Simple Summary
Zoonotic diseases—diseases that can be transmitted from animals to humans and vice versa—pose a major health risk in sub-Saharan Africa due to close human-animal contact and limited diagnostic tools. This study examined 47 patients in Zambia suspected of having COVID-19, collecting samples from November 2020 to February 2021 at two major hospitals in Lusaka. Using advanced genetic testing (mNGS), researchers detected a variety of bacteria, viruses, fungi, and parasites, with 57.4% of patients carrying zoonotic pathogens. Some key infections included anthrax, plague, brucellosis, leptospirosis, and rickettsial diseases. However, no strong links were found between infections and factors like age, gender, or underlying health conditions. The study underscores the need for more research to better understand and prevent zoonotic disease transmission in Zambia.
Abstract
Zoonotic diseases present a growing public health challenge, particularly in sub-Saharan Africa (SSA) due to close interactions between humans and animals and poor diagnostic capacity. This pilot study investigated human exposure to zoonotic pathogens in Zambia among 47 suspected COVID-19 patients from whom nasopharyngeal samples were collected between November 2020 and February 2021 at two major COVID-19 referral centers in Lusaka. Using metagenomic next-generation sequencing (mNGS), the study identified a diverse range of pathogens, including bacterial, fungal, viral, and parasitic species. The prevalence of zoonotic pathogens was 57.4%. Noteworthy zoonoses included Bacillus anthracis, Sporothrix schenckii, Listeria monocytogenes, Yersinia pestis, Streptococcus suis, Vibrio parahaemolyticus, Brucella melitensis, Rickettsia prowazekii, Shewanella algae, Rickettsia japonica, Coxiella burnetii, Leptospira borgpetersenii, Erysipelothrix rhusiopathiae, Brucella abortus, Bartonella quintana, Banna virus, Vibrio alginolyticus, Bartonella clarridgeiae, Rickettsia canadensis, Leishmania braziliensis, Trypanosoma brucei, Pasteurella multocida, and Arcobacter butzleri. Despite moderate diversity in the microbial community, no significant demographic or health-related factors, including age, gender, or comorbidities such as HIV, were found to be statistically associated with zoonotic pathogen infection. The findings provide valuable data on the presence of zoonotic pathogens in humans in Zambia and highlight the need for more comprehensive research into zoonotic diseases in both clinical and non-clinical settings.
1. Introduction
Zoonotic diseases, which are transmitted between animals and humans, represent a major and growing public health challenge, particularly in sub-Saharan Africa (SSA) [1]. The close interaction between humans, livestock, and wildlife in this region creates a perfect environment for pathogen spill-over [2,3,4,5]. A key concern in SSA is the potential for zoonotic coinfections, where the presence of multiple pathogens in an individual can complicate diagnosis and worsen disease outcomes. For instance, co-infections with malaria—a leading endemic disease in SSA—and zoonotic pathogens like leptospirosis or rickettsiosis can result in more severe clinical manifestations, making it difficult to pinpoint the underlying cause and delaying timely treatment [1,6]. Similarly, co-infection between zoonotic agents and emerging diseases like the 2019 coronavirus disease 2019 (COVID-19) and others can exacerbate disease severity and increase mortality rates [1,6,7,8].
This challenge is compounded by the limitations of current diagnostic strategies, particularly the reliance on syndromic diagnosis. This often fails to account for the wide diversity of pathogens that could be causing illness, leading to misdiagnosis or under-diagnosis [9]. Lack of comprehensive surveillance and pathogen-specific diagnostics may hinder timely interventions [10,11]. In SSA, including Zambia, the full range of zoonotic pathogens contribution to human illness outside large outbreaks remains poorly understood. A few studies have reported a 63% increase in zoonotic disease outbreaks between 2012 and 2022 [3]. Additionally, between 2000 and 2022, some countries in SSA (Cameroon, Central African Republic, Cote d’Ivoire, Democratic Republic of Congo, Gabon, Ghana, Kenya, Liberia, Madagascar, Mauritania, Mozambique, Namibia, Nigeria, Senegal, Sierra Leone, South Africa, South Sudan, Sudan, Tanzania, Uganda, Zambia, and Zimbabwe) reported a combined 28,934 human cases of zoonotic diseases, with 1182 associated deaths (a fatality rate of 345.4 per 1000 population) [3]. Pathogens such as Rickettsia sp. (770.4 per 1000), Toxoplasma sp. (678.11 per 1000), influenza A (523.81 per 1000), Coxiella burnetii (489.04 per 1000), and Brucella sp. (484.00 per 1000) had some of the highest attack rates in the region, while filoviruses (>600 per 1000) and Leptospira sp. (500 per 1000) were most frequently associated with fatalities [3].
Despite some reports of zoonotic diseases in humans in SSA, much of the research has focused on their presence in domestic and wild animals, with less attention paid to their impact on human health [12,13,14,15]. In Zambia, for instance, zoonoses are often neglected in diagnostic protocols at public health facilities, contributing to a lack of awareness and poor recognition of their impact on human morbidity and mortality [13]. This gap in knowledge is particularly concerning given Zambia’s vulnerability to emerging infectious diseases and the increasing risks of pathogen spill-over. A more comprehensive understanding of zoonotic diseases in humans is crucial not only to observe trends but also to actively intervene and mitigate these threats. By identifying the most common pathogens, determining infection hotspots, and pinpointing vulnerable populations, we can use this data to design pre-emptive, effective public health and clinical interventions and prevention strategies.
