Immune Responses Against West Nile Virus and Mosquito Salivary Proteins in Wild Birds from St. Tammany Parish, Louisiana
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Aedes albopictus and Culex quinquefasciatus Mosquito Rearing and Salivary Gland Extract (SGE) Preparation
2.3. ELISA Testing Against Culex quinquefasciatus and Aedes albopictus Salivary Gland Extract
2.4. ELISA Testing Against West Nile Virus Whole-Cell Lysate Antigen
2.5. Mosquito SGE Protein Electrophoresis and Immunoblotting
2.6. In-Gel Digestion and Liquid Chromatography-Mass Spectrometry (LCMS) Preparation
2.7. Protein Identification
2.8. Data Analysis
3. Results
3.1. Description of the Study Population
3.2. WNV-Infected Mosquito Pools
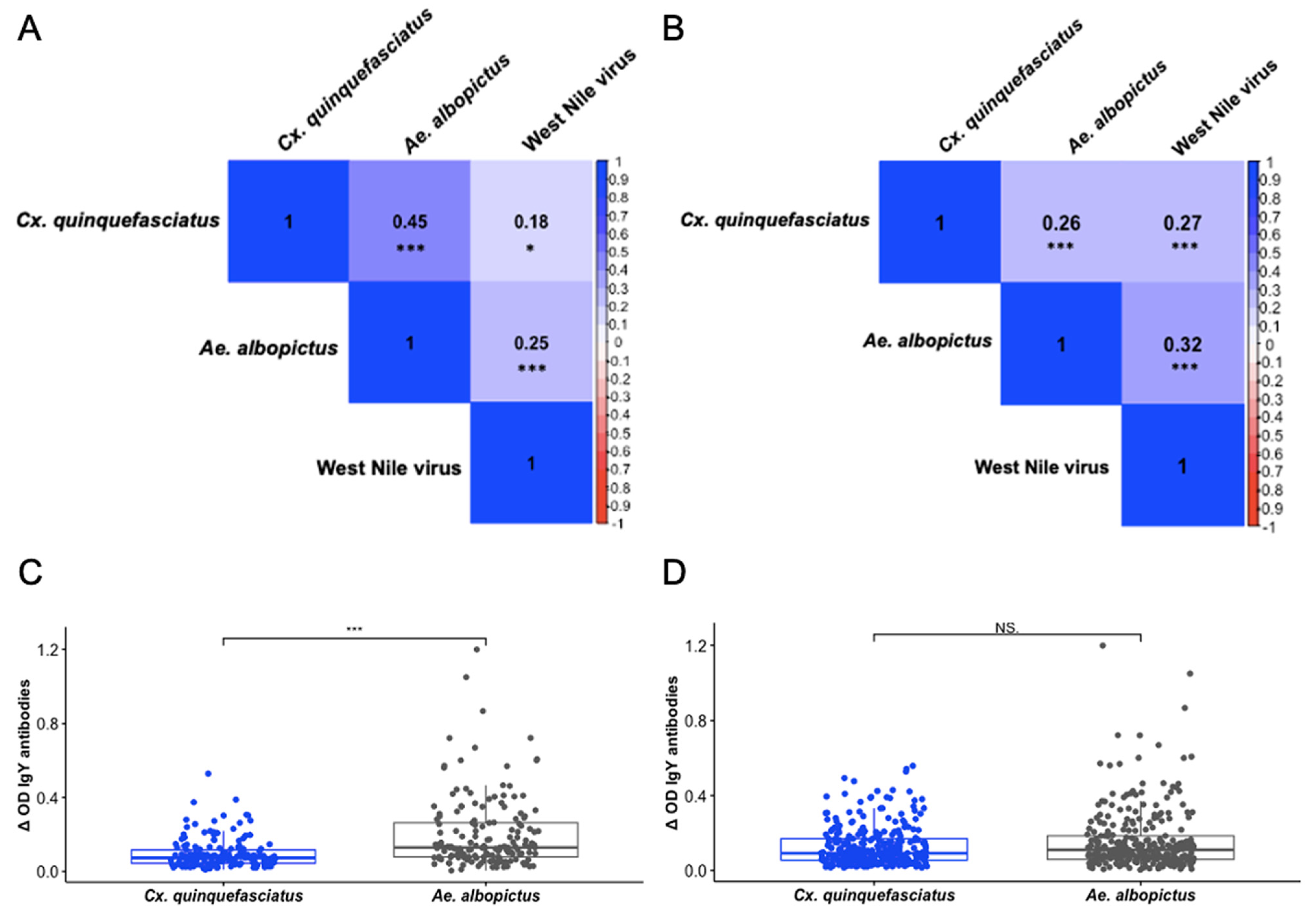
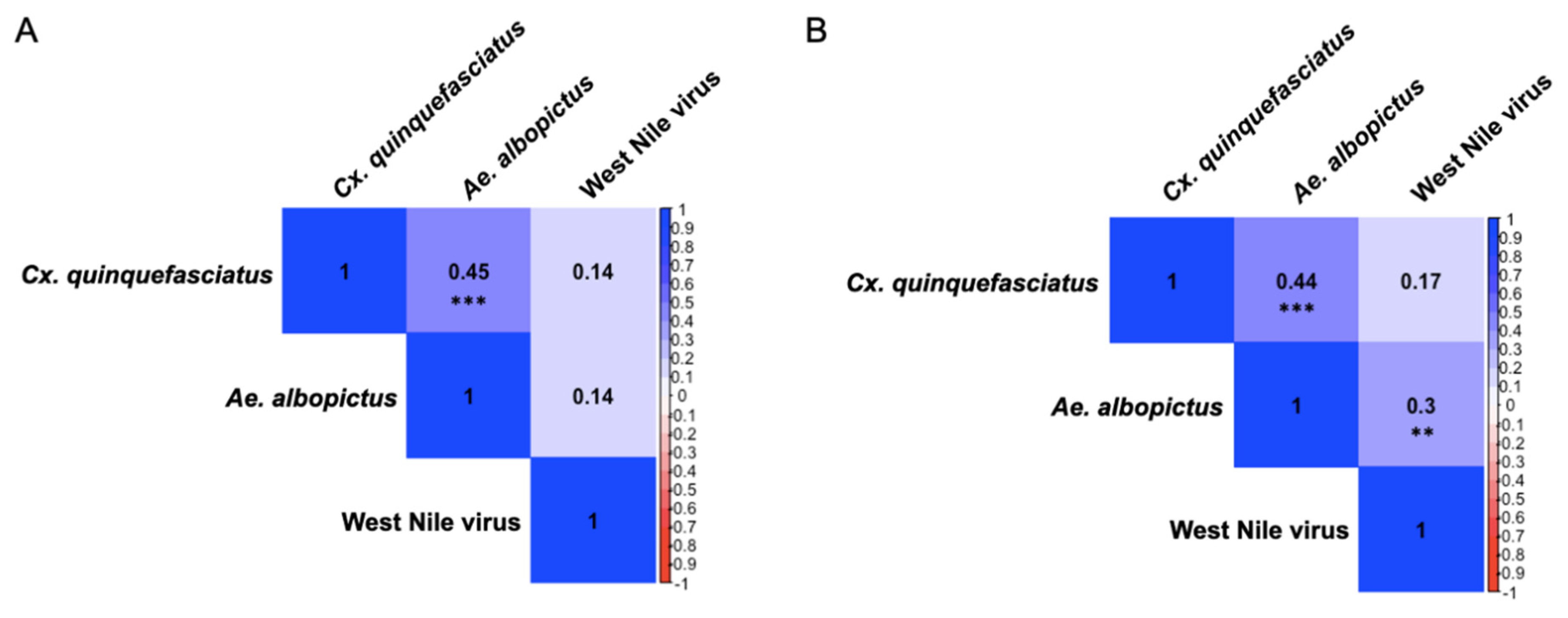
3.3. IgY Responses Are Associated Among Culex quinquefasciatus, Aedes albopictus, and West Nile Virus
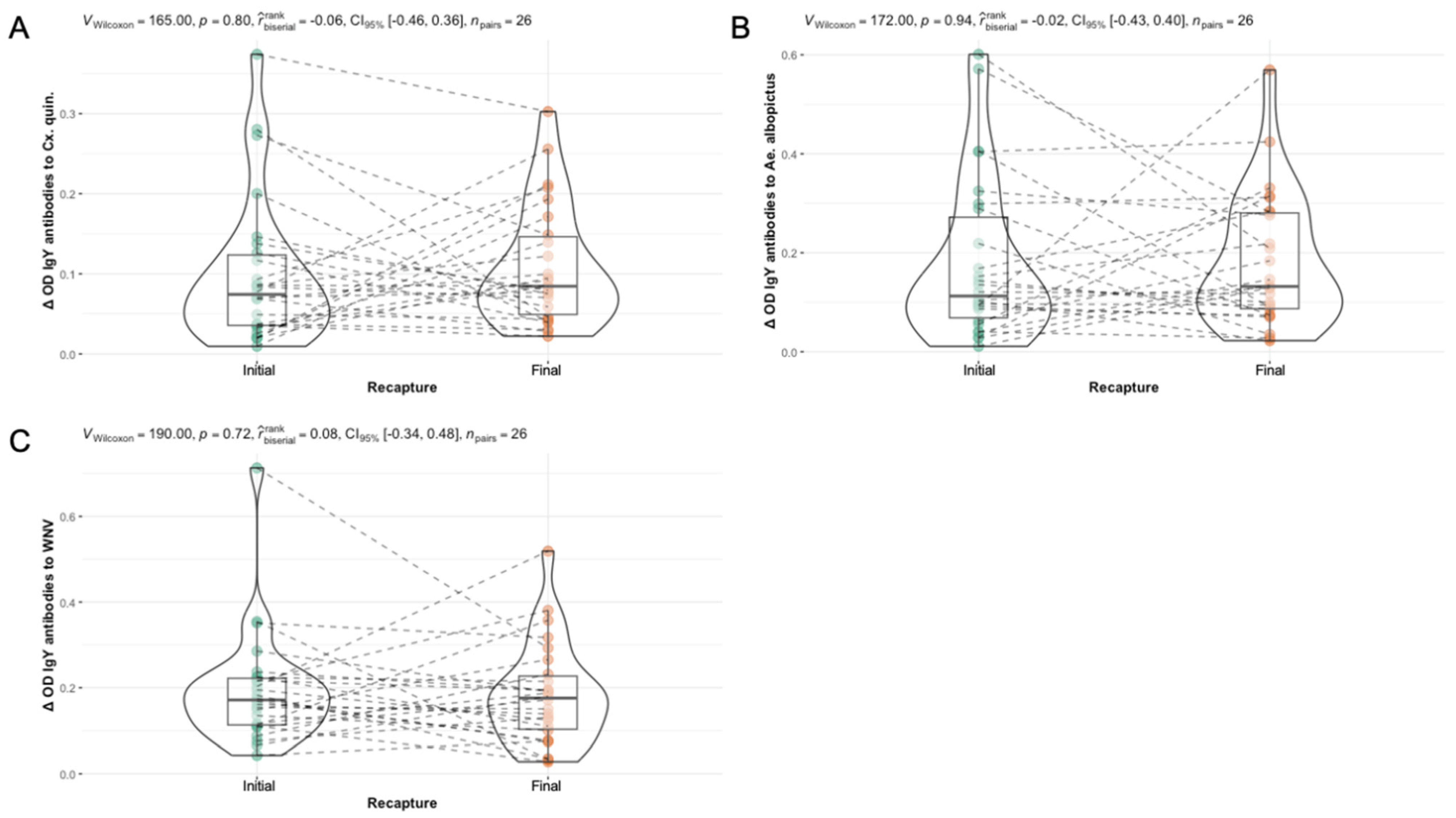
