Phytochemical Profile, Antioxidant and Antimicrobial Activity of Two Species of Oak: Quercus sartorii and Quercus rysophylla
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Extraction
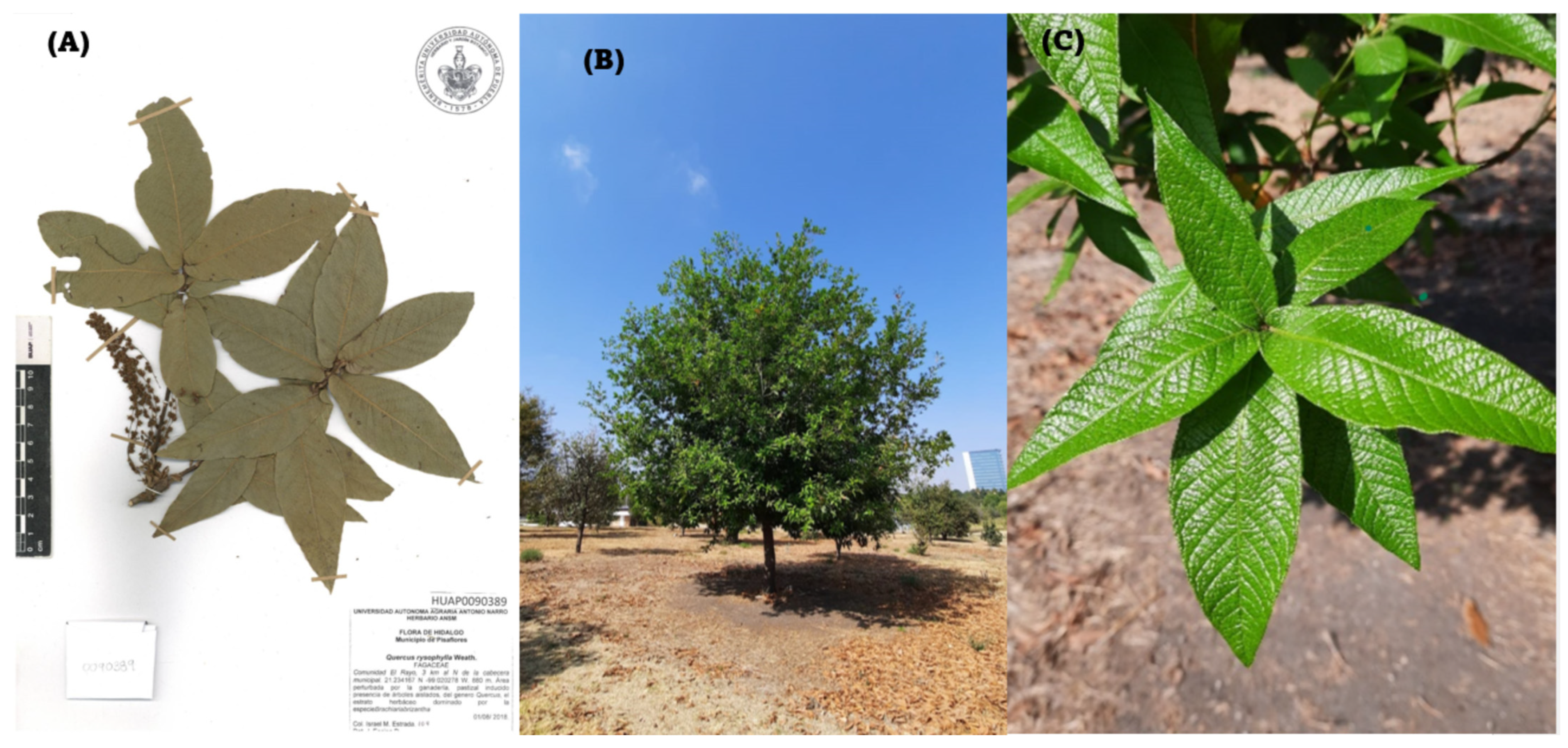
2.1.1. Collection of Leaves of the Species: Quercus sartorii and Quercus rysophylla
2.1.2. Differential Extraction by the Soxhlet Method
2.2. Phytochemical Testing
2.2.1. Lieberman–Burchard (Steroids and Terpenes)
2.2.2. Shinoda Test (Flavonoid)
2.2.3. Salkowsky Test (Steroid and Flavone Identification)
2.2.4. Dragendorf (Alkaloid Detection)
2.2.5. Baljet Test (Sesquiterpenlactones)
2.2.6. Phenolic Hydroxyls Test
2.2.7. Saturations- KMnO4 Test
2.2.8. Molish Test—For Sugars
2.2.9. Coumarins (NaOH Test)
2.2.10. Saponin Test (Foam Test)
2.3. Antioxidant Studies
2.3.1. Total Phenolic Content
2.3.2. Total Flavonoid Content
2.3.3. DPPH (2,2-Diphenyl-1-picrylhydracil) Scavenging
2.4. Antimicrobial Activity
2.4.1. Antibiogram
2.4.2. Minimal Inhibitory Concentration (MIC)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Profile
3.2. Antioxidant Profile
3.2.1. DPPH (2,2-Diphenyl-1-picrylhydracil) Scavenging Results
3.2.2. Total Flavonoid and Total Phenolic Content
3.3. Antimicrobial Activity of Extracts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal Uses, Phytochemistry, and Pharmacological Activities of Quercus Species. Evid.-Based Complement. Altern. Med. eCAM 2020, 2020, 1920683. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gutiérrez, J.O.; Alvarado-Segura, A.A.; Machuca-Velazco, R.; Borja-de-la-Rosa, A. Caracterización anatómica y propiedades físicas de la madera de monte bajo de dos especies de Quercus del volcán Popocatépetl. Madera Bosques 2023, 29, e2911580. [Google Scholar] [CrossRef]
- Red de Herbarios Mexicanos Herbario del Jardín Botánico BUAP, Puebla, Mexico. Available online: https://www.herbanwmex.net/portal/collections/misc/collprofiles.php?collid=484 (accessed on 29 November 2024).
- Rocha, N.; González-Laredo, R.; Vázquez-Cabral, B.; Moreno-Jiménez, M.; Gallegos-Infante, J.; Gamboa-Gómez, C.; Flores-Rueda, A. Oak Leaves as a New Potential Source for Functional Beverages: Their Antioxidant Capacity and Monomer Flavonoid Composition. In Functional and Medicinal Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 381–411. ISBN 978-0-12-816397-9. [Google Scholar]
