Tickborne Colpodella Species Infections: Time for a New Integrated Approach to Understand Transmission and Pathogenicity
Simple Summary
Abstract
1. Introduction
2. Methods
3. Overview of Colpodella Species
3.1. Distribution of Colpodella Species in Different Geographic Areas, Hosts and Vectors
3.2. Colpodella Species Infections in Human and Animal Hosts
4. Is Colpodella Species an Opportunistic or Zoonotic Parasite?
4.1. Colpodellosis in the Making
4.2. From Free-Living Predators to Opportunistic Parasites
4.3. Pathogenic Protists Are Vectors for Other Pathogens
4.4. Culturing Colpodella Species for Identification of Virulence Markers
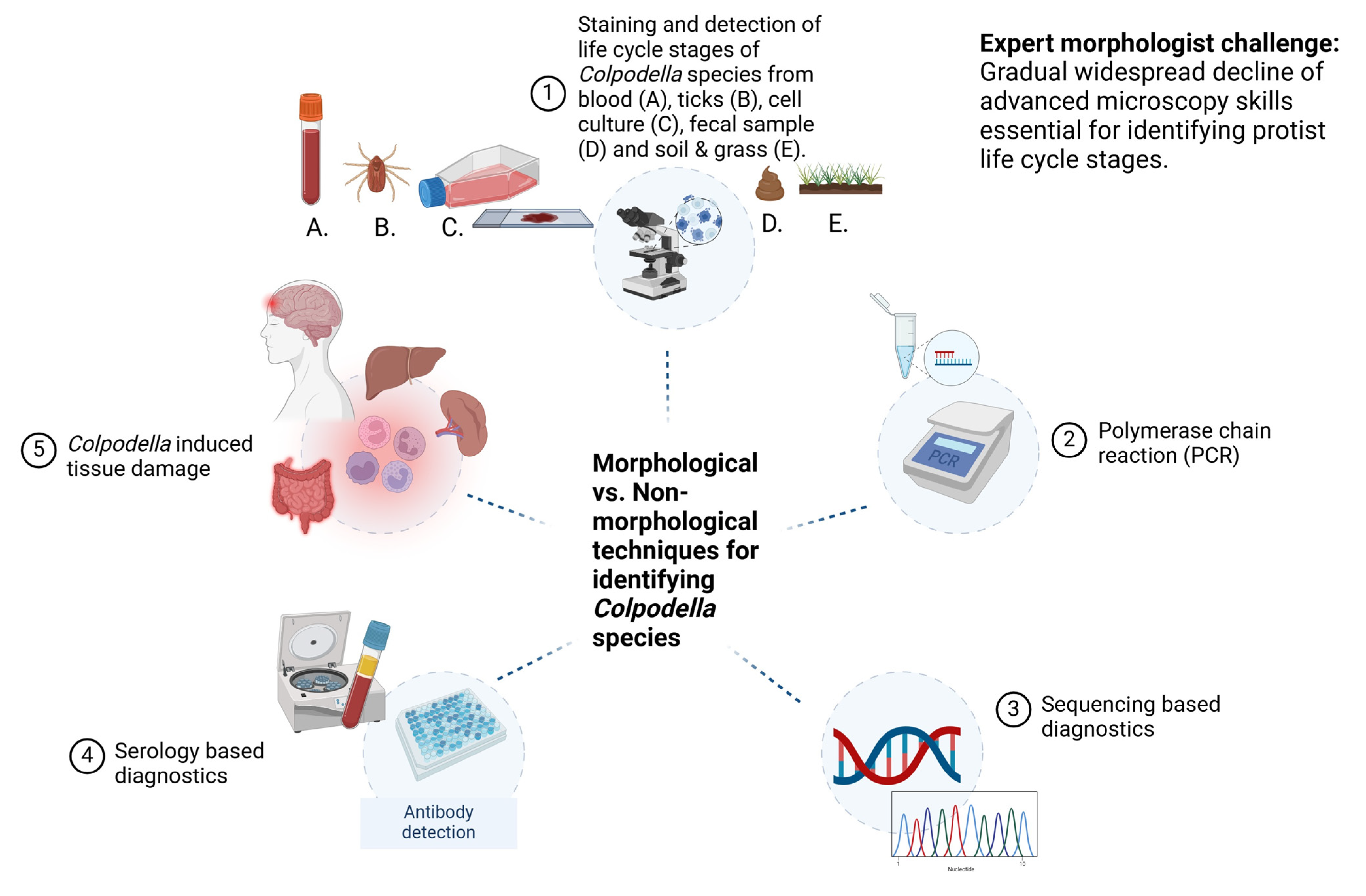
5. Techniques for Detecting Life Cycle Stage Markers of Colpodella spp. in Arthropod and Vertebrate Hosts
5.1. Mechanisms of Pathogenesis in Colpodellosis—What Do We Know?
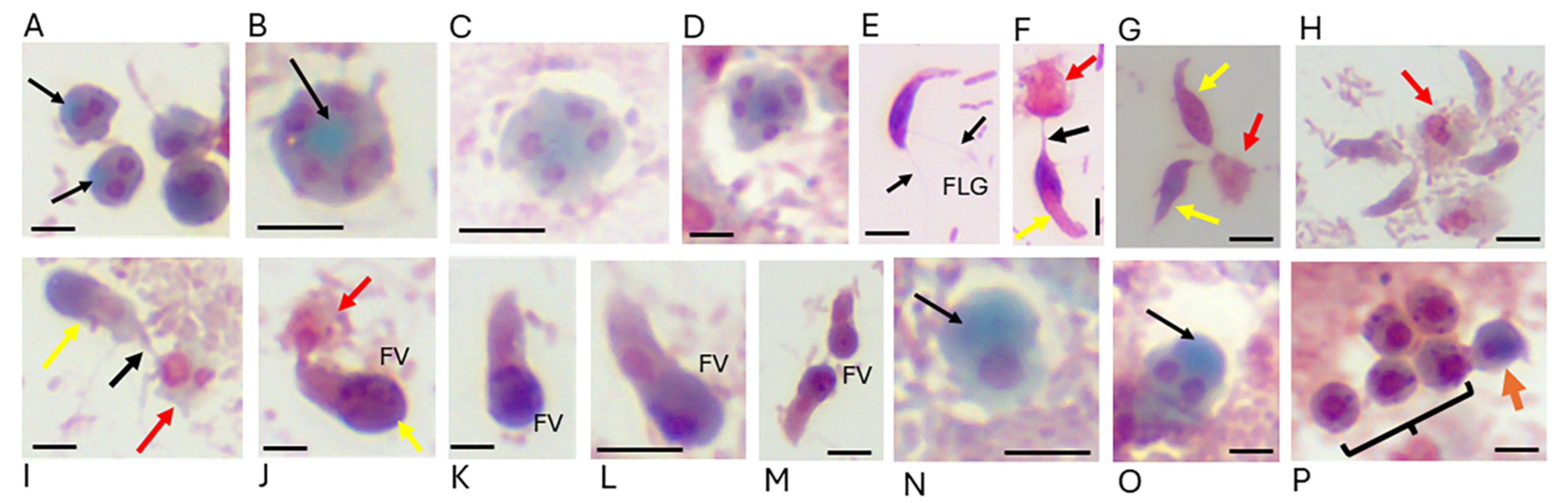
5.2. Microscopy: The Gold Standard for Parasite Identification
5.3. Light, Differential Interference Contrast (DIC) Microscopy and Electron Microscopy (EM)
5.4. Morphology-Based Diagnostic Techniques
5.5. Parasite-Induced Tissue Damage
5.6. Expert Morphologist Challenge
5.7. Polymerase Chain Reaction (PCR) and Sequencing-Based Diagnostics
5.8. Serology-Based Diagnostics
5.9. Cost Effectiveness
6. Culture Conditions for Colpodellids and Chromerids
Culture Media for Cultivating Colpodella Species
7. Colpodella spp. in Coinfections
7.1. Colpodella spp. Detected in Blood from Horses
7.2. Colpodella spp. Detected in Blood and Ticks from Cattle and Goats
7.3. Colpodella spp. Detected in Blood from Dogs, Cats and Ticks
7.4. Colpodella spp. Detected in Camel Ticks, Cattle and Wildlife
7.5. Colpodella spp. Identified in Fecal Samples from Sheep, Goats, Cattle, Duck and Eurasian Coot
7.6. Colpodella spp. Detected in Pangolin Ticks
8. Colpodella spp. in Single Infections
8.1. Colpodella spp. in Human Infections
8.2. Colpodella spp. Detected in Fecal Samples from Large Cats
9. Conclusions and Recommendations
- (1)
- When Colpodella spp. are identified in arthropods, and in human and animal specimens, the specimens should be stained for microscopy to identify life cycle stages of Colpodella spp. Staining protocols have been developed that can distinguish Colpodella spp. life cycle stages from those of prey that may be present in specimens.