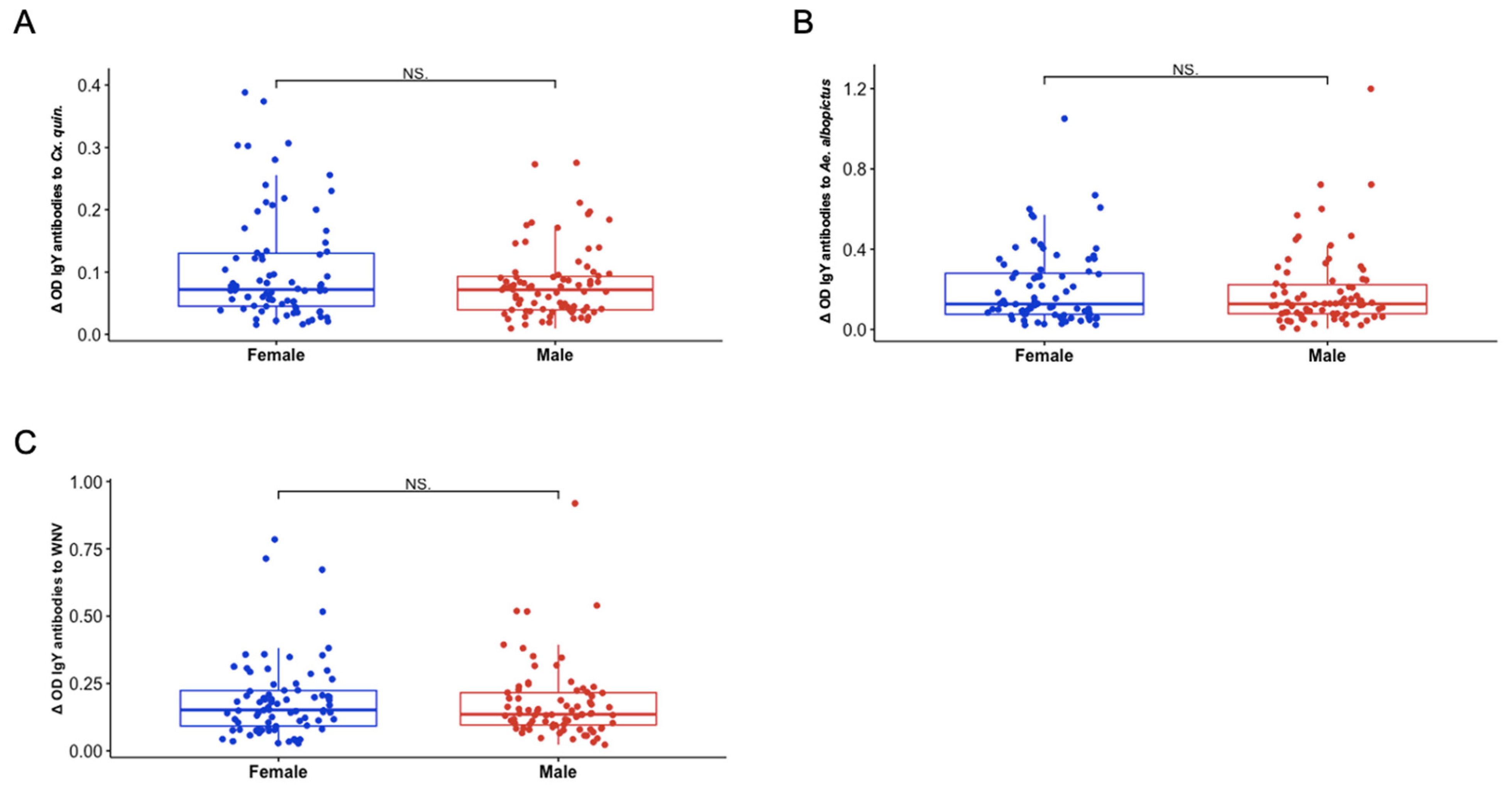
3.4. Presence of Sex-Specific Associations of IgY Response to Aedes albopictus and West Nile Virus
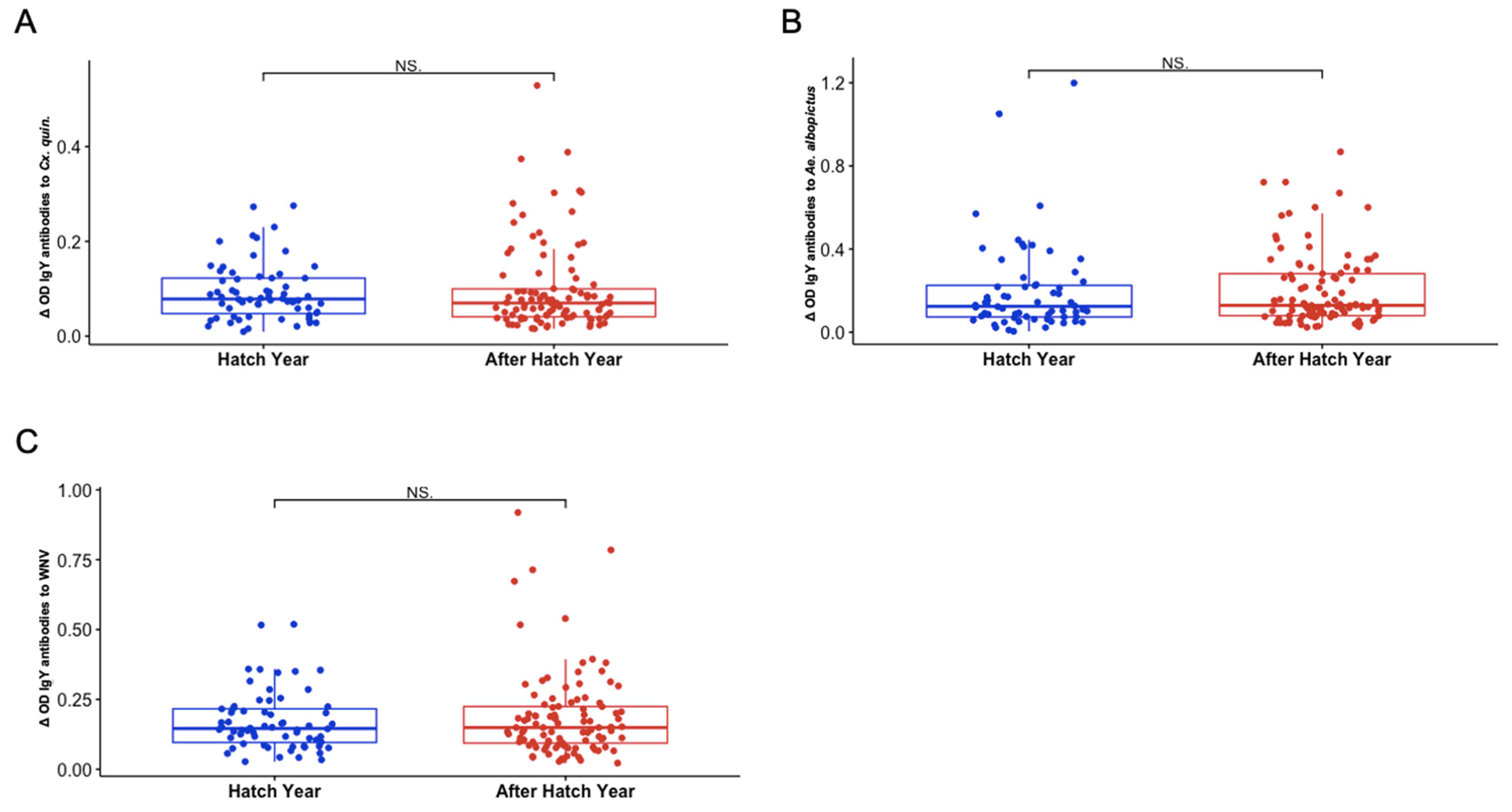
3.5. Sex and Hatch Year Are Not Important Variables Defining Exposure to Aedes albopictus or Culex quinquefasciatus Mosquito Bites Among Northern Cardinals
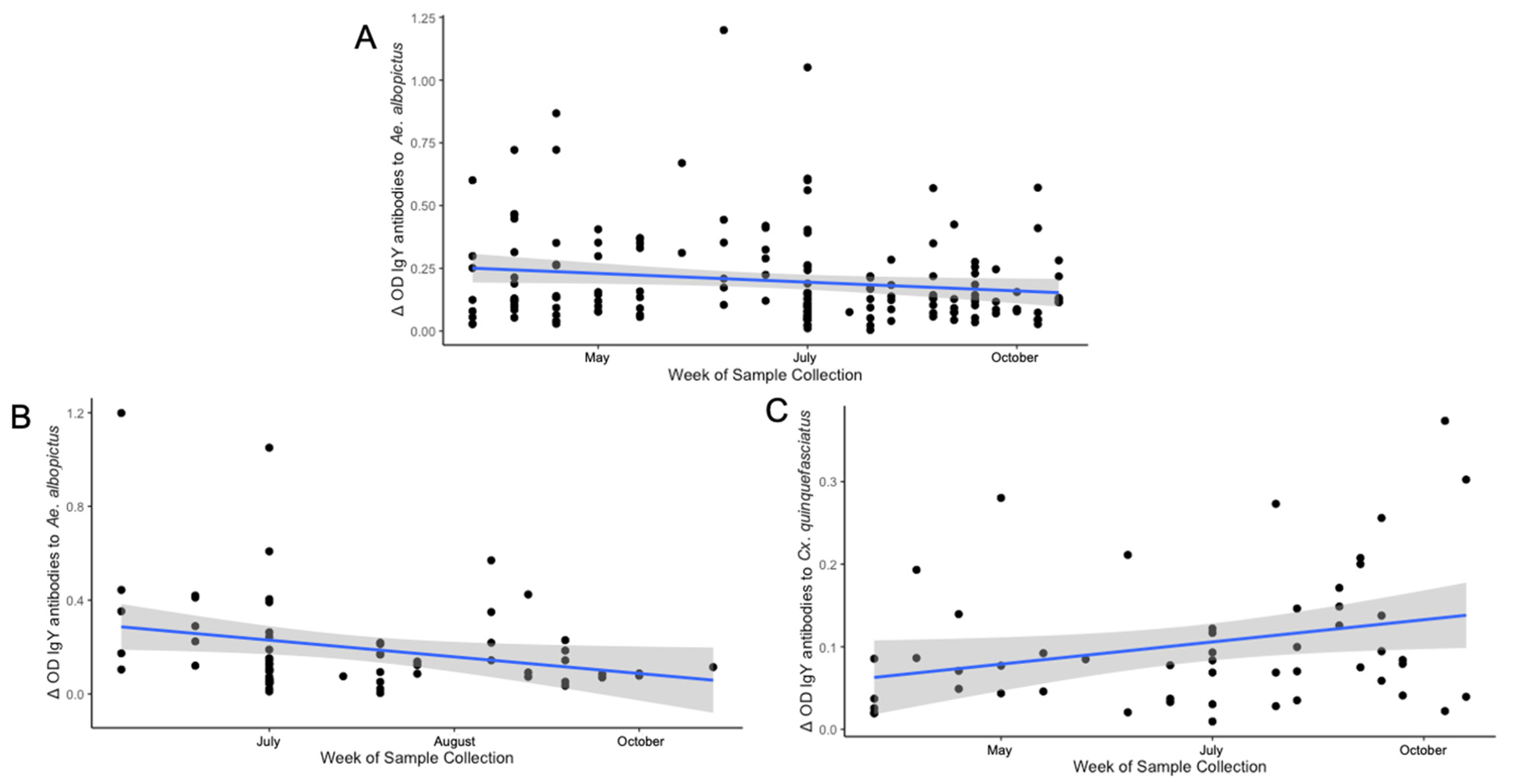
3.6. Differences in Seasonal Exposure to Aedes albopictus and Culex quinquefasciatus Among Northern Cardinals
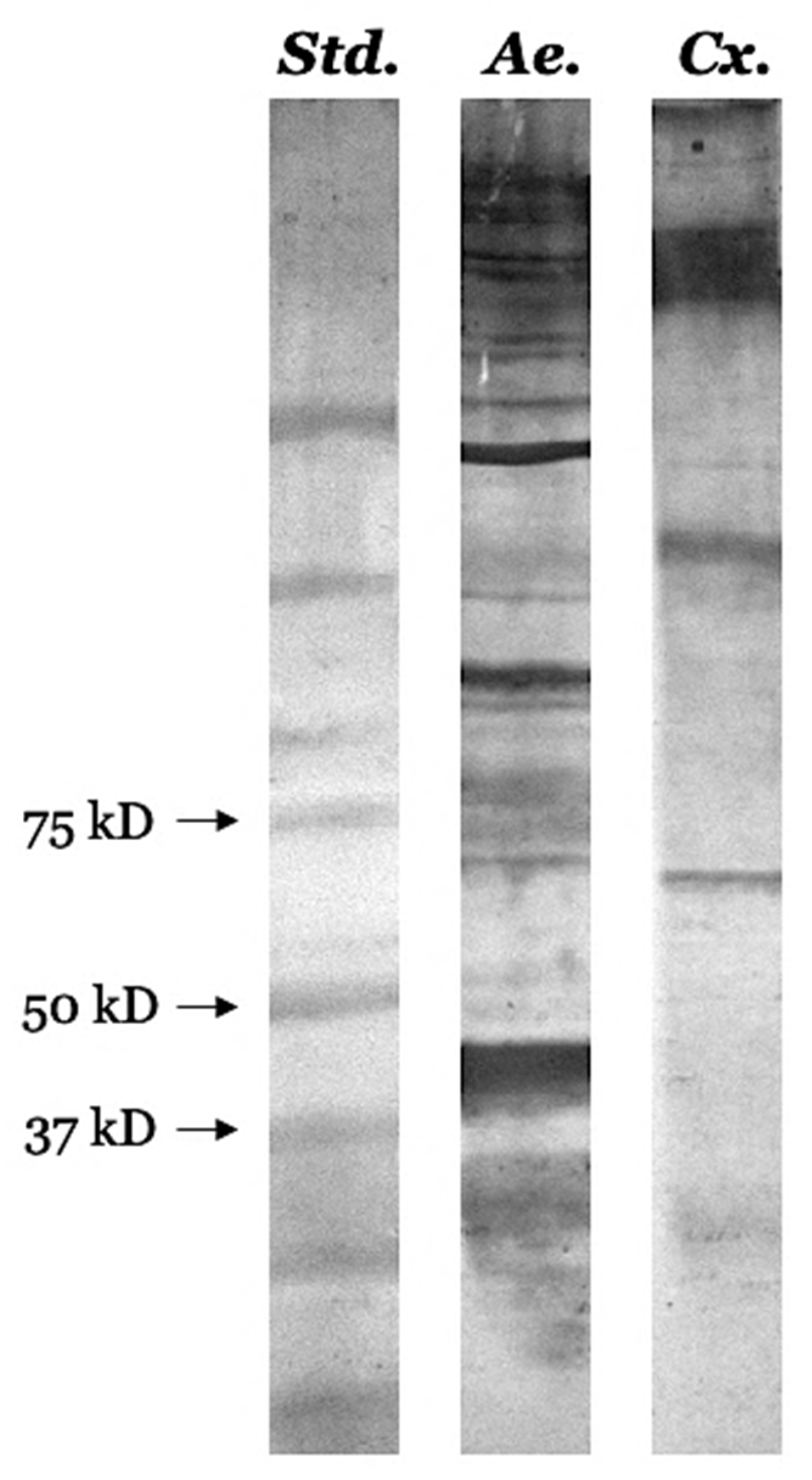
3.7. Identification of Several Pharmacologically Active Immunogenic Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | Ammonium bicarbonate |
| ACN | Acetonitrile |
| Ae. | Aedes |
| Cx. | Culex |
| ELISA | Enzyme-linked immunosorbent assay |
| FA | Ferulic acid |
| IAA | Indole-3-acetic acid |
| IgY | Immunoglobulin Y |
| LCMS | Liquid chromatography–mass spectrometry |
| OD | Optical density |
| PBS | Phosphate buffered saline |
