Abstract
This study investigated the recovery of polyphenolic compounds such as punicalagin, punicalin, and ellagic acid via solid-state fermentation (SSF)-assisted extraction from pomegranate peel (Punica granatum L.) using Aspergillus niger GH1 and Saccharomhyces cerevisiae. Food processing has contributed to the increase in agroindustrial wastes, which has become a global concern due to environmental protection. However, these wastes can be valorized via the extraction of high-value components such as bioactive compounds. Ellagitannins extracted during the bioprocesses were identified via the HPLC–MS technique and quantified via total polyphenols (hydrolyzable and condensed assays). Enzymatic activities were tested. HPLC–MS analysis showed a decrease in the levels of punicalagin, the formation of punicaline, and the accumulation of ellagic acid during fermentation kinetics. The present study compares two different bioprocesses in order to obtain, from agroindustrial wastes, high-added-value compounds using SSF-.
1. Introduction
Pomegranate (Punica granatum L.) is a plant and fruit grown mainly in ancient Egypt, Italy, and Greece, though recently, it has spread to Asia, North Africa, and Europe [1]. The most important producers are India, Iran, Turkey China, the United States of America, Argentina, and Brazil, among others. [2]. Pomegranate has been investigated for its high content of polyphenolic compounds such as ellagitannins [3,4,5,6]. The antioxidative activity of pomegranate has been attributed to bioactive compounds that exhibit diverse medicinal properties and health benefit effects. Nowadays, pomegranate is recognized as an important fruit with a high percentage of antioxidant activity [7]. It has been reported that pomegranate peel (PP) has higher concentrations of bioactive compounds and exhibits a higher antioxidant activity than the rest of the fruit [8]. PP represents approximately 50% of the total weight of the fruit and is an important source of bioactive compounds such as ellagitannins [9]—mainly punicalin, pedunculagin, and punicalagin [5]. In 2017, a world production of 3.8 million tons of pomegranate peel was recorded [2]. The peel of the fruit is rich in phenolics acid such as gallic, ellagic, and caffeic acids. It has been reported that PP has a higher concentration of phenolic compounds than pomegranate juice and allows for a higher antioxidant activity [10]. Hydrolyzable tannins could be ellagitannins and gallotannins. The first ones are esters of hexahydroxydiphenic acid (HHDP) bound to a polyol and, when it is exposed to strong acids or bases, ester bonds hydrolyze and HHDP reorganizes into a dilactone, producing ellagic acid [11,12]. Ellagitannins can be found in grapes, rambutan, strawberries, cranberries, blueberries, guavas, and raspberries, among others [13]
Food processing has increased the by-products or agroindustrial wastes, which are produced along the food supply chain in stages such as agricultural production, manufacturing, processing, and distribution [14]. Recently, the use of agroindustrial wastes has become a global concern due to environmental protection; following this, residues can be valorized by extracting high-value compounds which can be reused as functional and nutraceutical ingredients; for example: proteins, polysaccharides, fibers, flavoring compounds, and phytochemicals [15].
Bioactive compounds have been recovered via various techniques and are mainly based on solvent extraction, supercritical fluid extraction, subcritical water extraction, enzymes, ultrasounds, and microwaves. These techniques allow for the recovery of specific bioactive compounds [14]. The extraction methods exhibit some disadvantages, such as their use of toxic solvents that damage the environment, their cost, their being difficult to implement, and their use high temperatures that can damage the bioactive compounds. Recently, ellagitannins have been extracted using emerging techniques to reduce the use of polluting solvents and obtain better extraction yields; for example: pressurized liquid extraction, ultrasound assisted extraction, microwave assisted simultaneous distillation, and supercritical fluid extraction [16].
