Enhanced Protein Digestibility and Amino Acid Profile of a Novel Legume (Inga paterno) Seed Flours: Evaluation of Proximal Composition Changes by Sprouting
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sprouting Treatment
2.3. Proximate Composition Analysis
2.4. In Vitro Protein Digestibility
2.5. Free Amino Acid Profile
2.6. Estimation of Nutritional Parameters
2.7. Statistical Analysis
3. Results
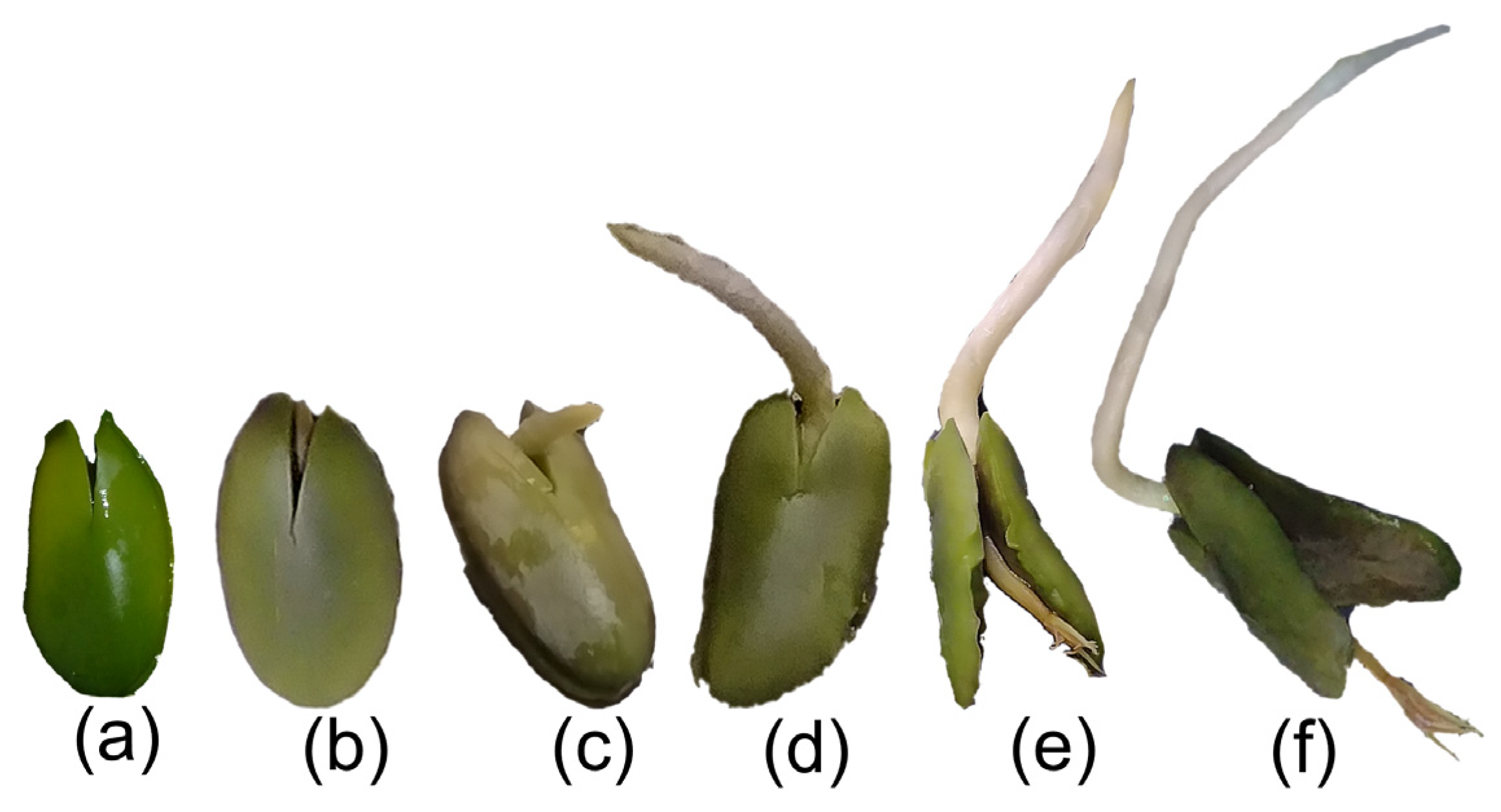
3.1. Radicle Growth
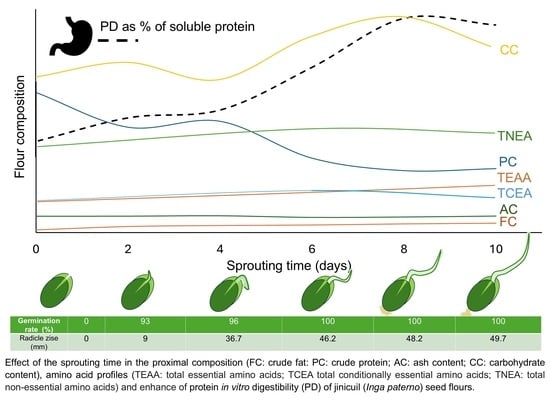
3.2. Chemical Composition Changes
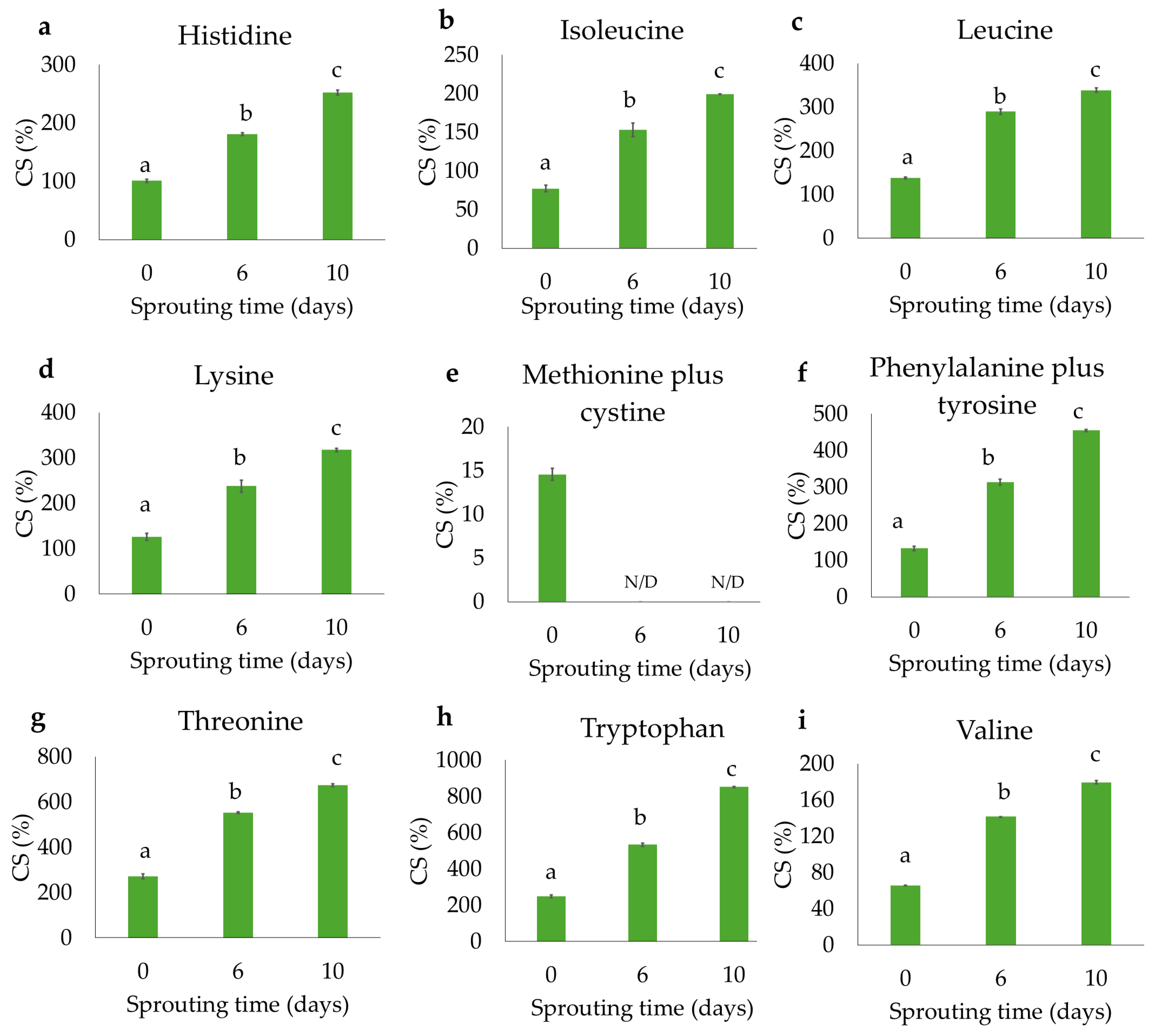
3.3. Flour Amino Acid Profile and Nutritional Parameters
3.4. Protein Digestibility and Free Amino Acids Release
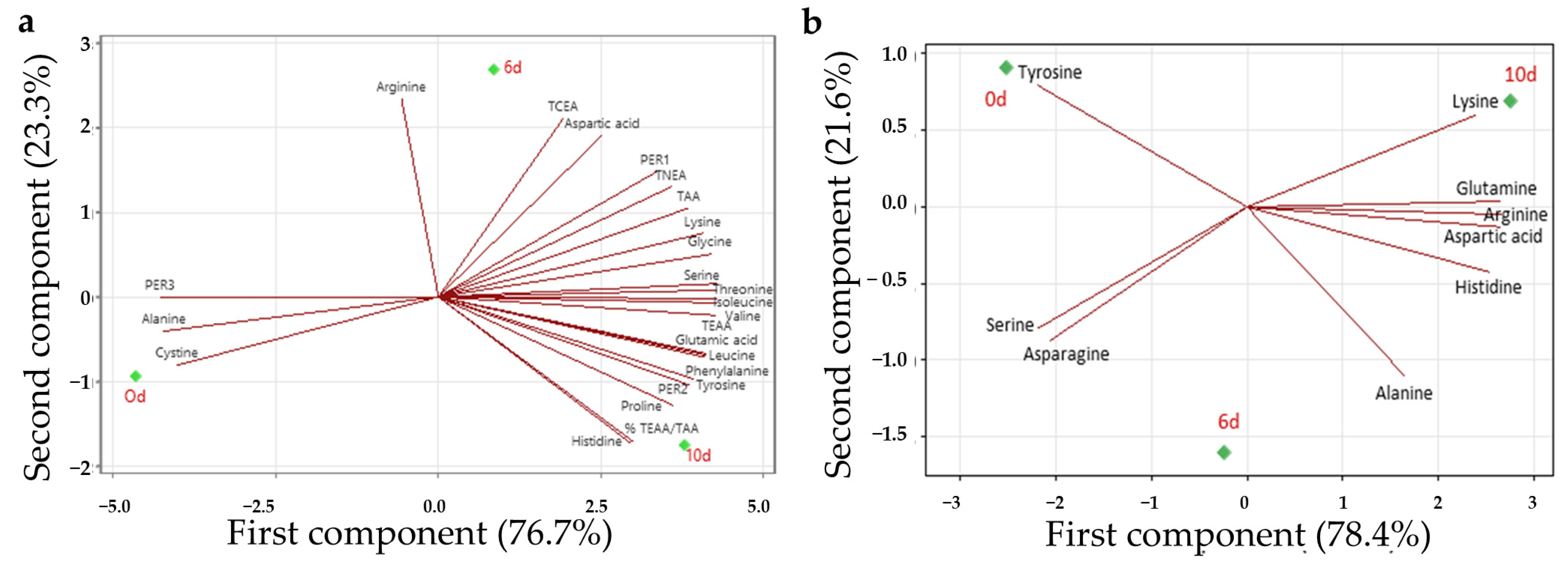
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAP | milligrams of amino acid in 1 g of test protein |
| AAR | milligrams of amino acid in 1 g of reference protein or FAO requirement pattern |
| BV | Biological value |
| CS | Chemical score |
| dm | Dry matter |
| D1 | Soluble protein of flours without enzyme addition |
| D2 | Soluble protein after buccal digestion |
| D3 | Soluble protein after gastric digestion |
