Abstract
This review provides an overview of the biotransformation of limonene and α-pinene, which are commonly found in wood residues and citrus fruit by-products, to produce high-value-added products. Essential oils derived from various plant parts contain monoterpene hydrocarbons, such as limonene and pinenes which are often considered waste due to their low sensory activity, poor water solubility, and tendency to autoxidize and polymerise. However, these terpene hydrocarbons serve as ideal starting materials for microbial transformations. Moreover, agro-industrial byproducts can be employed as nutrient and substrate sources, reducing fermentation costs, and enhancing industrial viability. Terpenes, being secondary metabolites of plants, are abundant in byproducts generated during fruit and plant processing. Microbial cells offer advantages over enzymes due to their higher stability, rapid growth rates, and genetic engineering potential. Fermentation parameters can be easily manipulated to enhance strain performance in large-scale processes. The economic advantages of biotransformation are highlighted by comparing the prices of substrates and products. For instance, R-limonene, priced at US$ 34/L, can be transformed into carveol, valued at around US$ 530/L. This review emphasises the potential of biotransformation to produce high-value products from limonene and α-pinene molecules, particularly present in wood residues and citrus fruit by-products. The utilisation of microbial transformations, along with agro-industrial byproducts, presents a promising approach to extract value from waste materials and enhance the sustainability of the antimicrobial, the fragrance and flavour industry.
1. Introduction
Essential oils can be derived from various plant parts, including flowers, fruits, leaves, buds, seeds, twigs, bark, herbs, wood, and roots. These oils are predominantly composed of monoterpene hydrocarbons [1]. These terpene hydrocarbons, such as limonene, pinenes, and terpenes, serve as ideal starting materials for microbial transformations to obtained high value molecules such as carveol, terpineol, verbenol and verbenol. Microorganisms and enzymes can act as catalysts in two different processes: de novo synthesis and biotransformation of natural precursors. De novo synthesis involves utilising the entire metabolic pathway of microorganisms to produce a combination of flavour compounds, whereas biotransformation focuses on specific reactions that lead to the production of a major compound. In de novo synthesis, microorganisms metabolise carbohydrates, fats, and proteins, converting the breakdown products into flavour components [2,3]. However, this process yields only trace amounts of flavours and is not economically viable for industrial production due to low concentrations of the desired compounds [4]. In biotransformation, a precursor is introduced into the process, inducing the microorganism to follow a specific pathway and produce the final product through one or two chemical reactions [5]. Additionally, agro-industrial byproducts can be utilised as nutrient and substrate sources in fermentation processes, reducing fermentation costs and enabling industrial viability [6]. Terpenes are noteworthy substrates for biotransformation, as they are secondary metabolites of plants and can be found in byproducts generated during fruit and plant processing. Monoterpenes (such as limonene, α-pinene, and β-pinene) and sesquiterpenes (such as valencene and farnesene) are common volatile compounds found in various essential oils. Structurally, they are closely related to aroma compounds of significant interest in the industry, requiring only a few chemical reactions to obtain high-value products [7] (Table 1). From an economic perspective, the advantages of biotransformation become evident when comparing the prices of substrates and products. For example, in 2015, R-limonene has a reference price of US$34/L, while its oxygenated form, carveol, is priced around US$530/L [7,8]. Selecting the appropriate biocatalyst is another crucial step in natural aroma production. Microbial cells offer advantages over enzymes, as they possess higher stability and do not require additional cofactors for the reaction to occur. Microorganisms also exhibit rapid growth rates, and fermentation parameters can be easily manipulated, allowing for genetic engineering to enhance strain performance in large-scale processes [9]. The objective of this review is to provide approaches and strategies to improve the biotransformation of two of the most promising biomolecules found in agroforestry residual biomass. The review will discuss general knowledge of terpenes, recent advances of biotransformation of limonene and alpha-pinene, strategies to improve the biotransformation of these two molecules using solid-state fermentation and finally various extraction technology to recover the produce molecules.

Table 1.
Chemical composition of balsam fir, black spruce, jack pine and citrus essential oils.
2. A Brief Description of Terpenes
Conifers are the most abundant source of terpene compounds in nature [13]. In conifers, volatile compounds are contained in the oleoresin. This is a mixture of volatile monoterpenoids and sesquiterpenoids (turpentine), and non-volatile diterpenoids which represent the resin acids [14]. Monoterpenoids are low molecular weight compounds (136 Da for hydrocarbons and up to 200 Da for oxygenated derivatives) (Figure 1). Highly volatile, they are also odorants, naturally emitted by plants to attract pollinators or repel predators. They can be captured by processes such as hydrodistillation, to form extracts known as essential oils. Terpenes are often removed from essential oils as undesirable components, whereas synthetic oxy-functionalised derivatives find broad applications in flavours and fragrances industries. In addition to their perfuming power, these molecules respectively possess medicinal properties, which in synergy make essential oils renowned in aromatherapy. Volatile terpenoids also appear to be involved in protecting plants against heat and oxidative stress [14]. Their role as a communication molecule is thought to attract pollinating insects, thus aiding reproduction, but they also act as “signal” molecules to preserve surrounding trees in the event of an attack [15]. Despite the diversity of terpene compounds, they share a common biosynthetic pathway. This pathway comprises four stages [16]. Briefly, the first involves the formation of isopentenyl pyrophosphate (IPP), the C5 unit of biological isoprene. The main route for the synthesis of IPP and its allylic isomer, dimethylallyl pyrophosphates (DMAPP), is via the mevalonate pathway. A second synthesis pathway is also present, the methylerythritol phosphate (MEP/DOXP) pathway. In the second step, C5 units condense to generate three larger prenylpyrophosphates (GPP, FPP and GGPP). Subsequently, in the third step, these three pyrophosphates undergo a wide range of cyclisation and rearrangement to produce the parent carbon skeletons for each terpene class. The final stage encompasses various oxidation, reduction, conjugation and other transformations that convert the carbon skeletons into thousands of distinct terpene metabolites. Although several in vitro studies have shown that many monoterpenes and diterpenes have significant bactericidal effects, it is not always easy to achieve the same efficacy in vivo due to the multiple presence of molecules present in extracted oils. As a result, microbial biotransformation is a pertinent strategy to overcome difficulties and problems arising from the chemical synthesis, to have access to regiospecifc and stereospecifc compounds and explain the inactivity of essential oils or chemicals against some microorganisms.
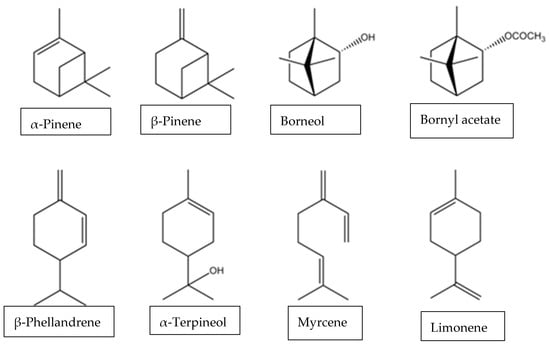
Figure 1.