| PVDF | Polyvinylidene fluoride |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SGE | Salivary gland extract |
| WNV | West Nile virus |
References
- Fay, R.L.; Keyel, A.C.; Ciota, A.T. Chapter Three—West Nile virus and climate change. In Advances in Virus Research; Roossinck, M.J., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 114, pp. 147–193. [Google Scholar] [CrossRef]
- Hayes, E.B.; Komar, N.; Nasci, R.S.; Montgomery, S.P.; O’Leary, D.R.; Campbell, G.L. Epidemiology and Transmission Dynamics of West Nile Virus Disease. Emerg. Infect. Dis. 2005, 11, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.S.; Mead, D.G.; Kitron, U.D. Limited Spillover to Humans from West Nile Virus Viremic Birds in Atlanta, Georgia. Vector Borne Zoonotic Dis. 2013, 13, 812–817. [Google Scholar] [CrossRef]
- Faraji, A.; Egizi, A.; Fonseca, D.M.; Unlu, I.; Crepeau, T.; Healy, S.P.; Gaugler, R. Comparative Host Feeding Patterns of the Asian Tiger Mosquito, Aedes albopictus, in Urban and Suburban Northeastern USA and Implications for Disease Transmission. PLoS Neglected Trop. Dis. 2014, 8, e3037. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, R.A.; West, P.A.; Lindsay, S.W. Suitability of two carbon dioxide-baited traps for mosquito surveillance in the United Kingdom. Bull. Entomol. Res. 2007, 97, 591–597. [Google Scholar] [CrossRef]
- Savage, H.M.; Niebylski, M.L.; Smith, G.C.; Mitchell, C.J.; Craig, G.B., Jr. Host-Feeding Patterns of Aedes albopictus (Diptera: Culicidae) at a Temperate North American Site. J. Med. Entomol. 1993, 30, 27–34. [Google Scholar] [CrossRef]
- Vouillon, A.; Barthelemy, J.; Lebeau, L.; Nisole, S.; Savini, G.; Lévêque, N.; Simonin, Y.; Garcia, M.; Bodet, C. Skin tropism during Usutu virus and West Nile virus infection: An amplifying and immunological role. J. Virol. 2023, 98, e01830-23. [Google Scholar] [CrossRef]
- Rossi, S.L.; Ross, T.M.; Evans, J.D. West Nile Virus. Clin. Lab. Med. 2010, 30, 47–65. [Google Scholar] [CrossRef]
- Ratard, R. West Nile Virus Annual Report. 2021. Available online: https://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/Annuals/2021/EncephalitisWNV_LaIDAnnual_2021.pdf (accessed on 16 May 2024).
- Rochlin, I.; Faraji, A.; Healy, K.; Andreadis, T.G. West Nile Virus Mosquito Vectors in North America. J. Med. Entomol. 2019, 56, 1475–1490. [Google Scholar] [CrossRef]
- West Nile Virus|La Dept. of Health. Available online: https://ldh.la.gov/page/west-nile-virus (accessed on 16 May 2024).
- Louisiana Arbovirus Surveillance Summary 2020. 2021. Available online: https://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/Arboviral/arboweekly/2020_wnv_reports/ARBO_2049.pdf (accessed on 16 May 2024).
- Louisiana Arbovirus Surveillance Summary 2023. 2024. Available online: https://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/Arboviral/arboweekly/2023_WNV_Reports/ARBO_2352.pdf (accessed on 16 May 2024).