- Decombeix, A.-L.; Harper, C.J.; Galtier, J.; Meyer-Berthaud, B.; Krings, M. Tyloses in Fossil Plants: New Data from a Mississippian Tree, with a Review of Previous Records. Bot. Lett. 2022, 169, 510–526. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. The Polyphenolic Profile of Oak (Quercus) Species: A Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Moctezuma Vega, B.X. Interacción Entre Adultos de Macrodactylus mexicanus (Coleoptera: Melolonthidae) con Quercus grahamii y Quercus sartorii (Fagaceae), Mediada por Compuestos Volátiles; Benemérita Universidad Autónoma de Puebla: Puebla, Mexico, 2022. [Google Scholar]
- Valencia-A, S.; Flores-Franco, G.; Jiménez-Ramírez, J.; Mora-Jarvio, M. Distribution and Diversity of Fagaceae in Hidalgo, Mexico. Bot. Sci. 2017, 95, 660–721. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Burlacu, E.; Nisca, A.; Tanase, C. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, S.; Wang, G.; Shao, X.; Wang, Y.; Li, Y.; Ding, Y. The Genus Quercus: Metabolites, Biological Activity and Mechanisms of Action. Phytochem. Rev. 2024, 24, 259–302. [Google Scholar] [CrossRef]
- Boeder, A.; Spiller, F.; Carlström, M.; Izídio, G. Enterococcus Faecalis: Implications for Host Health. World J. Microbiol. Biotechnol. 2024, 40, 190. [Google Scholar] [CrossRef]
- Elashiry, M.M.; Bergeron, B.E.; Tay, F.R. Enterococcus faecalis in Secondary Apical Periodontitis: Mechanisms of Bacterial Survival and Disease Persistence. Microb. Pathog. 2023, 183, 106337. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Pasachova Garzón, J.; Ramirez Martinez, S.; Muñoz Molina, L. Staphylococcus aureus: Generalidades, mecanismos de patogenicidad y colonización celular. Nova 2019, 17, 25–38. [Google Scholar] [CrossRef]
- Chávez-Jacobo, V.M. La batalla contra las superbacterias: No más antimicrobianos, no hay ESKAPE. TIP Rev. Espec. Cienc. Químico-Biológicas 2020, 23. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- da Fonseca, A.d.S.; Mencalha, A.L.; de Paoli, F. Antimicrobial Photodynamic Therapy against Acinetobacter baumannii. Photodiagnosis Photodyn. Ther. 2021, 35, 102430. [Google Scholar] [CrossRef]
- Wu, H.-J.; Xiao, Z.-G.; Lv, X.-J.; Huang, H.-T.; Liao, C.; Hui, C.-Y.; Xu, Y.; Li, H.-F. Drug-resistant Acinetobacter baumannii: From Molecular Mechanisms to Potential Therapeutics (Review). Exp. Ther. Med. 2023, 25, 209. [Google Scholar] [CrossRef]
- Alqethami, A.; Aldhebiani, A.Y. Medicinal Plants Used in Jeddah, Saudi Arabia: Phytochemical Screening. Saudi J. Biol. Sci. 2021, 28, 805–812. [Google Scholar] [CrossRef]
