3.1. Hormonal Regulation in Organogenesis
Plant cells and tissues exhibit totipotency, allowing them to regenerate into completely fertile plants under suitable in vitro conditions. This capability makes in vitro cultures crucial to plant biotechnology. These methods are rapidly evolving to refine processes and harness plant phenotypic plasticity for various applications in agriculture, industry, conservation, and research [
10,
11,
12]. The regeneration of genetically identical shoots from diverse tissue explants typically requires the addition of plant hormones to the culture media, particularly cytokinins (CKs) and auxin [
10,
11,
12]. Modifying the CK or auxin signaling pathways can markedly improve shoot regeneration, even in tissues that are traditionally difficult to regenerate [
10,
11,
12].
Under laboratory conditions, processes are initiated by wounding; incubation on an appropriate medium; or supplementation with phytohormones, typically auxins and cytokinins. Plant cells perceive these signals and subsequently alter their metabolic and genetic configurations to initiate reprogramming. However, the precise mechanisms by which these stimuli modulate development patterns and determine optimal regeneration pathways remain unclear [
12]. The initial stage of plant regeneration involves the wounding of the explants. Various wound signals, including electric current, hydraulic pressure, Ca
2+, reactive oxygen species (ROS), and metabolic alterations, play a vital role in this process (
Figure 1A). Although the examination of downstream genes of wound signaling indicates a significant impact on plant regeneration, comprehensive information regarding its effect on in vitro regeneration remains limited [
11]. Research has shown that oxidative stress contributes to plant regeneration, influencing phenomena such as programmed cell death, phytohormone signaling pathways, and cell differentiation through ROS [
11]. Organogenesis, a form of plant regeneration following tissue injury, involves the regeneration of organs, such as shoots and roots, from the site of the wound and detached organs. This process comprises three steps: (1) signaling to stimulate regeneration, (2) phytohormone, and (3) cell fate transition [
11,
12].
Auxin, uniquely among phytohormones, exhibits the capacity for polar transport and polarity induction, rendering it a critical regulator of in vitro plant morphogenesis, whereas cytokinin may induce auxin biosynthesis [
9,
10]. Shoot and root formation is supported by auxin canalizing through vascular tissues, whereas shoot development occurs when there is a balance between auxin production and active transport via carriers [
9,
10]. The IPA-mediated auxin synthesis pathway is vital in cell induction [
9,
10].
Shoot regeneration is influenced by endogenous hormones and environmental conditions, including exogenous plant growth regulators (PGRs) [
10]. The signaling pathways of auxin and CK (as an auxin biosynthesis inducer) interact not only with each other but also with other pathways [
10]. CK and auxin signaling are crucial for micropropagation, with auxin predominating in early organogenesis stages and CK predominating in later stages (shooting) [
10,
12]. Root growth is enhanced when the ratio of auxin to CK is elevated, whereas shoot development is promoted when the CK-to-auxin ratio is high [
10,
12]. Organogenesis is genetically complex and involves numerous genes, such as transcription factors, hormonal response regulators, transporters, efflux–influx carrier genes and epigenetic regulators involved in hormonal metabolism and transport, and cell-cycle genes, all of which are influenced by the concentration and distribution of endogenous hormones [
10,
11,
12,
13]. The reduced regeneration capacity with age may result from decreased phytohormone responsiveness [
11].
Physiologically, roots exhibit a natural means of canalization of the shoot-derived auxin. Successful rooting necessitates elevated auxin production in specific xylem-associated stem cells [
13]. Vessels (sieve elements) are crucial for rooting, as they establish robust channels for auxin transport and facilitate root canalization [
9,
12,
13]. Various in vitro root induction protocols employ pulse treatments with high auxin concentrations (IBA) and darkness [
9,
12,
13]. These protocols address natural rooting challenges by increasing auxin content in vessel element-rich tissue, thereby inducing a transient auxin peak to activate cell division in xylem-pole cells for root induction [
9,
12,
13].