Some examples of monoterpenoides.
3. Recent Advances in Biotransformation of Terpenes: Study Case of Limonene and Alpha Pinene
Currently, the primary production of aroma compounds relies on chemical methods due to the challenges associated with direct extraction from natural sources. Natural extraction often results in complex mixtures with low concentrations and limited availability based on geography and seasonality [17]. Essential oils typically yield quantities ranging from 0.005% to 10%, with many falling below 1% [18]. The composition of essential oils varies, and they can contain over 100 different components, with major compounds often comprising more than 85% of the oil. The increasing focus on health, nutrition, and the desire to avoid synthetic chemicals in food has spurred the development of biotechnological processes to produce flavour compounds that can be classified as “natural” [19]. Some companies are actively working on biotechnological methods for aroma production. For instance, the Amyris Company utilises a genetically modified strain of Saccharomyces cerevisiae to produce β-farnesene from sugarcane. This sesquiterpene can also serve as a precursor to produce other aroma compounds in the fragrance industry [8]. Evolva and Isobionics companies have reported the biotechnological production of valencene, which can be further oxidised to nootkatone. These companies’ market nootkatone as a flavouring agent and a potential natural insect repellent. Isobionics has developed efficient microbial strains for terpene biotransformation. Based on the above examples, bioprocessing of limonene and pinenes, mainly found in many essential oils and citrus fruits, offers significant potential for biotransformation [20].
3.1. Limonene Biotransformation
Limonene is the most abundant naturally occurring monoterpene found in orange peel oil, comprising up to 90% of its composition [21]. Due to its abundance and low cost, limonene has been extensively studied as a precursor to produce high-value derivatives, offering a potential strategy for enhancing the commercial value of agro-industrial residues like orange peel oil [22] (Figure 2). The applications of limonene as a flavour and fragrance additive in food products, cosmetics, household cleaning products, and textiles are well known [23]. Additionally, limonene finds utility as a solvent, a feedstock for fine chemicals, a precursor for polymeric biomaterials, and as an active ingredient in medicine [24]. Currently, most of the limonene is obtained from citrus rinds, a significant byproduct generated during fruit processing in citrus juice industries [25]. However, many citrus juice industries, particularly those in developing countries, lack the necessary infrastructure and technology for on-site recycling of large quantities of citrus waste [26]. Consequently, a substantial number of citrus rinds are disposed of in landfills rather than being utilised for limonene extraction. Limonene is extensively studied as a precursor in the biotechnological production of monoterpenoids, as it can be transformed into various value-added compounds such as carvone, carveol, perillyl alcohol, terpineols, menthol, and pinenes (Table 2). It serves as a cyclic monoterpene with multiple industrial applications as a bio-solvent, in surfactant formulations, as an antibacterial agent, and as a pesticide in nutrition, as well as a precursor for fine chemicals in the food, perfume, medicine, agrochemical, and oral hygiene industries [27]. Given the structural similarity between limonene and oxygenated monoterpenoids with pleasant fragrances, such as perillyl alcohol, carveol, carvone, menthol, and α-terpineol, limonene can be used as a precursor for the synthesis of these flavour compounds [24]. Several studies have reported the biotransformation of limonene into α-terpineol, using microorganisms such as Cladosporium sp., Pseudomonas gladioli, and an α-terpineol dehydratase isolated from the same strain [28]. Orange peel oil analysis has revealed that D-limonene is the major component, accounting for 96.1% of the total content [29]. The biotransformation of D-limonene to a-terpineol was conducted using a strain of Penicillium digitatum NRRL 1202. Experiments employed two distinct media: malt yeast broth (MYB) and malt extract broth (MEB). Among them, the MYB medium resulted in the most efficient bioconversion of D-limonene to a-terpineol. Optimisation of the bioconversion process using synthetic media supplemented with orange peel oil demonstrated increased yields of α-terpineol, reaching 79% at 3 h of fermentation and 95.5% after 7 h with the addition of MYB in the medium. This demonstrates that supplementation of synthetic medium (medium rich of reducing sugar, minerals and organic nitrogen) allows the microorganism to perform and increase the biotransformation of limonene. However, it should be noted that the use of expensive synthetic media and the concentration of orange peel oil were limitations of this study as the cost to scale-up the process was high because of the high cost of nutrients. Instead, the use of residual medium as wastewater coming from agroindustry could be a way to decrease the cost of the process and maintain high performance of biotransformation [29]. Molina et al. [7] investigated the bioconversion of limonene using two isolated strains, resulting in the production of high-value derivatives carvone and α-terpineol. The aspergillus LB-2038 strain achieved a higher concentration of carvone (47 mg/L), while the Penicillium LB-2025 strain stood out in α-terpineol production (10 mg/L). However, when 1% limonene supplementation was attempted, it affected initial biomass development and the microorganisms were unable to metabolise the substrates, indicating the toxicity of a high concentration of limonene to microbial cells. To mitigate the toxicity, a two-phase system with an organic phase containing limonene and an aqueous phase for the catalyst was implemented in this study. It is worth noting that comparing different strains for biotransformation results could have provided valuable insights [30]. Roy and Jayati [12] infected mandarin orange peels with Aspergillus oryzae and Penicillium species, resulting in GC-MS analysis showing a ratio of 10% beta pinene and 70% limonene for the infected orange peels, and 30% beta pinene, 27% beta-myrcene, and 32% limonene for the Penicillium-infected peel oil. The extraction of pure limonene oil with petroleum ether was performed in this study, but conducting a solid-state fermentation with different particle sizes of orange peels could have been an alternative approach to avoid early solvent addition and limonene toxicity. Furthermore, the optimisation of inoculum size and the use of multiple strains could have been beneficial for the optimisation of limonene biotransformation. Another study conducted by Bicas et al. [6] demonstrated that Pseudomonas Rhodesia and Pseudomonas fluorescens can metabolise limonene, with the latter exhibiting versatility and the ability to synthesise α-terpineol at high concentrations (11 g/L) [6]. The conversion of refining limonene to α-terpineol using fungal biocatalysts has been reported, with Penicillium and Fusarium oxysporum achieving yields of 3.45 g/L and 2.4 g/L, respectively. The conversion of limonene obtained from citrus extraction to carvone and carveol by Pseudomonas aeruginosa and Rhodococcus opacus resulted in yields of 0.63 g/L and 2.4 g/L, respectively [31]. From an economic perspective, limonene has a weighted average price of US$ 34/L, while its oxygenated derivatives such as carveol, perillyl alcohol, and carvone have reference prices of US$ 529/L, US$ 405/L, and US$ 350/L, respectively [8]. These monoterpenoids are widely used aroma compounds found in natural sources at low concentrations, making biotechnological approaches crucial for large-scale production. Therefore, the utilisation of limonene as a substrate to produce value-added derivatives through biotransformation is economically promising.