SSF is a bioprocess whereby micro-organisms are allowed to grow in an environment without free water [17]. It has been demonstrated that this technique allows for higher yields of extraction and better product characteristics than submerged fermentation. SSF has great potential to recover bioactive compounds such as ellagitannins [18] since micro-organisms such as fungi naturally produce enzymes that degrade the cell wall, generating hydrolysis [19] and the mobilization of compounds towards the extraction solvent [20]. This bioprocess is low-cost and easy to implement since it requires small equipment and exhibits an important reduction in operating costs since it does not require sterilization, aeration, or agitation steps [17]. Several important factors must be considered in the development of SSF, the selection of the micro-organism strain, and the solid support. Bagasse, peels, seeds, and straw, among other examples, can be considered agroindustrial wastes. In the case of micro-organisms, filamentous fungi have been widely used since the technique mimics their natural habitat so they can synthetize enzymes and some other metabolites [21]. Moreover, the use of yeast has been recommended, which are able to grow over a material with low water activity and some bacteria.
Several in vivo and in vitro studies have reported that pomegranate presents antioxidant, antiviral, antibacterial, anti-inflammatory, antimicrobial, anticancer, and antiproliferative activity [7].
For this reason, PP is proposed, an agroindustrial waste that can be used due to its high content of bioactive compounds with highly relevant biological properties. In the present study, the SSF technique was applied to evaluate the ellagitannins extraction from PP using two different micro-organisms: Aspergillus niger GH1 and Saccharomyces cerevisiae.
2. Materials and Methods
Raw material: PP was collected from Cuatrociénegas, Coahuila, México. The peel was dehydrated for 48 h at 60 °C and grounded to powder (30-mesh particle size in an industrial homogenizer: 5 L; model LP12 Series 600-182, CDMX, México), stored in black plastics bags, and protected from light at room temperature.
Micro-organism and culture medium: In this study, we used the strains Aspergillus niger GH1 (collection DIA-UADEC, Saltillo, Coahuila, Mexico) and Saccharomyces cerevisiae 227 (collection of Instituto Tecnológico de Durango, México). Previously, the spores of A. niger GH1 were conserved in a cryoprotective solution at −55 °C (skimmed milk/glycerol 9:1). The strain GH1 of Aspergillus niger has been deposited in the Micoteca of the University of Minho, number MUM:23.16. Spores were reactivated in potato dextrose agar (PDA-Bioxon) at 30 °C for five days and then were recovered using 0.01% Tween 80, and spores/mL were counted in a Neubauer chamber.
SSF with Aspergillus niger GH1: The SSF was performed on a polypropylene flask with pomegranate peel powder (1.5 g) as support/substrate. The conditions were inoculum 1 × 106 spores/g, 80% of humidity, and culture medium of NaNO3 (15.6 g/L), KH2PO4 (3.04 g/L), KCl (1.52 g/L), and MgSO4 (1.52 g/L). The reactors were incubated at 30 °C for 48 h, taking data every 6 h. All tests were conducted in triplicate. These conditions corresponded to those previously reported by Sepúlveda et al. (2012) [22].
SSF with Saccharomyces cerevisiae: The SSF was carried out in a polypropylene flask where the pomegranate peel powder (1.5 g) as support was inoculated with the yeast strain. The SSF conditions were temperature 25 °C, 70% humidity, inoculum 2 × 106, pH 5, peptone 10 g/L, extract of yeast 10 g/L, and NaCl 230 g/L. The reactors were incubated at 25 °C for 48 h, taking data every 6 h. All tests were conducted in triplicate. These conditions were previously reported by Moccia et al. (2012) [23].
Recovery fermentation extracts: All the fermentation extracts were recovered and filtered via manual compression every 6 h. Filtered extracts were protected from light at refrigerated at −20 °C until analytical determinations (total polyphenols, HPLC-ESI-MS analysis, and enzymatic activities).
Determination of total polyphenols: In both bioprocesses, condensed polyphenols present in PP fermentation extracts were determined via the HCl-butanol technique and ferric reagent [24]. Hydrolyzable polyphenols were determined via the Folin–Ciocalteu method [25]. The tests were conducted in triplicate, and the results were expressed as mg/g of filtered material. Total polyphenols are the result of adding the content of condensed and hydrolyzable polyphenols.
