Abstract
Heavy metal contamination in wastewater is a significant concern for human health and the environment, prompting increased efforts to develop efficient and sustainable removal methods. Despite significant efforts in the last few decades, further research initiatives remain vital to comprehensively address the long-term performance and practical scalability of various adsorption methods and adsorbents for heavy metal remediation. This article aims to provide an overview of the mechanisms, kinetics, and applications of diverse adsorbents in remediating heavy metal-contaminated effluents. Physical and chemical processes, including ion exchange, complexation, electrostatic attraction, and surface precipitation, play essential roles in heavy metal adsorption. The kinetics of adsorption, influenced by factors such as contact time, temperature, and concentration, directly impact the rate and effectiveness of metal removal. This review presents an exhaustive analysis of the various adsorbents, categorized as activated carbon, biological adsorbents, agricultural waste-based materials, and nanomaterials, which possess distinct advantages and disadvantages that are linked to their surface area, porosity, surface chemistry, and metal ion concentration. To overcome challenges posed by heavy metal contamination, additional research is necessary to optimize adsorbent performance, explore novel materials, and devise cost-effective and sustainable solutions. This comprehensive overview of adsorption mechanisms, kinetics, and diverse adsorbents lays the foundation for further research and innovation in designing optimized adsorption systems and discovering new materials for sustainable heavy metal remediation in wastewater.
1. Introduction
The discharge of toxic heavy metals into the environment poses a significant threat to the quality of water and aquatic ecosystems, endangering human health [1,2]. Trace elements such as arsenic (As), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), lead (Pb), mercury (Hg), nickel (Ni), and zinc (Zn) are recognized as metallic pollutants in wastewater, industrial effluent, and sewage sludge [3,4,5,6]. In surficial environments, the most inorganic stable forms of As and Cr occur as inorganic oxyanions but are frequently referred to as cationic species, e.g., As3+, As5+, Cr3+, and Cr6+ [7,8,9,10]. In general, complete or partial toxic heavy metals removal from wastewater and polluted water is essential to prevent potential health and environmental problems and ensure ecosystem sustainability [11,12]. The World Health Organization has established maximum contaminant or permissible limits for As, Cu, Pb, Cd, Cr, Co, Hg, Ni, and Zn in drinking water at 0.01, 2.5, 0.05, 0.003, 0.05, 0.1, 0.001, 2.0, and 5.0 mg/L, respectively [13]. Traditional methods for removing metal ions from effluents, such as chemical precipitation, lime coagulation, ion exchange, reverse osmosis, and solvent extraction, have limitations, such as insufficient metal removal, high reagent and energy requirements, and the production of noxious sludge or waste products that require proper disposal [13]. Therefore, it is necessary to devise efficient and environmentally friendly methods for reducing heavy metal content.
Among various methods for removing metal ions, adsorption is regarded as the most promising due to its simplicity of use, high removal efficiency across a wide pH range, and low cost [14]. However, the production of suitable adsorbent materials can be costly, and certain materials, such as commercial activated carbons, cannot be regenerated after use, rendering large-scale applications unsustainable [15]. In order to promote sustainable treatment methods, it is crucial to develop and implement readily available, inexpensive, and renewable adsorbents [16]. The conversion of agricultural waste and residues into value-added sorbents aligns with the circular bioeconomy and green chemistry principles, providing a renewable and environmentally beneficial approach [17]. In this context, the investigation of agricultural, biological, and industrial byproducts as potential metal adsorbents has been motivated by the search for inexpensive and readily available sorbents.
Bioadsorbents have several advantages over traditional techniques. These inexpensive biofilter materials have a high affinity and capacity for metal ions, and they are readily available. Some bioadsorbents exhibit a broad spectrum of metal ion-binding capabilities, whereas others are selective towards particular metal ion types [18]. The use of biological organisms as adsorbents is limited by their intolerance for low pH or high concentrations of toxic metal ions [19]. Plant fibers, in contrast, are chemically and physically more robust, making them appropriate for sorption applications [20]. Metal ions can be effectively adsorbed by plant fibers, which are predominantly made up of cellulose, hemicelluloses, lignin, pectin, and other plant extracts. Metal ions are bonded predominantly to chemical functional groups, such as carboxylic (predominant in hemicelluloses, pectin, and lignin), phenolic (lignin and extractives), hydroxylic (cellulose, hemicelluloses, lignin, and pectin), and carbonyl (lignin and extractives) groups. Through complexation and ion exchange, hydroxyl, carboxyl, and phenolic groups frequently form strong bonds with metal ions [21,22].
Though there are several prospects of bioadsorbents and nanomaterials, such as environmental sustainability, biodegradability, eco-friendliness, high adsorption capacity, etc., issues such as regeneration and reusability, selectivity and specificity, scale-up and practical applications in real-world scenarios, competitive adsorption, and long-term stability and durability still need to be addressed. Moreover, understanding the adsorption process and mechanism, evaluating adsorption kinetics and thermodynamics, designing effective adsorbents, and assessing adsorption capacity are also important in biosorption studies.
This study summarizes the mechanisms, kinetics, and applications of various adsorbents for the removal of heavy metals from an effluent. Bioadsorbents, which include both non-living detritus and living plants and microorganisms, are also studied as alternatives to conventional practices. A schematic representation of this article is presented in Figure 1.
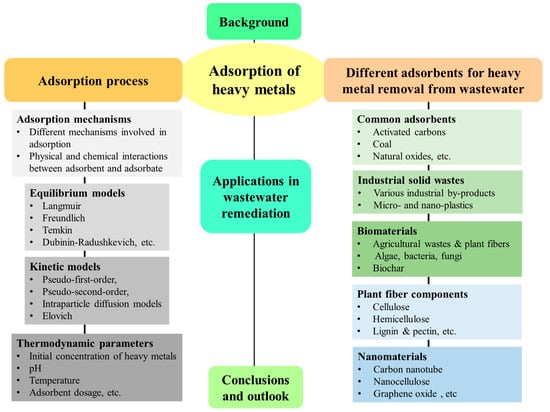
Figure 1.
A schematic representation of the current review.
2. Adsorption Processes of Heavy Metals
2.1. Adsorption Mechanisms
The adsorption process forms a layer of adsorbate (metal ions) on the surface of adsorbents. Adsorption can be reproduced for multiple applications via a desorption method (reverse adsorption in which adsorbate ions are transported from the adsorbent surface) because adsorption is a reversible process in certain circumstances [23]. Adsorption onto a solid adsorbent includes three major steps: transportation of the pollutant to the adsorbent surface from aqueous solution, adsorption onto the solid surface, and transport within the adsorbent particle. Generally, electrostatic attraction causes charged pollutants to adsorb on differently charged adsorbents because heavy metals have a vigorous affinity for hydroxyl (OH−) or other functional group surfaces [24].
Adsorption is mainly classified into two types: physical adsorption and chemisorption (described as activated adsorption as well). Physical adsorption is the adhesion of an adsorbent to the surface of an adsorbate because of the nonspecific (i.e., independent of the nature of the material) van der Waals force, whereas chemisorption occurs while chemical bonding creates strong attractive forces, i.e., chemical adsorption constructs ionic or covalent bonds through chemical reactions. Nevertheless, physical adsorption is a reversible process but less specific, whereas chemisorption is irreversible but more specific [25]. When adsorption occurs over biological systems, the process is referred to as biosorption. Biosorption is a process that combines metal removal and recovery. Biosorption is effective due to the adsorbents’ low cost and ease of regeneration. Bacteria, fungi, algae, industrial waste, agricultural waste, natural residues, and other biological materials have all been widely used to adsorb heavy metals from wastewater [26]. Physical adsorption, chemisorption, electrostatic interactions, simple diffusion, intra-particle diffusion, hydrogen bonding, redox interactions, complexation, ion exchange, precipitation, and pore adsorption are all possible mechanisms to adsorb heavy metal ions onto bioadsorbents [27,28]. Figure 2 illustrates several possible mechanisms of metal adsorption onto the biosorbents.
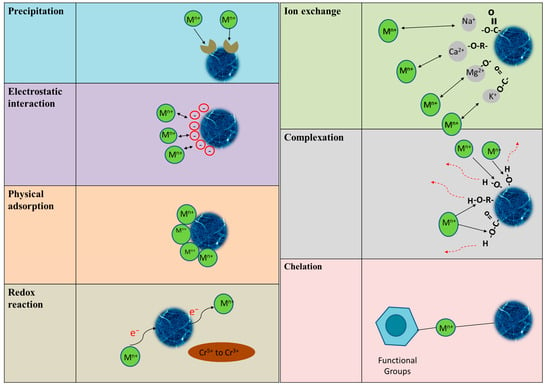
Figure 2.
Different mechanisms of cationic heavy metals adsorption by several types of biosorbents. Adapted from [29,30].
Biowaste materials contain a variety of functional groups, the majority of which are negatively charged, such as hydroxyl, carboxyl, carbonyl, and amino groups. Bioadsorbents, such as plant fibers or other biomass-based materials, often have a porous structure with various cavities and surface sites where metal ions can bind. The presence of these pores and cavities increases the surface area available for adsorption, providing more opportunities for metal ions to interact with and be retained by the biosorbent [31]. As a result, the porous nature of bioadsorbents contributes to their high metal ion adsorption capacity and efficiency in wastewater treatment applications. Adsorption of contaminants from effluents is a process that involves the diffusion of pollutant molecules and their electrostatic attractions to the surface. The adsorption of potentially toxic elements onto wood biochar, for example, can be described by a variety of mechanisms [32]. Possibly, the electrostatic attractions between positively charged metal ions and negatively charged functional groups of bioadsorbents successfully promote the adsorption capacity. Additionally, attractive forces such as hydrophobic interactions, van der Waal forces, and hydrogen bonding could be involved in the process of metal adsorption on the surface of biosorbents [33]. Additionally, complexation and chelation are other known mechanisms in the adsorption process. Generally, complexation is a process that occurs when multiple species combine, whereas chelation is a specific case of complexation that results in the development of rings [34]. A metal surrounded by ligands takes the central position in the complexation process and forms mononuclear complexes. Polynuclear complexes are created when two or more metals are bound together by ligands in the central position. Likewise, polydentate ligands could be used in the chelation to aid a stable structure formation via multiple bonding [31].
2.2. Equilibrium Models
Adsorption at a given temperature can be quantified using mathematical equations in the form of an adsorption isotherm that relates the amount of adsorbate retained by the adsorbent (qe) to the concentration in solution at equilibrium (Ce) (Table 1). The two empirical models that are most frequently used for describing the heavy metal adsorption process at a certain temperature and on different bioadsorbent materials are the Freundlich and Langmuir isotherms [35,36,37]. In addition, Temkin, Dubinin–Radushkevich, Redlich–Peterson, Koble–Corrigan, and Toth isotherms are used to describe how toxic pollutants interact with adsorbent materials [38,39,40,41]. Adsorption isotherms play a vital role in interpreting the mechanism of metal ion adsorption onto different adsorbents [42]. These models shed light on the surface properties of adsorbents and the intermolecular interactions between adsorbed molecules and the adsorbent matrix [43]. Isotherm and kinetic models contribute to understanding the adsorption process, relying on various factors, including the adsorbent’s structure and the physical and chemical characteristics of the solute [42]. The Langmuir model finds application in solid–liquid systems, elucidating that all sites on the surface of the adsorbent have equal opportunities to be occupied by heavy metals. On the contrary, the Freundlich model characterizes a non-ideal process occurring on heterogeneous surfaces, often involving multilayer formation [44].

Table 1.
Commonly used isotherm models for heavy metal adsorption.
In a study on Pb2+ adsorption from aqueous solutions by raw and activated charcoals of Melocanna baccifera Roxburgh (bamboo), Lalhruaitluanga et al. utilized a combined Langmuir–Freundlich equation (L–F) to describe the equilibrium relationships between sorbent and Pb2+ ions in solution [66]. Reddy, Seshaiah, Reddy, Rao, and Wang evaluated the experimental equilibrium adsorption data using four widely employed two-parameter equations: the Langmuir, Freundlich, Dubinin–Radushkevich (D–R), and Temkin isotherms [38]. The findings indicated that the Freundlich model provided the best fit for the Pb2+ adsorption data on Moringa oleifera bark. Chen, Li, Li, Chen, Chen, Yang, Zhang, and Liu [36] observed that the chitosan fibers had better adsorption of Cu2+ ions than Cr4+. This might be due to the amino groups in chitosan fibers, which may have good chelation with Cu2+ ions. The Cu2+ ion adsorption process followed the quasi-second-order kinetic equation, and was compatible with the Langmuir isotherm.
