evid Based. -Complement. Altern. Med. 2021, 2021, 5511221. [Google Scholar] [CrossRef]
- Euphorbia. In Drugs and Lactation Database (LactMed®); National Institute of Child Health and Human Development: Bethesda, MD, USA, 2006.
- Naomi, R.; Bahari, H.; Yazid, M.D.; Othman, F.; Zakaria, Z.A.; Hussain, M.K. Potential Effects of Sweet Potato (Ipomoea batatas) in Hyperglycemia and Dyslipidemia—A Systematic Review in Diabetic Retinopathy Context. Int. J. Mol. Sci. 2021, 22, 10816. [Google Scholar] [CrossRef]
- Bhuyan, B.; Baishya, K.; Rajak, P. Effects of Alternanthera sessilis on Liver Function in Carbon Tetra Chloride Induced Hepatotoxicity in Wister Rat Model. Indian J. Clin. Biochem. 2018, 33, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, S.; Ibrahim, M.; Abdel Mohsen, M.; Abou-Setta, L.; Sleem, A.; El-Missiry, M. The influence of the extraction method on polyphenols, flavonoids composition and anti-hyperlipidemic properties of papaya leaves (Carica papaya Linn.). Bull. Natl. Res. Cent. 2021, 45, 85. [Google Scholar] [CrossRef]
- Sui, X.; Tsuji, K.; Ebihara, Y.; Tanaka, R.; Muraoka, K.; Yoshida, M.; Yamada, K.; Yasukawa, K.; Taga, T.; Kishimoto, T.; et al. Soluble interleukin-6 (IL-6) receptor with IL-6 stimulates megakaryopoiesis from human CD34+ cells through glycoprotein (gp)130 signaling. Blood 1999, 93, 2525–2532. [Google Scholar] [CrossRef]
- Kaushansky, K.; Broudy, V.C.; Lin, N.; Jorgensen, M.J.; McCarty, J.; Fox, N.; Zucker-Franklin, D.; Lofton-Day, C. Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development. Proc. Natl. Acad. Sci. USA 1995, 92, 3234–3238. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Liu, Z.; Tariq, T.; Rabail, R.; Kowalczewski, P.Ł.; Lewandowicz, J.; Blecharczyk, A.; Abid, M.; Inam-Ur-Raheem, M.; Aadil, R.M. Delving into the Therapeutic Potential of Carica papaya Leaf against Thrombocytopenia. Molecules 2022, 27, 2760. [Google Scholar] [CrossRef]
- Kaser, A.; Brandacher, G.; Steurer, W.; Kaser, S.; Offner, F.A.; Zoller, H.; Theurl, I.; Widder, W.; Molnar, C.; Ludwiczek, O.; et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: Role in inflammatory thrombocytosis. Blood 2001, 98, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Hampilos, K.; Corn, J.; Hodsdon, W.; Wagner, P.; Roop, R.; Elise, A.; Troy, L. Effect Of Carica Papaya Leaf Extract On Platelet Count In Chronic Immune Thrombocytopenic Purpura: A Case Series. Integr. Med. 2019, 18, 30–35. [Google Scholar] [CrossRef]
- Anjum, V.; Arora, P.; Ansari, S.H.; Najmi, A.K.; Ahmad, S. Antithrombocytopenic and immunomodulatory potential of metabolically characterized aqueous extract of Carica papaya leaves. Pharm. Biol. 2017, 55, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, L.M.; Zain, Z.; Jalil, S.; Seman, Z.; Mansur, F.; Samseh Abdullah, N. The Potential Role of Thrombopoietin and Interleukin-6 in the Thrombocytosis Effect of Carica papaya Leaves. Int. J. Res. Pharm. Sci. 2020, 11, 1928–1934. [Google Scholar] [CrossRef]
- Saraf, M.; Kavimandan, B. Animal Trials of Carica Papaya Leaf Extracts for Increasing Platelet Count. Indian J. Public Health Res. Dev. 2017, 8, 782. [Google Scholar] [CrossRef]
- Kumar, M.S.; Geetha, M.; Shah, M.J.; Goyal, M.; Srinivas, L.D. Evaluation of efficacy of Carica papaya leaf extracts to increase platelet count in hydroxyurea induced thrombocytopenia in Albino rats. Int. J. Basic Clin. Pharmacol. 2017, 7, 173–178. [Google Scholar] [CrossRef][Green Version]