Analytical RP-HPLC–ESI-MS: Ellagitannins were quantified via HPLC–MS. The analysis via reverse phase-high performance liquid chromatography were performed on a Varian HPLC system with an autosampler (Varian ProStar 410, Palo Alto, CA, USA), a ternary pump (Varian ProStar 230I, Palo Alto, CA, USA), and a PDA detector (Varian ProStar 330, Palo Alto, CA, USA). All the samples (5 µL) were injected onto a Denali C18 column (150 mm × 2.1 mm, 3 µm, Grace, Palo Alto, CA, USA). The oven temperature was 30 °C. The eluents were formic acid (0.2%, v/v; solvent A) and acetonitrile (solvent B). Data were collected and processed using MS Workstation software (V 6.9). First, samples were analyzed in full-scan mode acquired in the m/z range of 50–2000 [26]. All the samples were filtered with 0.45 µm nylon membrane.
Enzymatic activities: The fermented extracts were used to determine the enzymatic activities reported as associated with the biotransformation of ellagitannins [27,28,29,30,31,32]. Spectrophotometric and chromatographic techniques determined the identification of these activities.
Ellagitannase [27]: TPP was used as substrate. We used a mixture of 1 mL of 1 mg/mL of TPP and 0.05 mL of enzymatic extract in citrate buffer 0.05 M at pH 5. It was incubated at 50 °C for 10 min. To stop the reaction, 0.02 mL of HCl 1.5 M was added. It was centrifugated at 6000 rpm for 30 min. The precipitated was suspended in 1.5 mL of ethanol and ultrasonicated for 30 min. The sample was filtered with a 0.45 µm nylon membrane and analyzed via HPLC. One enzyme activity unit was defined as the amount of enzyme that released 1 μmol of ellagic acid per min under the assay conditions.
Polyphenoloxidase [28]: A substrate solution was prepared with pyrocatechol 0.1 M in 0.05 M citrate buffer, pH 5, enzyme preparation, and 0.05 M citrate buffer, pH 5, and they were pre-incubated at 30 °C for 10 min before the assay. Then, 1.7 mL of buffer citrate and 0.5 mL of enzyme preparation were added into a test tube and incubated at 30 °C for 10 min. To stop the reaction, the test tube was boiled in a water bath for 2 min. The oxidized pyricatechol absorbance was read at 420 nm. The differences of absorbances were calculated via A420 = Atest − Acontrol. One unit of enzyme activity was defined as an increase in absorbance by 0.001 min−1.
Cellulase [29]: CM cellulose (0.2 mL) and enzyme solution (0.05 mL) were incubated at 30 °C for 10 min. To stop the reaction, 2 mL of 0.3 M tricholoroacetic acid (TCA) was used. CM cellulose was calculated via the DNS method. The results were expressed as glucose concentration. One enzyme activity unit was defined as the amount of enzyme that released 1 μmol of glucose per min under the assay conditions.
β—glucosidase [30]: A standard reaction mixture was used, with 0.1 mL of 9 mM r-nitrophenol β-D-glucopyranoside (pNPG), 0.8 mL of 200 mM sodium acetate buffer (pH 4.6), and 0.1 mL of enzyme solution. The mixture was incubated for 15 min at 50 °C. The reaction was stopped with 1 mL of 0.1 M sodium carbonate. The released p-nitrophenol was read at 400 nm. One unit of enzyme was defined as the amount of enzyme that releases 1 μmol p-nitrophenol per min at pH 4.6 at 50 °C under the assay conditions.
Tannase [31]: Methylgalate was used as substrate, a reaction mixture (0.5 mL) was made of enzymatic extract and 0.01 M suspension of metylgalate 0.5 M with a citrate buffer at pH 5. The mixture was incubated at 30 °C for 10 min. The reaction was stopped by adding 0.2 mL of 0.5 N KOH. One enzyme activity unit was defined as the amount of enzyme that released 1 μmol of galic acid from methylgalate per min under the assay conditions.
Xylanase [32]: Xylan was used as a substrate. A total of 0.5 mL of enzyme solution and 0.5 mL of 0.5% (w/v) suspension of xylan with 0.05 M citrate buffer, pH 5.4, was used. The reaction mixture was incubated at 50 °C for 10 min. The reaction was stopped by adding 2 mL of 0.3 N TCA. One enzyme activity unit was defined as the amount of enzyme that released 1 μmol xylose from xylan per min under the assay conditions.