- (2)
- Blood should be collected from humans and animals infected to obtain antiserum to be used for the evaluation of antibodies specific to Colpodella spp. antigens. The culture of specimens containing Colpodella spp. will provide cell pellets that can be extracted for protein, DNA or RNA and for immunological and molecular biology investigations.
- (3)
- Primers targeting the 18S rRNA genes of the bodonid, algae and ciliate prey species should also be used for PCR amplification to determine the presence of the prey species in arthropods and host samples.
- (4)
- Specimens containing Colpodella spp. should be cultured to propagate the identified species. The prey species for Colpodella spp. may be present in the specimen and can be cultured along with Colpodella spp. Media can be bacterized before use and Bodo spp. and Parabodo spp. can be obtained in monoprotist cultures from the ATCC and added to the culture to maintain growth of Colpodella spp.
- (5)
- When epidemiological screenings are performed for tickborne bacteria, piroplasms or for Cryptosporidium, the presence of Colpodella spp. should be suspected and screened.
- (6)
- Prolonged symptoms non-responsive to conventional treatments following tick bite or biting flies with unknown etiology in humans or animals should be evaluated for Colpodella spp. Similarly, symptoms of diarrhea in suspected cases of cryptosporidiosis should be evaluated for the presence of Colpodella spp.
- (7)
- Proper prevention, management, diagnosis and treatment of colpodellosis will require an integrated approach that includes staining and microscopy, morphological characterization, nucleic acid amplification and immunoassays.
Funding
Conflicts of Interest
References
- Kuvardina, O.N.; Leander, B.S.; Aleshin, V.V.; Myl’nikov, A.P.; Keeling, P.J.; Simdyanov, T.G. The phylogeny of colpodellids (Alveolata) using small subunit rRNA gene sequences suggests they are the free-living sister group to apicomplexans. J. Eukaryot. Microbiol. 2002, 49, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Patterson, D. Ultrastructure and the identification of the predatory flagellate Colpodella pugnax Cienkowski (Apicomplexa) with a description of Colpodella turpis n. sp. and a review of the genus. Syst. Parasitol. 1996, 33, 187–198. [Google Scholar] [CrossRef]
- Brugerolle, G. Colpodella vorax: Ultrastructure, predation, life-cycle, mitosis, and phylogenetic relationships. Europ. J. Protistol. 2002, 38, 113–125. [Google Scholar] [CrossRef]
- Mylnikov, A.P. Ultrastructure and phylogeny of colpodellids (Colpodellida, Alveolata). Biol. Bull. Russ. Acad. Sci. 2009, 36, 582–590. [Google Scholar] [CrossRef]
- Mylnikov, A.P.; Mylnikova, Z.M. Feeding spectra and pseudoconoid structure in predatory alveolate flagellates. Inland. Water Biol. 2008, 1, 210–216. [Google Scholar] [CrossRef]
- Cavalier-Smith, T.; Chao, E.E. Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom. Nov.). Eur. J. Protistol. 2004, 40, 185–212. [Google Scholar] [CrossRef]
- Sam-Yellowe, T.Y.; Getty, T.A.; Addepalli, K.; Walsh, A.M.; Williams-Medina, A.R.; Fujioka, H.; Peterson, J.W. Novel life cycle stages of Colpodella sp. (Apicomplexa) identified using Sam-Yellowe’s trichrome stains and confocal and electron microscopy. Int. Microbiol. 2022, 25, 669–678. [Google Scholar] [CrossRef]
- Getty, T.A.; Peterson, J.W.; Fujioka, H.; Walsh, A.M.; Sam-Yellowe, T.Y. Colpodella sp. (ATCC 50594) Life Cycle: Myzocytosis and Possible Links to the Origin of Intracellular Parasitism. Trop. Med. Infect. Dis. 2021, 6, 127. [Google Scholar] [CrossRef]
- Matsimbe, A.M.; Magaia, V.; Sanches, G.S.; Neves, L.; Noormahomed, E.; Antunes, S.; Domingos, A. Molecular detection of pathogens in ticks infesting cattle in Nampula province, Mozambique. Exp. Appl. Acarol. 2017, 73, 91–102. [Google Scholar] [CrossRef]
- Jimale, K.A.; Bezzera-Santos, M.A.; Mendoza-Roldan, J.A.; Latrofa, M.S.; Baneth, G.; Otrano, D. Molecular detection of Colpodella sp. and other tick-borne pathogens in ticks of ruminants, Italy. Acta Tropica 2024, 257, 107306. [Google Scholar] [CrossRef]
- Li, B.; Zhai, J.Q.; Wu, Y.J.; Shan, F.; Zou, J.J.; Hou, F.H.; Que, T.C.; Chen, W. Molecular identification of tick-borne Rickettsia, Anaplasma, Ehrlichia, Babesia, and Colpodella in confiscated Malayan pangolins. PLoS Negl. Trop. Dis. 2024, 18, e0012667. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.C.; Sun, X.; Bao, Y.; Fu, W.; Lin, K.; Chen, T.; Zheng, C.; Li, S.; Chen, W.; Huang, C. Molecular identification of Colpodella sp. of South China tiger Panthera tigris amoyensis (Hilzheimer) in the Meihua Mountains, Fujian, China. Folia Parasitol. 2022, 69, 2022.019. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Mahmoud, H.Y.A.H.; Hifumi, T.; Tanaka, T. Discovery of Colpodella spp. in ticks (Hyalomma dromedarii) infesting camels in southern Egypt. Ticks Tick-Borne Dis. 2024, 15, 102352. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, J.; Lu, N.; Qi, X.; Yang, C.; Liu, B.; Lu, Y.; Gu, Y.; Tan, W.; Zhu, C.; et al. Potential novel Colpodella spp. (phylum Apicomplexa) and high prevalence of Colpodella spp. in goat-attached Haemaphysalis longicornis ticks in Shandong province, China. Ticks Tick-Borne Dis. 2024, 15, 102328. [Google Scholar] [CrossRef]
- Phetkarl, T.; Fungwithaya, P.; Udompornprasith, S.; Amendt, J.; Sontigun, N. Preliminary study on prevalence of hemoprotozoan parasites harbored by Stomoxys (Diptera: Muscidae) and tabanid flies (Diptera: Tabanidae) in horse farms in Nakhon Si Thammarat province, Southern Thailand. Vet. World 2023, 16, 2128–2134. [Google Scholar] [CrossRef]