- Gheith, I.; El-Mahmoudy, A. Hemogram and iron indices in renal anemia and the amelioration with Carica papaya leaf extract applied on albino rat model. Biosci. Rep. 2019, 39, BSR20181699. [Google Scholar] [CrossRef] [PubMed]
- Sundarmurthy, D.; Jayanthi, R.; Kuntegowdanahalli, L. Effect of Carica papaya leaf extract on platelet count in chemotherapy-induced thrombocytopenic patients: A preliminary study. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1. [Google Scholar] [CrossRef]
- Hussain, S.M.; Sohrab, M.; Al-Mahmood, A.; Shuayb, M.; Al-Mansur, M.; Hasan, C. Clinical use of Carica papaya leaf extract in chemotherapy induced thrombocytopaenia. Int. J. Clin. Exp. Med. 2017, 10, 3752–3756. [Google Scholar]
- Babu, R. A randomized, double-blind, placebo-controlled, proof of concept study to assess the safety and efficacy of Carica papayaand Tinospora cordifolia leaf extract (Thrombobliss) in subjects undergoing chemotherapy treatment and subjects with systemic microbial infection andsubsequent reduction in platelet count. Int. J. Clin. Trials 2017, 4, 116–121. [Google Scholar] [CrossRef]
- Tiwari, R.; Mandal, D.; Patel, J. A post marketing randomized placebo controlled study to evaluate the efficacy of study product UPLAT® (Carica papaya leaf extract + Tinospora cordifolia extract) in the cancer patients with thrombocytopenia induced by chemotherapy. Int. J. Clin. Trials 2018, 5, 170–176. [Google Scholar] [CrossRef][Green Version]
- Panda, A.; Bhuyan, G.C.; Dighe, D.; Rao, M. Phyto extracts of Carica papaya and Tinospora cordifolia can correct thrombocytopenia in alcoholic decompensate liver cirrhosis: Case Series. J. Ayurveda Integr. Med. Sci. 2018, 3, 99–101. [Google Scholar] [CrossRef]
- Sreelatha, P.; Jose, W. Efficacy of Carica Papaya Leaf Extract in Reducing Treatment-Delay Secondary to Chemotherapy induced Thrombocytopenia. J. Clin. Diagn. Res. 2020, 14, XC09–XC12. [Google Scholar] [CrossRef]
- Gerber, D.E.; Grossman, S.A.; Zeltzman, M.; Parisi, M.A.; Kleinberg, L. The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas. Neuro-Oncology 2007, 9, 47–52. [Google Scholar] [CrossRef] [PubMed]
- de Guzman, G.; Dacanay, A.; Andaya, B.; Alejandro, G.J. Ethnopharmacological studies on the uses of Euphorbia hirta in the treatment of dengue in selected indigenous communities in Pangasinan (Philippines). J. Intercult. Ethnopharmacol. 2016, 5, 1. [Google Scholar] [CrossRef]
- Tayone, W.; Tayone, J.; Hashimoto, M. Isolation and Structure Elucidation of Potential Anti-Dengue Metabolites from Tawa-tawa (Euphorbia hirta Linn.). Walailak J. Sci. Technol. 2014, 11, 825–832. [Google Scholar] [CrossRef]
- Bai, X.; Li, L.; Wu, Y.; Jie, B. Flavonoids of Euphorbia hirta inhibit inflammatory mechanisms via Nrf2 and NF-κB pathways. Cell Biochem. Biophys. 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.F.; Nabavi, S.M. Flavonoids and platelet aggregation: A brief review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef]
- Perera, S.D.; Jayawardena, U.A.; Jayasinghe, C.D. Potential Use of Euphorbia hirta for Dengue: A Systematic Review of Scientific Evidence. J. Trop. Med. 2018, 2018, 2048530. [Google Scholar] [CrossRef]
- dos Santos Alves, C.F.; Bonez, P.C.; de Souza Ebling, M.; Casagrande, C.; Freitas, L.; Dolwitsch, C.; Pires, F.; Sagrillo, M.R.; de Brum, G.F.; de Campos, M.M.A.; et al. Antimicrobial, Cyto and Genotoxic Activities of Equisetum hyemale. Pharmacogn. J. 2019, 11, 1–9. [Google Scholar] [CrossRef]