In recent years, synthetic auxin and cytokinin derivatives, including meta-topolin (mT), indole 3-butyric acid (IBA), thidiazuron (TDZ), 6-benzylaminopurine (BAP), and 1-naphthalene acetic acid (NAA), have been evaluated to enhance tissue growth control, thus demonstrating the potential of novel synthetic phytohormone derivatives in cannabis clonal propagation (
Table 1) [
3,
4,
5,
14,
15].
Fiber-type hemp exhibits apical dominance [
16], promoting the development of taller plants with elongated fibers that flourish at higher densities. Consequently, hemp with prominent apical dominance was cultivated selectively. Apical dominance, characterized by the suppression of axillary bud growth by the main shoot, is a significant factor contributing to the challenge associated with shoot proliferation in industrial hemp [
16]. The development of buds is controlled by a sophisticated interplay between internal hormones, including auxin and cytokinins. Auxin, produced in young leaves at the apical meristem, is employed by the shoot apex and moves downward through the polar auxin pathway. This process inhibits the lateral buds by limiting their ability to access this transport pathway [
16].
The auxin signaling pathway involves nuclear Transport Inhibitor Response 1/Auxin Signaling F-box protein (TIR1/AFB) auxin receptors, which interact with auxin and act as F-box ubiquitin ligases to facilitate ubiquitination and breakdown of Aux/IAA transcriptional suppressors [
15]. These suppressors control auxin response factors (ARFs), which recognize auxin response elements (AREs) in the promoter regions of genes regulated by auxin [
15]. Recent findings indicate that certain processes previously thought to be governed by the TIR1/AFB pathway, such as root growth regulation, occur too swiftly to involve transcription and protein synthesis, suggesting the existence of a potential non-transcriptional branch of TIR1/AFB signaling (
Figure 1B). Specific auxin antagonists, “including 4-(2,4-dimethylphenyl)-2-(1H-indol-3-yl)-4-oxobutanoic acid (auxinole) and 2-(1H-indol-3-yl)-4-oxo-4-phenylbutanoic acid (PEO-IAA) [
15], can bind to TIR1, inhibiting the formation of the TIR1/AFB–IAA–Aux/IAA complex” and consequently suppressing the expression of auxin-responsive genes [
15].
Two recent studies explored the combination of auxin polar transport inhibitors, including “N-1-naphtylphtalamic acid (NPA), 2,3,5-triiodobenzoic acid (TIBA), and PEO-IAA, with the cytokinin N-benzyl-9-(tetrahydro-2H-pyran-2-yl) adenine (BAP9THP)”. This combination was employed to boost shoot regeneration in
Cannabis sativa by inhibiting apical dominance [
15,
16].
3.2. Carbon Source
Carbon (C) is essential for the majority of macromolecules in plants, including cell walls, proteins, DNA, and RNA, and carbon deprivation is lethal for seedlings [
9]. In vitro plant cells, tissues, and organ cultures require carbohydrates in the culture medium because of the lack of complete autotrophy/heterotrophy. These carbohydrates are essential for maintaining osmotic potential and act as reservoirs of energy and carbon, supporting energy-intensive processes such as shoot proliferation, root induction, embryogenesis, organogenesis, and cryopreservation [
17]. The requirement for a carbon source in plant culture media is attributed to the heterotrophic nature of cultured cells and cannot be substituted by any other element [
17]. Plants utilize various sugars in their metabolic processes, including monosaccharides, disaccharides, trisaccharides, and sugar alcohols [
17]. These exogenous sugars are crucial for plant growth and development because they influence nutritional value, osmotic potential, and cell division [
18]. They supply energy to explants that are incapable of photosynthesis under heterotrophic or mixotrophic in vitro conditions [
17].