- Janouškovec, J.; Tikhonenkov, D.V.; Burki, F.; Howe, A.T.; Kolísko, M.; Mylnikov, A.P.; Keeling, P.J. Factors mediating plastid dependency and the origins of parasitism in apicomplexans and their close relatives. Proc. Natl. Acad. Sci. USA 2015, 112, 10200–10207. [Google Scholar] [CrossRef]
- Hussein, S.; Li, X.; Bukharr, S.M.; Zhou, M.; Ahmad, S.; Amhad, S.; Javid, A.; Guan, C.; Hussain, A.; Ali, W.; et al. Cross-genera amplification and identification of Colpodella sp. with Cryptosporidium primers in fecal samples of zoo felids from northeast China. Braz. J. Biol. 2021, 83, e247181. [Google Scholar] [CrossRef]
- Elochukwu, C.V.; Nnabuife, H.E.; Nicodemus, M.; Ogo, N.I.; Sylvanus, O.S.; Cornelius, J.O.; Kamani, J.; Maxwell, O.N. Molecular detection of Colpodella sp. using Cryptosporidium primers in faecal samples of small ruminants in FCT and Plateau State, Nigeria. J. Vet. Biomed. Sci. 2024, 6, 112–122. [Google Scholar]
- Hasapis, K.A.; Charalambidou, I.; Phanis, C.O.; Kazamia, S.; Kassinis, N.; Schou, C.; Karanis, P. First Detection and Molecular Characterization of Colpodella in Goats, Foxes, and Birds. Acta Parasitol. 2025, 70, 22. [Google Scholar] [CrossRef]
- Neupane, S.; Saski, C.; Nayduch, D. House fly larval grazing alters dairy cattle manure microbial communities. BMC Microbiol. 2021, 21, 346. [Google Scholar] [CrossRef]
- Myshrall, K.L.; Mobberley, J.M.; Green, S.J.; Visscher, P.T.; Havemann, S.A.; Reid, R.P.; Foster, J.S. Biogeochemical cycling and microbial diversity in the thrombolitic microbialites of Highborne Cay, Bahamas. Geobiology 2010, 8, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.L.; Keeling, P.J.; Krause, P.J.; Horak, A.; Bent, S.; Rollend, L.; Hua, X.G. Colpodella spp.–like Parasite Infection in Woman, China. Emerg. Infect. Dis. 2012, 18, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-F.; Jiang, R.-R.; Chang, Q.-C.; Zheng, Y.-C.; Jiang, B.-G.; Sun, Y.; Jia, N.; Wei, R.; Bo, H.-B.; Huo, Q.-B.; et al. Potential novel tick-borne Colpodella species parasite infection in patient with neurological symptoms. PLoS Negl. Trop. Dis. 2018, 12, e0006546. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hu, Y.; Qiu, H.; Wang, J.; Jiang, J. Colpodella sp. (Phylum Apicomplexa) Identified in Horses Shed Light on Its Potential Transmission and Zoonotic Pathogenicity. Front. Microbiol. 2022, 13, 857752. [Google Scholar] [CrossRef]
- Wheatley, M.A.; Shamoun, J.; Maggi, R.; Breitschwerdt, E.B.; Sommer, S.L.; Cullen, J.M.; Stowe, D.M. Eosinophilic pericardial effusion and pericarditis in a cat. JFMS Open Rep. 2023, 9, 20551169231213498. [Google Scholar] [CrossRef]
- Neculicioiu, V.S.; Colosi, I.A.; Toc, D.A.; Lesan, A.; Costache, C. When a Ciliate Meets a Flagellate: A Rare Case of Colpoda spp. and Colpodella spp. Isolated from the Urine of a Human Patient. Case Report and Brief Review of Literature. Biology 2021, 10, 476. [Google Scholar] [CrossRef]
- Solarz, W.; Najberek, K.; Wilk-Woźniak, E.; Biedrzycka, A. Raccoons foster the spread of freshwater and terrestrial microorganisms—Mammals as a source of microbial eDNA. Divers. Distrib. 2020, 26, 453–459. [Google Scholar] [CrossRef]
- Stoeck, T.; Kasper, J.; Bunge, J.; Leslin, C.; Ilyin, V.; Epstein, S. Protistan diversity in the Arctic: A case of paleoclimate shaping modern biodiversity? PLoS ONE 2007, 2, e728. [Google Scholar] [CrossRef]
- Solon, A.J.; Mastrangelo, C.; Vimercati, L.; Sommers, P.; Darcy, J.L.; Gendron, E.M.S.; Porazinska, D.L.; Schmidt, S.K. Gullies and Moraines Are Islands of Biodiversity in an Arid, Mountain Landscape, Asgard Range, Antarctica. Front. Microbiol. 2021, 12, 654135. [Google Scholar] [CrossRef]
- Heidelberg, K.B.; Nelson, W.C.; Holm, J.B.; Eisenkolb, N.; Andrade, K.; Emerson, J.B. Characterization of eukaryotic microbial diversity in hypersaline Lake Tyrrell, Australia. Front. Microbiol. 2013, 4, 115. [Google Scholar] [CrossRef]
- Egizi, A.; Morin, P.J.; Fonseca, D.M. Unraveling microbe-mediated interactions between mosquito larvae in a laboratory microcosm. Aquat. Ecol. 2014, 48, 179–189. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; Zowalaty, M.E.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Sam-Yellowe, T.Y.; Roy, A.; Nims, T.; Qaderi, S.; Peterson, J.W. Detection of Kelch13 and Coronin Genes in Colpodella sp. ATCC 50594. Parasitologia 2025, 5, 5. [Google Scholar] [CrossRef]
- Olmo, J.L.; Esteban, G.F.; Finlay, B.J. New records of the ectoparasitic flagellate Colpodella gonderi on non-Colpoda ciliates. J. Int. Microbiol. 2011, 14, 207–211. [Google Scholar]
- Sam-Yellowe, T.Y.; Asraf, M.M.; Peterson, J.W.; Fujioka, H. Fluorescent Nanoparticle Uptake by Myzocytosis and Endocytosis in Colpodella sp. ATCC 50594. Microorganisms 2023, 11, 1945. [Google Scholar] [CrossRef]
- Wu, S.; Meng, J.; Yu, F.; Zhou, C.; Yang, B.; Chen, X.; Yang, G.; Sun, Y.; Cao, W.; Jiang, J.; et al. Molecular epidemiological investigation of piroplasms carried by pet cats and dogs in an animal hospital in Guiyang, China. Front. Microbiol. 2023, 14, 1266583. [Google Scholar] [CrossRef]
- Huggins, L.G.; Colella, V.; Koehler, A.V.; Schunack, B.; Traub, R.J. A multipronged next-generation sequencing metabarcoding approach unearths hyperdiverse and abundant dog pathogen communities in Cambodia. Transbound. Emerg. Dis. 2022, 69, 1933–1950. [Google Scholar] [CrossRef]
- Squarre, D.; Nakamura, Y.; Hayashida, K.; Kawai, N.; Chambaro, H.; Namangala, B.; Sugimoto, C.; Yamagishi, J. Investigation of the piroplasm diversity circulating in wildlife and cattle of the greater Kafue ecosystem, Zambia. Parasites Vectors 2020, 13, 599. [Google Scholar] [CrossRef]
- Salazar-Ardiles, C.; Paredes Valencia, K.; Andrade, D.C. Amoebas: The omnipotent organism and silent assassin. Mol. Biol. Rep. 2025, 52, 160. [Google Scholar] [CrossRef]