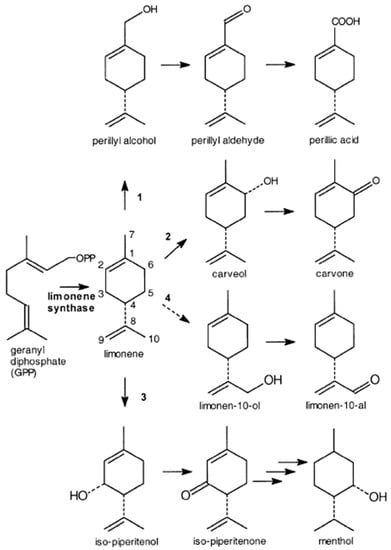
Figure 2.
Biosynthetic pathways in plants starting from limonene.

Table 2.
Monoterpenes used as substrates in bioprocesses for the production of natural flavour compounds.
3.2. Biotransformation of α-Pinene
One of the prominent antimicrobial monoterpenes found in various essential oils is α-pinene. This natural compound holds significant importance as it is widely utilised in the food and fragrance industries, pharmaceuticals, fine chemicals, and as a renewable fuel source [33]. Currently, industrial production of α-pinene primarily relies on tree tapping (gum turpentine) or as a byproduct of paper pulping (crude sulfate turpentine, CST). However, extracting α-pinene from trees is laborious, inefficient, and depletes natural resources due to its low content [34]. Hence, exploring sustainable technologies for the biotransformation of α-pinene would be a promising avenue (Figure 3). In a study conducted by [35], a cold-adapted fungus, Chrysosporium pannorum, was identified as a biocatalyst for the oxidation of refining α-pinene. Gas chromatography-mass spectrometry (GC-MS) analysis of the fermentation process revealed the production of two commercially valuable molecules, verbenol and verbenone, through the biotransformation of α-pinene. The oxidative activity of C. pannorum was observed over a wide temperature range (5–25 °C), with the optimum temperature being 10 °C to produce these bioactive compounds. By sequentially adding the substrate over a period of three days, the yield of verbenol and verbenone significantly increased to 722 mg/L and 176 mg/L, respectively, resulting in a twofold enhancement compared to a single supply of α-pinene. The total concentration of conversion products in the culture medium reached 1.33 g/L. Another study by [36] focused on the biotransformation of α-pinene to (+)-α-terpineol using Candida tropicalis MTCC 230. Under continuous agitation at 30 °C for 96 h, a concentration of 0.5 g/L of (+)-α-terpineol was obtained. The strain demonstrated efficient bioconversion capabilities, achieving a conversion rate of 77% and a production of 0.43 g/L under the specified conditions. Rottava et al. [37] reported the metabolism of α-pinene by the yeast Hormonema sp. UOFS Y-0067, isolated from pine tree samples. After 72 h, this yeast produced transverbenol (0.4 g/L) and verbenone (0.3 g/L) through α-pinene biotransformation. It is worth noting that all these experiments were conducted using pure or refine α-pinene. To the best of our knowledge, no research has been conducted on the biotransformation of α-pinene using residual wood, such as tree bark. Further investigations should include GC-MS analysis of different tree species or parts to identify sources with high α-pinene content. Based on the market value of the molecules obtained by the transformation of α-pinene, it would be important to study the technological feasibility of the biotransformation of α-pinene contained in wood residues rich in pinene. The key parameters for a good microbial fermentation in a semi-solid medium are widely studied in the literature. The particle size and specific surface area are key parameters to ensure a good contact surface between the microorganisms and the molecules to be biotransformed. Also, the particle size is an important factor in oxygen transfer during solid or semi-solid fermentation. Other important factors are the moisture content, the inoculum size and the fermentation temperature [38]. Regarding terpenes, the volatilisation of molecules and the presence of lignin can be factors slowing down the yield of molecules obtained. The following parts will discuss in detail the current practices to address these challenges.
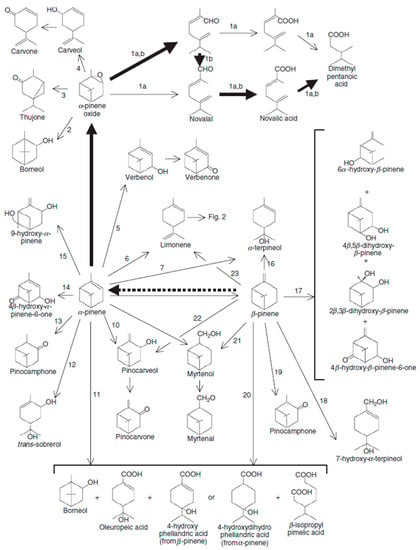
Figure 3.
Degradation pathways of alpha pinene by Pseudomonas.
4. Avenues and Approaches for Improving the Biotransformation of Limonene and Alpha Pinene
4.1. Application of Solid-State Fermentation for Biotransformation from Filamentous Fungi
Solid-state fermentation corresponds to the growth of aerobic or anaerobic microorganisms on moist solid particles in the absence or near absence of free water [39]. These microorganisms grow on a solid matrix to which a liquid phase is bound, and a gaseous phase is trapped within or between these particles. Most solid-state fermentation processes involve filamentous fungi. However, there are a few processes that incorporate bacteria and yeast. Solid-state fermentation can involve a pure culture of organisms or the cultivation of several strains inoculated simultaneously or successively [40]. Current work on solid-state fermentation focuses on the application of these processes in the development of bioprocesses, i.e., bioremediation or the production of high value-added products. The latter are composed of biologically active secondary metabolites including antibiotics, aromatic compounds (limonene and pinene derivatives), alkaloids, biofertilizers, enzymes, and so on. In the case of the biotransformation of limonene or alpha pinene, it would be very interesting to carry out fermentation in a solid medium, as this brings the molecules present in the substrate into contact with the biotransforming strains. Also, the possibility of carrying out mixed cultures would enable them to produce different products and increase the yield of the process. Last but not least, this strategy avoids the need to use synthetic media to initiate the fermentation of the microorganisms and reduces or eliminates the use of solvents at the start of the process to extract the oil or juice containing the molecules to be biotransformed. Solid-state fermentation-based processes offer potential advantages in the biotransformation of bioactive molecules contained in forest and agricultural residues.