- Cortes-Torres, A.G.; López-Castillo, G.N.; Marín-Torres, J.L.; Portillo-Reyes, R.; Luna, F.; Baca, B.E.; Sandoval-Ramírez, J.; Carrasco-Carballo, A. Cymbopogon Citratus Essential Oil: Extraction, GC–MS, Phytochemical Analysis, Antioxidant Activity, and In Silico Molecular Docking for Protein Targets Related to CNS. Curr. Issues Mol. Biol. 2023, 45, 5164–5179. [Google Scholar] [CrossRef]
- Marami, L.M.; Dilba, G.M.; Babele, D.A.; Sarba, E.J.; Gizaw, A.; Bune, W.M.; Bayu, M.D.; Admasu, P.; Mekbeb, A.; Tadesse, M.; et al. Phytochemical Screening and In-Vitro Evaluation of Antibacterial Activities of Echinops Amplexicaulis, Ruta Chalepensis and Salix Subserrata Against Selected Pathogenic Bacterial Strains in West Shewa Zone, Ethiopia. J. Exp. Pharmacol. 2021, 13, 511–520. [Google Scholar] [CrossRef]
- Zulfisa, Z.; Fika, R.; Agusfina, M.; Yonrizon, Y.; Muhsanah, A. Determination of Total Phenolic Content of Ethanol Extract of Broken Bone Twigs (Euphorbia tirucalli Linn.) by Folin-Ciocalteu Method Spectrophotometrically. J. EduHealth 2023, 14, 1326–1331. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Carrasco-Sandoval, J.; Falcó, I.; Sánchez, G.; Fabra, M.J.; López-Rubio, A.; Rodriguez, A.; Henríquez-Aedo, K.; Aranda, M. Multivariable Optimization of Ultrasound-Assisted Extraction for the Determination of Phenolic and Antioxidants Compounds from Arrayan (Luma apiculata (DC.) Burret) Leaves by Microplate-Based Methods and Mass Spectrometry. J. Appl. Res. Med. Aromat. Plants 2022, 28, 100356. [Google Scholar] [CrossRef]
- Saraluz, S.-O.; Alatriste, V.; Lucina, M.-T.J.; Paulina, M.-S.I.; Alan, C.-C. Loeselia mexicana: Antioxidant and Antimicrobial Properties by Soxhlet Differential Extraction. GSC Biol. Pharm. Sci. 2023, 23, 115–120. [Google Scholar] [CrossRef]
- Alami, M.M.; Guo, S.; Mei, Z.; Yang, G.; Wang, X.; Alami, M.M.; Guo, S.; Mei, Z.; Yang, G.; Wang, X. Environmental Factors on Secondary Metabolism in Medicinal Plants: Exploring Accelerating Factors. Med. Plant Biol. 2024, 3, e016. [Google Scholar] [CrossRef]
- Wahnou, H.; Liagre, B.; Sol, V.; El Attar, H.; Attar, R.; Oudghiri, M.; Duval, R.E.; Limami, Y. Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment. Cancers 2023, 15, 3826. [Google Scholar] [CrossRef]
- Wahnou, H.; Limami, Y.; Oudghiri, M. Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management. BioChem 2024, 4, 38–61. [Google Scholar] [CrossRef]

| Bacterium | Gram | Characteristics | Habitat/ Ecological Niche | Associated Infections | References |
|---|---|---|---|---|---|