Exogenous carbohydrate supplements enhance cell division in plant embryos by promoting cell expansion and reserve accumulation, mediated by various sugar-sensing systems such as (1) hexokinase (HXK); (2) hexose transport-associated sensor (SUCROSE-NONFERMENTATION1-RELATED PROTEIN KINASE1 (SnRK1); (3) TARGET OF RAPAMYCIN (TOR) kinase; and (4) the Suc-specific pathway, possibly involving a signaling Suc transporter (
Figure 2) [
17]. Studies have demonstrated crosstalk between phytohormone signaling, particularly auxin and cytokinin biosynthesis [
10] and sugar sensing, regulating developmental processes at transcriptional, post-transcriptional, and post-translational levels [
9,
10]. Sugars can also influence phytohormone responses by modifying their levels, localization, and transport [
9]. Recent developments in plant propagation techniques, including photoautotrophic micropropagation, have offered a novel framework with numerous beneficial features. These include CO
2 enrichment within culture vessels, reduction or elimination of sugars from growth media, and optimization of in vitro culture conditions for effective micropropagation [
17]. Carbohydrates are primarily utilized as transport sugars in phloem sap and have distinct effects on morphogenesis through vascular differentiation, necessitating the evaluation of their specific influences on different species and growth stages. The effectiveness of a carbon source is influenced by its nature, quantity, and interactions, and inappropriate applications may result in slowed morphogenesis and physiological abnormalities, such as vitrification/hyperhydricity [
9,
17,
18].
An examination of various sucrose concentrations (0, 1.5, 3.0, 4.5, and 6.0 wt/vol) demonstrated that
Cannabis sativa plants achieved superior fresh weight, shoot lengths, and overall quality when grown in 1.5% and 3.0% (
Table 1) [
5]. In a separate study, researchers successfully developed a technique for inducing in vitro flowering induction in cannabis by utilizing DKW medium supplemented with 2% sucrose in a filtered vessel (
Table 1) [
1].
3.3. Mineral Additives
Despite substantial progress in understanding the molecular mechanisms of plant regeneration, the role of mineral nutrients as morphogenic stimulants in culture media is often underestimated. Each ion has potential morphogenic properties. Recent research has shown that epigenetic regulation influences most nutrient signaling pathways. Appropriate nutrition is vital for thriving plant tissue culture, and media selection should consider the morphogenic impact of individual elements and ions, drawing upon the principles of classical plant physiology [
19].
Nitrogen (N) is essential for plant nutrition, being the second most abundant element after carbon, and it is a significant constituent of amino acids and other biological macromolecules [
9]. The optimal form of N for plants is significantly dependent on the pH [
9]. Predominantly absorbed as nitrate and ammonium from the soil, nitrogen influences plant growth and cell division, with high crop yields heavily dependent on N fertilization [
19]. Key regulatory processes, including auxin-, cytokinin-, and strigolactone-mediated cell division in the shoot apical meristem and gibberellin-regulated shoot organogenesis, govern plant architecture in response to N supply [
9,
19]. Furthermore, amino acids transport, as a signaling molecule, has been implicated in the regulation of plant architecture [
9,
19].
In a recent study on
Cannabis sativa, microcutting cultures with 500 mgL
−1 ammonium nitrate demonstrated optimal shoot extension and leaf development [
4]. Nitrogen additives also enhance shoot culture performance and mitigate vitrification issues [
2]. Potassium (K), although not a structural element like nitrogen, is crucial for plant development, functioning as a symporter with hydrogen and facilitating auxin transport. The relationship between auxin distribution, initially identified in 1962 [
9], was subsequently found to be significantly affected by potassium deficiency via various transporters [
9]. Potassium is also utilized for agar solidification, reducing hyperhydricity and promoting cell division, particularly under auxin deficiency [
9].