| Hys | Histidine |
| Leu | Leucine |
| Met | Methionine |
| PCA | Principal component analysis |
| PC1 | First component |
| PC2 | Second component |
| PD | Protein digestibility |
| PDCAAS | Protein Digestibility Corrected Amino Acid Score |
| PER | Protein efficiency ratio |
| Pro | Proline |
| SP | Soluble protein |
| Tyr | Tyrosine |
| TAA | Total amino acid |
| TCEA | Total conditionally essential amino acids |
| TEAA | Total essential amino acids |
| TNEA | Total non-essential amino acids |
References
- Kamboj, R.; Nanda, V. Proximate Composition, Nutritional Profile and Health Benefits of Legumes—A Review. Legume Res. 2018, 41, 325–332. [Google Scholar] [CrossRef]
- Martínez, M.Á.; Evangelista, V.; Basurto, F.; Mendoza, M.; Cruz-Rivas, A. Flora Útil de Los Cafetales En La Sierra Norte de Puebla, México Useful Plants of the Sierra Norte de Puebla, Mexico. Rev. Mex. Biodivers. 2007, 78, 15–40. [Google Scholar]
- Padilla, E.V.; Cuevas, Y.; Arturo Solís, M.R.G. Inga colimana (Leguminosae) una especie nueva del occidente de México. Acta Botánica Mex. 2005, 72, 33–38. [Google Scholar] [CrossRef]