2.3. Kinetic Models
Kinetic adsorption models describe the mechanism of adsorption of heavy metal ions by biosorbents and particularly determine the rate of biosorption during the removal of heavy metals from wastewater on an industrial scale to optimize the design parameters, including the adsorbate residence time and reactor dimension [67]. In experimental works, the kinetic description of adsorption processes and the removal efficiency of heavy metals by various adsorbents have frequently been evaluated by several mathematical models [68,69,70], most of which are empirical equations [71,72,73]. The common kinetics models (pseudo-first order, pseudo-second order, intra-particle diffusion kinetic, and Elovich), which were used to fit experimental data, are expressed in Table 2. The choice between these equations is frequently based on the goodness-of-fit, as judged by the coefficient of determination (R2) [73,74]. The use and misuse of adsorption kinetic data and linear forms of pseudo-first-order and pseudo-second-order equations were discussed in [75,76]. Recently, Bullen et al. revised the pseudo-order models and proposed the following equation (Equation (1)), called the ‘revised rate equation’ (rPSO) [77]:
where k′ is the revised pseudo-order rate constant (k’ = k2q2/C0), C0 is the initial adsorbate concentration in the solution, and Ct is the adsorbate concentration at time t. They stipulated that this rPSO provides the first-order and zero-order dependencies upon C0 and Ct (amount of adsorbate adsorbed onto adsorbent).

Table 2.
The kinetic models used for heavy metal adsorption.
Wattanakornsiri et al. investigated the removal of Pb2+ and Cd2+ ions from waste aqueous solutions using naturally modified biosorbents derived from three local fruit peels: dragon fruit peel, rambutan peel, and passion fruit peel [88]. The study found that adsorption followed the pseudo-second-order kinetic model, and the adsorption data fit well with both the Freundlich and Langmuir isotherm models. However, the Langmuir model demonstrated the best fit.
2.4. Thermodynamic Parameters
A thermodynamic study offers insights into the minimum kinetic energy necessary for the adsorbate to become bound to the adsorption site [89]. The nature of the adsorption process (spontaneity, randomness, endothermicity, or exothermicity) can be evaluated by estimating thermodynamic parameters [72,90] such as the Gibbs free energy change (ΔG°, kJ/mol), the standard enthalpy change (ΔH°, kJ/mol), and the standard entropy change (ΔS°, J/mol K−1) [91]. These parameters can be calculated by the following equations [72,78,92,93] stated as (Equations (2)–(5)):
where R represents the universal (ideal) gas constant (8.314 J/mol K), T is the temperature in Kelvin (K); Kc is the apparent equilibrium adsorption [72] or the Langmuir isothermal constant [92]; Cad,e and Ce are the concentration (mg/L) of heavy metal in adsorbent and in solution, respectively [78]. ∆H0 and ∆S0 values can be calculated at different temperatures, assuming these parameters to be independent of temperature [90]. A negative or positive value ΔG° at a known temperature confirms the spontaneity (or non-spontaneity) of the adsorption process, a positive value of ΔH° suggests the endothermic nature of the adsorption of a pollutant by the sorbent, and a positive value of ΔS° illustrates an increase in the randomness of the adsorption process [92,93].
The thermodynamic properties associated with the removal of metal ions exhibit variation due to the adsorbent’s composition, structure, and surface characteristics, leading to differing affinities for metal ion removal. Enhanced surface area and a porous structure can promote interactions and subsequent adsorption [91]. Factors like the presence of competing ions and shifts in pH can modify the charge distribution on both the metal ion and adsorbent surfaces, thereby influencing thermodynamic equilibrium. Additionally, distinct metal ions possess varying thermodynamic affinities due to their unique electronic configurations and charge densities [22,94].
3. Different Adsorbents for Heavy Metal Removal from Wastewater
Numerous adsorbents were used in their native or modified forms to remove metallic trace elements (e.g., heavy metals and metalloids) from wastewater (Table 3). Activated carbons (AC), zeolites, clay minerals, nanosized metal oxides, animal-based wastes, agricultural and food wastes, industrial waste materials, and various advanced adsorbents have been tested for multi-metal removal from wastewaters and aqueous solutions [23].

Table 3.
Commonly used adsorbents and their performance.
3.1. Industrial Solid Wastes
Industrial waste can encompass a wide range of materials, including leftover raw materials, production residues, processed by-products, and pollutants generated during manufacturing, processing, or other industrial activities. Various industrial solid wastes showed a significant capacity for adsorption, which could be used to remove metal ions from wastewater. Due to their by-product status, they are readily available and particularly cost-effective. Traditionally, the byproducts like fly ash [109,110], blast furnace sludge [111], waste slurry [112], lignin [113], Fe(OH)3 [114], and red mud [115] have been utilized as effective adsorbents due to their technological viability to remove heavy metal ions from polluted water [116]. Among the industrial by-products, red mud is considered inexpensive and readily available, as well as highly efficient in metal adsorption. However, the difficulty of disposing of wastewater that is generated during its activation prior to application and its recovery after application has limited its practicability [117]. Several other industrial wastes, including sawdust [118], areca waste [119], tea factory waste [120], battery industry waste [121], waste biogas residual slurry [122], sea nodule residue [123], and grape stalk waste [124] have been used as low-cost adsorbents to remove heavy metals from contaminated water.
Recently, micro- and nano-plastics such as polyamide (nylon), polyester, polypropylene, polyethylene, and polyvinyl chloride were reported to have the ability of heavy metal adsorption. Polypropylene, among the other polymers, showed a higher capacity for adsorption [125]. Godoy et al. studied the adsorption of several heavy metals (Cd, Co, Cu, Cr, Ni, Pb, and Zn) on different types of microplastics [126]. They found that the polyethylene and polyvinyl chloride showed a higher ability to adsorb Pb, Cr, and Zn, while the low adsorption capacity was related to polyethylene terephthalate. Fu et al. described that microplastics have the ability to adsorb heavy metals, and the adsorption process can be influenced by many factors, such as particle size, type of microplastics, and the type and concentration of metal ions [127]. Zon et al. studied the adsorption of Cr by polyethylene micro-beads in seawater [128]. The process was influenced by surface area, reactivity of metals, particle size, and pH. Dong et al. reported the adsorption mechanism of As3+ by polystyrene microplastic particles [129]. They described that electrostatic forces and non-covalent interactions might be the key mechanisms for As3+ adsorption.
3.2. Biomaterials as Metal Biosorbents
Biosorbents, where the biological matrix acts as an active binding site [130,131], are typically obtained from three sources: (i) non-living biomass such as bark, crab shells, shrimp shells, fish scales, krill, lignin, and squid, (ii) algal biomass including micro- and macro-algae, and algal-derived biochar [41], and (iii) living or dead microbial biomass like bacteria, fungi, and yeast [23,132].
Numerous cheap and non-living plant-based materials [133], including potato peels [134], seed shells [135], coffee husks [136], crude olive stones [137], apple peel bead [138], citrus peels [139], shells of hazelnut and almond [140], chemically modified orange peel [141], banana peels and chemically modified banana peels [142], peels from banana, orange, and potato immobilized on sodium alginate beads [143], physic seed hull [144], rice husk [145], millet and Sorghum vulgare (Guinea corn) husks [146], rice and corn husk biochar [147], peanut husk [35], coconut husk [148], palm fruit fiber [149], neem bark [150], sugarcane bagasse [151], Rosa damascena leaf powder [152], and ajwa date pits [92], watermelon rind [130], etc., have been extensively investigated as prospective heavy metal adsorbents (Table 4).

Table 4.
Commonly used agricultural wastes for heavy metal removal from wastewater.
In a study, Strychnos potatorum seeds with chemically modified surfaces (SMSP) were examined for their ability to remove Pb2+ ions from aqueous solutions [164]. The experimental adsorption isotherm data were analyzed, and it was determined that the Freundlich adsorption isotherm model provided a better fit. SMSP exhibited a maximal adsorption capacity of 166.67 mg/g for Pb2+ ions under optimal conditions comprising a pH of 5.0, a contact time of 30 min, a dosage of 2 g/L, and a temperature of 30 °C. In addition, the adsorption kinetics of SMSP for the removal of Pb2+ ions was consistent with the pseudo-second-order kinetic model. In a separate study, Shukla et al. assessed the ability of coir, a low-cost lignocellulosic fiber, to remove heavy metal ions such as Ni2+, Zn2+, and Fe2+ from aqueous solutions [165]. Langmuir-type adsorption was accomplished using coir fibers. The modified coir fibers (oxidized with hydrogen peroxide) adsorbed 4.33, 7.88, and 7.49 mg/g of Ni2+, Zn2+, and Fe2+, respectively, whereas the unmodified coir fibers adsorbed 2.51, 1.83, and 2.84 mg/g [165].
Algae, a renewable biomass that grows universally and amply in the world’s littoral zones, have piqued the interest of numerous researchers as potential new adsorbents for metal ion removal. Several advantages of algae involve their widespread accessibility, low cost, and relatively consistent features [23]. Bioadsorption of Cu2+ and Zn2+ using dried marine green macroalgae, Chaetomorpha linum [166]; Cu2+, Zn2+, Cd2+, and Pb2+ by Caulerpa lentillifera [167] are examples demonstrating the effectiveness of algae as heavy metal adsorbents (Table 5). Nowadays, metal ion removal by microorganisms has been considered extremely efficient. For instance, Bacillus cereus [168], Escherichia coli [169], Pseudomonas aeruginosa [170], etc., have all been studied for their ability to bind heavy metals in aqueous solutions. Additionally, several species of bacteria, including Bacillus sp., Micrococcus luteus, Pseudomonas cepacia, Bacillus subtilis, and Streptomyces coelicolor have been successfully employed to remove Cu2+, Zn2+, Cd2+, and Ni2+ from an effluent [171,172,173].

Table 5.
Biosorption of heavy metal ions using microorganisms.
Fungi are another frequently used biosorbent, which is easy to grow to produce a high yield of biomass in a short time and can be simply manipulated genetically and morphologically [190,191]. Among the fungi used as biosorbents, Aspergillus niger [192], Saccharomyces cerevisiae [193], Lentinus edodes [193], etc., are the most common species. Bhainsa and D’souza [194] studied Cu2+ ion removal using Rhizopus oryzae biomass modified with NaOH and achieved a maximum adsorption of 43.7 mg Cu2+/g. Although fungal biosorbents have a wide range of sources, are inexpensive, and exhibit rapid adsorption, their separation following the process can be difficult [23]. Yeast is also used as an adsorbent, which is a fungus larger than bacteria. Like other eukaryotic organisms, it contains a nucleus and related cytoplasmic organelles [190,195]. For living cells, the cytoplasm is critical because it interacts with metal ions and separates into compartments to remove them once they enter the cells [23]. Han et al. described that waste beer yeast from the brewing industry could be a promising adsorbent to remove Cu2+ (1.45 mg/g) within 30 min [196].
Apart from the aforementioned biosorbents, biochar prepared from lignocellulosic materials and microalgae can prevent pollutants from reaching organisms via soil or water and reduce bioavailability through adsorption because of its graphene-like carbon matrix, large surface area, high porosity, and increased cation and anion exchange capacity [24]. Biochar has been widely used in anaerobic digestion and in wastewater treatment processes for eliminating pathogens, trace metals, and suspended matter [197]. The adsorption mechanism of biochar depends on the chemical properties of the biochar surface and the nature of pollutants. Generally, three major types of adsorption for biochar are: (i) physical passage, in which pollutants settle on the adsorbent surface; (ii) pore filling, in which adsorbate condenses into the pores of biochar; and (iii) precipitation, in which adsorbate forms layers on the adsorbent surfaces [198]. In fact, the dissociation of O2-containing functional groups creates a negatively charged biochar surface, which facilitates electrostatic attraction between cations and biochar. Removal of Cu2+ from aqueous media was studied using rice straw biochar, which indicated that the ion exchange of native cations with Cu2+ might be the dominant mechanism for heavy metal adsorption [199]. Microalgae-derived biochar possesses an irregular porosity of 1 mm, which enables it to act as an adsorbent; however, it has a lower cation exchange capacity (CEC) compared to lignocellulose-derived biochar [200]. Van Hien, Valsami-Jones, Vinh, Phu, Tam, and Lynch applied biochar from biomass residue for remediating Zn-contaminated water and observed that the biomass dose, contact time, and metal concentration had a great effect on heavy metal uptake [59].