Deficiency of both magnesium (Mg) and sulfur (S) severely impairs plant development because Mg functions as a cofactor and S serves as a structural component, as in amino acids and thiols [
9]. Calcium (Ca) operates as a signal transducer, regulating kinases, cell wall structure, and responses to stimuli, thus playing a crucial role in plant tissue culture [
9] through various signaling processes [
9]. For macroelements (excluding carbon), it is recommended to utilize potassium nitrate (KNO
3), ammonium phosphate monobasic (NH
4H
2PO
4), calcium nitrate (Ca(NO
3)
2), and magnesium sulphate (MgSO
4) to achieve optimal ion concentrations in the medium (
Figure 3) [
9], including for in vitro micropropagation in
Cannabis sativa (
Table 1) [
4].
3.4. pH of Medium
The pH level heavily influences plant development, particularly its impact on auxin transport. Most plant cellular processes are sensitive to pH, making it crucial to maintain a precise pH equilibrium. PTC has three pH types: cytoplasmic (approximately 7, highly buffered), extracellular (medium), and vacuolar (
Figure 3). Three distinct vacuolar types are categorized based on their pH levels: alpha type (PSV, protein storage associated with stem cells), beta type (found in dividing cells), and gamma type (lytic, present in expanding/apoptotic cells) [
9]. Shoot stem-cell formation is linked to the transition from beta- to alpha-type vacuoles [
9]. Local auxin accumulation may induce PSV, whereas chloride promotes lytic vacuole formation, acidifying the vacuole and increasing water uptake [
9]. The medium pH is the most adjustable, and artificial acidification enhances plant morphogenesis [
9]. The classification of plants as acidic, neutral, or alkaline is determined by their preferred growing conditions, with most plants requiring an acidic environment to facilitate nutrient absorption under typical circumstances [
9]. Recent studies have emphasized the critical role of pH homeostasis in plant morphogenesis [
9]. In plant tissue culture, chemical buffers maintain the pH of the media within the physiological range [
9]. During micropropagation of
Cannabis sativa, various media pH levels (4.0, 5.0, 5.8, 6.0, and 7.0) were examined. The results showed no significant differences in fresh weights, shoot lengths, or quality ratings across these pH levels. Nevertheless, 5.8, 6.0, and 7.0 pH values yielded more lateral nodes (
Table 1) [
5].
3.6. Surface Sterilization
Plants harbor diverse endophytic/epiphytic microbes that colonize plant tissues without compromising plant health [
20]. Recently, plant–endophyte associations have garnered significant attention owing to their applications in enhancing plant growth, mitigating biotic and abiotic stresses, and producing valuable metabolites [
20]. Endophytes also provide mechanisms against pathogens with varying virulence. However, they can contaminate culture media [
21], presenting a challenge for successful cultivation. Effective sterilization protocols are essential to minimize endophytic harm and phytotoxicity. Typical surface sterilization involves washing the plant material; chemical sterilization with ethanol, sodium hypochlorite, and mercuric chloride; rinsing; and inoculating sterile explants on suitable media. Protocols vary based on plant parts, endophytic, sterilant, concentration, exposure time, and tissue type. Commonly, sterilants include sodium hypochlorite, ethanol, mercuric chloride, formaldehyde, and, more recently, hydrogen peroxide [
3,
22]. Combinations of sterilants and surfactants such as Triton X-100, Tween 80, Tween 20, and Teepol enhance disinfection efficacy by oxidizing cellular components and denaturing proteins and lipids [
20].
Cannabis (
Cannabis sativa sub sp. sativa) also hosts beneficial microbial communities in its tissues and seeds [
23]. Stage I disinfection with 20%, 40%, and 60% bleach (7.5% sodium hypochlorite) for 10 min demonstrated no differences in contamination rates and caused no damage to explants in micropropagation studies [
5].