- Reagan, K.L.; Machain-Williams, C.; Wang, T.; Blair, C.D. Immunization of Mice with Recombinant Mosquito Salivary Protein D7 Enhances Mortality from Subsequent West Nile Virus Infection via Mosquito Bite. PLoS Neglected Trop. Dis. 2012, 6, e1935. [Google Scholar] [CrossRef]
- Londono-Renteria, B.L.; Eisele, T.P.; Keating, J.; James, M.A.; Wesson, D.M. Antibody Response Against Anopheles albimanus (Diptera: Culicidae) Salivary Protein as a Measure of Mosquito Bite Exposure in Haiti. J. Med. Entomol. 2010, 47, 1156–1163. [Google Scholar] [CrossRef]
- Londono-Renteria, B.; Cardenas, J.C.; Cardenas, L.D.; Christofferson, R.C.; Chisenhall, D.M.; Wesson, D.M.; McCracken, M.K.; Carvajal, D.; Mores, C.N. Use of Anti-Aedes aegypti Salivary Extract Antibody Concentration to Correlate Risk of Vector Exposure and Dengue Transmission Risk in Colombia. PLoS ONE 2013, 8, e81211. [Google Scholar] [CrossRef]
- Maldonado-Ruiz, L.P.; Montenegro-Cadena, L.; Blattner, B.; Menghwar, S.; Zurek, L.; Londono-Renteria, B. Differential Tick Salivary Protein Profiles and Human Immune Responses to Lone Star Ticks (Amblyomma americanum) From the Wild vs. A Laboratory Colony. Front. Immunol. 2019, 10, 1996. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.I.; Gould, H.J.; Sutton, B.J.; Calvert, R.A. Avian IgY Binds to a Monocyte Receptor with IgG-like Kinetics Despite an IgE-like Structure. J. Biol. Chem. 2008, 283, 16384–16390. [Google Scholar] [CrossRef]
- Lee, W.; Syed Atif, A.; Tan, S.C.; Leow, C.H. Insights into the chicken IgY with emphasis on the generation and applications of chicken recombinant monoclonal antibodies. J. Immunol. Methods 2017, 447, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, B.L.B.; Nogueira, C.P.; Venancio, E.J. IgY Antibodies from Birds: A Review on Affinity and Avidity. Animals 2023, 13, 3130. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, L.; Schal, C.; Wesson, D.M.; Arellano, C.; Apperson, C.S. Oviposition responses of Aedes mosquitoes to bacterial isolates from attractive bamboo infusions. Parasites Vectors 2015, 8, 486. [Google Scholar] [CrossRef]
- Londono-Renteria, B.; Patel, J.C.; Vaughn, M.; Funkhauser, S.; Ponnusamy, L.; Grippin, C.; Jameson, S.B.; Apperson, C.; Mores, C.N.; Wesson, D.M.; et al. Long-Lasting Permethrin-Impregnated Clothing Protects Against Mosquito Bites in Outdoor Workers. Am. J. Trop. Med. Hyg. 2015, 93, 869–874. [Google Scholar] [CrossRef]
- Cardenas, J.C.; Drame, P.M.; Luque-Burgos, K.A.; Berrio, J.D.; Entrena-Mutis, E.; González, M.U.; Carvajal, D.J.; Gutiérrez-Silva, L.Y.; Cardenas, L.D.; Colpitts, T.M.; et al. IgG1 and IgG4 antibodies against Aedes aegypti salivary proteins and risk for dengue infections. PLoS ONE 2019, 14, e0208455. [Google Scholar] [CrossRef]
- Robinson, J.E.; Holton, D.; Liu, J.; McMurdo, H.; Murciano, A.; Gohd, R. A novel enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to HIV-1 envelope glycoproteins based on immobilization of viral glycoproteins in microtiter wells coated with concanavalin A. J. Immunol. Methods 1990, 132, 63–71. [Google Scholar] [CrossRef]
- Londono-Renteria, B.; Drame, P.M.; Montiel, J.; Vasquez, A.M.; Tobón-Castaño, A.; Taylor, M.; Vizcaino, L.; Lenhart, A.E. Identification and Pilot Evaluation of Salivary Peptides from Anopheles albimanus as Biomarkers for Bite Exposure and Malaria Infection in Colombia. Int. J. Mol. Sci. 2020, 21, 691. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Huang, Y.; Kilgore, M.D.; Thurmon, A.N.; Vaasjo, L.O.; Galazo, M.J.; Xu, X.; Cao, J.; Wang, X.; Ning, B.; et al. Mapping dynamic molecular changes in hippocampal subregions after traumatic brain injury through spatial proteomics. Clin. Proteom. 2024, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Londono-Renteria, B.; Drame, P.M.; Weitzel, T.; Rosas, R.; Gripping, C.; Cardenas, J.C.; Alvares, M.; Wesson, D.M.; Poinsignon, A.; Remoue, F.; et al. An gambiae gSG6-P1 evaluation as a proxy for human-vector contact in the Americas: A pilot study. Parasites Vectors 2015, 8, 533. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Moore, C.G.; Francy, D.B.; Eliason, D.A.; Monath, T.P. Aedes albopictus in the United States: Rapid spread of a potential disease vector. J. Am. Mosq. Control Assoc. 1988, 4, 356–361. [Google Scholar] [PubMed]
- Palmisano, C.T.; Taylor, V.; Caillouet, K.; Byrd, B.; Wesson, D.M. Impact of West Nile virus outbreak upon St. Tammany Parish Mosquito Abatement District. J. Am. Mosq. Control Assoc. 2005, 21, 33–38. [Google Scholar] [CrossRef]
- Willis, F.S.; Nasci, R.S. Aedes albopictus (Diptera: Culicidae) Population Density and Structure in Southwest Louisiana. J. Med. Entomol. 1994, 31, 594–599. [Google Scholar] [CrossRef]
- Tesh, R.B.; Parsons, R.; Siirin, M.; Randle, Y.; Sargent, C.; Guzman, H.; Wuithiranyagool, T.; Higgs, S.; Vanlandingham, D.L.; Bala, A.A.; et al. Year-round West Nile Virus Activity, Gulf Coast Region, Texas and Louisiana. Emerg. Infect. Dis. 2004, 10, 1649–1652. [Google Scholar] [CrossRef]
- Armbruster, P.A. Photoperiodic Diapause and the Establishment of Aedes albopictus (Diptera: Culicidae) in North America. J. Med. Entomol. 2016, 53, 1013–1023. [Google Scholar] [CrossRef]
- Hawley, W.A. The biology of Aedes albopictus. J. Am. Mosq. Control. Assoc. 1988, 1, 1–39. [Google Scholar]
- Obenauer, P.J.; Kaufman, P.E.; Allan, S.A.; Kline, D.L. Host-Seeking Height Preferences of Aedes albopictus (Diptera: Culicidae) in North Central Florida Suburban and Sylvatic Locales. J. Med. Entomol. 2009, 46, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.R.; Debboun, M. Chapter 2—Mosquito Species of Texas. In Mosquitoes, Communities, and Public Health in Texas; Debboun, M., Nava, M.R., Rueda, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 9–167. [Google Scholar] [CrossRef]
- Diniz, D.F.A.; de Albuquerque, C.M.R.; Oliva, L.O.; de Melo-Santos, M.A.V.; Ayres, C.F.J. Diapause and quiescence: Dormancy mechanisms that contribute to the geographical expansion of mosquitoes and their evolutionary success. Parasites Vectors 2017, 10, 310. [Google Scholar] [CrossRef]