- Mathison, B.A.; Sapp, S.G.H. An annotated checklist of the eukaryotic parasites of humans, exclusive of fungi and algae. ZooKeys 2021, 1069, 1–313. [Google Scholar] [CrossRef]
- Contreras-Ferro, R.; Trueba, J.M.; Sánchez-Mora, P.; Escudero, R.; Sánchez-Seco, M.P.; Montero, E.; Negredo, A.; González, L.M.; Dashti, A.; Llorente, M.T.; et al. Why an Integrated Approach to Tick-Borne Pathogens (Bacterial, Viral, and Parasitic) Is Important in the Diagnosis of Clinical Cases. Trop. Med. Infect. Dis. 2024, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Fu, S.; Jiang, J.F.; Feng, H.; Liu, Z.; Sun, Y.; Li, M. Persistent human babesiosis with low-grade parasitemia, challenges for clinical diagnosis and management. Heliyon 2024, 10, e39960. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Nachum-Biala, Y.; Dvorkin, A.; Arogeti, I.; Amiel, S.; Soueid, Y.; Shwartz, D.; Mumcuoglu, K.Y.; Salant, H. Description of Babesia galileei sp. nov. A Piroplasmid species causing severe disease in domestic cats. Parasit. Vectors 2024, 17, 297. [Google Scholar] [CrossRef] [PubMed]
- Olano, J.P.; Weller, P.F.; Guerrant, R.L.; Walker, D.H. Principles of Parasitism: Host–Parasite Interactions. In Tropical Infectious Diseases: Principles, Pathogens and Practice; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–7. [Google Scholar]
- Roberts, L.S.; Janovy, J. Roberts Foundation of Parasitology, 9th ed; McGraw Hill Publishers: New York, NY, USA, 2012. [Google Scholar]
- Jones, A.W. Introduction to Parasitology; Addison Wesley: Boston, MA, USA, 1967. [Google Scholar]
- Zeeshan, I.; Ijaz, A.; Maghsi, I.A.; Qasim, M.; Amin, S.B. Navigating Naegleria fowleri: Understanding pathogenesis, causes and preventive measures. Med. Clin. Case Rep. J. 2023, 1, 166–167. [Google Scholar]
- Cope, J.R.; Ratard, R.C.; Hill, V.R.; Sokol, T.; Causey, J.J.; Yoder, J.S.; Mirani, G.; Mull, B.; Mukerjee, K.A.; Narayanan, J.; et al. The first association of a primary amebic meningoencephalitis death with culturable Naegleria fowleri in tap water from a US treated public drinking water system. Clin. Infect. Dis. 2015, 60, e36–e42. [Google Scholar] [CrossRef]
- Aurongzeb, M.; Fatima, S.Z.; Hussain, S.I.; Rashid, Y.; Aziz, T.; Alhomrani, M.; Alsanie, W.F.; Alamri, A.S. Detection and identification of Naegleria species along with Naegleria fowleri in the tap water samples. BMC Med. Genom. 2025, 18, 6. [Google Scholar] [CrossRef]
- Aykur, M.; Dagci, H. Evaluation of molecular characterization and phylogeny for quantification of Acanthamoeba and Naegleria fowleri in various water sources, Turkey. PLoS ONE 2021, 16, e0256659. [Google Scholar] [CrossRef]
- Dereeper, A.; Allouch, N.; Guerlais, V.; Garnier, M.; Ma, L.; De Jonckheere, J.F.; Joseph, S.J.; Ali, I.K.M.; Talarmin, A.; Marcelino, I. Naegleria genus pangenome reveals new structural and functional insights into the versatility of these free-living amoebae. Front. Microbiol. 2023, 13, 1056418. [Google Scholar] [CrossRef]
- Phung, N.T.N.; Pham, H.T.; Tran, T.T.; Dinh, V.H.; Tran, N.M.; Tran, N.A.N.; Ngo, M.Q.N.; Nguyen, H.T.T.; Tran, D.K.; Le, T.K.T.; et al. Naegleria fowleri: Portrait of a Cerebral Killer. Diagnostics 2025, 15, 89. [Google Scholar] [CrossRef]
- Aykur, M.; Dirim Erdogan, D.; Selvi Gunel, N.; Guler, A.; Biray Avci, C.; Celebisoy, N.; Gunduz, C.; Dagci, H. Genotyping and Molecular Identification of Acanthamoeba Genotype T4 and Naegleria fowleri from Cerebrospinal Fluid Samples of Patients in Turkey: Is it the Pathogens of Unknown Causes of Death? Acta Parasitol. 2022, 67, 1372–1383. [Google Scholar] [CrossRef]
- Borecka, A.; Bielawska-DrÓzd, A.; Skotarczak, B.; Adamska, M.; CieŚlik, P.; Antos-Bielska, M.; SkopiŃska-RÓŻewska, E.; Donskow-Łysoniewska, K. Acanthamoeba—pathogen and vector of highly pathogenic bacteria strains to healthy and immunocompromised individuals. Cent. Eur. J. Immunol. 2020, 45, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Ramos, D.; Reyes-Batlle, M.; Bellini, N.K.; Rodríguez-Expósito, R.L.; Piñero, J.E.; Lorenzo-Morales, J. Naegleria australiensis isolated from a wastewater treatment station in Santiago Island, Cape Verde. J. Water Health 2023, 21, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Walochnik, J.; Wylezich, C.; Michel, R. The genus Sappinia: History, phylogeny and medical relevance. Exp. Parasitol. 2010, 126, 4–13. [Google Scholar] [CrossRef]
- Otero-Ruiz, A.; Gonzalez-Zuñiga, L.D.; Rodriguez-Anaya, L.Z.; Lares-Jiménez, L.F.; Gonzalez-Galaviz, J.R.; Lares-Villa, F. Distribution and Current State of Molecular Genetic Characterization in Pathogenic Free-Living Amoebae. Pathogens 2022, 11, 1199. [Google Scholar] [CrossRef]
- Rojo, J.U.; Rajendran, R.; Salazar, J.H. Laboratory Diagnosis of Primary Amoebic Meningoencephalitis. Lab. Med. 2023, 54, e124–e132. [Google Scholar] [CrossRef]
- Shaukat, A.; Khaliq, N.; Riaz, R.; Munsab, R.; Ashraf, T.; Raufi, N.; Shah, H. Noninvasive diagnostic biomarkers, genomic profiling, and advanced microscopic imaging in the early detection and characterization of Naegleria fowleri infections leading to primary amebic meningoencephalitis (PAM). Ann. Med. Surg. 2024, 86, 2032–2048. [Google Scholar] [CrossRef]
- El-Sayed, N.M.; Hikal, W.M. Several staining techniques to enhance the visibility of Acanthamoeba cysts. Parasitol. Res. 2015, 114, 823–830. [Google Scholar] [CrossRef]
- Gunarathna, N.; Amarasinghe, A.; Wijesundara, S.; Iddawela, D.; Wickramasinghe, S. Isolation, molecular characterization and phylogeny of Naegleria species in water bodies of North-Western Province, Sri Lanka. PLoS ONE 2021, 16, e0248510. [Google Scholar] [CrossRef]