3.7. Hyperhydricity
Disturbances in tissue structure and biochemical changes in hyperhydric plants primarily affect gas exchange and photosynthesis [
24,
25,
26]. In vitro cultivation exposes plants to elevated humidity, insufficient illumination, excess sugars and minerals, hormonal imbalances, and poor aeration (accumulation of CO
2 and ethylene), leading to various disorders [
24,
25,
26]. The most common abnormality observed in plants cultivated in vitro is hyperhydricity, also known as vitrification or glassiness. This condition is characterized by thickened stems; short internodes; and fragile, elongated, and twisted leaves [
24,
25,
26]. This condition also manifests through various indicators, including a deficiency in chlorophyll, oversized starch particles within plastids, excessive intercellular fluid, reduced cellular adhesion, hypolignification, fewer epicuticular layers on leaves, altered enzyme activity, and disrupted protein synthesis [
24,
25,
26]. Plants affected by hyperhydricity also exhibit membrane damage, reduced cell wall thickness, decreased mitochondria, enlarged spaces between cells, increased vacuole formation, and deterioration of vascular tissues. Another symptom is reduced lignin content due to decreased enzyme activity in its biosynthesis and increased phenolic compounds [
24,
25,
26]. The causes and solutions for hyperhydricity are species-specific [
24,
25,
26]. Approaches include adjusting the medium’s mineral and hormonal composition, utilizing exogenous additives, improving aeration, and specific lighting; however, not all methods are universally effective [
24,
25,
26]. Reducing ethylene levels with silver nitrate [
9], increasing air exchange, balancing nutrition by lowering nitrogen and chloride, and reducing exogenous cytokinin to decrease auxin biosynthesis are potential methods [
9]. Hemp micropropagation faces challenges such as hyperhydricity and culture decline of microshoots [
6].
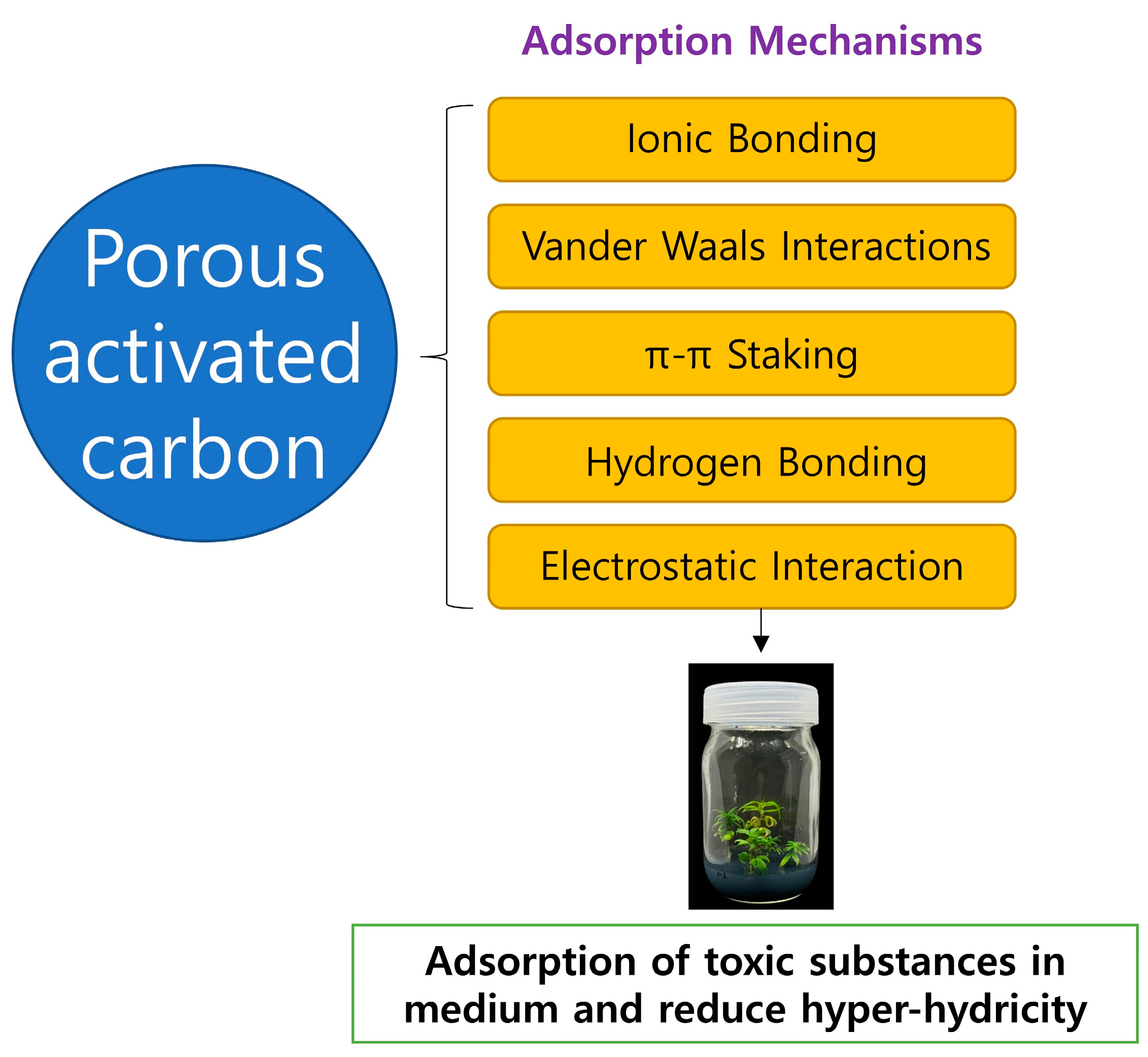
Activated carbon (AC) is extensively utilized for gas and water treatment in various industries globally, due to its efficacy, reliability, and accessibility [