4.2. Selection of Microorganisms
Synthetic limonene or pinenes are often used instead of residues for biotransformation [41]. It would be of interest to use known strains to carry out solid or semi-solid fermentations of residual media containing limonene (citrus like orange, lemon) or pinene (white birch bark). This practice, rather than the use of oils, would minimise the toxicity of pinene or limonene at the start of fermentation. In addition, the use of solvents for juice extraction will be eliminated at the start of the process. Another possible step would be to isolate microbial strains from the residual wood and orange media. Indeed, the natural presence of strains in these residues testifies to their ability to withstand toxic doses of the molecules in question, as well as their capacity to metabolise them. Among strategies, bioprospecting is used by some authors in an attempt to identify new strains of microorganisms for the production of bioaromas [42]. Particularly, soil samples have been frequently used for the prospecting of microorganisms that are potential bio-transformers of terpene compounds. To illustrate this approach, some examples of microorganisms isolated from soil may be cited: Bacillus fusiformis, which was able to convert isoeugenol to vanillin (production of 8.10 g L1 after 72 h) [43]; Bacillus pumilus, which was also able to convert isoeugenol to vanillin (production of 3.75 g L1 after 150 h) [44]; Pseudomonas putida, which was able to convert isoeugenol to vanillic acid (98% of molar conversion after 40 min); Bacillus subtillis, which was able to convert isoeugenol to vanillin (production of 0. 9 g L1 after 48 h) [45] and Chrysosporium pannorum, which was able to convert a-pinene to verbenone and verbenol. Rottava et al. [37] have isolated strains from effluents of citrus industry as well as other samples (soil from the plantation of citrus, citrus fruits, and citrus leaves). Of the 405 strains isolated [37], eight were able to bioconvert limonene and fifteen converted pinene, generating α-terpineol as a product in both cases. The transformation of limonene to α-terpineol typically involves the oxidation of limonene. The first step is the hydroxylation of limonene, which can occur at different positions to yield a variety of alcohols. This is typically catalysed by cytochrome P450 monooxygenases and the formation of Limonene-1,2-diol: One of the primary alcohols formed from the hydroxylation of limonene is limonene-1,2-diol. Then the conversion to Terpineol: Limonene-1,2-diol can then be dehydrated to yield α-terpineol. This step can be enzymatically catalysed by dehydratase enzymes [46]. While the biotransformation of α-Pinene occurs typically with the oxidation of α-pinene to yield α-pinene oxide. This reaction is facilitated by the enzyme cytochrome P450 monooxygenase or similar enzymes present in Pseudomonas or other fungi. The α-pinene oxide can undergo a rearrangement to form generally verbenol. The verbenol formed can then be hydroxylated to produce terpineol. This hydroxylation can be mediated by specific hydroxylase enzymes present in fungi [46].
Bicas et al. [9] have obtained 248 microorganisms, of which seventy were developed in a medium containing limonene as the sole source of carbon. However, as reported by Molina et al. [30], there are several challenges to be overcome to enable the production of aromas by biotransformation, among them the high toxicity of both the substrate and the product and the low yields obtained. According to these authors, such difficulties can be overcome with the isolation and selection of strains as the studies mentioned above.
4.3. Pathways of Terpenes Biotransformation
When bacteria encounter monoterpenes, they must address the toxic effects associated with them [47]. To prevent the accumulation of monoterpenes in the cell and cytoplasmic membrane, bacteria modify their membrane lipids, transform monoterpenes, and employ active transport via efflux pumps [48]. At subtoxic concentrations, microorganisms utilise monoterpenes as their sole carbon and energy source. While numerous microbial cultures have reported monoterpene transformations over the past 50 years, the biochemical pathways involved have often been undisclosed. Furthermore, only a small fraction of the investigated strains has been deposited in culture collections. Without detailed knowledge of the genes or the availability of strains, the observations from biotransformation experiments hold limited value for future studies.
4.3.1. Bicyclic Monoterpenes
The isomers α-pinene and β-pinene (C10H16) are the primary components of wood resins, particularly conifers. Pseudomonas Rhodesia (CIP 107491) and P. fluorescens (NCIMB 11671) were found to grow on α-pinene as the sole carbon source. α-pinene undergoes oxidation to α-pinene oxide via a NADH-dependent α-pinene oxygenase and then experiences ring cleavage by a specific α-pinene oxidelyase, yielding isonovalal, which is subsequently isomerised to novalal [6]. The cleavage reaction of α-pinene oxide has also been observed in Nocardia sp. strain P18.3 [37]. An alternative degradation pathway for pinene via a monocyclic p-menthene derivative has been described in Pseudomonas sp. strain PIN [49]. Bacillus pallidus BR425 degrades α-pinene and β-pinene, yielding limonene and pinocarveol. While α-pinene is transformed into limonene and pinocarveol, β-pinene exclusively produces pinocarveol. Both intermediates can be further converted into carveol and carvone. Although the activity of specific monooxygenases has been suggested, experimental evidence is lacking [5]. Serratia marcescens utilises α-pinene as the sole carbon source, leading to the formation of trans-verbenol. In glucose and nitrogen-supplemented medium, this strain produces α-terpineol. The two oxidation products are considered dead-end products as they accumulate in cultures [6]. It should be noted that for many biotransformation studies, caution must be exercised as monoterpenes often contain impurities and oxidation products that can serve as substrates, resulting in traces of monoterpene and monoterpenoid transformation products that are not further metabolised.
4.3.2. Monocyclic Monoterpenes
Limonene (C10H16) is the most abundant monocyclic monoterpene and the second most abundant volatile organic compound (VOC) indoors, after toluene [23]. It is a major component of essential oils derived from citrus plants such as lemon and orange. Biotransformation of limonene start with activation of geranyldiphosphate by linalool synthase, which triggers ionization and rearrangement of gernayldiphosphate. The positively charged geranyl cation will be hydrolyzed by to form linalool and myrcene. For GPP to form limonene or terpineol, it must be activated in its cyclic form to give alpha terpinyl cation [50]. Rhodococcus erythropolis DCL14 transforms limonene into limonene-1,2-epoxide, which is then converted to limonene-1,2-diol by a limonene-1,2-monooxygenase and a limonene-1,2-epoxide hydrolase, respectively. A specific dehydrogenase forms the ketone, 1-hydroxy-2-oxolimonene, which is further oxidised to a lactone by a 1-hydroxy-2-oxolimonene 1,2-monooxygenase. Enzyme activities were only detected in limonene-induced cells, indicating tight regulation of limonene degradation. R. erythropolis DCL14 possesses a second pathway for limonene degradation in which limonene is hydroxylated to trans-carveol by a NADPH-dependent limonene 6-monooxygenase. R. opacus PWD4 follows the same pathway, converting limonene to trans-carveol. Biomass from a glucose-toluene chemostat culture of R. opacus PWD4 transformed limonene into trans-carveol, which was further oxidised to carvone by a trans-carveol dehydrogenase [31]. In Pseudomonas gladioli, studies on limonene metabolism identified α-terpineol and perillyl alcohol as major metabolites. However, none of the involved enzymes have been purified or further characterised. A α-terpineol dehydratase from P. gladioli was isolated and partially purified. The hydration reaction of limonene to form α-terpineol as the sole product was observed [51]. Geobacillus stearothermophilus (formerly Bacillus) has been shown to grow on limonene as the sole carbon source. The main transformation product of limonene in this case is perillyl alcohol, while α-terpineol and perillyl aldehyde are present in minor concentrations.