- Al-Snafi, A. The pharmacology of Equisetum arvense—A review. IOSR J. Pharm. 2017, 7, 31–42. [Google Scholar] [CrossRef]
- Mir, M.; Khurshid, R.; Aftab, R. Management of thrombocytopenia and flu-like symptoms in dengue patients with herbal water of Euphorbia hirta. J. Ayub Med. Coll. Abbottabad 2012, 24, 6–9. [Google Scholar] [PubMed]
- Bangayan, J.; Palattao, J.; Chua, J.M.; Capili, J.; Ramirez, R. Evaluation of Equisetum hyemale and Euphorbia hirta leaf extracts in increasing platelet count of sprague dawley rats. Int. J. Biosci. 2020, 17, 58–65. [Google Scholar] [CrossRef]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as Anti-Inflammatory Agents in Animal Models of Prevalent Inflammatory Diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Khan, H.; Gowrishankar, S.; Lagoa, R.J.L.; Mahomoodally, F.M.; Khan, Z.; Suroowan, S.; Tewari, D.; Zengin, G.; Hassan, S.T.S.; et al. The role of flavonoids in autoimmune diseases: Therapeutic updates. Pharmacol. Ther. 2019, 194, 107–131. [Google Scholar] [CrossRef]
- Dewi, N.K.S.M.; Ramona, Y.; Saraswati, M.R.; Wihandani, D.M.; Wirasuta, I.M.A.G. The Potential of the Flavonoid Content of Ipomoea batatas L. as an Alternative Analog GLP-1 for Diabetes Type 2 Treatment-Systematic Review. Metabolites 2023, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.A.; Lozano, M.L.; Castillo, J.; Benavente-Garcia, O.; Vicente, V.; Rivera, J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J. Thromb. Haemost. 2005, 3, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Alam, M. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Koffuor, G. Haematopoietic Effect of an Ethanolic Leaf Extract of Ipomoea involucrata P. Beauv in Phlebotomized New Zealand White Rabbits. J. Med. Biomed. Sci. 2012, 1, 10–16. [Google Scholar]
- Li, Q.; He, D.; He, Y. Study on the protective effect of flavonoids extracted from Jatropha curcas leaves against radiation damage in mice. Heliyon 2024, 10, e39403. [Google Scholar] [CrossRef]
- Mashhadi Akbar Boojar, M. An Overview of the Cellular Mechanisms of Flavonoids Radioprotective Effects. Adv. Pharm. Bull. 2020, 10, 13–19. [Google Scholar] [CrossRef]
- Bisol, Â.; de Campos, P.S.; Lamers, M.L. Flavonoids as anticancer therapies: A systematic review of clinical trials. Phytother. Res. 2020, 34, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Ao, J.; Li, Y.; Zhang, J.; Duan, C. Exploring the protective mechanisms of total tannins from Geum japonicum var. chinense F.Bolle in mice with hematopoietic dysfunction via the JAK2/STAT3/5 signaling pathway. J. Ethnopharmacol. 2022, 296, 115507. [Google Scholar] [CrossRef] [PubMed]
- Isenburg, J.C.; Karamchandani, N.V.; Simionescu, D.T.; Vyavahare, N.R. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials 2006, 27, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Boukhers, I.; Morel, S.; Kongolo, J.; Domingo, R.; Servent, A.; Ollier, L.; Kodja, H.; Petit, T.; Poucheret, P. Immunomodulatory and Antioxidant Properties of Ipomoea batatas Flour and Extracts Obtained by Green Extraction. Curr. Issues Mol. Biol. 2023, 45, 6967–6985. [Google Scholar] [CrossRef]
- Hasan, Z.; Quadir, R.; Eva, E.O.; Khanam, M. Platelet Raising Effect of Ipomoea batatas (Sweet Potato) Leaf Extract in Rats. East. Med. Coll. J. 2016, 1, 9–12. [Google Scholar]