3.3. Activated Carbon
In recent times, the wastewater treatment industry has shown significant interest in activated carbon (AC) due to its remarkably high adsorption capacity for heavy metals. AC’s porous structure, small particle size, high surface area, active free valences, appropriate surface functional groups, and affinity for adsorbing various substances make it a valuable resource with significant adsorption potential in diverse applications [201]. AC is derived from various naturally produced waste materials, including rubber wood sawdust [202], rice husk [203], coconut shell [204], hazelnut shell [205], palm shell [206], apricot stone [207], eucalyptus bark [208], soybean hulls [209], bamboo [210], etc., and has been investigated to extract metal ions from wastewater. Additionally, AC that is altered with alginate [211], tannic acid [212], magnesium nitrate [213], and surfactants [214] might be a viable alternative to remove heavy metals from wastewaters and aqueous solutions.
The mechanism of heavy metal adsorption by AC involves a combination of multiple mechanisms, including physical adsorption, electrostatic adsorption, ion exchange, reduction, complexation, and precipitation [215]. Ongoing advancements have focused on augmenting the adsorption efficacy of AC through modifications employing physical, chemical, organic, and inorganic loading techniques. Physical modification techniques, including microwave heating, ultrasound irradiation, steam activation, and non-thermal plasma technology, have been explored to enhance active sites [215]. Furthermore, chemical modification methods involving acidic and alkaline treatments have also been used [215,216]. While AC is primarily utilized to eliminate unpleasant color, odor, taste, and other organic impurities from water/wastewater, the adsorption capacity of AC is limited by several factors, including loss of adsorption efficacy upon regeneration, the need for regeneration following exhaustion, and the possibility of secondary contamination due to pollutants being separated from the AC but not eliminated [24]. Therefore, research and the use of alternative adsorbents are imperative.
3.4. Plant Fiber Components
The use of plant fiber-based food wastes, particularly agro-waste materials, as biosorbents for the removal of heavy metals from aqueous solutions is gaining momentum. Numerous studies have examined the potential use of plant fiber components such as hemicelluloses, cellulose, pectin, and lignin in heavy metal adsorption from an effluent (Table 6). Cellulose is the most prevalent organic compound on Earth, and its high surface area, hydroxyl groups, and porous structure allow for efficient adsorption of heavy metals [217]. Heavy metal removal has been studied using cellulose-based materials such as cellulose nanofibers, cellulose derivatives, and cellulose-based composites. Through surface complexation, ion exchange, and electrostatic interactions, they can absorb heavy metals [218].

Table 6.
Plant fiber components used for removing heavy metals from wastewater.
Several agro-waste materials (agave bagasse, sorghum straw, oats straw) and their fractions to identify functional groups with hydroxyl, carboxyl, and nitrogen-containing compounds were observed [230,231]. They observed that the lignin exhibited a higher contribution than hemicelluloses regarding the adsorption capacity of Cr3+ in sorghum straw and oats straw. In the case of agave bagasse, lignin was found to be the primary fraction responsible for Cr3+ adsorption. In their study, the primary contributors to Cr3+ removal from an aqueous solution were identified as hemicelluloses and lignin, whereas cellulose present in the studied agro-waste adsorbents did not appear to play a significant role in this process, although cellulose constitutes the largest proportion (greater than 46%) of the agro-waste materials compared to hemicelluloses (12–26%), lignin (3–10%), and other compounds (22–30%). In contrast, Pejic et al. investigated the sorption capacity of waste short hemp fibers for Pb2+, Cd2+, and Zn2+ ions in aqueous mediums [232]. They demonstrated that by gradually reducing the amount of lignin or hemicelluloses in hemp fibers via chemical treatment, the sorption characteristics of hemp fibers improved. Short hemp fibers can sorb metal ions (Pb2+, Cd2+, and Zn2+) from both individual and combined metal solutions. The maximum total adsorption capacities for Pb2+, Cd2+, and Zn2+ ions were the same in single solutions, which was 0.078 mmol/g. However, in ternary mixtures, their adsorption capabilities differed, with values of 0.074 mmol/g for Pb2+ and 0.035 mmol/g for both Cd2+ and Zn2+ [232]. Hu et al. reported that the amount of metal ions attached to rice bran fibers varies [233]. The maximum metal ion (Cd2+, Cu2+, and Pb2+) binding capacity was demonstrated by soluble hemicellulose: 76.3 mg/g for Pb, 68.5 mg/g for Cu, and 59.1 mg/g for Cd. In contrast, insoluble fiber removed 32.5 mg/g of Pb, 10.6 mg/g of Cu, and 18.3 mg/g of Cd. Rice bran cellulose exhibited low binding capacities: 20.5 mg/g for Pb, 13.6 mg/g for Cu, and 9.9 mg/g for Cd. Al-Ghouti et al. discovered that raw date pits (RDP) could be used as a solid adsorbent to remove copper ions, cadmium ions, and methylene blue (MB) [234]. They discovered two methods for MB adsorption: hydrogen bonding and electrostatic attraction. They observed that the most common method for Cd2+ to bind in the cellulose/lignin unit was via two OH- groups. In another study, the ability of spruce, coconut coir, sugarcane bagasse, kenaf bast, kenaf core, and cotton to remove Cu2+, Ni2+, and Zn2+ ions from aqueous solutions was investigated [235]. The purpose of the study was to determine the relationship between the lignin content of these substances and their capacity to absorb these metal ions. Kartel et al. observed that beet pectin had a high affinity for Pb2+ and Cu2+, citrus pectin had a high affinity for Ni2+, and apple pectin had a high affinity for Co2+ [236]. This could be due to the substantial structural differences between pectin sources. According to Khotimchenko et al., pectin with an esterification level close to “0” binds the most Zn2+. The degree of methylation, which varies between 1 and 60%, is the primary factor influencing heavy metal adsorption [237].
It has been well-established that plant fiber components exhibit effective heavy metal removal from wastewater. The adsorption capacities are influenced by various factors, such as contact time, pH, concentration of heavy metals, adsorbent dosage, and temperature [13,22,238]. To better understand the adsorption behavior of these components, models of adsorption isotherms, kinetics, and thermodynamics have been utilized. Furthermore, numerous modification techniques, including chemical modification, surface functionalization, and composite formation, have been investigated to enhance the adsorption efficiency of these plant fiber materials.
3.5. Nanomaterials
Conventional adsorbents, some of which are depicted above, typically have an insufficient capacity for metal adsorption and thus cannot efficiently remove the majority of heavy metals in wastewater treatment [23]. In this concern, researchers investigated how to develop novel adsorbents with enhanced properties and functionalities. These days, there is a high demand for highly porous nanostructures such as graphene [239], fullerene [240], nanosized metal oxides and MXene [24], graphitic carbon nitride (g-C3N4) and metal-organic frameworks [23], halloysite particles [216] and especially, carbon nanotubes [241] have been reported as substitute adsorbents to remove metal ions from wastewater considering the advantages of nanotechnology (Table 7). They are usually strong, resistant, electrically conductive, non-corrosive, and thermally stable. Additionally, high surface areas and large pore volumes of these nanomaterials, when associated with different types of intermolecular interactions, enable effective adsorption in a variety of systems [24]. By and large, these nanomaterials outperform conventional adsorbents such as titanium dioxide, activated carbon, and iron oxide. Nevertheless, novel materials also have shortcomings, and therefore, scientific and technological research must address issues of durability and functionalities, which are critical in environmental applications [24].

Table 7.
Some commonly used nano-adsorbents for heavy metal removal.
Nanosheets composed of two-dimensional nanomaterials, comprised of Ca2+ (Ca) and Y3+ (Y) cations along with carbonate [CO32−] anions, referred to as CaY–CO32− layered double-hydroxide (LDH) materials, exhibit exceptional affinity and selectivity for toxic transition metal ions such as Cr3+, Ni2+, Cu2+, Zn2+, Pb2+, Cd2+, as well as metalloid As3+ [10]. Furthermore, there is an emerging focus on the use of nanoparticles derived from food wastes, particularly nano-cellulose, as potential adsorbents for heavy metal removal [253,254]. A review by Fayaz et al. explored the utilization of nano-cellulose obtained from food waste through various treatment processes [255]. When subjected to specific treatments, nano-cellulose can be modified to enhance its adsorption capacity for metal ions in wastewater treatment [256]. These modified nano-cellulose materials show promise as they possess inherent advantages like renewability, biodegradability, and low cost, given their origin from food waste. However, to fully harness their potential, further research and development efforts are required to optimize their durability and functionalities in environmental applications. The exploration of nano-cellulose and its derivatives from food waste as adsorbents represents a sustainable and innovative approach toward mitigating heavy metal pollution in water resources.
4. Conclusions and Outlook
The use of various non-toxic adsorbents has proven to be a successful approach for the simultaneous removal of multiple heavy metals, metalloids, and other pollutants from polluted water and wastewater. This method is highly effective, practical, economical, and environmentally friendly [39,257]. To achieve optimal results, mathematical tools [258], such as machine learning algorithms [259], can be employed to assess and optimize the adsorption processes’ isotherms, thermodynamics, kinetics, and operational parameters. In this regard, low-cost and locally available agro-biowastes have emerged as promising candidates for heavy metal and metalloid removal on an industrial scale. These biowastes, such as plant fibers, fruit and vegetable peels, and byproducts from food processing industries, possess large multi-chemical functional groups, surface area, cation-exchange capacity, and controllable pore structures [30,88]. Their physical preparation as adsorbents adheres to the principles of green chemistry. Additionally, these bioadsorbents can be reused for multiple cycles, further enhancing their cost-effectiveness and sustainability [39,260,261]. Despite these advancements, further research is necessary to optimize the performance of adsorbents, explore new materials, gain deeper insights into the underlying mechanisms, and develop more cost-effective and sustainable methods for heavy metal removal from wastewater. An outline diagram for the challenges and potential opportunities associated with the present topic is presented in Figure 3 and described as follows:
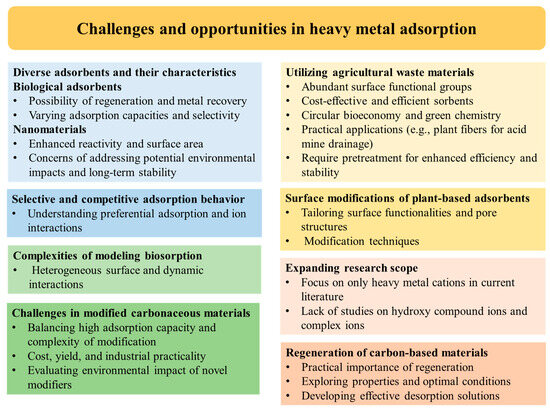
Figure 3.
Outline diagram for the challenges and potential opportunities of heavy metal removal from wastewater.
- Biological adsorbents, including microbial biomass, algae, and fungi, utilize living or nonliving biomass to bind heavy metals. These bioadsorbents are eco-friendly, easily accessible, and can be regenerated through biomass regeneration or metal recovery procedures. However, their adsorption capacities and selectivity may vary depending on the biomass source and pretreatment techniques. In contrast, nanomaterials, such as nanoparticles and nanocomposites, offer distinct advantages for heavy metal adsorption due to their small dimension, large surface area, and enhanced reactivity. Through magnetic or functionalized modifications, their recyclability and reusability can be improved. Nevertheless, concerns about potential environmental impacts and long-term stability must be addressed;
- Inexpensive adsorbents like agricultural waste materials, such as vegetable and fruit peels, have gained popularity due to their abundant surface functional groups, leading to high metal adsorption capacities. These readily accessible plant-based materials, including plant fibers and other detritus, offer cost-effective and efficient sorbents for metal ions. Utilizing plant-based byproducts as metal sorbents aligns with circular bioeconomy and green chemistry principles, offering economically viable and eco-friendly solutions. However, their application may require pretreatment to enhance adsorption efficiency and stability. A practical application involves using plant fibers as low-cost biowaste for adsorbing heavy metals from polluted water or acid mine drainage generated by mining industries. In this method, fibers can act as adsorbents in a bioreactor, attracting and retaining heavy metal ions (Figure 4);
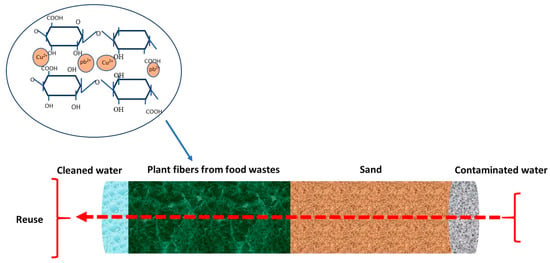 Figure 4. Biofilter for removing heavy metals from contaminated water.