To address these knowledge gaps, this study investigated human exposure to zoonotic pathogens in Lusaka, Zambia using samples from suspected COVID-19 patients. The findings are intended to raise awareness of zoonotic pathogens among public health decision-makers and clinicians and lay the groundwork for larger, collaborative investigations into the broader landscape of clinically relevant zoonotic diseases in human populations across Zambia.
2. Materials and Methods
2.1. Study Population and Participant Enrollment
This mNGS-based pilot study was conducted on bio-banked nasopharyngeal samples previously used to evaluate the effectiveness of rapid COVID-19 test kits against polymerase chain reaction during the pandemic [16]. A total of 47 suspected COVID-19 samples were simply randomly selected into the study from 244 bio-banked samples for a pilot study of zoonotic pathogens in humans. A sample of 30 is usually recommended for pilot studies, but we opted for 47, as some researchers recommend a sample size of more than 30 [17]. The sample size was not meant to provide statistical power for testing a hypothesis but to create a study design basis for a future large-scale study on zoonoses in humans across Zambia. The study was also meant to provide relevant preliminary data indicating the prevalence of zoonotic pathogens in humans in Zambia. The samples came from patients presenting at two major COVID-19 referral centers in Lusaka, Zambia, the University Teaching Hospital (UTH) and Levy Mwanawasa University Teaching Hospital (LMUTH), between November 2020 and February 2021, as previously reported [16]. All of any gender individuals aged 18 years and older presenting to the emergency department with suspected SARS-CoV-2 infection were eligible for participation. No exclusion criterion was applied. Following informed consent, participants were enrolled and completed a detailed questionnaire capturing their demographic information, clinical symptoms, pre-existing health conditions, and comorbidities (HIV, diabetes, hypertension, cancer, chronic respiratory disease).
2.2. Specimen Collection and Laboratory Testing
Each participant provided a nasopharyngeal swab. The nasopharyngeal swab was placed into 3 mL of viral transport medium for both rapid antigen and reverse transcriptase polymerase chain reaction (RT-PCR) testing. The RealStar SARS-CoV-2 RT-PCR kit (Altona Diagnostics GmbH, Hamburg, Germany) was used for RT-PCR testing. The positivity of samples and presence of zoonotic pathogens were also evaluated using metagenomic next-generation sequencing (mNGS) through the NovaSeq 6000 (Illumina, San Diego, CA, USA) PCR machine, as previously reported [14,18,19].
2.3. Metagenomic Next-Generation Sequencing
RNA extracted from each of the nasopharyngeal swabs from 47 patients was subjected to mNGS for microbiome analysis. The library preparation was performed using the NEBNext Ultra II RNA protocol (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s guidelines. In brief, RNA extracts were fragmented and spiked with the External RNA Controls 103 Consortium (ERCC) collection (ThermoFisher, Waltham, MA, USA), followed by complementary DNA (cDNA) synthesis. The cDNA was then ligated with adaptors, which were subsequently digested. The DNA was barcoded and purified using a magnetic rack. Library size and concentration were assessed using the 4150 Tapestation system (Agilent, Boston, MA, USA). The flow cell was washed to remove salts, and the library was denatured with sodium hydroxide (NaOH). PhiX was included as a calibration control. Sequencing was performed on the NovaSeq 6000 platform (Illumina, San Diego, CA, USA). The sequencing approach was a paired-end approach, and the expected maximum read length was 2 × 150 bp.
2.3.1. Processing and Analysis of mNGS Data
Sequencing data from the NovaSeq 6000 platform (Illumina, San Diego, CA, USA) were processed in the CZ pipeline. The pipeline processed raw, short-read RNA-seq data, which were directly uploaded from Illumina’s BaseSpace. Analysis proceeded in three main stages: (i) host filtering and quality control (QC), (ii) assembly-based alignment, and (iii) reporting and visualization. QC began with validating paired-end FASTA files from the short-read libraries. Host sequences were filtered using STAR by aligning reads to the human HG38 reference genome; matches were discarded. Further QC included adapter trimming as well as removal of low-quality, duplicate, and low-complexity reads [20]. Residual human reads were excluded via Bowtie2 and gmap-gsnap. Throughout, the remaining read counts and key QC metrics (e.g., non-host read totals, QC pass rate, compression ratio) were calculated and shared via the user CZ pipeline interface. The download option of the metrics was available.
For taxonomic classification, reads were aligned to NCBI’s nucleotide (nt) and protein (nr) databases using GSNAPL and RAPsearch2. Each read was linked to potential taxonomic identifiers (taxIDs) using accession2taxid and BLAST version 1.4.0. In parallel, reads were assembled into contigs with SPAdes, then re-mapped with Bowtie2 to associate raw reads with contigs. Contigs were aligned to candidate accessions from the BLAST step, improving alignment precision, especially for ambiguous regions. Reads were assigned taxIDs based on alignment: ties were resolved randomly. Final species- and genus-level counts were aggregated from NT and NR assignments.