- Osime, E.O.; Ediale, G.E.; Omoti, C.; Famodu, A.A. Effect of Sweetpotato Leaf (Ipomoea Batatas) Extract On Some Haematological Parameters Using Rabbits. J. Med. Biomed. Res. 2009, 7, 5–11. [Google Scholar] [CrossRef]
- Sivakumar, R.; Sunmathi, D. Phytochemical Screening and Antimicrobial Activity of Ethanolic Leaf Extract of Alternanthera sessilis (L.) R.Br. Ex Dc and Alternanthera Philoxeroides (Mart.) Griseb. Eur. J. Pharm. Sci. 2018, 3, 409–412. [Google Scholar]
- Arollado, E.C.; Peňaa, I.G.; Dahilig, V.R.A. Platelet Augmentation Activity of Selected Philippine Plants. Int. J. Pharm. Phytopharm. Res. 2013, 3, 121–123. [Google Scholar]
- Kota, S.; Govada, V.R.; Anantha, R.K.; Verma, M.K. An Investigation into phytochemical constituents, antioxidant, antibacterial and anti-cataract activity of Alternanthera sessilis, a predominant wild leafy vegetable of South India. Biocatal. Agric. Biotechnol. 2017, 10, 197–203. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Yang, J.J.; Lin, C.C. Effects of oleanolic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice. Cancer Lett. 1997, 111, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Michel, C. Chemistry of the antioxidant effect of polyphenols. Ann. N. Y. Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Marcińczyk, N.; Gromotowicz-Poplawska, A.; Tomczyk, M.; Chabielska, E. Tannins as Hemostasis Modulators. Front. Pharmacol. 2022, 12, 806891. [Google Scholar] [CrossRef]
- Guerrero, J.A.; Navarro-Nuñez, L.; Lozano, M.L.; Martínez, C.; Vicente, V.; Gibbins, J.M.; Rivera, J. Flavonoids inhibit the platelet TxA2 signalling pathway and antagonize TxA2 receptors (TP) in platelets and smooth muscle cells. Br. J. Clin. Pharmacol. 2007, 64, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. β-Sitosterol Alleviates Inflammatory Response via Inhibiting the Activation of ERK/p38 and NF-κB Pathways in LPS-Exposed BV2 Cells. Biomed. Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef]
- Zakaria, M.; Beshir, M.; Hassan, T.; Esh, A.; Abdelaziz, E.; Tayib, R.; Nafea, A. Role of interleukin 4 (IL4) and interleukin 6 (IL6) in the pathogenesis and prognosis of childhood primary immune thrombocytopenia. Eur. J. Pediatr. 2023, 182, 3129–3138. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.B.N. Anti-Inflammatory and Antipyretic Activities of β-Sitosterol. Planta Med. 2008, 39, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, N.; Xue, M.; Zhang, M.; Liu, W.; Xu, C.; Fan, Y.; Meng, Y.; Zhang, Q.; Zhou, Y. Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). Antioxidants 2023, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Mr, N.; Patil, D.; Aithal, S.; Bm, A. Evaluation of Activity of Alternanthera Sessilis Leaves Aqueous Extract on Platelet Count In Drug-Induced Thrombocytopenia in Albino Rats. Asian J. Pharm. Clin. Res. 2023, 16, 157–162. [Google Scholar] [CrossRef]
- Perumal, V.; Khatib, A.; Uddin Ahmed, Q.; Fathamah Uzir, B.; Abas, F.; Murugesu, S.; Zuwairi Saiman, M.; Primaharinastiti, R.; El-Seedi, H. Antioxidants profile of Momordica charantia fruit extract analyzed using LC-MS-QTOF-based metabolomics. Food Chem. 2021, 2, 100012. [Google Scholar] [CrossRef] [PubMed]
- Trakoolthong, P.; Ditthawuttikul, N.; Sivamaruthi, B.S.; Sirilun, S.; Rungseevijitprapa, W.; Peerajan, S.; Chaiyasut, C. Antioxidant and 5α-Reductase Inhibitory Activity of Momordica charantia Extract, and Development and Characterization of Microemulsion. Appl. Sci. 2022, 12, 4410. [Google Scholar] [CrossRef]