Figure 4. Biofilter for removing heavy metals from contaminated water. - Current research in the field of heavy metal adsorption and removal from water primarily focuses on the adsorption of heavy metal cations. However, there is a noticeable lack of specific studies on the adsorption of hydroxy compound ions or other complex ions. In most documents, the adsorption of complex ions is only briefly mentioned or addressed in a limited manner;
- Modeling the biosorption process is challenging due to the diverse physical and chemical processes involved. The nature of active sites in bioadsorbents varies significantly based on their source, making characterization difficult. While successful in validating experimental data for single-component biosorption, real-life scenarios often involve multiple components adsorbing simultaneously on a heterogeneous biosorbent surface. This complexity leads to dynamic interactions between metal ions and functional groups, making the modeling of such systems more intricate;
- Plant-based adsorbents require surface modifications to enhance their surface functionalities and develop suitable pores for effective adsorption. Surface oxidation, sulfonation, amination, and adjustments to pore structures are crucial processes in achieving improved adsorption performance. Various modification procedures, such as chemical, mechanical, thermal, gasification, and combinations of these techniques, are available to tailor the adsorbent properties according to specific contaminant removal needs. By exploring and optimizing these modification methods, plant-based adsorbents can be fine-tuned to be efficient and versatile tools for water and wastewater treatment, contributing to sustainable and eco-friendly solutions;
- Modified carbonaceous materials have shown great promise in achieving high adsorption capacity and efficient removal of heavy metals. However, the process of modification, especially through chemical means, can be quite intricate. This complexity, along with considerations of cost, yield, and operational practicality, poses challenges for their application on an industrial scale. Additionally, the use of some novel modifiers might introduce new sources of pollution, making it essential to carefully assess their environmental impact;
- Further study of selective adsorption and competitive adsorption behavior among heavy metal ions is highly valuable. Understanding the mechanisms that govern the preferential adsorption of specific metal ions and how different ions interact and compete for adsorption sites can significantly enhance the development of efficient and targeted remediation strategies for contaminated water and wastewater;
- In future research, the regeneration of carbon-based materials, particularly plant fibers, holds significant practical importance. Exploring the properties of these materials and understanding the optimal operational conditions for the regeneration process are essential steps. Additionally, the development of effective desorption solutions is crucial for ensuring the full utilization and reusability of carbon-based adsorbents.
Author Contributions
Z.R.: writing—original draft, writing—review and editing; A.K. (Ahasanul Karim): conceptualization, writing—original draft, writing—review and editing; A.K. (Antoine Karam): supervision, writing—review and editing; S.K.: conceptualization, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The project was funded by the Natural Sciences and Engineering Research Council of Canada (CRSNG RDC 538873-19).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 227. [Google Scholar] [CrossRef]
- Türkmen, D.; Bakhshpour, M.; Akgönüllü, S.; Aşır, S.; Denizli, A. Heavy metal ions removal from wastewater using cryogels: A review. Front. Sustain. 2022, 3, 765592. [Google Scholar] [CrossRef]
- Kerur, S.; Bandekar, S.; Hanagadakar, M.S.; Nandi, S.S.; Ratnamala, G.; Hegde, P.G. Removal of hexavalent Chromium-Industry treated water and Wastewater: A review. Mater. Today Proc. 2021, 42, 1112–1121. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Nejad, M.S.; Sheibani, H. Super-efficient removal of arsenic and mercury ions from wastewater by nanoporous biochar-supported poly 2-aminothiophenol. J. Environ. Chem. Eng. 2022, 10, 107363. [Google Scholar] [CrossRef]
- Staszak, K.; Wieszczycka, K. Recovery of metals from wastewater—State-of-the-art solutions with the support of membrane technology. Membranes 2023, 13, 114. [Google Scholar] [CrossRef]
- Martin, D.P.; Seiter, J.M.; Lafferty, B.J.; Bednar, A.J. Exploring the ability of cations to facilitate binding between inorganic oxyanions and humic acid. Chemosphere 2017, 166, 192–196. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Q.; Huang, X.; Li, T.; Yang, G. Microscopic understanding about adsorption and transport of different Cr (VI) species at mineral interfaces. J. Hazard. Mater. 2021, 414, 125485. [Google Scholar] [CrossRef]
- Zahir, M.H.; Irshad, K.; Rahman, M.M.; Shaikh, M.N.; Rahman, M.M. Efficient capture of heavy metal ions and arsenic with a CaY–carbonate layered double-hydroxide Nanosheet. ACS Omega 2021, 6, 22909–22921. [Google Scholar] [CrossRef]
- Zlati, M.L.; Georgescu, L.P.; Iticescu, C.; Ionescu, R.V.; Antohi, V.M. New approach to modelling the impact of heavy metals on the European Union’s water resources. Int. J. Environ. Res. Public Health 2022, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Shaker, O.A.; Safwat, S.M.; Matta, M.E. Nickel removal from wastewater using electrocoagulation process with zinc electrodes under various operating conditions: Performance investigation, mechanism exploration, and cost analysis. Environ. Sci. Pollut. Res. 2023, 30, 26650–26662. [Google Scholar] [CrossRef]
- Karim, A.; Raji, Z.; Karam, A.; Khalloufi, S. Valorization of fibrous plant-based food waste as biosorbents for remediation of heavy metals from wastewater—A review. Molecules 2023, 28, 4205. [Google Scholar] [CrossRef] [PubMed]
- Madhubashani, A.; Giannakoudakis, D.A.; Amarasinghe, B.; Rajapaksha, A.U.; Kumara, P.T.P.; Triantafyllidis, K.S.; Vithanage, M. Propensity and appraisal of biochar performance in removal of oil spills: A comprehensive review. Environ. Pollut. 2021, 288, 117676. [Google Scholar] [PubMed]
- Chikri, R.; Elhadiri, N.; Benchanaa, M. Efficiency of sawdust as low-cost adsorbent for dyes removal. J. Chem. 2020, 2020, 8813420. [Google Scholar] [CrossRef]
- Bello, O.S.; Bello, I.A.; Adegoke, K.A. Adsorption of dyes using different types of sand: A review. S. Afr. J. Chem. 2013, 66, 117–129. [Google Scholar]
- Anastopoulos, I.; Pashalidis, I. Environmental applications of Luffa cylindrica-based adsorbents. J. Mol. Liq. 2020, 319, 114127. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.; Jeevanantham, S.; Harikumar, P.; Priyanka, G.; Devakirubai, D.R.A. A comprehensive review on sources, analysis and toxicity of environmental pollutants and its removal methods from water environment. Sci. Total Environ. 2022, 812, 152456. [Google Scholar]
- Fouda-Mbanga, B.; Prabakaran, E.; Pillay, K. Carbohydrate biopolymers, lignin based adsorbents for removal of heavy metals (Cd2+, Pb2+, Zn2+) from wastewater, regeneration and reuse for spent adsorbents including latent fingerprint detection: A review. Biotechnol. Rep. 2021, 30, e00609. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A review of water interactions, applications in composites, and water treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar]
- Dey, T.; Bhattacharjee, T.; Nag, P.; Ghati, A.; Kuila, A. Valorization of agro-waste into value added products for sustainable development. Bioresour. Technol. Rep. 2021, 16, 100834. [Google Scholar]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. A review on the heavy metal adsorption capacity of dietary fibers derived from agro-based wastes: Opportunities and challenges for practical applications in the food industry. Trends Food Sci. Technol. 2023, 137, 74–91. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Tripathi, A.; Ranjan, M.R. Heavy metal removal from wastewater using low cost adsorbents. Bioremediat. Biodegrad. 2015, 6, 1000315. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent advances in removal techniques of Cr (VI) toxic ion from aqueous solution: A comprehensive review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Nakano, Y.; Takeshita, K.; Tsutsumi, T. Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res. 2001, 35, 496–500. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Sarangi, B.; Mishra, S.P. A glance at the potential of Artocarpus genus fruit peels and its derivatives as adsorbent. Bioresour. Technol. Rep. 2023, 21, 101363. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Shah, M.U.H. Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Niazi, N.K.; Hassan, N.E.; Bibi, I.; Wang, H.; Tsang, D.C.; Ok, Y.S.; Bolan, N.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2019, 64, 216–247. [Google Scholar] [CrossRef]
- Huang, D.; Li, B.; Ou, J.; Xue, W.; Li, J.; Li, Z.; Li, T.; Chen, S.; Deng, R.; Guo, X. Megamerger of biosorbents and catalytic technologies for the removal of heavy metals from wastewater: Preparation, final disposal, mechanism and influencing factors. J. Environ. Manag. 2020, 261, 109879. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M. A review on valorization of biomass in heavy metal removal from wastewater. J. Water Process Eng. 2020, 38, 101602. [Google Scholar] [CrossRef]
- Abdelfattah, I.; Ismail, A.A.; Al Sayed, F.; Almedolab, A.; Aboelghait, K. Biosorption of heavy metals ions in real industrial wastewater using peanut husk as efficient and cost effective adsorbent. Environ. Nanotechnol. Monit. Manag. 2016, 6, 176–183. [Google Scholar] [CrossRef]
- Chen, L.; Li, Z.; Li, W.; Chen, Z.; Chen, G.; Yang, W.; Zhang, X.; Liu, X. Investigation of adsorption kinetics and the isotherm mechanism of manganese by modified Diatomite. ACS Omega 2021, 6, 16402–16409. [Google Scholar] [CrossRef]
- Umeh, T.C.; Nduka, J.K.; Akpomie, K.G. Kinetics and isotherm modeling of Pb (II) and Cd (II) sequestration from polluted water onto tropical ultisol obtained from Enugu Nigeria. Appl. Water Sci. 2021, 11, 65. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.; Rao, M.M.; Wang, M. Biosorption of Pb2+ from aqueous solutions by Moringa oleifera bark: Equilibrium and kinetic studies. J. Hazard. Mater. 2010, 174, 831–838. [Google Scholar] [CrossRef]
- Zhai, M.; Fu, B.; Zhai, Y.; Wang, W.; Maroney, A.; Keller, A.A.; Wang, H.; Chovelon, J.-M. Simultaneous removal of pharmaceuticals and heavy metals from aqueous phase via adsorptive strategy: A critical review. Water Res. 2023, 236, 119924. [Google Scholar] [CrossRef]
- Maity, S.; Patil, P.B.; SenSharma, S.; Sarkar, A. Bioremediation of heavy metals from the aqueous environment using Artocarpus heterophyllus (jackfruit) seed as a novel biosorbent. Chemosphere 2022, 307, 136115. [Google Scholar] [CrossRef]
- Khan, A.A.; Naqvi, S.R.; Ali, I.; Arshad, M.; AlMohamadi, H.; Sikandar, U. Algal-derived biochar as an efficient adsorbent for removal of Cr (VI) in textile industry wastewater: Non-linear isotherm, kinetics and ANN studies. Chemosphere 2023, 316, 137826. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, L.; Pei, Z.; Li, C.; Lv, J.; Xie, J.; Wen, B.; Zhang, S. Adsorption kinetics, isotherms and thermodynamics of Cr (III) on graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 100–106. [Google Scholar] [CrossRef]
- Wibowo, Y.G.; Safitri, H.; Ramadan, B.S. Adsorption test using ultra-fine materials on heavy metals removal. Bioresour. Technol. Rep. 2022, 19, 101149. [Google Scholar] [CrossRef]