The CZ portal offered several tools for result visualization. It provided QC metrics, internal control estimates, and stepwise read counts. Single-sample reports summarized taxon-specific metrics, including read counts and contig stats (where available), as previously reported [18,21]. Reads, metrics, and where-provided contigs for any taxon were downloadable with one click as CSV files. Coverage depths and breadth of the taxons were available for viral taxons only.
2.3.2. Quality Control Metrics
To help identify contaminants, background models were generated. The background correction model was applied to eliminate taxa that may have been prevalent in water controls and passed through filtration. This model was generated using sequence data from negative water controls. The water used for RNA extraction served as the control. In accordance with the model’s application, the Z-score metric was set at 100 to account for potential background contamination from the water controls, ensuring that only microbes present in the samples were included in the analysis. The threshold for nucleotide reads per million (NT_rPM) was set to a minimum of 5 to comprehensively assess the mosquito microbiome. NT_rPM is a scaled metric that reflects the abundance of taxa, with smaller values indicating a lower likelihood of a particular organism’s presence in the sample. To maintain specificity, the NT_rPM threshold was set to include only reads specific to particular taxa.
2.3.3. Bioinformatic Analysis
The relative abundance of microbes was assessed using the NT_rPM metric within the CZ ID pipeline. Heatmaps were generated to present the relative abundance of zoonotic microbial species only, as the dataset was too large to display all heatmaps. Microbial diversity across all microbes and within specific categories (bacteria, fungi, parasites, and viruses) was measured using the Shannon diversity index, calculated with the AL Young biodiversity calculator (https://www.alyoung.com/labs/biodiversity_calculator.html?srsltid=AfmBOoo7BjD_UuM24Illq1LMqUJKqZL2xm1DwMeLuAnfsQMUOP7NQSlH, accessed on: 16 January 2025) [22] and manual data manipulation in Excel. Shannon index values typically range from 0 (no diversity) to 4.5 (high diversity). The dominance of microbial species (proportion of most abundant species in a community) was evaluated using the Berger–Parker Index. The Berger–Parker Index is represented by 0 (perfect evenness, where all species are equally represented) and 1 (one species dominates entirely) [23,24].
2.4. Statistical Analysis
Descriptive data were analyzed in Excel, and results were presented in tables and graphs. Differences in species diversity and abundance among bacteria, fungi, parasites, and viral microbes were assessed using the Kruskal–Wallis test in SPSS Ver. 21 (IBM Corp, Armonk, NY, USA). A p-value of less than 0.05 was considered statistically significant. To examine associations between demographic and health-related factors (age, gender, HIV status, hypertension status, diabetes status, cancer status, and CRD status) and positivity for zoonotic pathogens, the Pearson chi-square test was applied. The association was considered significant if p < 0.05. A multivariable logistic regression analysis was then conducted to identify independent factors associated with zoonotic pathogen positivity. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to assess the strength of these associations. Independent variables were considered significant risk factors when p < 0.05.
2.5. Ethical Considerations
Ethical approval for this study was granted by the Excellence in Research Ethics and Science (ERES) Converge Ethics Committee IRB (Ref. No. 2020-JUL-07). Additionally, consent was obtained from all participants and/or their guardians. All participants were informed about the study procedures through an information sheet and asked to sign or provide a thumbprint on an informed consent form before sample collection. Participant information was kept anonymous using codes.
3. Results
3.1. Demographic Characteristics
A total of 47 study participants were enrolled, consisting of 42.6% females (20/47) and 57.4% males (27/47). Among the participants, 17.0% (8/47) were living with HIV, while 19.1% (9/47) had various other comorbidity conditions. In terms of zoonotic pathogen positivity, 57.4% (27/47) of participants tested positive for at least one zoonotic pathogen. Among those with comorbidities (17/47), 52.9% (9/17) tested positive for at least one zoonotic pathogen. The majority of these individuals were HIV positive, with 66.7% (6/9) of the zoonotic pathogen-positive participants being HIV-infected.
3.2. Diversity and Dominance of Microbes
RNAs from a total of 3171 microbes, of which 87 were pathogenic, were detected in all 47 samples, representing 50.9% (1615/3171) bacterial species, 47.9% (1519/3171) fungi, 0.79% (25/3171) viruses, and 0.38% (12/3171) parasites. A total of 723,813 microbial sequencing reads were obtained, with bacteria comprising 67.95% (491,807.1) of the reads, followed by fungi at 28.36% (20,294.11), viruses at 3.52% (25,453.25), and parasites at 0.17% (1259.52). The reads specific to zoonotic pathogens per patient are available in Supplementary Table S1. In terms of overall species diversity across all microbial categories, the Shannon index (H’) of 2.393 indicated moderate biodiversity within the microbial community. There was a significant difference in the diversity of microbes across different microbial groups (p = 0.00002). Bacterial microbes exhibited the highest diversity (H’ = 3.221, d = 0.080), followed by fungi (H’ = 3.421, d = 0.071). In contrast, viruses and parasites displayed low species richness and uneven distribution, with H’ values of 1.029 (d = 0.470) and 1.900 (d = 0.274), respectively.