- Savage, H.M.; Anderson, M.; Gordon, E.; Mcmillen, L.; Colton, L.; Delorey, M.; Sutherland, G.; Aspen, S.; Charnetzky, D.; Burkhalter, K.; et al. Host-Seeking Heights, Host-Seeking Activity Patterns, and West Nile Virus Infection Rates for Members of the Culex pipiens Complex at Different Habitat Types Within the Hybrid Zone, Shelby County, TN, 2002 (Diptera: Culicidae). J. Med. Entomol. 2008, 45, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Darbro, J.M.; Harrington, L.C. Bird-baited traps for surveillance of West Nile mosquito vectors: Effect of bird species, trap height, and mosquito escape rates. J. Med. Entomol. 2006, 43, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Halkin, S.L.; Linville, S.U. Northern cardinal: (Cardinalis cardinalis). In The Birds of North America, No. 440; The Birds of North America, Inc.: Philadelphia, PA, USA, 1999; pp. 1–32. [Google Scholar]
- Laskey, A.R. A Study of the Cardinal in Tennessee. Wilson Bull. 1944, 56, 27–44. [Google Scholar]
- Arcà, B.; Struchiner, C.J.; Pham, V.M.; Sferra, G.; Lombardo, F.; Pombi, M.; Ribeiro, J.M.C. Positive selection drives accelerated evolution of mosquito salivary genes associated with blood-feeding. Insect Mol. Biol. 2014, 23, 122–131. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C. Blood-feeding in mosquitoes: Probing time and salivary gland anti-haemostatic activities in representatives of three genera (Aedes, Anopheles, Culex). Med. Vet. Entomol. 2000, 14, 142–148. [Google Scholar] [CrossRef]
- Doucoure, S.; Mouchet, F.; Cournil, A.; Goff, G.L.; Cornelie, S.; Roca, Y.; Giraldez, M.G.; Simon, Z.B.; Loayza, R.; Misse, D.; et al. Human Antibody Response to Aedes aegypti Saliva in an Urban Population in Bolivia: A New Biomarker of Exposure to Dengue Vector Bites. Am. J. Trop. Med. Hyg. 2012, 87, 504–510. [Google Scholar] [CrossRef]
- Montiel, J.; Carbal, L.F.; Tobón-Castaño, A.; Vásquez, G.M.; Fisher, M.L.; Londono-Rentería, B. IgG antibody response against Anopheles salivary gland proteins in asymptomatic Plasmodium infections in Narino, Colombia. Malar. J. 2020, 19, 42. [Google Scholar] [CrossRef]
- Buezo Montero, S.; Gabrieli, P.; Montarsi, F.; Borean, A.; Capelli, S.; De Silvestro, G.; Forneris, F.; Pombi, M.; Breda, A.; Capelli, G.; et al. IgG Antibody Responses to the Aedes albopictus 34k2 Salivary Protein as Novel Candidate Marker of Human Exposure to the Tiger Mosquito. Front. Cell. Infect. Microbiol. 2020, 10, 377. [Google Scholar] [CrossRef]
- Moise, I.K.; Riegel, C.; Muturi, E.J. Environmental and social-demographic predictors of the southern house mosquito Culex quinquefasciatus in New Orleans, Louisiana. Parasites Vectors 2018, 11, 249. [Google Scholar] [CrossRef]
- Hamer, G.L.; Walker, E.D.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Goldberg, T.L.; Schotthoefer, A.M.; Brown, W.M.; Wheeler, E.; Kitron, U.D. Rapid amplification of West Nile virus: The role of hatch-year birds. Vector Borne Zoonotic Dis. 2008, 8, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Soltész, Z.; Erdélyi, K.; Bakonyi, T.; Barna, M.; Szentpáli-Gavallér, K.; Solt, S.; Horváth, É.; Palatitz, P.; Kotymán, L.; Dán, Á.; et al. West Nile virus host-vector-pathogen interactions in a colonial raptor. Parasites Vectors 2017, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, J.O.; Halimubieke, N.; Lendvai, Á.Z.; Figuerola, J.; Eichhorn, G.; Székely, T. Seasonal variation in sex-specific immunity in wild birds. Sci. Rep. 2021, 11, 1349. [Google Scholar] [CrossRef]
- Owen, J.C.; Nakamura, A.; Coon, C.A.; Martin, L.B. The effect of exogenous corticosterone on West Nile virus infection in Northern Cardinals (Cardinalis cardinalis). Vet. Res. 2012, 43, 34. [Google Scholar] [CrossRef] [PubMed]
- Lampman, R.L.; Krasavin, N.M.; Ward, M.P.; Beveroth, T.A.; Lankau, E.W.; Alto, B.W.; Muturi, E.; Novak, R.J. West Nile Virus Infection Rates and Avian Serology in East-Central Illinois. J. Am. Mosq. Control Assoc. 2013, 29, 108–122. [Google Scholar] [CrossRef]
- Gibbs, S.E.J.; Allison, A.B.; Yabsley, M.J.; Mead, D.G.; Wilcox, B.R.; Stallknecht, D.E. West Nile Virus Antibodies in Avian Species of Georgia, USA: 2000–2004. Vector-Borne Zoonotic Dis. 2006, 6, 57–72. [Google Scholar] [CrossRef]
- Lamb, J.S.; Tornos, J.; Lejeune, M.; Boulinier, T. Rapid loss of maternal immunity and increase in environmentally mediated antibody generation in urban gulls. Sci. Rep. 2024, 14, 4357. [Google Scholar] [CrossRef]
- McKee, E.M.; Walker, E.D.; Anderson, T.K.; Kitron, U.D.; Brawn, J.D.; Krebs, B.L.; Newman, C.; Ruiz, M.O.; Levine, R.S.; Carrington, M.E.; et al. West Nile virus antibody decay rate in free-ranging birds. J. Wildl. Dis. 2015, 51, 601–608. [Google Scholar] [CrossRef]
- Orlandi-Pradines, E.; Almeras, L.; Denis de Senneville, L.; Barbe, S.; Remoué, F.; Villard, C.; Cornelie, S.; Penhoat, K.; Pascual, A.; Bourgouin, C.; et al. Antibody response against saliva antigens of Anopheles gambiae and Aedes aegypti in travellers in tropical Africa. Microbes Infect. 2007, 9, 1454–1462. [Google Scholar] [CrossRef]
- Kaspers, B.; Göbel, T.W.; Schat, K.A.; Vervelde, L. Avian Immunology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Tiawsirisup, S.; Platt, K.B.; Evans, R.B.; Rowley, W.A. A Comparision of West Nile Virus Transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse). Vector-Borne Zoonotic Dis. 2005, 5, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Root, J.J.; Bosco-Lauth, A.M. West Nile Virus Associations in Wild Mammals: An Update. Viruses 2019, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Egizi, A.M.; Farajollahi, A.; Fonseca, D.M. Diverse Host Feeding on Nesting Birds May Limit Early-Season West Nile Virus Amplification. Vector-Borne Zoonotic Dis. 2014, 14, 447–453. [Google Scholar] [CrossRef]
- Komar, N.; Panella, N.A.; Langevin, S.A.; Brault, A.C.; Amador, M.; Edwards, E.; Owen, J.C. Avian Hosts for West Nile Virus in St. Tammany Parish, Louisiana, 2002. Am. J. Trop. Med. Hyg. 2005, 73, 1031–1037. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Taff, C.C.; Reisen, W.K.; Townsend, A.K. Mosquito blood-feeding patterns and nesting behavior of American crows, an amplifying host of West Nile virus. Parasites Vectors 2021, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain-Auger, J.A.; Auger, P.J.; Strauss, E.G. Breeding Biology of American Crows. Wilson Bull. 1990, 102, 615–622. [Google Scholar]
- Levine, R.S.; Mead, D.G.; Hamer, G.L.; Brosi, B.J.; Hedeen, D.L.; Hedeen, M.W.; McMillan, J.R.; Bisanzio, D.; Kitron, U.D. Supersuppression: Reservoir Competency and Timing of Mosquito Host Shifts Combine to Reduce Spillover of West Nile Virus. Am. J. Trop. Med. Hyg. 2016, 95, 1174–1184. [Google Scholar] [CrossRef]
- Louisiana Arbovirus Surveillance Summary 2021. Available online: https://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/Arboviral/arboweekly/2021_WNV_Reports/ARBO_2152.pdf (accessed on 1 January 2022).