- Philippe, N.; Shukla, A.; Abergel, C.; Bisio, H. Genetic manipulation of giant viruses and their host, Acanthamoeba castellanii. Nat. Protoc. 2024, 19, 3–29. [Google Scholar] [CrossRef]
- Guimaraes, A.J.; Gomes, K.X.; Cortines, J.R.; Peralta, J.M.; Peralta, R.H. Acanthamoeba spp. as a universal host for pathogenic microorganisms: One bridge from environment to host virulence. Microbiol. Res. 2016, 193, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Arthofer, P.; Panhölzl, F.; Delafont, V.; Hay, A.; Reipert, S.; Cyran, N.; Wienkoop, S.; Willemsen, A.; Sifaoui, I.; Arberas-Jiménez, I.; et al. A giant virus infecting the amoeboflagellate Naegleria. Nat. Commun. 2024, 15, 3307. [Google Scholar] [CrossRef] [PubMed]
- Sam-Yellowe, T.Y.; Yadavalli, R.; Fujioka, H.; Peterson, J.W.; Drazba, J.A. RhopH3, rhoptry gene conserved in the free-living alveolate flagellate Colpodella sp. (Apicomplexa). Eur. J. Protistol. 2019, 71, 125637. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, J.; Scharf, S.; Schmidt, S.; Jonscher, E.; Hoeijmakers, W.A.M.; Flemming, S.; Toenhake, C.G.; Schmitt, M.; Sabitzki, R.; Bergmann, B.; et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science 2020, 367, 51–59. [Google Scholar] [CrossRef]
- Behrens, H.M.; Schmidt, S.; Spielmann, T. The newly discovered role of endocytosis in artemisinin resistance. Med. Res. Rev. 2021, 41, 2998–3022. [Google Scholar] [CrossRef]
- Sharma, A.I.; Shin, S.H.; Bopp, S.; Volkman, S.K.; Hartl, D.L.; Wirth, D.F. Genetic background and PfKelch13 affect artemisinin susceptibility of PfCoronin mutants in Plasmodium falciparum. PLoS Genet. 2020, 16, e1009266. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; Boonhok, R.; Cabrera, M.; Mbenda, H.G.N.; Wang, M.; Min, H.; Liang, X.; Qin, J.; Zhu, X.; Miao, J.; et al. Role of Plasmodium falciparum Kelch 13 Protein Mutations in P. falciparum Populations from Northeastern Myanmar in Mediating Artemisinin Resistance. mBio 2020, 11, e01134-19. [Google Scholar] [CrossRef]
- Gnädig, N.F.; Stokes, B.H.; Edwards, R.L.; Kalantarov, G.F.; Heimsch, K.C.; Kuderjavy, M.; Crane, A.; Lee, M.C.S.; Straimer, J.; Becker, K.; et al. Insights into the intracellular localization, protein associations and artemisinin resistance properties of Plasmodium falciparum K13. PLoS Pathog. 2020, 16, e1008482. [Google Scholar] [CrossRef]
- Demas, A.R.; Sharma, A.I.; Wong, W.; Early, A.M.; Redmond, S.; Bopp, S.; Neafsey, D.E.; Volkman, S.K.; Hartl, D.L.; Wirth, D.F. Mutations in Plasmodium falciparum actin-binding protein coronin confer reduced artemisinin susceptibility. Proc. Natl. Acad. Sci. USA 2018, 115, 12799–12804. [Google Scholar] [CrossRef]
- Bane, K.S.; Lepper, S.; Kehrer, J.; Sattler, J.M.; Singer, M.; Reinig, M.; Klug, D.; Heiss, K.; Baum, J.; Mueller, A.K.; et al. The Actin Filament-Binding Protein Coronin Regulates Motility in Plasmodium Sporozoites. PLoS Pathog. 2016, 12, e100571. [Google Scholar] [CrossRef]
- Chan, K.T.; Creed, S.J.; Bear, J.E. Unraveling the enigma: Progress towards understanding the coronin family of actin regulators. Trends Cell Biol. 2011, 21, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Sam-Yellowe, T.Y.; Fujioka, H.; Peterson, J.W. Ultrastructure of Myzocytosis and Cyst Formation, and the Role of Actin in Tubular Tether Formation in Colpodella sp. (ATCC 50594). Pathogens 2022, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Nakada-Tsukui, K.; Nozaki, T. Trogocytosis in Unicellular Eukaryotes. Cells 2021, 10, 2975. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Li, Y.; Jing, S.; Han, S.; He, H. Trichomonas gallinae Kills Host Cells Using Trogocytosis. Pathogens 2023, 12, 1008. [Google Scholar] [CrossRef]
- Salti, M.I.; Sam-Yellowe, T.Y. Are Colpodella Species Pathogenic? Nutrient Uptake and Approaches to Diagnose Infections. Pathogens 2024, 13, 600. [Google Scholar] [CrossRef]
- Vandersea, M.W.; Birkenheuer, A.J.; Litaker, R.W.; Vaden, S.L.; Renschler, J.S.; Gookin, J.L. Identification of Parabodo caudatus (class Kinetoplastea) in urine voided from a dog with hematuria. J. Vet. Diagn. Investig. 2015, 27, 117–120. [Google Scholar] [CrossRef]
- Bradbury, R.S.; Sapp, S.G.H.; Potters, I.; Mathison, B.A.; Frean, J.; Mewara, A.; Sheorey, H.; Tamarozzi, F.; Couturier, M.R.; Chiodini, P.; et al. Where Have All the Diagnostic Morphological Parasitologists Gone? J. Clin. Microbiol. 2022, 60, e0098622. [Google Scholar] [CrossRef]
- Rosenblatt, J.E. Laboratory diagnosis of infections due to blood and tissue parasites. Clin. Infect. Dis. 2009, 49, 1103–1108. [Google Scholar] [CrossRef]
- Ricciardi, A.; Ndao, M. Diagnosis of Parasitic Infections: What’s Going On? J. Biomol. Screen. 2015, 20, 6–21. [Google Scholar] [CrossRef]
- Leander, B.S.; Kuvardina, O.N.; Aleshin, V.V.; Mylnikov, A.P.; Keeling, P.J. Molecular phylogeny and surface morphology of Colpodella edax (Alveolata): Insights into the phagotrophic ancestry of apicomplexans. J. Eukaryot. Microbiol. 2003, 50, 334–340. [Google Scholar] [CrossRef]
- Li, X.; Dang, Z.; Tang, W.; Zhang, H.; Shao, J.; Jiang, R.; Zhang, X.; Huang, F. Detection of Parasites in the Field: The Ever-Innovating CRISPR/Cas12a. Biosensors 2024, 14, 145. [Google Scholar] [CrossRef]
- Sam-Yellowe, T.Y.; Addepalli, K.; Yadavalli, R.; Peterson, J.W. New trichrome stains identify cysts of Colpodella sp. (Apicomplexa) and Bodo caudatus. Int. Microbiol. 2020, 23, 303–311. [Google Scholar] [CrossRef]
- Aikawa, M. Variations in structure and function during the life cycle of malarial parasites. Bull. World Health Organ. 1977, 55, 139–156. [Google Scholar]
- Mackenstedt, U.; Brockelman, C.R.; Mehlhorn, H.; Raether, W. Comparative morphology of human and animal malaria parasites. I. Host-parasite interface. Parasitol. Res. 1989, 75, 528–535. [Google Scholar] [CrossRef]