| Enterococcus spp./Enterococcus faecalis | (+) | Opportunistic, facultative anaerobic | Gut microbiota, soil, water, and food products | Endocarditis, meningitis, pneumonia, peritonitis, internal abscesses, urinary tract infections, and sepsis | [12,13] |
| Staphylococcus aureus | (+) | β hemolytic, catalase, and coagulase-positive, aerobic, or anaerobic. | Skin, external mucous membranes | Osteomyelitis, infective endocarditis, bacteremia, and pneumonia | [14,15] |
| Klebsiella pneumoniae | (−) | Opportunistic, virulent bacillus due to adhesins and capsules that acts as an antiphagocytic factor. | Gut microbiota, soil, and water | Urinary tract and respiratory tract infections | [16] |
| Acinetobacter baumanii | (−) | Pleomorphic, opportunistic, trictly aerobic, immobile, non-fermenting lactose, catalase-positive, and oxidase-negative | Hospital environments, soil, water, and skin | Infections of the respiratory tract, bloodstream, skin and soft tissues, urinary tract, and central nervous system | [17,18,19] |
| Pseudomonas aeruginosa | (−) | Facultative anaerobic, opportunistic | Animals, plants, soil | Nosocomial infections, especially in patients on ventilators | [16] |
| Enterobacter spp. | (−) | Bacillus, facultative anaerobic, encapsulated | Gut, water, and soils | Urinary tract infections, respiratory tract infections, sepsis in immunocompromised patients | [16] |
| Test | Metabolite | Q. sartorii | Q. rysophylla |
|---|---|---|---|
| Lieberman—Burchard | Steroids | +++ | +++ |
| Shinoda | Flavonoids | − | − |
| Salkowsky | Flavones | +++ | +++ |
| Dragendorf | Alkaloids | − | − |
| Baljet | Sesquiterpenlactones | − | − |
| Ferric chloride | Phenolic oxidyls | + | + |
| KMnO4 | Saturations | +++ | +++ |
| Molish | Carbohydrates | +++ | +++ |
| NaOH | Coumarins | − | − |
| Foam | Saponins | − | − |
| Extract | Q. sartorii | Q. rysophylla |
|---|---|---|
| Hexane | 44.09 ± 18.6 B | 41.50 ± 9.6 C |
| AcOEt | 67.66 ± 8.4 A | 79.87 ± 4.2 D |
| DCM | 72.92 ± 4.6 A | 51.73 ± 10.2 B |
| Ethanol | 79.99 ± 2.2 D | 82.60 ± 2.7 E |
| Methanol | 81.14 ± 1.7 D | 80.82 ± 3.2 DE |
| Water | 77.03 ± 3.04 C | 68.71 ± 5.9 A |
| Total Flavonoids (mg QE/kg of Plant) | ||
|---|---|---|
| Extract | Q. sartorii | Q. rysophylla |
| Hexane | 139.58 ± 21.2 B | 159.40 ± 38.6 C |
| AcOEt | 505.76 ± 23.3 A | 920.96 ± 70.4 A |
| DCM | 161.37 ± 17.05 B | 48.70 ± 1.6 B |
| Ethanol | 24587.42 ± 996.3 C | 6005.36 ± 387.8 E |
| Methanol | 11844.08 ± 708.4 D | 19875.66 ± 2754.01 D |