- Innih, S.O.; Eze, I.G.; Omage, K. Evaluation of the haematinic, antioxidant and anti-atherosclerotic potential of Momordica charantia in cholesterol-fed experimental rats. Toxicol. Rep. 2022, 9, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.; Suhimi, A.; Yahya, T. Anticoagulant Evaluation of Momordica charantia Fruit Flesh Extract on Prothrombin Time and Activated Partial Prothrombin Time Test. Biomed. Pharmacol. J. 2023, 16, 2205–2212. [Google Scholar] [CrossRef]
- Murakami, T.; Emoto, A.; Matsuda, H.; Yoshikawa, M. Medicinal foodstuffs. XXI. Structures of new cucurbitane-type triterpene glycosides, goyaglycosides-a, -b, -c, -d, -e, -f, -g, and -h, and new oleanane-type triterpene saponins, goyasaponins I, II, and III, from the fresh fruit of Japanese Momordica charantia L. Chem. Pharm. Bull. 2001, 49, 54–63. [Google Scholar] [CrossRef]
- Parkash, A.; Ng, T.B.; Tso, W.W. Purification and characterization of charantin, a napin-like ribosome-inactivating peptide from bitter gourd (Momordica charantia) seeds. J. Pept. Res. 2002, 59, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Gabardi, S.; Ulbricht, C. Bitter melon (Momordica charantia): A review of efficacy and safety. Am. J. Health Syst. Pharm. 2003, 60, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Muanda, F.; Koné, D.; Dicko, A.; Soulimani, R.; Younos, C. Phytochemical composition and antioxidant capacity of three malian medicinal plant parts. Evid Based.-Complement. Altern. Med. 2011, 2011, 674320. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, S.E. Exploring the potential of Beta vulgaris L. Against thrombocytopenia: In vitro, in silico, phytochemicals, and antioxidant analysis. J. Med. Pharm. Allied Sci. 2024, 13, 6464–6471. [Google Scholar] [CrossRef]
- Ahamad, A.; Ibrahim, M.; Ansari, S.; Taleuzzaman, M. Antithrombocytopenic potential of metabolically characterized extract and fractions of Momordica charantia L. fruits by in vivo study. Ann. Phytomed. Int. J. 2021, 10, 264–272. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Liu, T.; Wang, Y.; Wei, X.; Qi, S.; Gu, B. Effects of Momordica charantia exosomes on platelet activation, adhesion, and aggregation. Blood Coagul. Fibrinolysis 2022, 33, 372–380. [Google Scholar] [CrossRef]
| Drug | Model | Tx | Dose (mg/kg) | Duration | PC0 | PC1 | Sig |
|---|---|---|---|---|---|---|---|
| CycP [50] | Wistar Rats | CPLE | 150 | 14 days | 408.5 | 1014.83 | p < 0.001 |
| Busulfan [51] | Sprague-Dawley Rats | CPLE | 600 | 7 days | 267 | 608 1 | p < 0.00 |
| Carboplatin [52] | Sprague-Dawley Rats | CPLE | 400 | 21 days 2 | 241 | 973 | - |
| Hydroxyurea [53] | Albino Rats | CPLE | 3300 | 5 days | 273 | 766 | p < 0.001 |
| Gentamycin [54] | Albino Rats | CPLE | 100 | 21 days | 481 | 720 | p < 0.05 |
| Patient | Day 0 | Day 15 | Day 30 | Day 60 | Day 90 |
|---|---|---|---|---|---|
| Patient #1 | 60 | 90 | 117 | 128 | 155 |
| Patient #2 | 33 | 56 | 72 | 92 | 180 |
| Patient #3 | 38 | 52 | 82 | 98 | 167 |
| Herbal Extract | Study/Reference | Trial Type | Sample Size | Key Findings | Country |
|---|---|---|---|---|---|
| Carica papaya L. | Hamplios et al. [49] | Case series (Chronic ITP) | 4 patients | Significant platelet increase in steroid-refractory ITP patients | USA |
| - | Sundarmurthy et al. [55] | Randomized controlled trial (CIT) | 40 patients | Significant platelet increase post-chemo in intervention group | India |
| - | Hussain et al. [56] | Randomized controlled trial (CIT) | 60 patients | Significant platelet increase; no adverse effects reported | Pakistan |