- Sánchez Mendoza, N.A.; Jiménez Martínez, C.; Cardador Martínez, A.; Martín del Campo Barba, S.; Dávila Ortiz, G. Caracterización Física, Nutricional y No Nutricional de Las Semillas de Inga Paterno. Rev. Chil. Nutr. 2016, 43, 400–407. [Google Scholar] [CrossRef]
- Miyahira, R.F.; de Oliveira Lopes, J.; Antunes Costa, E.A. The Use of Sprouts to Improve the Nutritional Value of Food Products: A Brief Review. Plant Foods Hum. Nutr. 2021, 76, 143–152. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, J.H.; Xu, Y.; Chen, Y.; Qian, K.; Zhang, Y. Effect of Germination on Nutritional Quality of Soybean. Food Sci. Technol. 2023, 43, e008323. [Google Scholar] [CrossRef]
- Rodríguez, C.; Frías, J.; Vidal-Valverde, C.; Hernández, A. Total Chemically Available (Free and Intrachain) Lysine and Furosine in Pea, Bean, and Lentil Sprouts. J. Agric. Food Chem. 2007, 55, 10275–10280. [Google Scholar] [CrossRef]
- Winarsi, H.; Septiana, A.T.; Wulandari, S.P. Germination Improves Sensory, Phenolic, Protein Content and Anti-Inflammatory Properties of Red Kidney Bean (Phaseolus vulgaris L.) Sprouts Milk. Food Res. 2020, 4, 1921–1928. [Google Scholar] [CrossRef]