4.4. Improvements in Physical Pretreatment of Wood and Citrus Residues
4.4.1. Pretreatment and Conditioning
Pretreatment of forest and agricultural residual materials is an important link in the emerging circular economy in general and bioprocessing. Optimising this component will enable us to expand the range of high value-added products. Pretreatment methods can be thermal, chemical, physical or biological. Whatever the method, pretreatments aim to make biomass manageable during mechanical, microbiological or chemical transformations [52]. Pretreatment of forest and agricultural residues has an influence on the quantity and quality of terpenes extracted. Indeed, grinding is a technique that reduces the size of the biomass to improve the contact surface with steam during extraction. However, there may be losses in terms of terpenes yield due to adsorption on the mill walls or that may be volatilised in the open air due to reaching an equilibrium point with ambient temperature [53]. Nevertheless, the conditioning of fragmented forest and agricultural residues is accompanied by exothermic reactions. These reactions lead to material losses through fungal and bacterial attack that can reach around 25% and generate a reduction in energetic power [54]. Degradation phenomena generally depend on forest species, moisture content, particle size and storage time. The study by Douville et al. [54] showed that fungal attacks lead to weight losses in wood, as they degrade chemical compounds such as lignin and cellulose to consume their carbon molecules. Consequently, it is necessary to process the biomass immediately after harvesting and, to avoid the risk of bacterial or fungal degradation of the biomass and inhibit enzymatic activity after harvesting [55].
4.4.2. Thermal Pretreatment
According to several studies, torrefaction and pyrolysis are promising options for thermal pretreatment [56]. Torrefaction is a form of thermal pretreatment that produces a finished product characterised by low moisture and high calorific value compared with fresh biomass [57]. The biomass resulting from this process contains 70% of its initial weight and 90% of its energy content. Torrefaction is therefore a cost-effective and efficient method. The cost of torrefaction depends on plant costs (39%) and equipment costs (31%). However, pyrolysis is a form of thermochemical conversion that requires advanced technologies to produce a higher-quality end product [57].
4.4.3. Chemical Pretreatment
Chemical pretreatments use chemical reagents and solvents to modify or extract the major components of lignocellulosic biomass, such as cellulose, hemicellulose and lignin. This makes it possible to transform lignocellulosic biomass into interesting and environmentally friendly products [58]. The most commonly used processes for chemical pretreatment are alkaline pretreatment, acid dilution, steam explosion and the use of low-boiling organic solvents such as methanol and ethanol [59]. These methods aim to increase the internal surface area of the biomass and reduce the degree of polymerisation and crystallinity of the cellulose. For example, alkaline pretreatments degrade the lignin structure and break bonds with carbohydrate fractions [58]. Steam pretreatment, on the other hand, is energy-efficient and chemical-free, while avoiding dilution of the sugars. In fact, after steam pretreatment, the biomass slurry is often washed or diluted to remove the inhibitors formed during pretreatment (like furfural and HMF). This can result in a diluted sugar solution. The extent of dilution depends on the washing protocol and the intended downstream process. Also, as the biomass undergoes steam pretreatment, a portion of the hemicellulose fraction is solubilized, leading to the formation of sugars. The concentration of these sugars is affected by the severity of the pretreatment (combination of temperature, time, and pressure). In addition, this process can lead to condensation of soluble lignin, making BFR less digestible [60].
4.4.4. Biological Pre-Treatments
These are activities associated mainly with antifungal action, and have the power to degrade lignin, hemicellulose and cellulose. This process differs from the chemical and thermal processes and requires large amounts of energy [60]. In fact, biological pretreatment is an eco-responsible method that avoids toxic discharges into the environment. For example, white-rot fungi are responsible for lignin degradation and require less energy (oxygen, water and nutrients) during their reactions [61]. Two other enzymes derived from basidiomycetes: manganese peroxidase and laccase are responsible for hemicellulose and lignin degradation. On the one hand, these methods are relevant, as they improve productivity and avoid damaging the ecosystem through rejects. However, these methods require long reaction times to achieve relevant results. Admittedly, this is time-consuming for manufacturers [62]. On a large scale, biological pretreatment requires costly processes, as it requires a sterile environment. In addition, the microorganisms used during this process consume some of the biomass carbohydrates, reducing the efficiency of this method [61].
4.4.5. Extrusion
The lignocellulosic biomass consists mainly of lignin, cellulose, and hemicellulose, which form a strong and complex three-dimension structure [63]. To use lignocellulosic biomass as a feedstock for microbial biotransformation, it needs to be pretreated. The aim of this pretreatment is to break or weaken the lignocellulosic structure to extract/recover the compounds of interest. Usually, the target is to recover cellulose, hemicellulose and terpenes. But lignin blocks the access to those compounds [64]. Extrusion is a thermo-mechanical process that applies high shearing forces to a material (a solid or a semi-solid) for different purposes such as blending, compounding, mixing, disruption, compaction, pelletisation, etc. Therefore, extrusion is used in a wide range of domains: plastics, polymers, food, pharmaceuticals, ceramics, metal, biorefinery, paper industry, etc. They need to be moistened by a liquid. Adding water in non-optimal condition and ratios (solid/liquid) can cause biomass clogging problems inside the barrel due to evaporation and low viscosity. Compared to other physical pretreatments, extrusion is regarded as a low-energy consumption technology. Furthermore, extrusion does not need significant downstream operations because it does not produce inhibitors and does not use high water amounts as hydrothermal pretreatments do. The short pretreatment time is one of the main benefits of extrusion: while biological, chemical and some physico-chemical pretreatments may last from 30 min to several days, one extrusion pretreatment lasts a few minutes (usually around 2 or 3 min) [38].
5. Existing Technology for Terpenes Extraction
There are different types of extraction processes for volatile compounds such as terpenes which are based on essential oils extraction. These vary according to the needs of producers who are always seeking energy efficiency. Table 3 presents the principle and parameters affecting the extraction process (temperature, pressure, solvent usage), as well as the advantages and disadvantages of each extraction method in terms of yield, quality of essential oils, and environmental impact. The extraction of essential oils from plant tissues is ensured through the phenomenon of diffusion, which is a time-consuming process. The entrainment of volatile compounds depends on several factors, such as the quantity of the plant’s lipid fraction, the degree of solubility of the volatile compounds, the gradients of total pressure, and the temperature gradients during the extraction process [65]. The extraction phenomenon follows the principle of Fick’s law (Equation (1)). Diffusivity in Fick’s law in Equation (1) is an important property that indicates the rate of mass transfer and it is useful for equipment design [66]:
where F (mol.m−2.s−1) or (kg.m−2.s−1) is the mass flux of the solute, N (mol/m3) or (kg/m3) is the concentration of the solute in the solid particle, D (m2.s−1) is known as the diffusivity or diffusion coefficient for the solute in the solvent, and x (m) is the distance in the direction of the transfer.
5.1. Steam Distillation
This process involves an external steam source that diffuses through the raw material placed in the vessel, carrying away the volatile molecules. When the raw material is fragile, it is loaded onto plates (also called false bottoms) to create multiple stages. The advantage here is to maximise the exchange surface between the plant material and the steam, and also to avoid compaction [67]. For whole plants like lavender or branches such as fir, the raw material is loaded directly into the vessel. Steam distillation is the most commonly used method because it avoids overheating and reduces distillation times. Steam distillation is a gentler technique recommended for fragile raw materials such as flowers or leaves. However, it is not suitable for powders (such as ground wood or bark) as the steam tends to agglomerate them [68].