3.3. Prevalence and Abundance of Zoonotic Pathogens
Pathogenic microbes made up a small proportion of the total microbes, at 2.7% (87/3171). Of the 87 pathogenic microbes, 27.6% (24/87) were of zoonotic origin (Figure 1). The overall prevalence of zoonotic pathogens in the study population was 57.4% (27/47). The prevalence among males was 55.6% (15/27) compared to 60% (12/20) among females. The most prevalent zoonotic pathogens were Staphylococcus pseudintermedius (21.3%), Bacillus anthracis (8.5%), Sporothrix schenckii (8.5%), Arcobacter butzleri (6.4%), Listeria monocytogenes (4.3%), Yersinia pestis (4.3%), and Coxiella burnetii (4.3%) (Figure 1). Meanwhile, B. anthracis and Pasteurella multocida were the most abundant organisms. The abundance and distribution of zoonotic pathogens are shown in the heatmap in Figure 2.
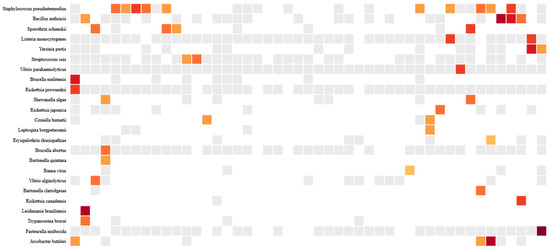
Figure 1.
Heatmap of zoonotic pathogens detected in 27 human samples. Microbes that were not present in the sample are represented by white and gray cells. White cells mean that the pathogens were completely absent, while gray cells mean that the pathogens were filtered out after application of the background model and other metrics as defined in the methods. For the other colors, a denser shade indicates a higher likelihood of high pathogen abundance. Color of boxes indicates number of reads, increasing from beige to light orange to red  .
.
 .
.

Figure 2.
Prevalence and relative abundance of zoonotic pathogens.
3.4. Risk Factors for Infection with Zoonotic Pathogens
Although the sample size was low, the chi-square (χ2) test and multivariate logistic regression analysis were used to assess the potential influence of demographic and health-related factors on infection with zoonotic pathogens. There was no association between the factors and infection with zoonotic pathogens, and none of the variables (gender, age, comorbidities, or HIV status) were found to be statistically significant predictors of zoonotic pathogen infection (p > 0.05) (Table 1).

Table 1.
Likelihood estimates for occurrence of zoonoses in humans.
4. Discussion
This pilot study explored the presence and diversity of zoonotic pathogens in patients presenting with suspected SARS-CoV-2 infection at two major COVID-19 referral hospitals in Lusaka, Zambia. By utilizing metagenomic next-generation sequencing (mNGS), 24 zoonotic pathogens were identified in nasopharyngeal samples from 47 patients. Despite the high prevalence of zoonotic pathogens, no significant associations were found between demographic factors (such as gender, age) or health-related conditions (including HIV status and comorbidities) and the likelihood of infection with zoonotic pathogens.
This is the first comprehensive insight into the diversity of zoonotic pathogens in humans in Zambia. Prior to this, most studies largely focused on domestic and wildlife reservoirs, with limited understanding of the role such pathogens play in human illness [12,13,14,15,18,19,25,26,27,28]. Therefore, the study fills a crucial gap in the current literature. The high prevalence of zoonotic pathogens could be indicative of under-recognized clinically relevant zoonotic risks in Zambia and may call for changes in public health guidelines concerning diagnosis and treatment [29].
A range of zoonotic pathogens were identified. Among these, Coxiella burnetii, known to cause Q fever, was detected for the first time in humans in the country and prior to this in rodents [28]. C. burnetii is associated with severe respiratory illness and is a known zoonotic threat [5,30]. Other notable first-time reports included S. schenckii and Bartonella species, recently reported in rodents by Munjita and collaborators, further highlighting the diverse range of zoonotic pathogens circulating in the human and animal population [14]. S. schenckii is a dimorphic fungus commonly associated with zoonotic transmission through contact with infected dogs and cats [31]. It is known for causing sporotrichosis, a condition that can manifest as skin lesions and occasionally spread to the lymphatic system, bones, and other organs [31].
Bacillus anthracis, which causes anthrax, was detected during a period when Zambia was experiencing sporadic outbreaks of the disease. We postulate that there has been low-level transmission occurring within the population, which culminated into a major outbreak in 2023 [32]. This temporal overlap suggests that the potential for zoonotic pathogens to cause public health crises remains high, particularly in areas with significant livestock populations. Additionally, pathogens commonly associated with seafood consumption, such as Vibrio parahaemolyticus and Vibrio alginolyticus, were identified, indicating potential risks linked to the consumption of marine products [33]. While Zambia is a landlocked country with limited direct access to marine seafood, it is important to consider that seafood consumption, although potentially lower than in coastal regions, may still pose a risk. This could occur through the importation of seafood products, particularly in urban areas or markets where fish and other marine foods are available. Moreover, the risk may be compounded by environmental factors, such as contamination of freshwater sources with Vibrio sp., which could increase the potential for exposure through indirect means, such as through water or imported aquatic animals.
Banna virus, often associated with encephalitis in humans [34], was detected for the first time in human samples from Zambia. This has added to the growing list of emerging viral zoonoses that have been identified in the region. Banna virus is an arbovirus that circulates in mosquitoes [34]. Therefore, there is a need to investigate the epidemiology of this virus in mosquitoes and related potential vectors in order to determine possible sources of this pathogen.