| Water | 22947.13 ± 2326.1 C | 17289.96 ± 3014.8 D |
| Total Phenolic Content (mg GAE/kg of Plant) | ||
|---|---|---|
| Extract | Q. sartorii | Q. rysophylla |
| Hexane | 32.55 ± 12.7 C | 2.87 ± 0.9 C |
| AcOEt | 446.73 ± 175.3 A | 885.67 ± 106.5 A |
| DCM | 163.55 ± 43 B | 5.99 ± 1.9 B |
| Ethanol | 12864.62 ± 552.2 E | 3468.8 ± 702.7 E |
| Methanol | 6212.35 ± 2210.7 F | 4619.36 ± 587.9 F |
| Water | 22342.10 ± 3076.5 D | 17747.14 ± 1139.9 D |
| Bacteria | Extract | Q. sartorii | Q. rysophylla |
|---|---|---|---|
| Acinetobacter baumanni | Antibiotic | 7.04 B | 5.5 C |
| Hexane | - | 0.6 A | |
| AcOEt | 1.02 A | 0.7 A | |
| DCM | 1.20 A | 0.5 A | |
| Ethanol | - | 1.5 B | |
| Methanol | - | - | |
| Water | - | - | |
| Staphylococcus aureus | Antibiotic | 8.01 B | 6.9 C |
| Hexane | - | 1.3 B | |
| AcOEt | 0.58 A | 0.7 A | |
| DCM | 0.51 A | 0.5 A | |
| Ethanol | 0.60 A | - | |
| Methanol | 0.55 A | - | |
| Water | - | - | |
| Enterococcus faecalis | Antibiotic | 3.55 | 6.1 |
| Hexane | - | - | |
| AcOEt | - | - | |
| DCM | - | - | |
| Ethanol | - | - | |
| Methanol | - | - | |
| Water | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coyotl-Martinez, E.; Hernández-Rivera, J.A.; Parra-Suarez, J.L.A.; Reyes-Carmona, S.R.; Carrasco-Carballo, A. Phytochemical Profile, Antioxidant and Antimicrobial Activity of Two Species of Oak: Quercus sartorii and Quercus rysophylla. Appl. Biosci. 2025, 4, 13. https://doi.org/10.3390/applbiosci4010013
Coyotl-Martinez E, Hernández-Rivera JA, Parra-Suarez JLA, Reyes-Carmona SR, Carrasco-Carballo A. Phytochemical Profile, Antioxidant and Antimicrobial Activity of Two Species of Oak: Quercus sartorii and Quercus rysophylla. Applied Biosciences. 2025; 4(1):13. https://doi.org/10.3390/applbiosci4010013
Chicago/Turabian StyleCoyotl-Martinez, Elizabeth, Juan Alex Hernández-Rivera, José L. Arturo Parra-Suarez, Sandra Raquel Reyes-Carmona, and Alan Carrasco-Carballo. 2025. "Phytochemical Profile, Antioxidant and Antimicrobial Activity of Two Species of Oak: Quercus sartorii and Quercus rysophylla" Applied Biosciences 4, no. 1: 13. https://doi.org/10.3390/applbiosci4010013
APA StyleCoyotl-Martinez, E., Hernández-Rivera, J. A., Parra-Suarez, J. L. A., Reyes-Carmona, S. R., & Carrasco-Carballo, A. (2025). Phytochemical Profile, Antioxidant and Antimicrobial Activity of Two Species of Oak: Quercus sartorii and Quercus rysophylla. Applied Biosciences, 4(1), 13. https://doi.org/10.3390/applbiosci4010013