- Fuehrer, H.P.; Campino, S.; Sutherland, C.J. The primate malaria parasites Plasmodium malariae, Plasmodium brasilianum and Plasmodium ovale spp.: Genomic insights into distribution, dispersal and host transitions. Malar. J. 2022, 21, 138. [Google Scholar] [CrossRef]
- Antunes, S.; Rosa, C.; Couto, J.; Ferrolho, J.; Domingos, A. Deciphering Babesia-Vector Interactions. Front. Cell Infect. Microbiol. 2017, 7, 429. [Google Scholar] [CrossRef]
- Lares-Jiménez, L.F.; Borquez-Román, M.A.; Alfaro-Sifuentes, R.; Meza-Montenegro, M.M.; Casillas-Hernández, R.; Lares-Villa, F. Detection of serum antibodies in children and adolescents against Balamuthia mandrillaris, Naegleria fowleri and Acanthamoeba T4. Exp. Parasitol. 2018, 189, 28–33. [Google Scholar] [CrossRef]
- Orfano, A.S.; Nacif-Pimenta, R.; Duarte, A.P.; Villegas, L.M.; Rodrigues, N.B.; Pinto, L.C.; Campos, K.M.; Pinilla, Y.T.; Chaves, B.; Barbosa Guerra, M.G.; et al. Species-specific escape of Plasmodium sporozoites from oocysts of avian, rodent, and human malarial parasites. Malar. J. 2016, 15, 394. [Google Scholar] [CrossRef]
- de Souza, W. Contribution of microscopy to a better understanding of the anatomy of pathogenic protists. Proc. Natl. Acad. Sci. USA 2024, 121, e2321515121. [Google Scholar] [CrossRef]
- Kuupiel, D.; Bawontuo, V.; Mashamba-Thompson, T.P. Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low- and Middle-Income Countries: Lean and Agile Supply Chain Management. Diagnostics 2017, 7, 58. [Google Scholar] [CrossRef]
- von Philipsborn, P.; Steinbeis, F.; Bender, M.E.; Regmi, S.; Tinnemann, P. Poverty-related and neglected diseases—an economic and epidemiological analysis of poverty relatedness and neglect in research and development. Glob. Health Action 2015, 8, 25818. [Google Scholar] [CrossRef] [PubMed]
- Getty, T. Life Cycle and Morphological Characterization of Colpodella sp. (ATCC 50594) in Hay Medium. Master’s Thesis, Cleveland State University, Cleveland, OH, USA, December 2020. [Google Scholar]
- Sam-Yellowe, T.Y.; Yadavalli, R. Voromonas pontica Identified by Giemsa Staining and AntiRhopH3 Protein Reactivity. J. Microbiol. Modern Tech. 2019, 4, 103. [Google Scholar]
- Oborník, M.; Modrý, D.; Lukeš, M.; Cernotíková-Stříbrná, E.; Cihlář, J.; Tesařová, M.; Kotabová, E.; Vancová, M.; Prášil, O.; Lukeš, J. Morphology, ultrastructure and life cycle of Vitrella brassicaformis n. sp., n. gen., a novel chromerid from the Great Barrier Reef. Protist 2012, 163, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Oborník, M.; Vancová, M.; Lai, D.H.; Janouškovec, J.; Keeling, P.J.; Lukeš, J. Morphology and ultrastructure of multiple life cycle stages of the photosynthetic relative of apicomplexa, Chromera velia. Protist 2011, 162, 115–130. [Google Scholar] [CrossRef]
- Sam-Yellowe, T.Y.; Salti, M.I.; Adeloye, O.E. Sam-Yellowe’s Trichrome Staining Identifies Life Cycle Stages of Free-Living Colpodellids. J. Appl. Microb. Res. 2024, 7, 1–9. [Google Scholar]
- Füssy, Z.; Masařová, P.; Kručinská, J.; Esson, H.J.; Oborník, M. Budding of the Alveolate Alga Vitrella brassicaformis Resembles Sexual and Asexual Processes in Apicomplexan Parasites. Protist 2017, 168, 80–91. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, S.; Huang, J.L.; Tambo, E.; Zhuge, H.X.; Zhou, X.N. Human babesiosis, an emerging tick-borne disease in the People’s Republic of China. Parasites Vectors 2014, 7, 509. [Google Scholar] [CrossRef]
- Kumar, A.; O’Bryan, J.; Krause, P.J. The Global Emergence of Human Babesiosis. Pathogens 2021, 10, 1447. [Google Scholar] [CrossRef]
- Sanchez-Vicente, S.; Tokarz, R. Tick-Borne Co-Infections: Challenges in Molecular and Serologic Diagnoses. Pathogens 2023, 12, 1371. [Google Scholar] [CrossRef]
- Obaid, M.K.; Lan, X.; Ren, Q.; Zeb, J.; Luo, J.; Yang, J.; Jia, W.; Zan, X.; Yin, H.; Rashid, M.; et al. Molecular insights into Rickettsiales in blood and ticks of two-humped camels at Gansu Province, China: With an accidental detection of Colpodella sp. Vet. Microbiol. 2025, 305, 110528. [Google Scholar] [CrossRef]
- Templeton, T.J.; Pain, A. Diversity of extracellular proteins during the transition from the ‘proto-apicomplexan’ alveolates to the apicomplexan obligate parasites. Parasitology 2015, 143, 1–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okamoto, N.; Keeling, P.J. A Comparative Overview of the Flagellar Apparatus of Dinoflagellate, Perkinsids and Colpodellids. Microorganisms 2014, 2, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, K.V.; Janouškovec, J.; Tikhonenkov, D.V.; Mirzaeva, G.S.; Diakin, A.Y.; Simdyanov, T.G.; Mylnikov, A.P.; Keeling, P.J.; Aleoshin, V.V. A complex distribution of elongation family GTPases EF1A and EFL in basal alveolate lineages. Genome Biol. Evol. 2014, 6, 2361–2367. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Organism Name | Source | Vertebrate Host | Tick/Biting Flies | Country |
|---|---|---|---|---|
| Ticks infesting goat and dog | Goat | Haemaphysalis longicornis | China: Yiyuan County, Shangdong |
| Ticks infesting goat | Goat | Haemaphysalis longicornis | China: Yiyuan County, Shangdong |
| Blood | Horse | N/A | China |
| Blood | Horse | N/A | China |
| N/A | N/A | N/A | China |
| N/A | N/A | N/A | China |
| Woman with neurological symptoms | N/A | Tick | China |
| Ticks infesting dog | Dog | Haemaphysalis longicornis | China: Yiyuan County, Shandong |
| Blood | N/A | N/A | (China) |
| Blood | Dog | N/A | China: Guiyang |
| Blood | Cat | N/A | China: Guiyang |
| N/A | N/A | N/A | China |
| N/A | N/A | N/A | China |
| N/A | Panthera tigris altaica (Amur tiger) | N/A | China |
| N/A | Panthera tigris altaica (Amur tiger) | N/A | China |