Rickettsial pathogens, which have been reported in humans in Zambia before [27], were also detected in a number of individuals in this study. The high presence of rickettsiae in this study mirrors similar findings from other African countries, which have increasingly recognized rickettsial diseases as important causes of febrile illness [3,35]. These glaring findings suggest the need to include rickettsial pathogens in diagnostic protocols in hospitals.
Meanwhile, detection of Leptospira borgpetersenii in this study also marked the first report of a Leptospira sp. in humans in Zambia. Previous reports were in rodents and bats [36,37]. Leptospira sp. cause leptospirosis in humans, a zoonotic disease that is transmitted through contact with water or soil contaminated by the urine of infected animals [4]. Leptospirosis is often associated with flooding and environmental contamination, and the detection of L. borgpetersenii in Zambia is particularly noteworthy, as the country shares ecological and epidemiological links with regions such as Tanzania, which experience frequent outbreaks of leptospirosis [38].
This study also provides the first report of Leishmania braziliensis and A. butzleri in human samples in Zambia. The most recent report about Leishmania sp. was in dogs in the South of Zambia not long ago [39]. This study builds on that finding but this time in humans. L. braziliensis, a causative agent of cutaneous leishmaniasis, is a notable pathogen with significant public health implications, especially in tropical regions. The presence of this pathogen calls for increased vigilance in monitoring neglected tropical diseases that may emerge in Zambia.
A. butzleri, a bacterium associated with gastrointestinal infections, has been increasingly recognized as a zoonotic pathogen, particularly in the context of contaminated food and water [40]. Its identification in this study highlights the growing importance of foodborne pathogens in human health and emphasizes the need for comprehensive surveillance systems to detect and address these emerging threats. Additionally, L. monocytogenes, a pathogen typically associated with contaminated food sources, was also detected in this study. While L. monocytogenes has primarily been reported in foods in Zambia in the absence of outbreaks, its identification in active human infections is rare [41,42]. The discovery of L. monocytogenes warrants further investigation into its role in human infections in clinical and non-clinical settings, where asymptomatic or subclinical cases may go undiagnosed.
While the study’s findings are illuminating, there are some limitations to consider. First, all participants were enrolled at hospitals due to suspected COVID-19 infection, and it is possible that some individuals had self-treated with antibiotics prior to hospital visits. This may have reduced the abundance of bacterial pathogens detected by mNGS, as antibiotics could have suppressed microbial growth. Additionally, including suspected COVID-19 patients could lead to an overrepresentation of respiratory pathogens and an underestimation of other zoonotic threats in the general population. Furthermore, the lack of data regarding the specific geographic locations of participants makes it difficult to conduct future follow-up studies on zoonotic risks in particular hot spots. The relatively small sample size of 47 participants, though revealing a high prevalence of zoonotic pathogens, may have been insufficient to fully understand the risk factors for zoonotic pathogen infection in the broader population. Additionally, blood samples were not used in this study, meaning there could be under-reporting of most pathogens. Some of the pathogens may have come from the residues of food that patients may have eaten and not really an infection in the patients, an indication of possible modes of exposure to zoonoses and hence the title of this study. Despite these limitations, this study provides valuable data pointing towards the possible contribution of zoonotic pathogens to overall disease burden within the Zambian population. It highlights the need for more comprehensive robust studies with larger sample sizes in at-risk patient groups to elucidate the contribution to the overall disease burden of zoonotic disease in the light of poor health systems in low-income countries [43].
5. Conclusions
The detection of a diverse array of zoonotic pathogens, many of which are emerging or under-recognized, shows the importance of strengthened surveillance for zoonotic pathogens in humans. This is critical for mitigating risks associated with both known and unknown pathogens in this rapidly changing global health landscape amidst climate change.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/zoonoticdis5020013/s1: Table S1: The reads specific to zoonotic pathogens per patient.