- Devi, C.B.; Kushwaha, A.; Kumar, A. Sprouting Characteristics and Associated Changes in Nutritional Composition of Cowpea (Vigna unguiculata). J. Food Sci. Technol. 2015, 52, 6821–6827. [Google Scholar] [CrossRef]
- Elliott, H.; Woods, P.; Green, B.D.; Nugent, A.P. Can Sprouting Reduce Phytate and Improve the Nutritional Composition and Nutrient Bioaccessibility in Cereals and Legumes? Nutr. Bull. 2022, 47, 138–156. [Google Scholar] [CrossRef]
- He, Y.; Xiang, J.; Chen, J.; Fang, S.; Guo, Z.; Liang, X. Improving Bioaccessibility and Bioavailability of Isoflavone Aglycones from Chickpeas by Germination and Forming β-Cyclodextrin Inclusion Complexes. Pharmaceutics 2023, 15, 2684. [Google Scholar] [CrossRef]
- Chick, H.; Roscoe, M.H. The Biological Values of Proteins. Biochem. J. 1930, 24, 1780–1782. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency Calculating Protein Ratings Protein Efficiency Ratios. Available online: https://web.archive.org/web/20190428020406/http://www.inspection.gc.ca/food/requirements/labelling/industry/nutrition-labelling/elements-within-the-nutrition-facts-table/eng/1389206763218/1389206811747?chap=7 (accessed on 13 March 2024).