5.2. Solvent Extractions
Concretes, Resinoids, and Absolutes Extractions using nonpolar volatile solvents (hexane, diethyl ether, benzene, petroleum ether, dichloromethane, etc.) allow for the extraction of lipophilic molecules from the raw material. This includes volatile and non-volatile terpenoids, waxes, and fats. Extractions can be performed with hot solvents (as in the case of Soxhlet extraction) or at room temperature during cold maceration. In the essential oil industry, specific products are derived from these solvent extractions, namely concretes, resinoids, and absolutes [69]. The raw material loaded into tanks is “depleted” with the nonpolar solvent (often hexane). The solvent, enriched with lipophilic molecules, is evaporated at low temperatures. The resulting aromatic solid residue is called a concrete (or crude resinoid in the case of dry raw material or resin gum) [69]. To remove heavy molecules such as waxes and fats that do not contribute to the fragrance composition of the product, the concrete is extracted with ethanol at low temperatures under vigorous agitation. The resulting extract, after wax filtration, is called an absolute. These extractions of concretes, resinoids, and absolutes are used for fragile or low-yield raw materials (rose, jasmine, benzoin) and produce olfactively distinct products compared to essential oils obtained by hydrodistillation. They are primarily used in perfumery.
5.3. New Extraction Methods
5.3.1. Microwave-Assisted Extraction
Solvent-Free Microwave Extraction (SFME) allows for the extraction of essential oils from plants without the addition of water or steam, except for dry materials where a limited amount of water is added. The principle relies on the water naturally present in the raw material as a carrier for the volatile molecules, and microwave heating vaporise this water into steam which is carrying the essential oil. This technique is five to ten times faster than traditional distillation and allows extraction at temperatures below 100 °C, avoiding the thermal degradation of certain molecules [70]. Although more energy-efficient, this process is still less commonly used industrially compared to hydrodistillation [68].
5.3.2. CO2 Extraction or Supercritical Fluid Extraction
Supercritical CO2 is a fluid with high diffusion and density properties that can be used to extract volatile molecules from plants. The supercritical fluid passes through the raw material and collects the compounds. During expansion, the CO2 transitions into the gas phase, allowing for its separation from the extract [71]. This gentle extraction process preserves the integrity of the molecules, often resulting in extracts that closely resemble the fresh plant in terms of fragrance. CO2 extraction is advantageous due to its abundance, low cost, and non-toxic nature. However, this method requires significant investment in equipment and consumes a considerable amount of energy to reach the supercritical state (pressure conditions above 74 bars and a temperature of 31 °C) [72].
5.3.3. Subcritical Water Extraction
This process utilises hot water at its subcritical point, between 100 °C (boiling point) and 374.1 °C (critical point), maintained in a liquid state under high pressure (between 1 and 221 bars). In the subcritical state, water possesses different properties, allowing for the solubilisation of low-polarity molecules. Thus, the extraction of low-polarity or nonpolar volatile molecules, such as components of essential oils, is facilitated by reducing the polarity of water without the addition of organic solvents. This technique, which relies solely on water as the green solvent, is nevertheless costly in terms of equipment and energy [73].

Table 3.
Various extraction methods for volatile molecules as terpenes.
Table 3.
Various extraction methods for volatile molecules as terpenes.
| Extraction Methods | Principle | Advantages and Disadvantages | Reference |
|---|---|---|---|
| Steam distillation | The forest or agricultural residue is in direct contact with steam, which is then condensed. Recovery takes place in a separator, where the volatile molecules are dispersed in water. | Méthode très simple Very simple method No energy expenditure | [74] |
| Solvent extraction | The volatile molecules are separated from the solvent by evaporation of the solvent at high temperatures. | Efficient, slow and costly method Requires high temperatures (degradation of some constituents of volatile molecules) | [75] |
| Hydrodistillation | The residual material is submerged in water, which is then heated to boiling point. After passing through the cooler, the mixture is collected in an essencier. | Efficient, but slow method for 100 g (4 h) High water consumption | [76] |
| Supercritical fluid extraction | Extraction requires a supercritical fluid (CO2 in the presence of an organic solvent). | Efficient, low-cost method No oxidative degradation of lipids | [68] |
| Ultrasonic extraction | Sound waves exert vibrations on plant cell walls, improving extraction. | Reduced extraction time | [63] |
| Microwave extraction | The residual material is heated from the inside out, increasing the water pressure inside the cells and causing the cells to burst and spill their contents into the outside environment. | Environmental efficiency Fast method Saves time, water and residual solvent | [70] |
| Hydrodistillation combined with microwaves | For 100 g of plant material, this method requires power (1200 watts) and duration 15 min | Good yield, fast (75 min), low cost | [69] |
6. Conclusions
Limonene is the most abundant naturally occurring monoterpene in orange peel oil, which has been extensively studied as a precursor for high-value derivatives, including flavour and fragrance additives, solvents, feedstocks for fine chemicals, and active ingredients in medicine. Various microorganisms, such as Cladosporium sp., Pseudomonas gladioli, and Penicillium strains, have been employed for the biotransformation of refine limonene into compounds like α-terpineol and carvone, showcasing the potential for large-scale production of value-added derivatives. Similarly, α-pinene, a prominent antimicrobial monoterpene found in essential oils, has garnered attention for its applications in food, fragrance, pharmaceutical, and fine chemical industries. The current industrial production of α-pinene from natural sources is laborious and inefficient, highlighting the need for sustainable biotransformation technologies. Studies have identified biocatalysts such as Chrysosporium pannorum, Candida tropicalis, and Hormonema sp. UOFS Y-0067 for the oxidation and transformation of α-pinene into commercially valuable compounds like verbenol and α-terpineol. The main biotransformation study of limonene and α-pinene currently relies on refine or pure precursors, which have limitations such as solvent utilisation, high toxicity effect, low concentrations of products obtained. However, biotechnological approaches offer a promising solution by utilising microorganisms that can metabolite terpenes found in residual wood and citrus. These molecules are abundant in these feedstocks and could be biotransform with solid-state fermentation and high value industrial by-products could be achieved by improving the technology with strategy as solid-state fermentation and extrusion of the lignocellulosic biomass. Further research is needed to explore alternative sources of α-pinene, optimise fermentation conditions of limonene, and identify potential microbes for its biotransformation.