Author Contributions
Conceptualization, J.T., S.M.M., and M.B.; methodology, S.M.M. and J.T.; software, S.M.M. and J.T.; validation, J.T., S.M.M., W.M., and M.B; formal analysis, J.T., S.M.M., W.M., and M.B.; investigation, J.T., S.M.M., W.M., and M.B.; resources, J.T. and M.B.; data curation, J.T., S.M.M., W.M., and M.B.; writing—original draft preparation, S.M.M.; writing—review and editing, J.T., S.M.M., W.M., and M.B.; visualization, S.M.M.; supervision, M.B.; project administration, J.T.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by HerpeZ with a grant from the European and Developing Countries Clinical Trials Partnership (EDCTP2) programme under the PANDORA-ID-NET Consortium (EDCTP Reg/Grant RIA2016E-1609). The funders had no role in study design, collection, analysis and interpretation of data, manuscript writing, or decision to submit the article for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Excellence in Research Ethics and Science (ERES) Converge Ethics Committee IRB of Lusaka, Zambia (Ref. No. 2020-JUL-07).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to give special thanks to the Chan-Zuckerberg Biohub (CZ-Biohub) for the training in mNGS and processing of the samples. We also acknowledge the support of the Central Africa Network on Tuberculosis, HIV/AIDS, and Malaria (CANTAM), a network of excellence supported by EDCTP and NACCAP.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tagoe, J.N.A.; Yeboah, C.; Behene, E.; Kumordjie, S.; Nimo-Paintsil, S.; Attram, N.; Nyarko, E.O.; Carroll, J.A.; Fox, A.T.; Watters, C.; et al. Coinfection of Malaria and Bacterial Pathogens among Acute Febrile Patients in Selected Clinics in Ghana. Am. J. Trop. Med. Hyg. 2023, 109, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Salyer, S.J.; Silver, R.; Simone, K.; Barton Behravesh, C. Prioritizing Zoonoses for Global Health Capacity Building-Themes from One Health Zoonotic Disease Workshops in 7 Countries, 2014–2016. Emerg. Infect. Dis. 2017, 23, S55–S64. [Google Scholar] [CrossRef]
- Ateudjieu, J.; Siewe Fodjo, J.N.; Ambomatei, C.; Tchio-Nighie, K.H.; Zoung Kanyi Bissek, A.-C. Zoonotic Diseases in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Zoonotic Dis. 2023, 3, 251–265. [Google Scholar] [CrossRef]
- Allan, K.J.; Biggs, H.M.; Halliday, J.E.B.; Kazwala, R.R.; Maro, V.P.; Cleaveland, S.; Crump, J.A. Epidemiology of Leptospirosis in Africa: A Systematic Review of a Neglected Zoonosis and a Paradigm for ‘One Health’ in Africa. PLOS Neglected Trop. Dis. 2015, 9, e0003899. [Google Scholar] [CrossRef]
- Vanderburg, S.; Rubach, M.P.; Halliday, J.E.B.; Cleaveland, S.; Reddy, E.A.; Crump, J.A. Epidemiology of Coxiella Burnetii Infection in Africa: A OneHealth Systematic Review. PLOS Neglected Trop. Dis. 2014, 8, e2787. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Nahin, S.; Bonna, A.S.; Kabir Rozars, M.F.; Hossain Hawlader, M.D. Leptospirosis and COVID-19 Co-Infection Case in Bangladesh. Heliyon 2022, 8, e11828. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-Infections: Potentially Lethal and Unexplored in COVID-19. Lancet Microbe 2020, 1, e11. [Google Scholar] [CrossRef]
- Baral, B.; Saini, V.; Kandpal, M.; Kundu, P.; Dixit, A.K.; Parmar, H.S.; Meena, A.K.; Trivedi, P.; Jha, H.C. The Interplay of Co-Infections in Shaping COVID-19 Severity: Expanding the Scope beyond SARS-CoV-2. J. Infect. Public Health 2024, 17, 102486. [Google Scholar] [CrossRef]
- Bohl, J.A.; Lay, S.; Chea, S.; Ahyong, V.; Parker, D.M.; Gallagher, S.; Fintzi, J.; Man, S.; Ponce, A.; Sreng, S.; et al. Discovering Disease-Causing Pathogens in Resource-Scarce Southeast Asia Using a Global Metagenomic Pathogen Monitoring System. Proc. Natl. Acad. Sci. USA 2022, 119, e2115285119. [Google Scholar] [CrossRef]
- Kelly-Cirino, C.D.; Nkengasong, J.; Kettler, H.; Tongio, I.; Gay-Andrieu, F.; Escadafal, C.; Piot, P.; Peeling, R.W.; Gadde, R.; Boehme, C. Importance of Diagnostics in Epidemic and Pandemic Preparedness. BMJ Glob. Health 2019, 4, e001179. [Google Scholar] [CrossRef]
- Chidzwondo, F.; Mutapi, F. Challenge of Diagnosing Acute Infections in Poor Resource Settings in Africa. AAS Open Res. 2024, 4, 28. [Google Scholar]
- Moonga, L.C.; Hayashida, K.; Nakao, R.; Lisulo, M.; Kaneko, C.; Nakamura, I.; Eshita, Y.; Mweene, A.S.; Namangala, B.; Sugimoto, C.; et al. Molecular Detection of Rickettsia Felis in Dogs, Rodents and Cat Fleas in Zambia. Parasites Vectors 2019, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Mubemba, B.; Mburu, M.M.; Changula, K.; Muleya, W.; Moonga, L.C.; Chambaro, H.M.; Kajihara, M.; Qiu, Y.; Orba, Y.; Hayashida, K.; et al. Current Knowledge of Vector-Borne Zoonotic Pathogens in Zambia: A Clarion Call to Scaling-up “One Health” Research in the Wake of Emerging and Re-Emerging Infectious Diseases. PLOS Neglected Trop. Dis. 2022, 16, e0010193. [Google Scholar] [CrossRef]