27,
28]. It is frequently produced from lignocellulosic biomass-based agricultural waste such as coconut shells, rice husks, and palm kernel shells [
27,
28]. A significant advantage of activated carbon is its customizable pore structure, which can be achieved through chemical activation [
27,
28]. AC is highly porous, rendering it efficient for adsorbing various chemicals [
27,
28]. The adsorption mechanism is primarily attributable to micropores and weak Van der Waals forces that attract impurities [
27]. The extensive microporous structure of AC renders it suitable for diverse industrial applications, including chemical treatment, protective suits, water and air filtration, and environmental remediation (
Figure 4) [
27]. The primary mechanism by which AC enhances in vitro morphogenesis is through the permanent binding of inhibitory substances in the growth medium, thereby diminishing toxic metabolites, phenolic secretions, and accumulation of brown exudates [
28]. Moreover, AC participates in various stimulatory and inhibitory processes, including the emission of growth-enhancing compounds; modification and darkening of culture media; and adsorption of vitamins, metal ions, and plant growth regulators, such as abscisic acid and ethylene [
28]. The effect of AC concentration differs across plant species, media types, explants, and outcomes. The stimulatory or inhibitory role of AC is contingent on multiple factors [
28].
A recent study developed an in vitro micropropagation protocol for
Cannabis sativa ‘Cheungsam’. During the experimental procedure, the shoot tips and nodes exhibited blackening, and hyperhydricity increased. AC was incorporated into the medium to mitigate necrosis and hyperhydricity, which was hypothesized to be caused by ROS and phenolic compounds. Treatment with 0.5 g/L charcoal did not alleviate hyperhydricity and demonstrated no significant difference from the control [
3]. The addition of AC inhibited plant growth in all treatments compared with those without charcoal, suggesting that charcoal adsorbs both growth-inhibiting substances and essential nutrients, resulting in reduced growth [
3,
27,
28].