| N/A | Panthera tigris altaica (Amur tiger) | N/A | China |
| N/A | Panthera tigris altaica (Amur tiger) | N/A | China |
| N/A | Panthera tigris altaica (Amur tiger) | N/A | China |
| N/A | N/A | Rhipicephalus microplus | China: Shandong |
| N/A | N/A | Rhipicephalus microplus | China: Shandong |
| Rhipicephalus microplus | China: Shandong | ||
| N/A | Dermacentor everestianus | China: Qinghai | |
| N/A | N/A | Dermacentor nuttalli | China: Qinghai |
| N/A | N/A | Dermacentor nuttalli | China: Qinghai |
| N/A | N/A | Dermacentor nuttalli | China: Qinghai |
| N/A | N/A | Dermacentor everestianus | China: Qinghai |
| Haemaphysalis qinghiensis | China: Qinghai | ||
| Haemaphysalis qinghiensis | China: Qinghai | ||
| Man with relapsing fever | Homo sapiens | N/A | China |
| Woman with relapsing Babesia-like illness | Homo sapiens | N/A | China |
| Blood, ticks | Camel | China: Gansu | |
| Amblyomma javanense | Pangolin | Tick | China: Guangzhou |
| Amblyomma javanense | Pangolin | Tick | China: Guangzhou |
| Amblyomma javanense | Pangolin | Tick | China: Guangzhou |
| Amblyomma javanense | Pangolin | Tick | China: Guangzhou |
| Amblyomma javanense | Pangolin | Tick | China: Guangzhou |
| Amblyomma javanense | Pangolin | Tick | China: Guangzhou |
| Tick | China: Guiyang | ||
| N/A | Bos taurus | Tick | Pakistan |
| N/A | Bos taurus | Tick | Pakistan |
| N/A | Bos taurus | Tick | Pakistan |
| N/A | Bos taurus | Tick | Pakistan |
| N/A | Bos taurus | Tick | Pakistan |
| N/A | Bos taurus | Tick | Pakistan |
| Tick P03 collected from cattle | Cattle | Rhipicephalus (Boophilus) microplus; sex: female | Russia |
| N/A | N/A | N/A | Russia |
| N/A | Stomoxys indicus | Thailand: Nakhon Si Thammarat | |
| Blood | Dog | Cambodia | |
| N/A | Bos taurus | N/A | Japan |
| Tick | Rhipicephalus annulatus | Japan | |
| Feces | Fox (Vulpes vulpes indutus) | N/A | Nicosia, Cyprus |
| Feces | Duck Anas spp. | N/A | Nicosia, Cyprus |
| Feces | Duck Anas spp. | N/A | Nicosia, Cyprus |
| Feces | Eurasian Coot (Fulica atra) | ||
| Feces | Goat (Capra hircus) | N/A | Nicosia, Cyprus |
| N/A | N/A | N/A | Portugal |
| Identified with Amyloodinium ocellatum (dinoflagellate ectoparasite) | Sea bass Dicentrarchus labrax | N/A | Portugal |
| Skin, dried ear fragments, Warta Mouth National Park, western Poland | Procyon lotor | N/A | Poland |
| Skin, dried ear fragments, Warta Mouth National Park, western Poland | Procyon lotor | N/A | Poland |
| Slow sand filter column for wastewater treatment, Leipzig | N/A | N/A | Germany |
| Marine sample | N/A | Germany Helgoland | |
| Lake water filtered through 3 um from Lake Esch sur Sure, depth 0 m | N/A | N/A | Luxembourg |
| Ticks | Cattle and goats | Rhipicephalus bursa | Italy |
| Lake water | France | ||
| Feces of calves with diarrhea | N/A | Turkey: Nevsehir | |
| Woman with urinary tract infection (identified with Colpoda steinii) | N/A | Romania | |
| Whole body of Hyalomma dromedarii | Camel | Hyalomma dromedarii | Egypt: Luxor |
| Whole body of Hyalomma dromedarii | Camel | Hyalomma dromedarii | Egypt: Aswan |
| whole body of Hyalomma dromedarii | Camel | Hyalomma dromedarii | Egypt: Luxor |
| whole body of Hyalomma dromedarii | Camel | Hyalomma dromedarii | Egypt: Aswan |
| Feces | Sheep | N/A | Nigeria |
| Feces | Sheep | N/A | Nigeria |
| Feces | Sheep | N/A | Nigeria |
| Feces | Sheep | N/A | Nigeria |
| Feces | Sheep | N/A | Nigeria |
| Feces | Sheep | N/A | Nigeria |
| Tick P03 collected from cattle | Cattle | Rhipicephalus (Boophilus) microplus; sex: female | Mozambique |
| Blood | Cattle and wildlife | Zambia | |
| N/A | Bovine | N/A | Brazil |
| N/A | Bovine | N/A | Brazil |
| N/A | Bovine | N/A | Brazil |
| N/A | Bovine | N/A | Brazil |
| N/A | Bovine | N/A | Brazil |
| N/A | Bovine | N/A | Brazil |
| Tropical floodplain lake | N/A | N/A | Brazil |
| N/A | N/A | N/A | Costa Rica |
| Laboratory culture | N/A | N/A | Canada: Vancouver |
| Laboratory culture | N/A | N/A | Canada: Vancouver |
| Damp wood chip and surface sand, Locarno beach | N/A | N/A | Canada: Vancouver |
| Wood chip on the beach | N/A | N/A | Canada: Boundary Bay |
| Soil from UBC endowment lands | N/A | N/A | Canada: Vancouver |
| Cave and mine | N/A | N/A | New York, USA |
| Brown woodland soil, Gambrill Park | N/A | N/A | Maryland, USA |
| Freshwater laboratory dishes with mosquito larvae, Rutgers University | Mosquito larvae | N/A | New Jersey, USA |
| Mosquito larvae | N/A | New Jersey, USA | |
| Cat blood | N/A | N/A | North Carolina, USA |
| Mucus from Acropora formosa, Birch Aquarium | N/A | N/A | San Diego, California, USA |
| Anoxic marine sediment, Bolinas Tidal Flat | N/A | N/A | Bolinas, California, USA |
| Intertidal thrombolites | N/A | N/A | Florida, USA |
| Intertidal thrombolites | N/A | N/A | Florida, USA |
| Intertidal thrombolites | N/A | N/A | Florida, USA |
| Intertidal thrombolites | N/A | N/A | Florida, USA |
| Cattle manure, identified with Parabodo sp. | N/A | N/A | Kansas, USA |
| Soil, trembling aspen rhizosphere, elevated CO2 conditions | N/A | N/A | Michigan, USA |
| Button and pink thrombolithic mats | N/A | N/A | Bahamas |
| Hypersaline Lake Tyrrell | N/A | N/A | Australia |
| Wastewater | N/A | N/A | Australia |
| Wastewater | N/A | N/A | Australia |
| Wastewater | N/A | N/A | Australia |
| Wastewater | N/A | N/A | Australia |
| Wastewater | N/A | N/A | Australia |
| Megalapteryx didinus coprolite, sample 01098a, animal feces/manure Dart River Valley | N/A | N/A | New Zealand |
| Non-crust habitat, Asgard Range | N/A | N/A | Antarctica |
| Soil in front of the Brazilian Antarctic Station | N/A | N/A | Antarctica |
| Oxygen-depleted intertidal marine sediment, upper 2 cm, Greenland | N/A | N/A | Arctic |