- Mustapha, S.; Shuaib, D.; Ndamitso, M.; Etsuyankpa, M.; Sumaila, A.; Mohammed, U.; Nasirudeen, M. Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb (II), Cd (II), Zn (II) and Cu (II) ions from aqueous solutions using Albizia lebbeck pods. Appl. Water Sci. 2019, 9, 142. [Google Scholar] [CrossRef]
- Chen, Z.-l.; Zhang, J.-q.; Huang, L.; Yuan, Z.-h.; Li, Z.-j.; Liu, M.-c. Removal of Cd and Pb with biochar made from dairy manure at low temperature. J. Integr. Agric. 2019, 18, 201–210. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—Bamboo charcoal. J. Hazard. Mater. 2010, 177, 300–306. [Google Scholar] [CrossRef]
- Shen, L.; Li, J.; Lv, L.; Zhang, L.; Bai, R.; Zheng, T.; Zhang, Q. Comparison of functional and structural properties of ginkgo seed protein dried by spray and freeze process. J. Food Sci. Technol. 2021, 58, 175–185. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, H.; Guo, H.; Ling, C.; Yuan, X.; Li, P. Hydrated titanium oxide nanoparticles supported on natural rice straw for Cu (II) removal from water. Environ. Technol. Innov. 2020, 20, 101143. [Google Scholar] [CrossRef]
- Amar, M.B.; Walha, K.; Salvadó, V. Evaluation of olive stones for Cd (II), Cu (II), Pb (II) and Cr (VI) biosorption from aqueous solution: Equilibrium and kinetics. Int. J. Environ. Res. 2020, 14, 193–204. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Majeed, H.; Goff, H.D.; Rahman, M.R.T.; Zhong, F. Adsorption mechanism modeling using lead (Pb) sorption data on modified rice bran-insoluble fiber as universal approach to assess other metals toxicity. Int. J. Food Prop. 2019, 22, 1397–1410. [Google Scholar] [CrossRef]
- Liu, J.; Hu, C.; Huang, Q. Adsorption of Cu2+, Pb2+, and Cd2+ onto oiltea shell from water. Bioresour. Technol. 2019, 271, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Hu, W.; Guo, M.; Wang, Y.; Jia, J.; Hu, Z. Preparation of cotton-based fibrous adsorbents for the removal of heavy metal ions. Carbohydr. Polym. 2019, 225, 115218. [Google Scholar] [CrossRef] [PubMed]
- El-Sikaily, A.; El Nemr, A.; Khaled, A.; Abdelwehab, O. Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated carbon. J. Hazard. Mater. 2007, 148, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Baby, R.; Hussein, M.Z. Ecofriendly approach for treatment of heavy-metal-contaminated water using activated carbon of kernel shell of oil palm. Materials 2020, 13, 2627. [Google Scholar] [CrossRef] [PubMed]
- Kenessova, A.; Seilkhanova, G.; Rakhym, A.; Mastai, Y. Composite materials based on orange and pomegranate peels for Cu (II) and Zn (II) ions extraction. Int. J. Biol. Chem. 2020, 13, 154–160. [Google Scholar] [CrossRef]
- Özsin, G.; Kılıç, M.; Apaydın-Varol, E.; Pütün, A.E. Chemically activated carbon production from agricultural waste of chickpea and its application for heavy metal adsorption: Equilibrium, kinetic, and thermodynamic studies. Appl. Water Sci. 2019, 9, 56. [Google Scholar] [CrossRef]
- Ani, J.U.; Ochonogor, A.E.; Akpomie, K.G.; Olikagu, C.S.; Igboanugo, C.C. Abstraction of arsenic (III) on activated carbon prepared from Dialium guineense seed shell: Kinetics, isotherms and thermodynamic studies. SN Appl. Sci. 2019, 1, 1304. [Google Scholar] [CrossRef]
- Imran, M.; Anwar, K.; Akram, M.; Shah, G.M.; Ahmad, I.; Samad Shah, N.; Khan, Z.U.H.; Rashid, M.I.; Akhtar, M.N.; Ahmad, S. Biosorption of Pb (II) from contaminated water onto Moringa oleifera biomass: Kinetics and equilibrium studies. Int. J. Phytoremediat. 2019, 21, 777–789. [Google Scholar] [CrossRef]
- Van Hien, N.; Valsami-Jones, E.; Vinh, N.C.; Phu, T.T.; Tam, N.T.T.; Lynch, I. Effectiveness of different biochar in aqueous zinc removal: Correlation with physicochemical characteristics. Bioresour. Technol. Rep. 2020, 11, 100466. [Google Scholar] [CrossRef]
- Dabbagh, R.; Ashtiani Moghaddam, Z.; Ghafourian, H. Removal of cobalt (II) ion from water by adsorption using intact and modified Ficus carica leaves as low-cost natural sorbent. Desalination Water Treat. 2016, 57, 19890–19902. [Google Scholar] [CrossRef]
- Malik, R.; Dahiya, S. An experimental and quantum chemical study of removal of utmostly quantified heavy metals in wastewater using coconut husk: A novel approach to mechanism. Int. J. Biol. Macromol. 2017, 98, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Popoola, L.T.; Grema, A.S. Adsorption of Heavy Metals from Industrial Wastewater using Nanoparticles from Agro Wastes. In Nanopores; IntechOpen: London, UK, 2021. [Google Scholar]
- Van Suc, N.; Son, L.N. Mistletoe leaves as a biosorbent for removal of Pb (II) and Cd (II) from aqueous solution. Desalination Water Treat. 2016, 57, 3606–3618. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Competitive adsorption of cadmium (II) and nickel (II) metal ions from aqueous solution onto rice husk ash. Chem. Eng. Process. Process Intensif. 2009, 48, 370–379. [Google Scholar] [CrossRef]
- Emenike, P.; Omole, D.; Ngene, B.; Tenebe, I. Potentiality of agricultural adsorbent for the sequestering of metal ions from wastewater. Glob. J. Environ. Sci. Manag. 2016, 2, 411–442. [Google Scholar] [CrossRef]
- Lalhruaitluanga, H.; Jayaram, K.; Prasad, M.; Kumar, K. Lead (II) adsorption from aqueous solutions by raw and activated charcoals of Melocanna baccifera Roxburgh (bamboo)—A comparative study. J. Hazard. Mater. 2010, 175, 311–318. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Negm, N.A.; Hefni, H.H.; Wahab, M.M.A. Metal adsorption by agricultural biosorbents: Adsorption isotherm, kinetic and biosorbents chemical structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef]
- Mehmood, S.; Mahmood, M.; Núñez-Delgado, A.; Alatalo, J.M.; Elrys, A.S.; Rizwan, M.; Weng, J.; Li, W.; Ahmed, W. A green method for removing chromium (VI) from aqueous systems using novel silicon nanoparticles: Adsorption and interaction mechanisms. Environ. Res. 2022, 213, 113614. [Google Scholar] [CrossRef]
- Khamwichit, A.; Dechapanya, W.; Dechapanya, W. Adsorption kinetics and isotherms of binary metal ion aqueous solution using untreated venus shell. Heliyon 2022, 8, e09610. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous removal of organics and heavy metals from industrial wastewater: A review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Roby, O.; Hammoudan, I.; Saddik, R.; Garcia, Y.; Almarhoon, Z.M.; Mabkhot, Y.N. Kinetics, thermodynamics, equilibrium, surface modelling, and atomic absorption analysis of selective Cu (II) removal from aqueous solutions and rivers water using silica-2-(pyridin-2-ylmethoxy) ethan-1-ol hybrid material. RSC Adv. 2022, 12, 611–625. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Ji, H.; Guo, P.; Wan, D.; Li, B.; Sun, X. Adsorption of nitrate and nitrite from aqueous solution by magnetic Mg/Fe hydrotalcite. Water Supply 2021, 21, 4287–4300. [Google Scholar] [CrossRef]
- Bulut, E.; Özacar, M.; Şengil, İ.A. Adsorption of malachite green onto bentonite: Equilibrium and kinetic studies and process design. Microporous Mesoporous Mater. 2008, 115, 234–246. [Google Scholar] [CrossRef]
- Santhy, K.; Selvapathy, P. Removal of reactive dyes from wastewater by adsorption on coir pith activated carbon. Bioresour. Technol. 2006, 97, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Canzano, S.; Iovino, P.; Leone, V.; Salvestrini, S.; Capasso, S. Use and misuse of sorption kinetic data: A common mistake that should be avoided. Adsorpt. Sci. Technol. 2012, 30, 217–225. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Bullen, J.C.; Saleesongsom, S.; Gallagher, K.; Weiss, D.J. A revised pseudo-second-order kinetic model for adsorption, sensitive to changes in adsorbate and adsorbent concentrations. Langmuir 2021, 37, 3189–3201. [Google Scholar] [CrossRef]
- Manirethan, V.; Raval, K.; Rajan, R.; Thaira, H.; Balakrishnan, R.M. Kinetic and thermodynamic studies on the adsorption of heavy metals from aqueous solution by melanin nanopigment obtained from marine source: Pseudomonas stutzeri. J. Environ. Manag. 2018, 214, 315–324. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Abed, F.I.; Al-Musawi, T.J. Biosorption of Pb (II) from aqueous solution by spent black tea leaves and separation by flotation. Desalination Water Treat. 2016, 57, 2028–2039. [Google Scholar] [CrossRef]
- Aathithya, R.; Sowparnika, J.R.; Balakrishnan, V. Kinetic studies for the biosorption of chromium using cherry leaves (Muntingia calabura L.). Int. Lett. Nat. Sci. 2014, 20, 6–11. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, Y.; Zhang, M.; Ming, Z.; Yang, S.; Arkin, A.; Fang, P. Functionalized agricultural biomass as a low-cost adsorbent: Utilization of rice straw incorporated with amine groups for the adsorption of Cr (VI) and Ni (II) from single and binary systems. Biochem. Eng. J. 2016, 105, 27–35. [Google Scholar] [CrossRef]
- Batool, F.; Akbar, J.; Iqbal, S.; Noreen, S.; Bukhari, S.N.A. Study of isothermal, kinetic, and thermodynamic parameters for adsorption of cadmium: An overview of linear and nonlinear approach and error analysis. Bioinorg. Chem. Appl. 2018, 2018, 3463724. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Diao, Y.; Jain, R.; Rene, E.R.; Dutta, S. Adsorption of cadmium from aqueous solutions onto coffee grounds and wheat straw: Equilibrium and kinetic study. J. Environ. Eng. 2016, 142, C4015014. [Google Scholar] [CrossRef]
- Kamsonlian, S.; Suresh, S.; Ramanaiah, V.; Majumder, C.; Chand, S.; Kumar, A. Biosorptive behaviour of mango leaf powder and rice husk for arsenic (III) from aqueous solutions. Int. J. Environ. Sci. Technol. 2012, 9, 565–578. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Amini, M. Adsorption of copper (II) ions onto surfactant-modified oil palm leaf powder. J. Dispers. Sci. Technol. 2011, 32, 1641–1648. [Google Scholar] [CrossRef]
- Jain, C.K.; Malik, D.S.; Yadav, A.K. Applicability of plant based biosorbents in the removal of heavy metals: A review. Environ. Process. 2016, 3, 495–523. [Google Scholar] [CrossRef]
- Aryee, A.A.; Mpatani, F.M.; Du, Y.; Kani, A.N.; Dovi, E.; Han, R.; Li, Z.; Qu, L. Fe3O4 and iminodiacetic acid modified peanut husk as a novel adsorbent for the uptake of Cu (II) and Pb (II) in aqueous solution: Characterization, equilibrium and kinetic study. Environ. Pollut. 2021, 268, 115729. [Google Scholar] [CrossRef]
- Wattanakornsiri, A.; Rattanawan, P.; Sanmueng, T.; Satchawan, S.; Jamnongkan, T.; Phuengphai, P. Local fruit peel biosorbents for lead (II) and cadmium (II) ion removal from waste aqueous solution: A kinetic and equilibrium study. S. Afr. J. Chem. Eng. 2022, 42, 306–317. [Google Scholar] [CrossRef]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Babakhani, A.; Sartaj, M. Removal of cadmium (II) from aqueous solution using tripolyphosphate cross-linked chitosan. J. Environ. Chem. Eng. 2020, 8, 103842. [Google Scholar] [CrossRef]
- Akram, M.; Bhatti, H.N.; Iqbal, M.; Noreen, S.; Sadaf, S. Biocomposite efficiency for Cr (VI) adsorption: Kinetic, equilibrium and thermodynamics studies. J. Environ. Chem. Eng. 2017, 5, 400–411. [Google Scholar] [CrossRef]
- Azam, M.; Wabaidur, S.M.; Khan, M.R.; Al-Resayes, S.I.; Islam, M.S. Heavy metal ions removal from aqueous solutions by treated ajwa date pits: Kinetic, isotherm, and thermodynamic approach. Polymers 2022, 14, 914. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Kyzas, G.Z. Are the thermodynamic parameters correctly estimated in liquid-phase adsorption phenomena? J. Mol. Liq. 2016, 218, 174–185. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Choong, S.T. Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: An overview. Desalination 2005, 175, 305–316. [Google Scholar] [CrossRef]