3.8. Photoautotrophic Micropropagation
Ventilation, quantified as the air change rate per hour, represents the ratio of the total air exchanged between a zone and the external environment per unit of time to the zone’s volume, indicating air diffusion. Natural ventilation in smaller culture vessels occurs via gas-permeable films (0.2 to 0.5 μm pore size) and vessel–lid gaps, facilitating higher air change rates and maintaining sterility by preventing microorganism entry [
29]. An increased air change rate enhances CO
2 availability to explants, which is crucial for photosynthesis, photorespiration, and hormone biosynthesis, particularly photoautotrophic micropropagation (PAM) systems dependent on CO
2-derived carbohydrates [
29]. In photoautotrophic tissue culture, explant growth occurs on a sugar-free medium through photosynthesis and inorganic nutrient uptake [
29]. In cannabis micropropagation, the presence of axillary buds on the shoot indicates potential new shoot development, with the multiplication ratio being a key factor for efficiency [
29]. Elevated CO
2 resulting from increased air change rates can enhance axillary buds, leaf, and node numbers [
29]. Higher air change rates also reduce vessel humidity, enhance transpiration via water vapor diffusion, and mitigate inhibitory gases such as ethylene, thereby promoting explant growth [
29].
Photoautotrophic (sugar-free medium) micropropagation, introduced over two decades ago, remains a promising and evolving technology [
30]. The research and advancements in PAM emphasize the in vitro environmental features that enhance plant growth (
Figure 2) [
30]. PAM concepts and methodologies for optimizing in vitro environments to promote photosynthesis and biomass accumulation highlight the advantages of omitting organic carbon sources and utilizing various types of ventilated vessels with natural ventilation, as reported in several studies [
30].
Elevated CO
2 levels led to increased plant dry mass and more leaves. PAM offers several advantages over conventional tissue culture methods, including reducing culture time by approximately 30%, mitigating issues such as hyperhydricity, and a nearly 100% improvement in ex vivo survival rates. Additionally, PAM results in reduced labor and material expenses, making it superior to traditional tissue culture techniques [
29]. PAM also facilitates simultaneous root and shoot development, shortening the acclimation period and improving efficiency. Previous research on cannabis did not evaluate the use of small shoots or effective ventilation methods. For other species, various explant sizes have been used in PAM [
29].
The increased internode length observed with higher air change rates in PAM indicates that cannabis explants thrive under particular conditions, suggesting that elevated air exchange may require increased light for the photosynthetic process. The similarity in the total dry mass in PAM and shoot dry mass in TC (tissue culture) across various air change rates implies comparable growth rates between PAM and photomixotrophic (sugar and photosynthesis) TC. Enhanced photosynthesis in PAM, achieved through greater light exposure and CO
2 levels, could surpass that in TC. This assessment compares different air change rates by comparing traditional tissue cultures with PAM [
29].
For plants, light plays a vital role, affecting photosynthesis and governing various molecular, biochemical, and morphological processes that are fundamental to their growth and development. Key light attributes, such as fluorescence rate, wavelength regions, duration, and direction, drive photosynthesis and photomorphogenesis by selectively activating light receptors/photosensors. These photoreceptors process complex biochemical structures, capture photons, and detect light properties, transducing this information into biochemical and biological responses that regulate growth through hormonal signaling and homeostasis. Artificial light sources are exclusively used in plant tissue cultures [
31,
32].
In tissue culture, sucrose provides essential carbon for energy and growth, with light intensity promoting mixotrophic growth from both sucrose and photosynthesis. A recent study demonstrated that higher light intensity during flowering-promoting photoperiods resulted in fewer flowers owing to light-induced stress or photoinhibition [
1].
A groundbreaking study showed that
Cannabis can complete its entire life cycle in TC, yielding inflorescences and viable seeds in vitro. The study identified DKW medium with 2% sucrose in a filtered vessel with a lower relative humidity as the most effective condition. Notably, low light intensity during flower induction was essential for maximizing production. The plant’s moderate growth in a sugar-free medium suggests that it cannot achieve full photoautotrophy under passive ventilation, likely due to CO
2 limitation. CO
2 concentrations within filtered vessels containing plants fluctuated between 150 and 250 mmol/mol during the photoperiod, depending on the filter quantity, while ambient CO
2 levels ranged from 350 to 400 mmol/mol. Future research should investigate cannabis performance in forced ventilation systems [
1].