3. Results
3.1. SSF
A SSF kinetic was performed for 48 h, measuring fractions every 6 h, in order to obtain the highest concentration of polyphenols in SSF with A. niger GH1 and S. cerevisiae, using PP as the only carbon source. For comparative purposes, each fermented material was subjected to the same extraction procedure. The results show the polyphenolic content of the plant after fermentation. The maximum quantity of polyphenolic compounds was accumulated at 30 h with a value of 188.3 ± 9.5 mg/g of dry plant using Aspergillus niger GH1, and for Saccharomyces cerevisiae, the maximum value was 105.07 ± 8.4 mg/g of dry plant at 42 h of fermentation. The differences between the kinetic study with the fungus were statistically significant (p < 0.05).
3.2. RP-HPLC-ESI-MS Analysis
The recovered extracts were characterized via HPLC–MS. Table 1 reports the polyphenolic compounds found on the extracts of PP before and after (18 h) SSF with the A. niger GH1 and the Saccharomyces cerevisiae. Punicalagin is present in the unfermented material and on the yeast fermentation.

Table 1.
Identification of ellagitannins-related compounds in the extraction of unfermented and fermented PP.
Figure 1 shows the results obtained via HPLC of the main quantified components during SSF with Aspergillus niger GH1 and Saccharomyces cerevisiae at 0- and 24-h using PP as support. Data show high initial levels of punicalagin and an accumulation of punicalin and ellagic acid. In Figure 2, the chromatograms of fungus and yeast fermentations can be observed, where punicalagin only appears during yeast fermentation, and ellagic acid appears in both bioprocesses.
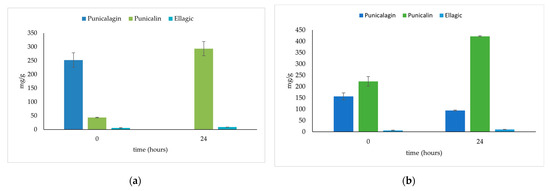
Figure 1.
Quantitative analysis of the main ellagitannins identified in the fermented/unfermented PP extracts: (a) 0 and 24 h of SSF with Aspergillus niger GH1; (b) 0 and 24 h with Saccharomyces cerevisiae.
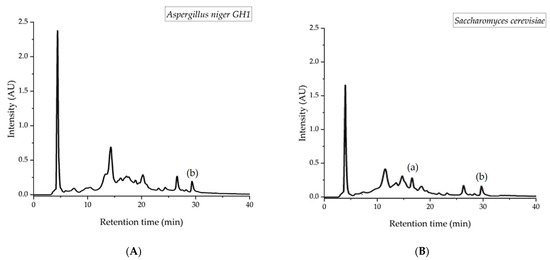
Figure 2.
Chromatograms of the main ellagitannins extracted from PP in SSF. Compound (A): punicalagine; compound (B): ellagic acid; Bioprocess (a): yeast fermentation; Bioprocess (b): chromatograms of fungus.
3.3. Enzymatic Activities
The ellagitannins have important biological potential, so it is important to establish the enzymes involved during the biodegradation of these compounds to obtain some other molecules. Table 2 reports several enzymatic activities of assayed ellagitannase [27], polyphenoloxidase [28], cellulase [29], β—glucosidase [30], tannase [31], and xylanase [32]. The results suggest that PP ellagitannins are hydrolyzed by enzymes.

Table 2.
Enzymatic activities evaluated.
4. Discussion
It has been reported that PP has numerous biological effects. It is an agroindustrial waste that can be used due its high content of bioactive compounds. SSF is an eco-friendly and low-cost technology. PP is a suitable support with which to perform an SSF, and the micro-organism was able to grow and invade the material.
SSF have been reported for the extraction of bioactive compounds, such as polyphenols, due to the ability of some micro-organisms to produce enzymes that allow for the bioavailability of compounds [11] and the mobilization of compounds towards the extraction solvent [20]. In this work, PP was used as a carbon and energy source for solid-state fermentation with fungus and yeast. The determination of total polyphenolic content involves the addition of total hydrolyzable polyphenols and total condensed polyphenols because the Folin–Ciocalteu reagent reacts mainly with the galloyl or HHDP groups, while the ferric reagent and hydrolysis with HCl-butanol act on compounds based on flavan-3-ol. The results show that the maximum accumulation time of polyphenolic compounds for A. niger GH1 was 188.3 mg/g, and for S. cerevisiae, it was 105.07 mg/g, indicating that SSF promotes the extraction of bioactive compounds from PP. There is a statistically significant difference between both micro-organisms, probably because they adapted differently to the support (PP) during fermentation.