- Jha, I.; Iyengar, L.; Rao, A.P. Removal of cadmium using chitosan. J. Environ. Eng. 1988, 114, 962–974. [Google Scholar] [CrossRef]
- Huang, C.; Chung, Y.-C.; Liou, M.-R. Adsorption of Cu (II) and Ni (II) by pelletized biopolymer. J. Hazard. Mater. 1996, 45, 265–277. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhang, L.; Yang, Y.; Liu, X. Diethylenetriaminepentaacetic acid–thiourea-modified magnetic chitosan for adsorption of hexavalent chromium from aqueous solutions. Carbohydr. Polym. 2021, 274, 118555. [Google Scholar] [CrossRef] [PubMed]
- Keng, P.-S.; Lee, S.-L.; Ha, S.-T.; Hung, Y.-T.; Ong, S.-T. Removal of hazardous heavy metals from aqueous environment by low-cost adsorption materials. Environ. Chem. Lett. 2014, 12, 15–25. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Gupta, G.; Prasad, G.; Singh, V. Removal of chrome dye from aqueous solutions by mixed adsorbents: Fly ash and coal. Water Res. 1990, 24, 45–50. [Google Scholar] [CrossRef]
- Panday, K.; Prasad, G.; Singh, V. Copper (II) removal from aqueous solutions by fly ash. Water Res. 1985, 19, 869–873. [Google Scholar] [CrossRef]
- Jahangiri, K.; Yousefi, N.; Ghadiri, S.K.; Fekri, R.; Bagheri, A.; Talebi, S.S. Enhancement adsorption of hexavalent chromium onto modified fly ash from aqueous solution; optimization; isotherm, kinetic and thermodynamic study. J. Dispers. Sci. Technol. 2018, 40, 1147–1158. [Google Scholar] [CrossRef]
- Lee, S.; Davis, A.P. Removal of Cu (II) and Cd (II) from aqueous solution by seafood processing waste sludge. Water Res. 2001, 35, 534–540. [Google Scholar] [CrossRef]
- Ajmal, M.; Khan, A.H.; Ahmad, S.; Ahmad, A. Role of sawdust in the removal of copper (II) from industrial wastes. Water Res. 1998, 32, 3085–3091. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhattacharjee, G.; Tyagi, R.; Pant, N.; Pal, N. Studies on the removal of some toxic metal ions from aqueous solutions and industrial waste. Part I (Removal of lead and cadmium by hydrous iron and aluminium oxide). Environ. Technol. Lett. 1988, 9, 1173–1185. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Cheng, Q.; Li, X.; Li, Z. Removal of toxic heavy metal ions (Pb, Cr, Cu, Ni, Zn, Co, Hg, and Cd) from waste batteries or lithium cells using nanosized metal oxides: A review. J. Nanosci. Nanotechnol. 2020, 20, 7231–7254. [Google Scholar] [CrossRef] [PubMed]
- Zamzow, M.; Eichbaum, B.; Sandgren, K.; Shanks, D. Removal of heavy metals and other cations from wastewater using zeolites. Sep. Sci. Technol. 1990, 25, 1555–1569. [Google Scholar] [CrossRef]
- Al-Zboon, K.; Al-Harahsheh, M.S.; Hani, F.B. Fly ash-based geopolymer for Pb removal from aqueous solution. J. Hazard. Mater. 2011, 188, 414–421. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhu, Z. Solid-state conversion of fly ash to effective adsorbents for Cu removal from wastewater. J. Hazard. Mater. 2007, 139, 254–259. [Google Scholar] [CrossRef]
- Srivastava, S.; Gupta, V.; Mohan, D. Removal of lead and chromium by activated slag—A blast-furnace waste. J. Environ. Eng. 1997, 123, 461–468. [Google Scholar] [CrossRef]
- Namasivayam, C.; Yamuna, R. Waste biogas residual slurry as an adsorbent for the removal of Pb (II) from aqueous solution and radiator manufacturing industry wastewater. Bioresour. Technol. 1995, 52, 125–131. [Google Scholar] [CrossRef]
- Carrott, P.; Carrott, M.R. Lignin from natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Namasivayam, C.; Ranganathan, K. Effect of organic ligands on the removal of Pb (II), Ni (II) and Cd (II) by ‘waste’Fe (III)/Cr (III) hydroxide. Water Res. 1998, 32, 969–971. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sharma, S. Removal of cadmium and zinc from aqueous solutions using red mud. Environ. Sci. Technol. 2002, 36, 3612–3617. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.M.; do Nascimento, R.A.; Amador, I.C.B.; de Sousa Santos, T.C.; Martelli, M.C.; de Faria, L.J.G.; da Paixão Ribeiro, N.F. Chemically activated red mud: Assessing structural modifications and optimizing adsorption properties for hexavalent chromium. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127325. [Google Scholar] [CrossRef]
- Zhu, C.; Luan, Z.; Wang, Y.; Shan, X. Removal of cadmium from aqueous solutions by adsorption on granular red mud (GRM). Sep. Purif. Technol. 2007, 57, 161–169. [Google Scholar] [CrossRef]
- Semerjian, L. Removal of heavy metals (Cu, Pb) from aqueous solutions using pine (Pinus halepensis) sawdust: Equilibrium, kinetic, and thermodynamic studies. Environ. Technol. Innov. 2018, 12, 91–103. [Google Scholar] [CrossRef]
- Subramani, B.S.; Shrihari, S.; Manu, B.; Babunarayan, K. Evaluation of pyrolyzed areca husk as a potential adsorbent for the removal of Fe2+ ions from aqueous solutions. J. Environ. Manag. 2019, 246, 345–354. [Google Scholar] [CrossRef]
- Nuhoğlu, Y.; Ekmekyapar Kul, Z.; Kul, S.; Nuhoğlu, Ç.; Ekmekyapar Torun, F. Pb (II) biosorption from the aqueous solutions by raw and modified tea factory waste (TFW). Int. J. Environ. Sci. Technol. 2021, 18, 2975–2986. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhang, H.; Li, Y.; Zhang, Z.; Zhang, W. Recycling spent lithium-ion battery as adsorbents to remove aqueous heavy metals: Adsorption kinetics, isotherms, and regeneration assessment. Resour. Conserv. Recycl. 2020, 156, 104688. [Google Scholar] [CrossRef]
- Bian, B.; Lv, L.; Yang, D.; Zhou, L. Migration of heavy metals in vegetable farmlands amended with biogas slurry in the Taihu Basin, China. Ecol. Eng. 2014, 71, 380–383. [Google Scholar] [CrossRef]
- Vu, N.H.; Kristianová, E.; Dvořák, P.; Abramowski, T.; Dreiseitl, I.; Adrysheva, A. Modified leach residues from processing deep-sea nodules as effective heavy metals adsorbents. Metals 2019, 9, 472. [Google Scholar] [CrossRef]
- Escudero, C.; Poch, J.; Villaescusa, I. Modelling of breakthrough curves of single and binary mixtures of Cu (II), Cd (II), Ni (II) and Pb (II) sorption onto grape stalks waste. Chem. Eng. J. 2013, 217, 129–138. [Google Scholar] [CrossRef]
- Selvam, S.; Jesuraja, K.; Venkatramanan, S.; Roy, P.D.; Kumari, V.J. Hazardous microplastic characteristics and its role as a vector of heavy metal in groundwater and surface water of coastal south India. J. Hazard. Mater. 2021, 402, 123786. [Google Scholar] [CrossRef] [PubMed]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef]
- Fu, Q.; Tan, X.; Ye, S.; Ma, L.; Gu, Y.; Zhang, P.; Chen, Q.; Yang, Y.; Tang, Y. Mechanism analysis of heavy metal lead captured by natural-aged microplastics. Chemosphere 2021, 270, 128624. [Google Scholar] [CrossRef]
- Zon, N.F.; Iskendar, A.; Azman, S.; Sarijan, S.; Ismail, R. Sorptive behaviour of chromium on polyethylene microbeads in artificial seawater. In Proceedings of the 12th International Civil Engineering Post Graduate Conference (SEPKA)—The 3rd International Symposium on Expertise of Engineering Design (ISEED) (SEPKA-ISEED 2018). In Proceedings of the MATEC Web of Conferences, Johor, Malaysia, 27–28 August 2018; p. 06001. [Google Scholar]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As (III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere 2020, 239, 124792. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ. Adv. 2020, 2, 100007. [Google Scholar] [CrossRef]
- Osman, A.I.; El-Monaem, E.M.A.; Elgarahy, A.M.; Aniagor, C.O.; Hosny, M.; Farghali, M.; Rashad, E.; Ejimofor, M.I.; López-Maldonado, E.A.; Ihara, I. Methods to prepare biosorbents and magnetic sorbents for water treatment: A review. Environ. Chem. Lett. 2023, 21, 2337–2398. [Google Scholar] [CrossRef]
- Kumar, M.; Kushwaha, A.; Goswami, L.; Singh, A.K.; Sikandar, M. A review on advances and mechanism for the phycoremediation of cadmium contaminated wastewater. Clean. Eng. Technol. 2021, 5, 100288. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Sundaram, V.; Sundaram, T.; Vo, D.-V.N. Biosorptive ascendency of plant based biosorbents in removing hexavalent chromium from aqueous solutions—Insights into isotherm and kinetic studies. Environ. Res. 2022, 210, 112902. [Google Scholar] [CrossRef] [PubMed]
- El-Azazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato peels as an adsorbent for heavy metals from aqueous solutions: Eco-structuring of a green adsorbent operating Plackett–Burman design. J. Chem. 2019, 2019, 4926240. [Google Scholar] [CrossRef]
- Maina, I.W.; Obuseng, V.; Nareetsile, F. Use of Moringa oleifera (Moringa) seed pods and Sclerocarya birrea (Morula) nut shells for removal of heavy metals from wastewater and borehole water. J. Chem. 2016, 2016, 9312952. [Google Scholar] [CrossRef]
- Thi Quyen, V.; Pham, T.-H.; Kim, J.; Thanh, D.M.; Thang, P.Q.; Van Le, Q.; Jung, S.H.; Kim, T. Biosorbent derived from coffee husk for efficient removal of toxic heavy metals from wastewater. Chemosphere 2021, 284, 131312. [Google Scholar] [CrossRef]
- Nieto, L.M.; Alami, S.B.D.; Hodaifa, G.; Faur, C.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Adsorption of iron on crude olive stones. Ind. Crops Prod. 2010, 32, 467–471. [Google Scholar] [CrossRef]
- Singh, R.; Martin, C.; Barr, D.; Rosengren, R. Immobilised apple peel bead biosorbent for the simultaneous removal of heavy metals from cocktail solution. Cogent Environ. Sci. 2019, 5, 1673116. [Google Scholar] [CrossRef]
- Šabanović, E.; Memić, M.; Sulejmanović, J.; Selović, A. Simultaneous adsorption of heavy metals from water by novel lemon-peel based biomaterial. Pol. J. Chem. Technol. 2020, 22, 46–53. [Google Scholar] [CrossRef]
- Bulut, Y.; Tez, Z. Adsorption studies on ground shells of hazelnut and almond. J. Hazard. Mater. 2007, 149, 35–41. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef]
- Massocatto, C.; Paschoal, E.; Buzinaro, N.; Oliveria, T.; Tarley, C.; Caetano, J.; Gonçalves, A., Jr.; Dragunski, D.; Diniz, K. Preparation and evaluation of kinetics and thermodynamics studies of lead adsorption onto chemically modified banana peels. Desalination Water Treat. 2013, 51, 5682–5691. [Google Scholar] [CrossRef]
- Nathan, R.J.; Martin, C.E.; Barr, D.; Rosengren, R.J. Simultaneous removal of heavy metals from drinking water by banana, orange and potato peel beads: A study of biosorption kinetics. Appl. Water Sci. 2021, 11, 116. [Google Scholar] [CrossRef]
- Mohammad, M.; Maitra, S.; Ahmad, N.; Bustam, A.; Sen, T.; Dutta, B.K. Metal ion removal from aqueous solution using physic seed hull. J. Hazard. Mater. 2010, 179, 363–372. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk-and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Jibril, H.B.; Salga, S.M.; Ahmed, S.; Saddiq, M. Application of agricultural wastes for the aqueous removal of heavy metals from waste water. Fudma J. Sci. 2021, 5, 231–236. [Google Scholar] [CrossRef]
- Sanka, P.M.; Rwiza, M.J.; Mtei, K. Removal of selected heavy metal ions from industrial wastewater using rice and corn husk biochar. Water Air Soil Pollut. 2020, 231, 244. [Google Scholar] [CrossRef]
- Abdulrasaq, O.O.; Basiru, O.G. Removal of copper (II), iron (III) and lead (II) ions from mono-component simulated waste effluent by adsorption on coconut husk. Afr. J. Environ. Sci. Technol. 2010, 4, 382–387. [Google Scholar] [CrossRef]
- Ideriah, T.; David, O.; Ogbonna, D. Removal of heavy metal ions in aqueous solutions using palm fruit fibre as adsorbent. J. Environ. Chem. Ecotoxicol 2012, 4, 82–90. [Google Scholar] [CrossRef]
- Maheshwari, U.; Gupta, S. Removal of Cr (VI) from wastewater using activated neem bark in a fixed-bed column: Interference of other ions and kinetic modelling studies. Desalination Water Treat. 2016, 57, 8514–8525. [Google Scholar] [CrossRef]