- Food and Agriculture Organization of the United Nations. Protein Quality Assessment in Follow-Up Formula for Young Children, Final Draft FAO Report; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-720-42%252FLinks%252FFAODraftReport_PDCAASComputinginFUF.pdf (accessed on 13 March 2024).
- Mansilla, W.D.; Marinangeli, C.P.F.; Cargo-Froom, C.; Franczyk, A.; House, J.D.; Elango, R.; Columbus, D.A.; Kiarie, E.; Rogers, M.; Shoveller, A.K. Comparison of Methodologies Used to Define the Protein Quality of Human Foods and Support Regulatory Claims. Appl. Physiol. Nutr. Metab. 2020, 45, 917–926. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Hayes, M. In Vitro Protein Digestibility of Selected Seaweeds. Foods 2022, 11, 289. [Google Scholar] [CrossRef]
- Xu, W.; Zhong, C.; Zou, C.; Wang, B.; Zhang, N. Analytical Methods for Amino Acid Determination in Organisms. Amino Acids 2020, 52, 1071–1088. [Google Scholar] [CrossRef]
- Vioque, J.; Alaiz, M.; Girón-Calle, J. Nutritional and Functional Properties of Vicia Faba Protein Isolates and Related Fractions. Food Chem. 2012, 132, 67–72. [Google Scholar] [CrossRef]
- Tedeschi, L.O. Mathematical Modeling in Ruminant Nutrition: Approaches and Paradigms, Extant Models, and Thoughts for Upcoming Predictive Analytics. J. Anim. Sci. 2019, 97, 1921–1944. [Google Scholar] [CrossRef]
- Saleh, H.M.; Hassan, A.A.; Mansour, E.H.; Fahmy, H.A.; El-Bedawey, A.E.F.A. Melatonin, Phenolics Content and Antioxidant Activity of Germinated Selected Legumes and Their Fractions. J. Saudi Soc. Agric. Sci. 2019, 18, 294–301. [Google Scholar] [CrossRef]
- Supap, N.; Pheeraya, C.; Anuchita, M. Melatonin, Its Precursors, Total Phenolic Content and Antioxidant Activity in Legumes Germinated under Normal and Saline Conditions. J. Sustain. Sci. Manag. 2021, 16, 54–67. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists (AOAC) Thirteenth: Washington, DC, USA, 1980. [Google Scholar]
- Milán-Noris, A.K.; Gutiérrez-Uribe, J.A.; Santacruz, A.; Serna-Saldívar, S.O.; Martínez-Villaluenga, C. Peptides and Isoflavones in Gastrointestinal Digests Contribute to the Anti-Inflammatory Potential of Cooked or Germinated Desi and Kabuli Chickpea (Cicer arietinum L.). Food Chem. 2018, 268, 66–76. [Google Scholar] [CrossRef]
- Metri-Ojeda, J.; Ramírez-Rodrigues, M.; Rosas-Ordoñez, L.; Baigts-Allende, D. Development and Characterization of a Low-Fat Mayonnaise Salad Dressing Based on Arthrospira platensis Protein Concentrate and Sodium Alginate. Appl. Sci. 2022, 12, 7456. [Google Scholar] [CrossRef]
- Baigts-Allende, D.K.; Pérez-Alva, A.; Ramírez-Rodrigues, M.A.; Palacios, A.; Ramírez-Rodrigues, M.M. A Comparative Study of Polyphenolic and Amino Acid Profiles of Commercial Fruit Beers. J. Food Compos. Anal. 2021, 100, 103921. [Google Scholar] [CrossRef]