| Composting diary manure, animal feces | N/A | N/A | |
| Composting diary manure, animal feces | |||
| Marine | N/A | N/A | |
| Animal feces/manure, pig manure storage pit | N/A | N/A | |
| Animal feces/manure, pig manure storage pit | N/A | N/A | |
| Animal feces/manure, pig manure storage pit | N/A | N/A |
| Source Found with Colpodella spp. | References |
|---|---|
| Ticks | |
| Ixodes persulcatus | [23] |
| Rhipicephalus (Boophilus) microplus | [9] |
| Rh. bursa | [10] |
| Rh. duttoni | [12] |
| Rh. haemaphysaloides | NCBI accession number MH208621 |
| Haemaphysalis longicornis | [12,14] |
| H. flava | [12] |
| H. bispinosa | [12] |
| H. hystricis | [12] |
| Hyalomma dromedarii | [13] |
| Hyalomma asiaticum | NCBI accession number PQ380976 |
| Dermacentor everestianus | NCBI accession number MH012047 |
| D. nuttalli | NCBI accession number MH012045 |
| D. andersoni | [12] |
| D. atrosignatus | [12] |
| D. taiwanensis | [12] |
| Amblyomma javanense | [11] |
| Biting fly | |
| Stomoxys indicus | [15] |
| Host tissue and body fluids | |
| Skin | [27] |
| Blood | [22,24,25,36,37,38] |
| Cerebrospinal fluid | [23] |
| Urine | [26] |
| Fecal samples | [17,18,19], NCBI accession number JN245625 |
| Sam-Yellowe’s Trichrome Staining | Application of Dyes in Order of Incubation |
|---|---|
| Sam-Yellowe’s trichrome A | 0.3% Methylene blue (1 min) 1% Brilliant green (5 min) 1% Neutral Red (1 min) Distilled water washes were performed in between each dye incubation. After the last wash, smears are air-dried before microscope observation using oil immersion at ×1000. |
| Sam-Yellowe’s trichrome D | 1% Crystal violet (30 s) 1% Brilliant green (2 min) 1% Neutral red (1 min) Distilled water washes were performed in between each dye incubation. After the last wash, smears are air-dried before microscope observation using oil immersion at ×1000. |
| Sam-Yellowe’s trichrome E | 1% Crystal violet (30 s) 1% Brilliant green (2 min) 1% Safranin (1 min) Distilled water washes were performed in between each dye incubation. After the last wash, smears are air-dried before microscope observation using oil immersion at ×1000. |
| Sam-Yellowe’s trichrome J | 0.3% Methylene blue (1 min) 0.5% Fast green in alcohol (5 min) 1% Neutral Red (1 min) Distilled water washes were performed in between each dye incubation. After the last wash, smears are air-dried before microscope observation using oil immersion at ×1000. |
| Colpodella spp. in Humans and Animals | Year | Country | Reference |
|---|---|---|---|
| Human, relapsing fever, non-tick-associated blood infection, single infection, female | 2012 | China | [22] |
| Human, relapsing fever, non-tick-associated, single infection, male | 2017 | China | NCBI accession number MF594625 |
| Cattle, tick-associated, coinfection | 2017 | Mozambique | [9] |
| Human, tickborne infection, neurological symptoms, single infection, female | 2018 | China | [23] |
| Raccoon, non-tick-associated Colpodella spp. in the skin of the ear, coinfection | 2019 | Poland | [27] |
| Cattle, non-tick-associated blood infection, coinfection | 2020 | Zambia | [38] |
| Human, urinary tract infection associated with Colpodella gonderi and its prey Colpoda steinii, female | 2021 | Romania | [26] |
| Large zoo felids, Colpodella spp. in fecal samples, coinfection | 2021 | China | [17] |
| Dog, non-tick-associated blood infection, coinfection | 2021 | Cambodia | [37] |
| Tiger (Panthera tigris amoyensis Hizheimer), in blood and ticks, tickborne Colpodella spp. infection, single infection, multiple-organ damage | 2022 | China | [12] |
| Horse, non-tick-associated blood infection, coinfection | 2022 | China | [24] |
| Cat, non-tick-associated blood infection, inflammation, tissue damage, single infection | 2023 | USA | [25] |
| Cats and dogs, non-tick-associated blood infection, coinfection | 2023 | China | [36] |
| Horse, Colpopdella spp. in infesting biting fly (Stomoxys indicus), coinfection | 2023 | Thailand | [15] |
| Goats and dogs, Colpodella spp. in ticks | 2024 | China | [14] |
| Camels, Colpodella spp. in infesting ticks | 2024 | Egypt | [13] |
| Cattle and goats, Colpodella spp. in infesting ticks | 2024 | Italy | [10] |
| Goats and sheep, non-tick-associated infection, Colpodella spp. in diarrhetic fecal samples, coinfection | 2024 | Nigeria | [18] |
| Pangolins, Colpodella spp. in infesting ticks, coinfection | 2024 | China | [11] |
| Goats, fox, duck, Eurasian Coot, non-tick-associated, Colpodella spp. in fecal samples | 2025 | Cyprus | [19] |
| Two-humped camels (Camelus bacterianus) | 2025 | China | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sam-Yellowe, T.Y.; Nims, T.; Qaderi, S.; Asraf, M.M. Tickborne Colpodella Species Infections: Time for a New Integrated Approach to Understand Transmission and Pathogenicity. Zoonotic Dis. 2025, 5, 14. https://doi.org/10.3390/zoonoticdis5020014
Sam-Yellowe TY, Nims T, Qaderi S, Asraf MM. Tickborne Colpodella Species Infections: Time for a New Integrated Approach to Understand Transmission and Pathogenicity. Zoonotic Diseases. 2025; 5(2):14. https://doi.org/10.3390/zoonoticdis5020014
Chicago/Turabian StyleSam-Yellowe, Tobili Y., Trinity Nims, Sona Qaderi, and Mary M. Asraf. 2025. "Tickborne Colpodella Species Infections: Time for a New Integrated Approach to Understand Transmission and Pathogenicity" Zoonotic Diseases 5, no. 2: 14. https://doi.org/10.3390/zoonoticdis5020014
APA StyleSam-Yellowe, T. Y., Nims, T., Qaderi, S., & Asraf, M. M. (2025). Tickborne Colpodella Species Infections: Time for a New Integrated Approach to Understand Transmission and Pathogenicity. Zoonotic Diseases, 5(2), 14. https://doi.org/10.3390/zoonoticdis5020014