Meanwhile, in order to learn the effect of SSF on the ellagitannins present in PP under the two evaluated conditions, the extracts were subjected to qualitative analysis via LC–MS. As expected, punicalagin is present in the unfermented material as well as in the material derived from S. cerevisiae. Punicalin, ellagic acid, and granatin B were found in all extracts. Moreover, some others ellagitannins were found on the evaluated material that corresponded to those reported as components of pomegranate ellagitannins generated via the hydrolysis of molecules with high weight [23]. PP is rich in phenolic acids such as gallic, ellagic, and caffeic acids. Ref. [33] reported that the most relevant bioactive compounds of pomegranate wine lees were hydrolyzable tannins: gallic acid, total punicalins, punicalagin, ellagic acid hexoside, and ellagic acid. Moreover, anthocyanins, flavonoids, and phenolic acids were detected. These results can be compared with those reported by [34], who studied the total polyphenolic content of pomegranate peel, where phenolic acids were the major phenolic compound, followed by flavonoids and tannins. Moreover, pomegranate peel has demonstrated a promising potential for biological activities, and it could be a source of functional food and nutraceuticals. Ref. [35] identified the phenolic compounds on ethanolic extracts of pomegranate peels via mass spectrometry (mainly punicalagin). The report shows that the punicalagin contained in the pomegranate peel improves the antimicrobial activity of the fruit.
As expected, the results suggest that PP wastes contain high levels of punicalagin. During the fermentation time with A. niger GH1, the punicalagin level drops from 0 to 24 h, though the levels of punicalin, an ellagic acid, increase. It has been reported that punicalagin leads to punicalin and the hexahydroxydiphenic acid group (HHDP), which, through spontaneous lactonization, converts to ellagic acid. On the other hand, for the S. cerevisiae fermented material, punicalagin is slowly reduced during the fermentation period, and an accumulation of punicalin and ellagic acid can be seen; this can be observed in Figure 2, where punicalagin can be detected under 18 h of bioprocessing with the yeast, whereas for the fungus, it is not detected. Ref. [23] reported that the pomegranate husk fermented with Aspergillus decreased the punicalagin levels in comparison to unfermented material. Moreover, with saccharomyces, the punicalagin was drastically reduced. Punicalagin is the main compound on PP and represents the hydrolase activity of the micro-organism as a pathway to accumulating ellagic acid.
In summary, punicalagin represents the main compound in the material, and the hydrolase activity of the micro-organism suggests a pathway for the formation of ellagic acid. The SSF with A. niger GH1 and S. cerevisiae promotes the hydrolysis of the ellagitannins to some other compounds, such as punicalin and ellagic acid, obtaining higher yields of extraction. The ellagic acid and punicalin are derived from the hydrolysis (partial or complete) of punicalagin [5].
Additionally, the biodegradation of pomegranate ellagitannins was evaluated. The maximum concentration of ellagic acid released was reached at 18 h of culture with a maximum value of 10.06 mg g−1. Ref. [32] reported that xylanase and cellulase activities were responsible for the degradation of ellagitannins and the accumulation of ellagic acid. However, [27] mentioned that xylanase and cellulase were detected at 24 and 6 h, respectively, and reported that these enzymes are not directly related to the accumulation of ellagic acid. Xylanase and tannase activities were detected in neither the fungus nor the yeast fermentation. β-Glucosidase activity was detected at 18 h of culture with the fungus, and it is directly related to the accumulation of ellagic acid. As [30] mentioned, this enzyme is associated with ellagitannins degradation. Ref. [36] reported the production of ellagic acid by cultivating fungi in the solid state, using polyurethane foam (PUF) as a support, and an aqueous extract obtained from pomegranate peel as the only source of carbon and energy. The authors determined that Aspergillus niger GH1 consumed ellagitannins during the first 36 h of culture, with the maximum concentration of ellagic acid being reached at 48 h. The authors attributed the biodegradation of ellagitannins to a new tannase, which is probably different from acyl hydrolase.