- Swieca, M.; Gawlik-Dziki, U.; Jakubczyk, A.; Bochnak, J.; Sikora, M.; Suliburska, J. Nutritional Quality of Fresh and Stored Legumes Sprouts—Effect of Lactobacillus plantarum 299v Enrichment. Food Chem. 2019, 288, 325–332. [Google Scholar] [CrossRef]
- Satyanarayana, B.; Subhashini Devi, P.; Arundhati, A. Biochemical Changes During Seed Germination of Sterculia urens Roxb. Not. Sci. Biol. 2011, 3, 105–108. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and Germination Improve Nutritional Value of Cereals and Legumes through Activation of Endogenous Enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zheng, Y. Integration of ABA, GA, and Light Signaling in Seed Germination through the Regulation of ABI5. Front. Plant Sci. 2022, 13, 1000803. [Google Scholar] [CrossRef]
- Dong, Y.; Tong, C.; Li, Q.; Zhang, L.; Gao, Y.; Yu, X. Dynamics of Composition, Structure, and Metabolism of Three Energy Substances in Flaxseed (Linum usitatissimum L.) during Germination. Food Chem. 2023, 410, 135344. [Google Scholar] [CrossRef]
- Oviedo-Rondón, E.O.; Toscan, A.; Fagundes, N.S.; Vidal, J.K.; Barbi, J.; Thiery, P. Soybean Meal Nutrient Composition, Amino Acid Digestibility, and Energy Content According to the Country of Origin and Year of Harvest Evaluated via NIRS. J. Appl. Poult. Res. 2024, 33, 100448. [Google Scholar] [CrossRef]
- Malama, F.; Nyau, V.; Marinda, P.; Munyinda, K. Effect of Sprouting on Selected Macronutrients and Physical Properties of Four Zambian Common Bean (Phaseolus vulgaris) Varieties. J. Food Nutr. Res. 2020, 8, 238–243. [Google Scholar]
- Shah, S.A.; Zeb, A.; Masood, T.; Noreen, N.; Abbas, S.J.; Samiullah, M.; Alim, A.; Muhammad, A. Effects of Sprouting Time on Biochemical and Nutritional Qualities of Mungbean Varieties. Afr. J. Agric. Res. 2011, 6, 5091–5098. Available online: https://academicjournals.org/article/article1380963999_Shah%20et%20al.pdf (accessed on 13 March 2024).
- Zhao, X.; Sun, L.; Zhang, X.; Wang, M.; Liu, H.; Zhu, Y. Nutritional Components, Volatile Constituents and Antioxidant Activities of 6 Chickpea Species. Food Biosci. 2021, 41, 100964. [Google Scholar] [CrossRef]
- Begum, N.; Khan, Q.U.; Liu, L.G.; Li, W.; Liu, D.; Haq, I.U. Nutritional Composition, Health Benefits and Bio-Active Compounds of Chickpea (Cicer arietinum L.). Front. Nutr. 2023, 10, 1218468. [Google Scholar] [CrossRef]
- Elobuike, C.S.; Idowu, M.A.; Adeola, A.A.; Bakare, H.A. Nutritional and Functional Attributes of Mungbean (Vigna radiata [L] Wilczek) Flour as Affected by Sprouting Time. Legume Sci. 2021, 3, e100. [Google Scholar] [CrossRef]
- Yoshari, R.M.; Astawan, M.; Prangdimurti, E.; Wresdiyati, T. The Production Process of Tempe Protein Isolate from Germinated Soybeans and Its Potential as an Antidiabetic. Food Res. 2023, 7, 71–79. [Google Scholar] [CrossRef]