Author Contributions
A.N.: Methodology, writing. K.A.: Reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Guerreiro, C.; Belo, I. Generation of flavors and fragrances through biotransformation and de novo synthesis. Food Bioprocess Technol. 2018, 11, 2217–2228. [Google Scholar] [CrossRef]
- Panakkal, E.J.; Kitiborwornkul, N.; Sriariyanun, M.; Ratanapoompinyo, J.; Yasurin, P.; Asavasanti, S.; Rodiahwati, W.; Tantayotai, P. Production of food flavouring agents by enzymatic reaction and microbial fermentation. Appl. Sci. Eng. Prog. 2021, 14, 297–312. [Google Scholar] [CrossRef]
- Gounaris, Y. Biotechnology for the production of essential oils, flavours and volatile isolates. A review. Flavour Fragr. J. 2010, 25, 367–386. [Google Scholar] [CrossRef]
- Vespermann, K.A.; Paulino, B.N.; Barcelos, M.C.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of α-and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef]
- Bicas, J.L.; Dionisio, A.P.; Pastore, G.M. Bio-oxidation of terpenes: An approach for the flavor industry. Chem. Rev. 2009, 109, 4518–4531. [Google Scholar] [CrossRef]
- Molina, G.; Pessôa, M.G.; Bicas, J.L.; Fontanille, P.; Larroche, C.; Pastore, G.M. Optimization of limonene biotransformation for the production of bulk amounts of α-terpineol. Bioresour. Technol. 2019, 294, 122180. [Google Scholar] [CrossRef]
- de Oliveira Felipe, L.; de Oliveira, A.M.; Bicas, J.L. Bioaromas–perspectives for sustainable development. Trends Food Sci. Technol. 2017, 62, 141–153. [Google Scholar] [CrossRef]
- Pessôa, M.G.; Vespermann, K.A.; Paulino, B.N.; Barcelos, M.C.; Pastore, G.M.; Molina, G. Newly isolated microorganisms with potential application in biotechnology. Biotechnol. Adv. 2019, 37, 319–339. [Google Scholar] [CrossRef]
- Poaty, B.; Lahlah, J.; Porqueres, F.; Bouafif, H. Composition, antimicrobial and antioxidant activities of seven essential oils from the North American boreal forest. World J. Microbiol. Biotechnol. 2015, 31, 907–919. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Roy, D.; Bhowal, J. Bioconversion of Mandarin Orange Peels by Aspergillus oryzae and Penicillium sp. In Advances in Bioprocess Engineering and Technology: Select Proceedings ICABET 2020; Springer: Berlin/Heidelberg, Germany, 2021; pp. 13–20. [Google Scholar]
- Francezon, N.; Stevanovic, T. Chemical composition of essential oil and hydrosol from Picea mariana bark residue. BioResources 2017, 12, 2635–2645. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, P.S.; Zerbe, P. Terpene synthases as metabolic gatekeepers in the evolution of plant terpenoid chemical diversity. Front. Plant Sci. 2019, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of agri-food waste: A promising route for the production of aroma compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef]
- Wenda, S.; Illner, S.; Mell, A.; Kragl, U. Industrial biotechnology—The future of green chemistry? Green Chem. 2011, 13, 3007–3047. [Google Scholar] [CrossRef]
- Pimentel, M.R.; Molina, G.; Dionísio, A.P.; Maróstica Junior, M.R.; Pastore, G.M. The use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol. Res. Int. 2011, 2011, 576286. [Google Scholar] [CrossRef]
- Sharma, P.; Vishvakarma, R.; Gautam, K.; Vimal, A.; Gaur, V.K.; Farooqui, A.; Varjani, S.; Younis, K. Valorization of citrus peel waste for the sustainable production of value-added products. Bioresour. Technol. 2022, 351, 127064. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.; Totti, B.; Rozza, A. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
- Maróstica Júnior, M.R.; Pastore, G.M. Biotransformação de limoneno: Uma revisão das principais rotas metabólicas. Química Nova 2007, 30, 382–387. [Google Scholar] [CrossRef]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Cara, P.D.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef]
- John, I.; Muthukumar, K.; Arunagiri, A. A review on the potential of citrus waste for D-Limonene, pectin, and bioethanol production. Int. J. Green Energy 2017, 14, 599–612. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Pahmeyer, M.J.; Assadpour, E.; Jafari, S.M. Extraction and purification of d-limonene from orange peel wastes: Recent advances. Ind. Crops Prod. 2022, 177, 114484. [Google Scholar] [CrossRef]
- Jongedijk, E.; Cankar, K.; Buchhaupt, M.; Schrader, J.; Bouwmeester, H.; Beekwilder, J. Biotechnological production of limonene in microorganisms. Appl. Microbiol. Biotechnol. 2016, 100, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Badee, A.; Helmy, S.A.; Morsy, N.F. Utilisation of orange peel in the production of α-terpineol by Penicillium digitatum (NRRL 1202). Food Chem. 2011, 126, 849–854. [Google Scholar] [CrossRef]
- Molina, G.; Pinheiro, D.M.; Pimentel, M.R.; dos Ssanros, R.; Pastore, G.M. Monoterpene bioconversion for the production of aroma compounds by fungi isolated from Brazilian fruits. Food Sci. Biotechnol. 2013, 22, 999–1006. [Google Scholar] [CrossRef]
- Duetz, W.A.; Fjällman, A.H.; Ren, S.; Jourdat, C.; Witholt, B. Biotransformation of D-limonene to (+) trans-carveol by toluene-grown Rhodococcus opacus PWD4 cells. Appl. Environ. Microbiol. 2001, 67, 2829–2832. [Google Scholar] [CrossRef]
- Toniazzo, G.; de Oliveira, D.; Dariva, C.; Oestreicher, E.G.; Antunes, O.A. Biotransformation of (−) β-pinene by Aspergillus niger ATCC 9642. In Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef] [PubMed]
- Karimkhani, M.M.; Nasrollahzadeh, M.; Maham, M.; Jamshidi, A.; Kharazmi, M.S.; Dehnad, D.; Jafari, S.M. Extraction and purification of α-pinene; a comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Trytek, M.; Jędrzejewski, K.; Fiedurek, J. Bioconversion of α-pinene by a novel cold-adapted fungus Chrysosporium pannorum. J. Ind. Microbiol. Biotechnol. 2015, 42, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; De, B.; Bhattacharyya, D. Microbial Oxidation of-Pinene to (+)-a-Terpineol by Candida tropicalis; NISCAIR-CSIR: New Delhi, India, 1999. [Google Scholar]
- Rottava, I.; Cortina, P.F.; Zanella, C.A.; Cansian, R.L.; Toniazzo, G.; Treichel, H.; Antunes, O.A.; Oestreicher, E.G.; de Oliveira, D. Microbial oxidation of (-)-α-pinene to verbenol production by newly isolated strains. Appl. Biochem. Biotechnol. 2010, 162, 2221–2231. [Google Scholar] [CrossRef]
- Konan, D.; Koffi, E.; Ndao, A.; Peterson, E.C.; Rodrigue, D.; Adjallé, K. An Overview of Extrusion as a Pretreatment Method of Lignocellulosic Biomass. Energies 2022, 15, 3002. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Yafetto, L. Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: A review and bibliometric analysis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef]