Otherwise, ellagitannase activity was detected at 18 h of culture with the fungus and 24 h with the yeast. Ascacio-Valdés et al. (2014) reported that this enzyme is directly related with the biodegradation of ellagitannins present in pomegranate peel, such as punicalagin and punicalin. They proposed the accumulation of ellagic acid because this enzyme degraded the ester bonds between glycosides and the HHDP group.
5. Conclusions
Many agroindustrial wastes have been used as substrates for SSF, such as sugarcane bagasse, corn cobs, coconut husks, grape wastes, candelilla stalks, and rambutan, among others. PP is also an example of agroindustrial waste, and it is an important source of bioactive compounds such as ellagitannins (mainly punicalagin), which present high antioxidant activity. SSF has been reported as a technique by which to extract bioactive compounds such as polyphenols. In this study, we are reporting on the extraction of bioactive compounds via SSF with A. niger GH1 and S. cerevisiae using pomegranate peel as a substrate. These bioprocesses allow for the biodegradation of ellagitannins (mainly punicalagin) of high molecular weight, accumulating bioactive compounds such as punicalin and ellagic acid. The time of maximum accumulation of polyphenolic compounds with the fungus at 24 h was higher than that with the yeast, and this can be achieved due to Aspergillus niger GH1 being a filamentous fungus that has the ability of grow on the used support. Enzymatic activities, particularly those of ellagitannase, are responsible for ellagitannins degradation in both bioprocesses. The extraction of bioactive compounds has a relevant role in biological activities, and the components in question can be used as nutraceutical components in the food industry.
Author Contributions
Conceptualization, J.A.A.-V.; methodology, A.L.I.-C., L.S. and M.L.C.-G.; formal analysis, C.N.A. and C.T.-L.; investigation, J.A.A.-V. and C.N.A.; writing—original draft preparation, A.L.I.-C.; writing—review and editing, A.L.I.-C., J.A.A.-V. and L.S.; supervision, J.A.A.-V. and C.N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Acknowledgments
Ana L. Izábal-Carvajal thanks CONAHCYT Mexico for scholarship support. The authors thank the School of Chemistry of the Autonomous University of Coahuila.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Evreinoff, V.A. Contribution à l’étude du Grenadier. J. Agric. Trop Bot. Appl. 1957, 4, 124–138. [Google Scholar] [CrossRef]
- Kahramanoglu, I. Trends in Pomegranate Sector: Production, Postharvest Handling and Marketing. Int. J. Agric. For. Life Sci. 2019, 3, 239–246. [Google Scholar]
- Bonzanini, F.; Bruni, R.; Palla, G.; Serlataite, N.; Caligiani, A. Identification and distribution of lignans in Punica granatum L. fruit endocarp, pulp, seeds, wood knots and commercial juices by GC-MS. Food Chem. 2009, 117, 745–749. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumäki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated Method for the Characterization and Quantification of Extractable and Nonextractable Ellagitannins after Acid Hydrolysis in Pomegranate Fruits, Juices, and Extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef]
- Seeram, N.; Lee, R.; Hardy, M.; Heber, D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep. Purif. Technol. 2005, 41, 49–55. [Google Scholar] [CrossRef]
- Pareek, S.; Valero, D.; Serrano, M. Postharvest biology and technology of pomegranate. J. Sci. Food Agric. 2015, 95, 2360–2379. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate fruit as a rich source of biologically active compounds. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.N.V.R. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006, 40, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Carbo, A.; Augur, C.; Prado-Barragan, L.A.; Favela-Torres, E.; Aguilar, C.N. Microbial production of ellagic acid and biodegradation of ellagitannins. Appl. Microbiol. Biotechnol. 2008, 78, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The Impact of Ellagitannins and Their Metabolites through Gut Microbiome on the Gut Health and Brain Wellness within the Gut–Brain Axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. J. Chromatogr. A 2017, 1514, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P.d.S. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Jamal, P.; Idris, Z.M.; Alam, M.Z. Effects of physicochemical parameters on the production of phenolic acids from palm oil mill effluent under liquid-state fermentation by Aspergillus niger IBS-103ZA. Food Chem. 2011, 124, 1595–1602. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; Correia, M.T.d.S.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Farinas, C.S. Developments in solid-state fermentation for the production of biomass-degrading enzymes for the bioenergy sector. Renew. Sustain. Energy Rev. 2015, 52, 179–188. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Aguilera-Carbó, A.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Martínez-Hernández, J.L.; Aguilar, C.N. Optimization of ellagic acid accumulation by Aspergillus niger GH1 in solid state culture using pomegranate shell powder as a support. Process Biochem. 2012, 47, 2199–2203. [Google Scholar] [CrossRef]
- Moccia, F.; Flores-Gallegos, A.C.; Chávez-González, M.L.; Sepúlveda, L.; Marzorati, S.; Verotta, L.; Panzella, L.; Ascacio-Valdes, J.A.; Aguilar, C.N.; Napolitano, A. Ellagic acid recovery by solid state fermentation of pomegranate wastes by aspergillus Niger and saccharomyces cerevisiae: A comparison. Molecules 2019, 24, 3689. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.T.; Hillis, W.E. The phenolic constituents of pronus domestica. J. Sci. Food Agric. 1945, 10, 63–68. [Google Scholar] [CrossRef]
- Makkar, H. Measurement of Total Phenolics and Tannins Using Folin-Ciocalteu Method; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Buenrostro, J.J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J. Basic Microbiol. 2016, 56, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ascacio-Valdés, J.A.; Buenrostro, J.J.; De la Cruz, R.; Sepúlveda, L.; Aguilera, A.F.; Prado, A.; Contreras, J.C.; Rodríguez, R.; Aguilar, C.N. Fungal biodegradation of pomegranate ellagitannins. J. Basic Microbiol. 2014, 54, 28–34. [Google Scholar] [CrossRef]
- Shi, B.; He, Q.; Yao, K.; Huang, W.; Li, Q. Production of ellagic acid from degradation of valonea tannins by Aspergillus niger and Candida utilis. J. Chem. Technol. Biotechnol. 2005, 80, 1154–1159. [Google Scholar] [CrossRef]
- Huang, W.; Niu, H.; Li, Z.; Lin, W.; Gong, G.; Wang, W. Effect of ellagitannin acyl hydrolase, xylanase and cellulase on ellagic acid production from cups extract of valonia acorns. Process. Biochem. 2007, 42, 1291–1295. [Google Scholar] [CrossRef]
- Vattem, D.A.; Shetty, K. Ellagic acid production and phenolic antioxidant activity in cranberry pomace (Vaccinium macrocarpon) mediated by Lentinus edodes using a solid-state system. Process. Biochem. 2003, 39, 367–379. [Google Scholar] [CrossRef]
- Sharma, S.; Bhat, T.K.; Dawra, R.K. A spectrophotometric method for assay of tannase using rhodanine. Anal. Biochem. 2000, 279, 85–89. [Google Scholar] [CrossRef]
- Huang, W.; Niu, H.; Li, Z.; He, Y.; Gong, W.; Gong, G. Optimization of ellagic acid production from ellagitannins by co-culture and correlation between its yield and activities of relevant enzymes. Bioresour. Technol. 2008, 99, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Ascacio-Valdés, J.A.; Gironés-Vilaplana, A.; Del Rio, D.; Moreno, D.A.; García-Viguera, C. Assessment of pomegranate wine lees as a valuable source for the recovery of (poly)phenolic compounds. Food Chem. 2014, 145, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ambigaipalan, P.; De Camargo, A.C.; Shahidi, F. Phenolic Compounds of Pomegranate Byproducts (Outer Skin, Mesocarp, Divider Membrane) and Their Antioxidant Activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef]
- Gosset-Erard, C.; Zhao, M.; Lordel-Madeleine, S.; Ennahar, S. Identification of punicalagin as the bioactive compound behind the antimicrobial activity of pomegranate (Punica granatum L.) peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Carbo, A.F.; Augur, C.; Prado-Barragan, L.A.; Aguilar, C.N.; Favela-Torres, E. Extraction and analysis of ellagic acid from novel complex sources. Chem. Pap. 2008, 62, 440–444. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).