- Chao, H.-P.; Chang, C.-C.; Nieva, A. Biosorption of heavy metals on Citrus maxima peel, passion fruit shell, and sugarcane bagasse in a fixed-bed column. J. Ind. Eng. Chem. 2014, 20, 3408–3414. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Al-Yasi, H.M.; Galal, T.M.; Hamza, R.Z.; Abdelkader, T.G.; Ali, E.F.; Hassan, S.H. Statistical optimization, kinetic, equilibrium isotherm and thermodynamic studies of copper biosorption onto Rosa damascena leaves as a low-cost biosorbent. Sci. Rep. 2022, 12, 8583. [Google Scholar] [CrossRef]
- Gryko, K.; Kalinowska, M.; Świderski, G. The Use of apple pomace in removing heavy metals from water and sewage. Environ. Sci. Proc. 2021, 9, 24. [Google Scholar]
- Ebrahimi, M.; Langeroodi, N.S.; Hooshmand, S. Biosorption of Fe (III) ions using carrot: Equilibrium, kinetics, and statistical analysis. Prot. Met. Phys. Chem. Surf. 2019, 55, 259–265. [Google Scholar] [CrossRef]
- Nasernejad, B.; Zadeh, T.E.; Pour, B.B.; Bygi, M.E.; Zamani, A. Camparison for biosorption modeling of heavy metals (Cr (III), Cu (II), Zn (II)) adsorption from wastewater by carrot residues. Process Biochem. 2005, 40, 1319–1322. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Akiode, O.K.; Oladipo, G.O.; Adedapo, A.E.; Adebayo, L.O.; Awotula, A.O. Application of raw and alkaline-modified coconut shaft as a biosorbent for Pb2+ removal. BioResources 2015, 10, 3462–3480. [Google Scholar] [CrossRef]
- Wang, H.; Huang, T.; Tu, Z.-c.; Ruan, C.-y.; Lin, D. The adsorption of lead (II) ions by dynamic high pressure micro-fluidization treated insoluble soybean dietary fiber. J. Food Sci. Technol. 2016, 53, 2532–2539. [Google Scholar] [CrossRef]
- Ahmad, S.; Zhu, X.; Wang, Q.; Wei, X.; Zhang, S. Microwave-assisted hydrothermal treatment of soybean residue and chitosan: Characterization of hydrochars and role of N and P transformation for Pb (II) removal. J. Anal. Appl. Pyrolysis 2021, 160, 105330. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.; Altaher, H.; Omar, W. Application of date palm trunk fibers as adsorbents for removal of Cd+2 ions from aqueous solutions. J. Water Reuse Desalination 2013, 3, 47–54. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, J.; Khare, R.; Cameotra, S.S.; Ali, A. Batch sorption dynamics, kinetics and equilibrium studies of Cr (VI), Ni (II) and Cu (II) from aqueous phase using agricultural residues. Appl. Water Sci. 2013, 3, 207–218. [Google Scholar] [CrossRef]
- Karnitz, O., Jr.; Gurgel, L.V.A.; De Melo, J.C.P.; Botaro, V.R.; Melo, T.M.S.; de Freitas Gil, R.P.; Gil, L.F. Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse. Bioresour. Technol. 2007, 98, 1291–1297. [Google Scholar] [CrossRef]
- El-Shafey, E. Removal of Zn (II) and Hg (II) from aqueous solution on a carbonaceous sorbent chemically prepared from rice husk. J. Hazard. Mater. 2010, 175, 319–327. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-Bandegharaei, A.; Giannakoudakis, D.A.; Robalds, A.; Usman, M.; Escudero, L.B.; Zhou, Y.; Colmenares, J.C.; Núñez-Delgado, A. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019, 295, 111684. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Senthamarai, C.; Sai Deepthi, A.; Bharani, R. Adsorption isotherms, kinetics and mechanism of Pb (II) ions removal from aqueous solution using chemically modified agricultural waste. Can. J. Chem. Eng. 2013, 91, 1950–1956. [Google Scholar] [CrossRef]
- Shukla, S.; Pai, R.S.; Shendarkar, A.D. Adsorption of Ni (II), Zn (II) and Fe (II) on modified coir fibres. Sep. Purif. Technol. 2006, 47, 141–147. [Google Scholar] [CrossRef]
- Ajjabi, L.C.; Chouba, L. Biosorption of Cu2+ and Zn2+ from aqueous solutions by dried marine green macroalga Chaetomorpha linum. J. Environ. Manag. 2009, 90, 3485–3489. [Google Scholar] [CrossRef] [PubMed]
- Pavasant, P.; Apiratikul, R.; Sungkhum, V.; Suthiparinyanont, P.; Wattanachira, S.; Marhaba, T.F. Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Bioresour. Technol. 2006, 97, 2321–2329. [Google Scholar] [CrossRef]
- Arivalagan, P.; Singaraj, D.; Haridass, V.; Kaliannan, T. Removal of cadmium from aqueous solution by batch studies using Bacillus cereus. Ecol. Eng. 2014, 71, 728–735. [Google Scholar] [CrossRef]
- Quintelas, C.; Rocha, Z.; Silva, B.; Fonseca, B.; Figueiredo, H.; Tavares, T. Removal of Cd (II), Cr (VI), Fe (III) and Ni (II) from aqueous solutions by an E. coli biofilm supported on kaolin. Chem. Eng. J. 2009, 149, 319–324. [Google Scholar] [CrossRef]
- Chellaiah, E.R. Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: A minireview. Appl. Water Sci. 2018, 8, 154. [Google Scholar] [CrossRef]
- Puyen, Z.M.; Villagrasa, E.; Maldonado, J.; Diestra, E.; Esteve, I.; Solé, A. Biosorption of lead and copper by heavy-metal tolerant Micrococcus luteus DE2008. Bioresour. Technol. 2012, 126, 233–237. [Google Scholar] [CrossRef]
- Abdel-Monem, M.; Al-Zubeiry, A.; Al-Gheethi, A. Biosorption of nickel by Pseudomonas cepacia 120S and Bacillus subtilis 117S. Water Sci. Technol. 2010, 61, 2994–3007. [Google Scholar] [CrossRef]
- Adel, A.; Lalung, J.; Efaq, A.; Ismail, N. Removal of cephalexin antibiotic and heavy metals from pharmaceutical effluents using Bacillus subtilis strain. Expert Opin. Environ. Biol. 2015, 4, 1000117. [Google Scholar] [CrossRef]
- Politaeva, N.; Smyatskaya, Y.A.; Tatarintseva, E. Using adsorption material based on the residual biomass of Chlorella sorokiniana microalgae for wastewater purification to remove heavy metal ions. Chem. Pet. Eng. 2020, 55, 907–912. [Google Scholar] [CrossRef]
- Sandau, E.; Sandau, P.; Pulz, O. Heavy metal sorption by microalgae. Acta Biotechnol. 1996, 16, 227–235. [Google Scholar] [CrossRef]
- Negm, N.A.; Abd El Wahed, M.G.; Hassan, A.R.A.; Abou Kana, M.T. Feasibility of metal adsorption using brown algae and fungi: Effect of biosorbents structure on adsorption isotherm and kinetics. J. Mol. Liq. 2018, 264, 292–305. [Google Scholar] [CrossRef]
- Lesmana, S.O.; Febriana, N.; Soetaredjo, F.E.; Sunarso, J.; Ismadji, S. Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochem. Eng. J. 2009, 44, 19–41. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Fan, L.; Cui, L.; Zhang, Y.; Cheng, J.; Wu, X.; Zeng, W.; Tian, Q.; Shen, L. Construction of fungi-microalgae symbiotic system and adsorption study of heavy metal ions. Sep. Purif. Technol. 2021, 268, 118689. [Google Scholar] [CrossRef]
- Srinivasa Rao, P.; Kalyani, S.; Suresh Reddy, K.; Krishnaiah, A. Comparison of biosorption of nickel (II) and copper (II) ions from aqueous solution by sphaeroplea algae and acid treated sphaeroplea algae. Sep. Sci. Technol. 2005, 40, 3149–3165. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Li, Z.; Fan, L.; Chen, R.; Wu, X.; Li, J.; Zeng, W. A high-efficiency Fe2O3@ Microalgae composite for heavy metal removal from aqueous solution. J. Water Process Eng. 2020, 33, 101026. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Khan, M.; Shamim, S. Unraveling the underlying heavy metal detoxification mechanisms of Bacillus species. Microorganisms 2021, 9, 1628. [Google Scholar] [CrossRef]
- Yue, Z.-B.; Li, Q.; Li, C.-c.; Chen, T.-h.; Wang, J. Component analysis and heavy metal adsorption ability of extracellular polymeric substances (EPS) from sulfate reducing bacteria. Bioresour. Technol. 2015, 194, 399–402. [Google Scholar] [CrossRef]
- Ansari, M.I.; Malik, A. Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater. Bioresour. Technol. 2007, 98, 3149–3153. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. Bacterial cellulose of Gluconoacetobacter hansenii as a potential bioadsorption agent for its green environment applications. J. Biomater. Sci. Polym. Ed. 2014, 25, 2053–2065. [Google Scholar] [CrossRef]
- Yousefi, N.; Jones, M.; Bismarck, A.; Mautner, A. Fungal chitin-glucan nanopapers with heavy metal adsorption properties for ultrafiltration of organic solvents and water. Carbohydr. Polym. 2021, 253, 117273. [Google Scholar] [CrossRef] [PubMed]
- Alsharari, S.F.; Tayel, A.A.; Moussa, S.H. Soil emendation with nano-fungal chitosan for heavy metals biosorption. Int. J. Biol. Macromol. 2018, 118, 2265–2268. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Bahkali, A.H.; Khiyami, M.A.; Alfadul, S.M.; Wabaidur, S.M.; Alam, M.; Alfarhan, B.Z. Low cost biosorbents from fungi for heavy metals removal from wastewater. Sep. Sci. Technol. 2020, 55, 1766–1775. [Google Scholar] [CrossRef]
- Kumar, R.; Bishnoi, N.R.; Bishnoi, K. Biosorption of chromium (VI) from aqueous solution and electroplating wastewater using fungal biomass. Chem. Eng. J. 2008, 135, 202–208. [Google Scholar] [CrossRef]
- Lu, N.; Hu, T.; Zhai, Y.; Qin, H.; Aliyeva, J.; Zhang, H. Fungal cell with artificial metal container for heavy metals biosorption: Equilibrium, kinetics study and mechanisms analysis. Environ. Res. 2020, 182, 109061. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef]
- Karim, A.; Islam, M.A.; Khalid, Z.B.; Yousuf, A.; Khan, M.M.R.; Faizal, C.K.M. Microbial lipid accumulation through bioremediation of palm oil mill effluent using a yeast-bacteria co-culture. Renew. Energy 2021, 176, 106–114. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, I.; Cárdenas-González, J.F.; Rodríguez Pérez, A.S.; Oviedo, J.T.; Martínez-Juárez, V.M. Bioremoval of different heavy metals by the resistant fungal strain Aspergillus niger. Bioinorg. Chem. Appl. 2018, 2018, 3457196. [Google Scholar] [CrossRef]
- Massoud, R.; Khosravi-Darani, K.; Sharifan, A.; Asadi, G.H. Lead bioremoval from milk by Saccharomyces cerevisiae. Biocatal. Agric. Biotechnol. 2019, 22, 101437. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’souza, S. Removal of copper ions by the filamentous fungus, Rhizopus oryzae from aqueous solution. Bioresour. Technol. 2008, 99, 3829–3835. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Yousuf, A.; Karim, A.; Pirozzi, D.; Khan, M.R.; Ab Wahid, Z. Bioremediation of palm oil mill effluent and lipid production by Lipomyces starkeyi: A combined approach. J. Clean. Prod. 2018, 172, 1779–1787. [Google Scholar] [CrossRef]
- Han, R.; Li, H.; Li, Y.; Zhang, J.; Xiao, H.; Shi, J. Biosorption of copper and lead ions by waste beer yeast. J. Hazard. Mater. 2006, 137, 1569–1576. [Google Scholar] [CrossRef]
- Tan, X.; Zhu, S.; Show, P.L.; Qi, H.; Ho, S.-H. Sorption of ionized dyes on high-salinity microalgal residue derived biochar: Electron acceptor-donor and metal-organic bridging mechanisms. J. Hazard. Mater. 2020, 393, 122435. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for wastewater treatment—Conversion technologies and applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Yu, K.L.; Show, P.L.; Ong, H.C.; Ling, T.C.; Lan, J.C.-W.; Chen, W.-H.; Chang, J.-S. Microalgae from wastewater treatment to biochar–feedstock preparation and conversion technologies. Energy Convers. Manag. 2017, 150, 1–13. [Google Scholar] [CrossRef]
- Angın, D.; Sarikulce, S. The effect of activation temperature on properties of activated carbon prepared from wine industry pressing waste. Desalination Water Treat. 2017, 73, 373–379. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Suratman, M.; Mohammed, N.; Ahmad, W.A. Chromium (VI) removal from aqueous solution by untreated rubber wood sawdust. Desalination 2009, 244, 109–121. [Google Scholar] [CrossRef]
- Wong, K.; Lee, C.; Low, K.; Haron, M. Removal of Cu and Pb by tartaric acid modified rice husk from aqueous solutions. Chemosphere 2003, 50, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Jimoh, A.; Odigure, J. Heavy metals removal from industrial wastewater by activated carbon prepared from coconut shell. Res. J. Chem. Sci. 2013, 3, 3–9. [Google Scholar]