- Munjita, S.M.; Kajihara, M.; Mubemba, B.; Kalonda, A.; Tembo, J.; Chitanga, S.; Changula, K.; Tato, C.; Vanaerschot, M.; Munsaka, S.; et al. Evidence of Multiple Bacterial, Viral and Parasitic Infectious Disease Agents of Public Health Importance in Mastomys Natalensis Rodents in Riverine Areas in Selected Parts of Zambia. Ecol. Epidemiol. 2024, 15. [Google Scholar] [CrossRef]
- Munjita, S.M.; Mubemba, B.; Changula, K.; Tembo, J.; Hamoonga, R.; Bates, M.; Chitanga, S.; Munsaka, S.; Simulundu, E. Unveiling the Hidden Threats: A Review of Pathogen Diversity and Public Health Risks from Bats, Rodents, and Non-Human Primates in Zambia (1990–2022). Front. Public Health 2024, 12, 1471452. [Google Scholar] [CrossRef]
- Tembo, J.; Egbe, N.F.; Maluzi, K.; Mulonga, K.; Chilufya, M.; Kapata, N.; Mukonka, V.; Simulundu, E.; Zumla, A.; Fwoloshi, S.; et al. Evaluation of SARS-CoV-2 Diagnostics and Risk Factors Associated with SARS-CoV-2 Infection in Zambia. Int. J. Infect. Dis. 2022, 120, 150–157. [Google Scholar] [CrossRef] [PubMed]
- In, J. Introduction of a Pilot Study. Korean J. Anesthesiol. 2017, 70, 601. [Google Scholar] [CrossRef]
- Munjita, S.M.; Mubemba, B.; Tembo, J.; Bates, M.; Munsaka, S. Rhipicephalus Simus Ticks: New Hosts for Phleboviruses. Parasitology 2024, 151, 962–970. [Google Scholar] [CrossRef]
- Munjita, S.M.; Moonga, G.; Mukubesa, A.N.; Ndebe, J.; Mubemba, B.; Vanaerschot, M.; Tato, C.; Tembo, J.; Kapata, N.; Chitanga, S.; et al. Luna Virus and Helminths in Wild Mastomys Natalensis in Two Contrasting Habitats in Zambia: Risk Factors and Evidence of Virus Dissemination in Semen. Pathogens 2022, 11, 1345. [Google Scholar] [CrossRef]
- Kalantar, K.L.; Carvalho, T.; de Bourcy, C.F.A.; Dimitrov, B.; Dingle, G.; Egger, R.; Han, J.; Holmes, O.B.; Juan, Y.-F.; King, R.; et al. IDseq—An Open Source Cloud-Based Pipeline and Analysis Service for Metagenomic Pathogen Detection and Monitoring. GigaScience 2020, 9, giaa111. [Google Scholar] [CrossRef]
- Munjita, S.M.; Abu, Y.E.; Mubemba, B. Total Microbiome of an African Filarial and Arbovirus Vector, Culex Quinquefasciatus: Insights into Composition and Prevalence of Human Pathogenic Microbes. J. Eur. Mosq. Control Assoc. 2025, 43, 1–9. [Google Scholar] [CrossRef]
- Young, T. Biodiversity Calculator for the Simpson and Shannon Indexes. Available online: https://www.alyoung.com/labs/biodiversity_calculator.html?srsltid=AfmBOor5T24oblgIGK-DVDa-O9GtqJ0uwfJshn3PtPU365GNfBdUAwmM (accessed on 14 December 2024).
- Caruso, T.; Pigino, G.; Bernini, F.; Bargagli, R.; Migliorini, M. The Berger–Parker Index as an Effective Tool for Monitoring the Biodiversity of Disturbed Soils: A Case Study on Mediterranean Oribatid (Acari: Oribatida) Assemblages. Biodivers. Conserv. Eur. 2008, 35–43. [Google Scholar]
- Beisel, J.-N.; Thomas, S.; Usseglio-Polatera, P.; Moreteau, J.-C. Assessing Changes in Community Structure by Dominance Indices: A Comparative Analysis. J. Freshw. Ecol. 1996, 11, 291–299. [Google Scholar] [CrossRef]
- Nyirenda, S.S.; Hang’ombe, B.M.; Mulenga, E.; Kilonzo, B.S. Serological and PCR Investigation of Yersinia Pestis in Potential Reservoir Hosts from a Plague Outbreak Focus in Zambia. BMC Res. Notes 2017, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Nakao, R.; Hang’ombe, B.M.; Sato, K.; Kajihara, M.; Kanchela, S.; Changula, K.; Eto, Y.; Ndebe, J.; Sasaki, M.; et al. Human Borreliosis Caused by a New World Relapsing Fever Borrelia–like Organism in the Old World. Clin. Infect. Dis. 2019, 69, 107–112. [Google Scholar] [CrossRef]
- Moonga, L.C.; Hayashida, K.; Mulunda, N.R.; Nakamura, Y.; Chipeta, J.; Moonga, H.B.; Namangala, B.; Sugimoto, C.; Mtonga, Z.; Mutengo, M.; et al. Molecular Detection and Characterization of Rickettsia Asembonensis in Human Blood, Zambia. Emerg. Infect. Dis. 2021, 27, 2237–2239. [Google Scholar] [CrossRef]
- Chitanga, S.; Simulundu, E.; Simuunza, M.C.; Changula, K.; Qiu, Y.; Kajihara, M.; Nakao, R.; Syakalima, M.; Takada, A.; Mweene, A.S.; et al. First Molecular Detection and Genetic Characterization of Coxiella Burnetii in Zambian Dogs and Rodents. Parasites Vectors 2018, 11, 40. [Google Scholar] [CrossRef]
- Simulundu, E.; Mweene, A.S.; Changula, K.; Monze, M.; Chizema, E.; Mwaba, P.; Takada, A.; Ippolito, G.; Kasolo, F.; Zumla, A.; et al. Lujo Viral Hemorrhagic Fever: Considering Diagnostic Capacity and Preparedness in the Wake of Recent Ebola and Zika Virus Outbreaks. Rev. Med. Virol. 2016, 26, 446–454. [Google Scholar] [CrossRef]
- National Center for Emerging and Zoonotic Infectious Diseases Respiratory Infections | CDC Yellow Book 2024. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/posttravel-evaluation/respiratory-infections (accessed on 11 January 2025).