| - | Babu et al. [57] | RCT (ThromboBliss, CPLE + T. cordifolia) | 250 patients | Significant platelet recovery; improved outcomes | India |
| - | Tiwari et al. [58] | RCT (UPLAT, CPLE + T. cordifolia) | 40 patients | Significant platelet increase in intervention group | India |
| - | Panda et al. [59] | Case series (Liver cirrhosis) | 3 patients | Sustained platelet recovery over 90 days | India |
| - | Sreelatha and Jose [60] | Retrospective study (CIT) | 50 patients | Platelet improvement in 88% of patients | India |
| - | Kumar et al. [53] | In vivo (Hydroxyurea-induced) | Albino rats | Significant platelet recovery post treatment | India |
| Euphorbia hirta L. | Mir et al. [69] | Clinical study (Dengue) | 125 patients | Platelet increase and improvement in flu-like symptoms | Pakistan |
| - | Bangayan et al. [70] | In vivo (Aspirin-induced thrombocytopenia) | Sprague-Dawley rats | Significant platelet increase comparable to steroids | Philippines |
| Equisetum hyemale L. | Bangayan et al. [70] | In vivo (Aspirin-induced thrombocytopenia) | Sprague-Dawley rats | Significant platelet recovery similar to Euphorbia hirta | Philippines |
| Ipomoea batatas (L.) Lam | Hasan et al. [83] | In vivo (Dose–response) | Rats | Dose-dependent significant platelet increase | Bangladesh |
| - | Koffuor et al. [76] | In vivo (Hematopoiesis study) | Rabbits | Significant platelet increase comparable to positive control | Ghana |
| - | Osime et al. [84] | In vivo (Dose–response) | Rabbits | Significant platelet increase at higher doses | Nigeria |
| Alternanthera sessilis (L.) | Nayana et al. [97] | In vivo (Cyclophosphamide-induced) | Albino rats | Significant platelet increase by day 11 | India |
| - | Arollado et al. [86] | In vivo (Anagrelide-induced) | Sprague-Dawley rats | Platelet increase observed but lower than Carica papaya | Philippines |
| Momordica charantia L. | Dar et al. [5] | In vivo (Rabbit study) | Rabbits | Significant platelet increase at low doses | Pakistan |
| - | Ahmad et al. [107] | In vivo (Busulfan-induced thrombocytopenia) | Rats | DCM fraction significantly restored platelet count | India |
| - | Zhang et al. [108] | In vitro/In vivo (Platelet function) | Platelets/Rats | Reduced platelet activation and thrombus formation | China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiggins, R.W.; Woo, J.; Cauba, J.N.; Mito, S. Evaluating the Potential of Herbal Extracts as Treatment in Immune Thrombocytopenia: A Review of Evidence and Limitations. Appl. Biosci. 2025, 4, 1. https://doi.org/10.3390/applbiosci4010001
Wiggins RW, Woo J, Cauba JN, Mito S. Evaluating the Potential of Herbal Extracts as Treatment in Immune Thrombocytopenia: A Review of Evidence and Limitations. Applied Biosciences. 2025; 4(1):1. https://doi.org/10.3390/applbiosci4010001
Chicago/Turabian StyleWiggins, Russell W., Jihoo Woo, John Nicholas Cauba, and Shizue Mito. 2025. "Evaluating the Potential of Herbal Extracts as Treatment in Immune Thrombocytopenia: A Review of Evidence and Limitations" Applied Biosciences 4, no. 1: 1. https://doi.org/10.3390/applbiosci4010001
APA StyleWiggins, R. W., Woo, J., Cauba, J. N., & Mito, S. (2025). Evaluating the Potential of Herbal Extracts as Treatment in Immune Thrombocytopenia: A Review of Evidence and Limitations. Applied Biosciences, 4(1), 1. https://doi.org/10.3390/applbiosci4010001