- Demirbas, E.; Dizge, N.; Sulak, M.; Kobya, M. Adsorption kinetics and equilibrium of copper from aqueous solutions using hazelnut shell activated carbon. Chem. Eng. J. 2009, 148, 480–487. [Google Scholar] [CrossRef]
- Issabayeva, G.; Aroua, M.K.; Sulaiman, N.M. Study on palm shell activated carbon adsorption capacity to remove copper ions from aqueous solutions. Desalination 2010, 262, 94–98. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Kongsuwan, A.; Patnukao, P.; Pavasant, P. Binary component sorption of Cu (II) and Pb (II) with activated carbon from Eucalyptus camaldulensis Dehn bark. J. Ind. Eng. Chem. 2009, 15, 465–470. [Google Scholar] [CrossRef]
- Khan, N.A.; Ibrahim, S.; Subramaniam, P. Elimination of heavy metals from wastewater using agricultural wastes as adsorbents. Malays. J. Sci. 2004, 23, 43–51. [Google Scholar]
- Lo, S.-F.; Wang, S.-Y.; Tsai, M.-J.; Lin, L.-D. Adsorption capacity and removal efficiency of heavy metal ions by Moso and Ma bamboo activated carbons. Chem. Eng. Res. Des. 2012, 90, 1397–1406. [Google Scholar] [CrossRef]
- Park, H.G.; Kim, T.W.; Chae, M.Y.; Yoo, I.-K. Activated carbon-containing alginate adsorbent for the simultaneous removal of heavy metals and toxic organics. Process Biochem. 2007, 42, 1371–1377. [Google Scholar] [CrossRef]
- Üçer, A.; Uyanik, A.; Aygün, Ş. Adsorption of Cu (II), Cd (II), Zn (II), Mn (II) and Fe (III) ions by tannic acid immobilised activated carbon. Sep. Purif. Technol. 2006, 47, 113–118. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Matsumoto, Y.; Machida, M. Adsorption of Zn (II) and Cd (II) ions onto magnesium and activated carbon composite in aqueous solution. Appl. Surf. Sci. 2010, 256, 1619–1623. [Google Scholar] [CrossRef]
- Ahn, C.K.; Park, D.; Woo, S.H.; Park, J.M. Removal of cationic heavy metal from aqueous solution by activated carbon impregnated with anionic surfactants. J. Hazard. Mater. 2009, 164, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; HPS, A.K.; Mistar, E.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Stor, M.; Czelej, K.; Krasiński, A.; Gradoń, L. Exceptional Sorption of Heavy Metals from Natural Water by Halloysite Particles: A New Prospect of Highly Efficient Water Remediation. Nanomaterials 2023, 13, 1162. [Google Scholar] [CrossRef]
- Karim, A.; Raji, Z.; Habibi, Y.; Khalloufi, S. A review on the hydration properties of dietary fibers derived from food waste and their interactions with other ingredients: Opportunities and challenges for their application in the food industry. Crit. Rev. Food Sci. Nutr. 2023, 1–35. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef]
- Fakhre, N.A.; Ibrahim, B.M. The use of new chemically modified cellulose for heavy metal ion adsorption. J. Hazard. Mater. 2018, 343, 324–331. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J. Environ. Sci. 2013, 25, 933–943. [Google Scholar] [CrossRef]
- Peng, X.-W.; Zhong, L.-X.; Ren, J.-L.; Sun, R.-C. Highly effective adsorption of heavy metal ions from aqueous solutions by macroporous xylan-rich hemicelluloses-based hydrogel. J. Agric. Food Chem. 2012, 60, 3909–3916. [Google Scholar] [CrossRef]
- Lian, Y.; Zhang, J.; Li, N.; Ping, Q. Preparation of hemicellulose-based hydrogel and its application as an adsorbent towards heavy metal ions. BioResources 2018, 13, 3208–3218. [Google Scholar] [CrossRef]
- Hernández-Martínez, A.R.; Molina, G.A.; Jiménez-Hernández, L.F.; Oskam, A.H.; Fonseca, G.; Estevez, M. Evaluation of inulin replacing chitosan in a polyurethane/polysaccharide material for Pb2+ removal. Molecules 2017, 22, 2093. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, T.-T.; Rao, Q.-Q.; Shui, S.-W.; Li, W.-W.; He, H.-B.; Yao, R.-S. Fabrication of mesoporous lignin-based biosorbent from rice straw and its application for heavy-metal-ion removal. J. Environ. Sci. 2017, 53, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, R.; Pang, Y.; Qiu, X.; Yang, D. Microwave-assisted synthesis of high carboxyl content of lignin for enhancing adsorption of lead. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 187–194. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, S.; Liu, P.; Gao, W.; Li, J.; Mo, L. Adsorption of Cu (II) ions in aqueous solution by aminated lignin from enzymatic hydrolysis residues. RSC Adv. 2017, 7, 44751–44758. [Google Scholar] [CrossRef]
- Shen, B.; Guo, Z.; Huang, B.; Zhang, G.; Fei, P.; Hu, S. Preparation of hydrogels based on pectin with different esterification degrees and evaluation of their structure and adsorption properties. Int. J. Biol. Macromol. 2022, 202, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Arachchige, M.P.M.; Mu, T.; Ma, M. Effect of high hydrostatic pressure-assisted pectinase modification on the Pb2+ adsorption capacity of pectin isolated from sweet potato residue. Chemosphere 2021, 262, 128102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yang, J.; Hu, D.; Wang, Z. Removing Pb2+ with a pectin-rich fiber from sisal waste. Food Funct. 2021, 12, 2418–2427. [Google Scholar] [CrossRef]
- Garcia-Reyes, R.B.; Rangel-Mendez, J.R. Contribution of agro-waste material main components (hemicelluloses, cellulose, and lignin) to the removal of chromium (III) from aqueous solution. J. Chem. Technol. Biotechnol. 2009, 84, 1533–1538. [Google Scholar] [CrossRef]
- Kumari, P.; Sharma, P.; Srivastava, S.; Srivastava, M. Arsenic removal from the aqueous system using plant biomass: A bioremedial approach. J. Ind. Microbiol. Biotechnol. 2005, 32, 521–526. [Google Scholar] [CrossRef]
- Pejic, B.; Vukcevic, M.; Kostic, M.; Skundric, P. Biosorption of heavy metal ions from aqueous solutions by short hemp fibers: Effect of chemical composition. J. Hazard. Mater. 2009, 164, 146–153. [Google Scholar] [CrossRef]
- Hu, G.; Huang, S.; Chen, H.; Wang, F. Binding of four heavy metals to hemicelluloses from rice bran. Food Res. Int. 2010, 43, 203–206. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Li, J.; Salamh, Y.; Al-Laqtah, N.; Walker, G.; Ahmad, M.N. Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J. Hazard. Mater. 2010, 176, 510–520. [Google Scholar] [CrossRef]
- Lee, B.-G.; Rowell, R.M. Removal of heavy metal ions from aqueous solutions using lignocellulosic fibers. J. Nat. Fibers 2004, 1, 97–108. [Google Scholar] [CrossRef]
- Kartel, M.T.; Kupchik, L.A.; Veisov, B.K. Evaluation of pectin binding of heavy metal ions in aqueous solutions. Chemosphere 1999, 38, 2591–2596. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, M.Y.; Kolenchenko, E.; Khotimchenko, Y.S. Zinc-binding activity of different pectin compounds in aqueous solutions. J. Colloid Interface Sci. 2008, 323, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Priya, A.; Kumar, P.S.; Hoang, T.K.; Sekar, K.; Chong, K.Y.; Khoo, K.S.; Ng, H.S.; Show, P.L. A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review. Chemosphere 2022, 303, 135146. [Google Scholar] [CrossRef] [PubMed]
- Melezhyk, A.; Kotov, V.; Tkachev, A. Optical properties and aggregation of graphene nanoplatelets. J. Nanosci. Nanotechnol. 2016, 16, 1067–1075. [Google Scholar] [CrossRef]
- Darwish, A.D. Fullerenes. Annu. Rep. Sect. A (Inorg. Chem.) 2013, 106, 356–375. [Google Scholar] [CrossRef]
- Burakov, A.; Romantsova, I.; Kucherova, A.; Tkachev, A. Removal of heavy-metal ions from aqueous solutions using activated carbons: Effect of adsorbent surface modification with carbon nanotubes. Adsorpt. Sci. Technol. 2014, 32, 737–747. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; González-Melgoza, L.L.; García-Depraect, O. Ongoing progress on novel nanocomposite membranes for the separation of heavy metals from contaminated water. Chemosphere 2021, 270, 129421. [Google Scholar] [CrossRef]
- Chen, M.; Shafer-Peltier, K.; Randtke, S.J.; Peltier, E. Modeling arsenic (V) removal from water by micellar enhanced ultrafiltration in the presence of competing anions. Chemosphere 2018, 213, 285–294. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Mazari, S.A.; Nizamuddin, S.; Karri, R.R. Magnetic nanoadsorbents’ potential route for heavy metals removal—A review. Environ. Sci. Pollut. Res. 2020, 27, 24342–24356. [Google Scholar] [CrossRef]
- Singh, N.; Nagpal, G.; Agrawal, S. Water purification by using adsorbents: A review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Abu-Nada, A.; McKay, G.; Abdala, A. Recent advances in applications of hybrid graphene materials for metals removal from wastewater. Nanomaterials 2020, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Mainali, B.; Angove, M.J. Manganese oxides and their application to metal ion and contaminant removal from wastewater. J. Water Process Eng. 2018, 26, 264–280. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Insight studies on metal-organic framework nanofibrous membrane adsorption and activation for heavy metal ions removal from aqueous solution. ACS Appl. Mater. Interfaces 2018, 10, 18619–18629. [Google Scholar] [CrossRef]
- Saleem, H.; Rafique, U.; Davies, R.P. Investigations on post-synthetically modified UiO-66-NH2 for the adsorptive removal of heavy metal ions from aqueous solution. Microporous Mesoporous Mater. 2016, 221, 238–244. [Google Scholar] [CrossRef]
- Khoso, W.A.; Haleem, N.; Baig, M.A.; Jamal, Y. Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFN’s). Sci. Rep. 2021, 11, 3790. [Google Scholar] [CrossRef]
- Houston, R.L.; Waclawik, E.R.; Sarina, S. Application of Alumina Nanofibers as Adsorbents for the Removal of Mercury (II) and Lead (II) from Aqueous Solutions. Minerals 2023, 13, 654. [Google Scholar] [CrossRef]
- Sharma, A.; Anjana; Rana, H.; Goswami, S. A comprehensive review on the heavy metal removal for water remediation by the application of lignocellulosic biomass-derived nanocellulose. J. Polym. Environ. 2022, 30, 1–18. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kadam, A.; Shinde, S.; Saratale, R.G.; Patra, J.; Ghodake, G. Recent developments in nanotechnology transforming the agricultural sector: A transition replete with opportunities. J. Sci. Food Agric. 2018, 98, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, G.; Soleimanian, Y.; Mhamadi, M.; Turgeon, S.L.; Khalloufi, S. The applications of conventional and innovative mechanical technologies to tailor structural and functional features of dietary fibers from plant wastes: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2149–2199. [Google Scholar] [CrossRef] [PubMed]
- Mautner, A. Nanocellulose water treatment membranes and filters: A review. Polym. Int. 2020, 69, 741–751. [Google Scholar] [CrossRef]
- Camparotto, N.G.; Paixão, G.R.; de Vargas Brião, G.; Oliveira, R.L.; Prediger, P.; Vieira, M.G.A. Comparative effect of mesoporous carbon doping on the adsorption of pharmaceutical drugs in water: Theoretical calculations and mechanism study. Environ. Toxicol. Pharmacol. 2023, 99, 104105. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Ali, S.S.; Hossain, S.S.; Asif, M. A review of the dynamic mathematical modeling of heavy metal removal with the biosorption process. Processes 2022, 10, 1154. [Google Scholar] [CrossRef]
- Hafsa, N.; Rushd, S.; Al-Yaari, M.; Rahman, M. A generalized method for modeling the adsorption of heavy metals with machine learning algorithms. Water 2020, 12, 3490. [Google Scholar] [CrossRef]
- Rani, L.; Kaushal, J.; Srivastav, A.L.; Mahajan, P. A critical review on recent developments in MOF adsorbents for the elimination of toxic heavy metals from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 44771–44796. [Google Scholar] [CrossRef]
- Dotto, G.L.; McKay, G. Current scenario and challenges in adsorption for water treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).