- Chieosilapatham, P.; Chuamanochan, M.; Chiewchavit, S.; Saikruatep, R.; Amornrungsun, E.; Preechasuth, K. Sporothrix Schenckii Sensu Stricto Related to Zoonotic Transmission in Thailand. Med. Mycol. Case Rep. 2023, 41, 44–47. [Google Scholar] [CrossRef]
- World Health Organisation Anthrax-Zambia. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON497 (accessed on 11 January 2025).
- Oberbeckmann, S.; Wichels, A.; Wiltshire, K.H.; Gerdts, G. Occurrence of Vibrio Parahaemolyticus and Vibrio Alginolyticus in the German Bight over a Seasonal Cycle. Antonie Van Leeuwenhoek 2011, 100, 291–307. [Google Scholar] [CrossRef]
- Nabeshima, T.; Nga, P.T.; Guillermo, P.; del Parquet, M.C.; Yu, F.; Thuy, N.T.; Trang, B.M.; Hien, N.T.; Nam, V.S.; Inoue, S.; et al. Isolation and Molecular Characterization of Banna Virus from Mosquitoes, Vietnam. Emerg. Infect. Dis 2008, 14, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.J.; Quan, V.; Frean, J.; Knobel, D.L.; Rossouw, J.; Weyer, J.; Marcotty, T.; Godfroid, J.; Blumberg, L.H. Prevalence of Selected Zoonotic Diseases and Risk Factors at a Human-Wildlife-Livestock Interface in Mpumalanga Province, South Africa. Vector-Borne Zoonotic Dis. 2018, 18, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Koizumi, N.; Ohnuma, A.; Mutemwa, A.; Hang’ombe, B.M.; Mweene, A.S.; Takada, A.; Sugimoto, C.; Suzuki, Y.; Kida, H.; et al. Molecular Epidemiology of Pathogenic Leptospira Spp. in the Straw-Colored Fruit Bat (Eidolon Helvum) Migrating to Zambia from the Democratic Republic of Congo. Infect. Genet. Evol. 2015, 32, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Hang’ombe, B.M.; Sawa, H.; Kobayashi, S.; Orba, Y.; Ishii, A.; Thomas, Y.; Isozumi, R.; Yoshimatsu, K.; Mweene, A.S.; et al. Cross-Reactivity of Secondary Antibodies against African Rodents and Application for Sero-Surveillance. J. Vet. Med. Sci. 2013, 75, 819–825. [Google Scholar] [CrossRef]
- Masunga, D.S.; Rai, A.; Abbass, M.; Uwishema, O.; Wellington, J.; Uweis, L.; El Saleh, R.; Arab, S.; Onyeaka, C.V.P.; Onyeaka, H. Leptospirosis Outbreak in Tanzania: An Alarming Situation. Ann. Med. Surg. 2022, 80, 104347. [Google Scholar] [CrossRef]
- Squarre, D.; Chambaro, H.M.; Hayashida, K.; Moonga, L.C.; Qiu, Y.; Goto, Y.; Oparaocha, E.; Mumba, C.; Muleya, W.; Bwalya, P.; et al. Autochthonous Leishmania Infantum in Dogs, Zambia, 2021. Emerg. Infect. Dis. 2022, 28, 888–890. [Google Scholar] [CrossRef]
- Prouzet-Mauléon, V.; Labadi, L.; Bouges, N.; Ménard, A.; Mégraud, F. Arcobacter Butzleri: Underestimated Enteropathogen. Emerg. Infect. Dis. 2006, 12, 307–309. [Google Scholar] [CrossRef]
- Mpundu, P.; Muma, J.B.; Mukubesa, A.N.; Kainga, H.; Mudenda, S.; Bumbangi, F.N.; Muleya, W.; Katemangwe, P.; Munyeme, M. Antibiotic Resistance Patterns of Listeria Species Isolated from Broiler Abattoirs in Lusaka, Zambia. Antibiotics 2022, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Mpundu, P.; Muma, J.B.; Mukumbuta, N.; Mukubesa, A.N.; Muleya, W.; Kapila, P.; Hang’ombe, B.M.; Munyeme, M. Isolation, Discrimination, and Molecular Detection of Listeria Species from Slaughtered Cattle in Namwala District, Zambia. BMC Microbiol. 2022, 22, 160. [Google Scholar] [CrossRef]
- Munjita, S.M.; Chileshe, M.; Mutemwa, S. Ebola Virus Disease in West Africa: A Call to Overhaul Health Systems in Sub-Saharan Africa. Int. J. Med. Sci. Public Health 2015, 4, 873–877. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).