- Bier, M.C.J.; Medeiros, A.B.P.; Soccol, C.R. Biotransformation of limonene by an endophytic fungus using synthetic and orange residue-based media. Fungal Biol. 2017, 121, 137–144. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Zhao, L.-Q.; Sun, Z.-H.; Zheng, P.; He, J.-Y. Biotransformation of isoeugenol to vanillin by Bacillus fusiformis CGMCC1347 with the addition of resin HD-8. Process Biochem. 2006, 41, 1673–1676. [Google Scholar] [CrossRef]
- Hua, D.; Ma, C.; Lin, S.; Song, L.; Deng, Z.; Maomy, Z.; Zhang, Z.; Yu, B.; Xu, P. Biotransformation of isoeugenol to vanillin by a newly isolated Bacillus pumilus strain: Identification of major metabolites. J. Biotechnol. 2007, 130, 463–470. [Google Scholar] [CrossRef]
- Gallage, N.J.; Møller, B.L. Vanillin–bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Ito, M. Bioconversion of essential oil components of Perilla frutescens by Saccharomyces cerevisiae. J. Nat. Med. 2020, 74, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, E.J.; Lin, G.-M.; Voigt, C.A.; Clardy, J. Bacterial terpene biosynthesis: Challenges and opportunities for pathway engineering. Beilstein J. Org. Chem. 2019, 15, 2889–2906. [Google Scholar] [CrossRef] [PubMed]
- Marmulla, R.; Harder, J. Microbial monoterpene transformations—A review. Front. Microbiol. 2014, 5, 346. [Google Scholar] [CrossRef]
- Yoo, S.; Day, D. Bacterial metabolism of α-and β-pinene and related monoterpenes by Pseudomonas sp. strain PIN. Process Biochem. 2002, 37, 739–745. [Google Scholar] [CrossRef]
- Sugiura, M.; Ito, S.; Saito, Y.; Niwa, Y.; Koltunow, A.M.; Sugimoto, O.; Sakai, H. Molecular cloning and characterization of a linalool synthase from lemon myrtle. Biosci. Biotechnol. Biochem. 2011, 75, 1245–1248. [Google Scholar] [CrossRef]
- Sales, A.; Felipe, L.d.O.; Bicas, J.L. Production, properties, and applications of α-terpineol. Food Bioprocess Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Tischer, B.; Vendruscolo, R.G.; Wagner, R.; Menezes, C.R.; Barin, C.S.; Giacomelli, S.R.; Budel, J.M.; Barin, J.S. Effect of grinding method on the analysis of essential oil from Baccharis articulata (Lam.) Pers. Chem. Pap. 2017, 71, 753–761. [Google Scholar] [CrossRef]
- Douville, J.; David, J.; Lemieux, K.M.; Gaudreau, L.; Ramotar, D. The Saccharomyces cerevisiae phosphatase activator RRD1 is required to modulate gene expression in response to rapamycin exposure. Genetics 2006, 172, 1369–1372. [Google Scholar] [CrossRef]
- Reinprecht, L.; Pánek, M. Ultrasonic technique for evaluation of bio-defects in wood: Part 1–Influence of the position, extent and degree of internal artificial rots. Int. Wood Prod. J. 2012, 3, 107–115. [Google Scholar] [CrossRef]
- Jung, C.G. Voies de traitements de déchets solides: Valorisation matière et énergie. Bull. Sci. Inst. Natl. Conserv. Nat. 2013, 50–54. [Google Scholar]
- Senneca, O.; Cerciello, F.; Russo, C.; Wütscher, A.; Muhler, M.; Apicella, B. Thermal treatment of lignin, cellulose and hemicellulose in nitrogen and carbon dioxide. Fuel 2020, 271, 117656. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Ilyas, R.A.; Nurazzi, N.M.; Rani, M.S.A.; Atikah, M.S.N.; Shazleen, S.S. Chemical pretreatment of lignocellulosic biomass for the production of bioproducts: An overview. Appl. Sci. Eng. Prog. 2021, 14, 588–605. [Google Scholar] [CrossRef]
- Eloutassi, N.; Louaste, B.; Boudine, L.; Remmal, A. Hydrolyse physico-chimique et biologique de la biomasse ligno-cellulosique pour la production de bio-éthanol de deuxième génération. Nat. Technol. 2014, 10, 10–14. [Google Scholar]
- Eloutassi, N.; Louaste, B.; Boudine, L.; Remmal, A. Valorisation de la biomasse lignocellulosique pour la production de bioéthanol de deuxième génération. J. Renew. Energ. 2014, 17, 600–609. [Google Scholar]
- Motte, J.-C. Digestion Anaérobie par voie Sèche de Résidus Lignocellulosiques: Etude Dynamique des Relations Entre Paramètres de Procédés, Caractéristiques du Substrat et Écosystème Microbien. Ph.D. Thesis, University of Montpellier, University of Montpellier, Montpellier, France, 2013. [Google Scholar]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech 2013, 3, 415–431. [Google Scholar] [CrossRef]
- Zheng, J.; Rehmann, L. Extrusion pretreatment of lignocellulosic biomass: A review. Int. J. Mol. Sci. 2014, 15, 18967–18984. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, M. Extrusion as a pretreatment for lignocellulosic biomass: Fundamentals and applications. Renew. Energy 2017, 114, 1427–1441. [Google Scholar] [CrossRef]
- Djerrari, A. Influence du Mode D’extraction et des Conditions de Conservation sur la Composition des Huiles Essentielles de Thym et de Basilic. PhD Thesis, Université des Sciences et Techniques du Languedoc, Montpellier, France, 1986. [Google Scholar]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C. Modeling and kinetics study of conventional and assisted batch solvent extraction. Chem. Eng. Res. Des. 2014, 92, 1169–1186. [Google Scholar] [CrossRef]
- Cassel, E.; Vargas, R.; Martinez, N.; Lorenzo, D.; Dellacassa, E. Steam distillation modeling for essential oil extraction process. Ind. Crops Prod. 2009, 29, 171–176. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Ferhat, A.; Kameli, A. Méthodes d’extraction et de distillation des huiles essentielles: Revue de littérature. Une 2019, 3, 1653–1659. [Google Scholar]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Yang, Y.; Kayan, B.; Bozer, N.; Pate, B.; Baker, C.; Gizir, A.M. Terpene degradation and extraction from basil and oregano leaves using subcritical water. J. Chromatogr. A 2007, 1152, 262–267. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochemistry 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Q. Comparisons of microwave-assisted extraction, simultaneous distillation-solvent extraction, Soxhlet extraction and ultrasound probe for polycyclic musks in sediments: Recovery, repeatability, matrix effects and bioavailability. Chromatographia 2011, 74, 489–495. [Google Scholar] [CrossRef]
- Lucchesi, M.-E. Extraction Sans Solvant Assistée par Micro-ondes Conception et Application à L’extraction des Huiles Essentielles. Ph.D. Thesis, Faculté des Sciences et Technologies, Université de la Réunion, Réunion, France, July 2005. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).