- Matilla, A.J. Seed Dormancy: Molecular Control of Its Induction and Alleviation. Plants 2020, 9, 1402. [Google Scholar] [CrossRef]
- Bueno, D.B.; da Silva Júnior, S.I.; Seriani Chiarotto, A.B.; Cardoso, T.M.; Neto, J.A.; Lopes dos Reis, G.C.; Gló-ria, M.B.A.; Tavano, O.L. The Germination of Soybeans Increases the Water-Soluble Components and Could Generate Innovations in Soy-Based Foods. LWT 2020, 117, 108599. [Google Scholar] [CrossRef]


| Sprouting Time (Days) | Crude Fat * | Crude Protein * | Ash * | Carbohydrates * |
|---|---|---|---|---|
| 0 | 0.45 ± 0.00 D | 23.23 ± 0.17 C | 2.71 ± 0.00 A | 67.81 ± 0.68 D |
| 2 | 1.01 ± 0.04 C | 17.46 ± 0.48 C | 2.72 ± 0.02 A | 69.80 ± 0.96 D |
| 4 | 1.16 ± 0.05 B | 18.45 ± 0.33 C | 2.79 ± 0.27 A | 67.44 ± 1.01 CD |
| 6 | 1.25 ± 0.05 B | 12.34 ± 0.67 B | 2.50 ± 0.13 A | 73.19 ± 0.22 AB |
| 8 | 1.34 ± 0.05 A | 10.27 ± 0.94 B | 2.57 ± 0.07 A | 76.00 ± 0.58 A |
| 10 | 1.54 ± 0.04 A | 10.60 ± 0.72 A | 2.74 ± 0.07 A | 71.94 ± 1.15 BC |
| Sprouting Time (Days) | D1 (% of Soluble Protein) | D2 (% of Soluble Protein) | D3 (% of Soluble Protein) |
|---|---|---|---|
| 0 | 31.77 ± 2.1.44 Dab | 29.37 ± 0.41 Db | 37.44 ± 1.95 Da |
| 2 | 36.44 ± 1.71 CDb | 33.64 ± 1.93 Cb | 45.86 ± 1.53 Ca |
| 4 | 40.03 ± 1.77 BCb | 39.12 ± 0.24 Bb | 48.68 ± 1.24 Ca |
| 6 | 40.88 ± 1.88 BCb | 39.56 ± 0.89 Bb | 64.12 ± 0.56 Ba |
| 8 | 44.73 ± 0.13 BCDa | 49.15 ± 0.40 Ab | 81.74 ± 1.05 Ac |
| 10 | 54.54 ± 1.72 Aa | 50.30 ± 0.17 Ab | 79.35 ± 1.78 Ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosas-Ordoñez, L.; Ramírez-Rodrigues, M.M.; Ramírez-Rodrigues, M.A.; Pereira, T.S.S. Enhanced Protein Digestibility and Amino Acid Profile of a Novel Legume (Inga paterno) Seed Flours: Evaluation of Proximal Composition Changes by Sprouting. Appl. Biosci. 2025, 4, 15. https://doi.org/10.3390/applbiosci4010015
Rosas-Ordoñez L, Ramírez-Rodrigues MM, Ramírez-Rodrigues MA, Pereira TSS. Enhanced Protein Digestibility and Amino Acid Profile of a Novel Legume (Inga paterno) Seed Flours: Evaluation of Proximal Composition Changes by Sprouting. Applied Biosciences. 2025; 4(1):15. https://doi.org/10.3390/applbiosci4010015
Chicago/Turabian StyleRosas-Ordoñez, Lizbeth, Milena M. Ramírez-Rodrigues, Melissa A. Ramírez-Rodrigues, and Taisa S. S. Pereira. 2025. "Enhanced Protein Digestibility and Amino Acid Profile of a Novel Legume (Inga paterno) Seed Flours: Evaluation of Proximal Composition Changes by Sprouting" Applied Biosciences 4, no. 1: 15. https://doi.org/10.3390/applbiosci4010015
APA StyleRosas-Ordoñez, L., Ramírez-Rodrigues, M. M., Ramírez-Rodrigues, M. A., & Pereira, T. S. S. (2025). Enhanced Protein Digestibility and Amino Acid Profile of a Novel Legume (Inga paterno) Seed Flours: Evaluation of Proximal Composition Changes by Sprouting. Applied Biosciences, 4(1), 15. https://doi.org/10.3390/applbiosci4010015