- Cendejas, P.M.; Goodman, A.G. Vaccination and Control Methods of West Nile Virus Infection in Equids and Humans. Vaccines 2024, 12, 485. [Google Scholar] [CrossRef]
- McMillan, J.R.; Hamer, G.L.; Levine, R.S.; Mead, D.G.; Waller, L.A.; Goldberg, T.L.; Walker, E.D.; Brawn, J.D.; Ruiz, M.O.; Kitron, U.; et al. Multi-Year Comparison of Community- and Species-Level West Nile Virus Antibody Prevalence in Birds from Atlanta, Georgia and Chicago, Illinois, 2005–2016. Am. J. Trop. Med. Hyg. 2023, 108, 366–376. [Google Scholar] [CrossRef]
- Magallanes, S.; Llorente, F.; Ruiz-López, M.J.; la Puente, J.M.; Ferraguti, M.; Gutiérrez-López, R.; Soriguer, R.; Aguilera-Sepúlveda, P.; Fernández-Delgado, R.; Jímenez-Clavero, M.Á.; et al. Warm winters are associated to more intense West Nile virus circulation in southern Spain. Emerg. Microbes Infect. 2024, 13, 2348510. [Google Scholar] [CrossRef]
- Pedersen, K.; Marks, D.R.; Wang, E.; Eastwood, G.; Weaver, S.C.; Goldstein, S.M.; Sinnett, D.R.; DeLiberto, T.J. Widespread Detection of Antibodies to Eastern Equine Encephalitis, West Nile, St. Louis Encephalitis, and Turlock Viruses in Various Species of Wild Birds from Across the United States. Am. J. Trop. Med. Hyg. 2016, 95, 206–211. [Google Scholar] [CrossRef] [PubMed]
| Site n (%) | 1 | 2 | 3 |
| 116 (70.3) | 11 (6.7) | 38 (23) | |
| Sex n (%) | Male | Female | Not available |
| 80 (48.5) | 78 (47.3) | 7 (4.2) | |
| Age n (%) | Hatch Year | After Hatch Year | Not available |
| 64 (38.8) | 101 (61.2) | 0 (0) | |
| Mass (g) | Average | Minimum | Maximum |
| 39.022 | 3 | 46 |
| Species | Number of Mosquitoes | Number Positive/Number of Pools Tested | MLE | 95% CI |
|---|---|---|---|---|
| Aedes albopictus | 2133 | 3/169 | 1.41 | 0.37–3.78 |
| Culex quinquefasciatus | 45,152 | 33/1155 | 0.75 | 0.52–1.04 |
| Culex nigripalpus | 18,887 | 5/580 | 0.27 | 0.10–0.59 |
| Culex salinarius | 60,824 | 2/1281 | 0.03 | 0.01–0.11 |
| Aedes vexans | 7031 | 0/374 | 0 | 0–0.54 |
| Culex erraticus | 14,824 | 0/571 | 0 | 0–0.26 |
| Culex restuans | 311 | 0/26 | 0 | 0–10.78 |
| Culiseta inornata | 33 | 0/3 | 0 | 0–62.88 |
| Culiseta melanura | 6 | 0/1 | 0 | 0–231.16 |
| Ochlerotatus triseriatus | 30 | 0/1 | 0 | 0–51.22 |
| Cx. quinquefasciatus | Ae. albopictus | West Nile Virus | |
|---|---|---|---|
| All Birds (n = 165) | 0.0797 p = 0.3087 | −0.1529 p = 0.04984 * | 0.0606 p = 0.4394 |
| After Hatch Year (n = 101) | 0.0599 p = 0.5513 | −0.0826 p = 0.4117 | 0.1215 p = 0.2262 |
| Hatch Year (n = 64) | 0.1546 p = 0.2226 | −0.2482 p = 0.04799 * | −0.0277 p = 0.828 |
| Male (n = 80) | −0.0277 p = 0.828 | −0.1709 p = 0.1295 | 0.0093 p = 0.9349 |
| Female (n = 78) | 0.1592 p = 0.1639 | −0.1185 p = 0.3015 | 0.1099 p = 0.3377 |
| Recaptured Birds (n = 52) 1 | 0.2770 p = 0.0468 * | −0.0132 p = 0.9259 | 0.1126 p = 0.4267 |
| Protein Name | ID | MW (Da) |
|---|---|---|
| Aedes albopictus ~38–44 kDa band | ||
| Uncharacterized protein (Aedes albopictus) | A0AAB0A6X6 | 38,495 |
| Uncharacterized protein (Aedes albopictus) | A0A182G0N4 | 41,526 |
| Pyruvate dehydrogenase E1 component subunit beta | A0A023ERL5 | 38,453 |
| Fructose-bisphosphate aldolase | A0A023EQM6 | 39,152 |
| Glycerol-3-phosphate dehydrogenase [NAD(+)] | A0A182GGV8 | 38,604 |
| Putative actin filament-coating protein tropomyosin | A0A023ETF0 | 43,859 |
| Aedes albopictus ~26–34 kDa band | ||
| 14-3-3 protein epsilon | A0A023ENU3 | 29,418 |
| Enoyl-CoA hydratase, mitochondrial | A0A023EPZ2 | 31,511 |
| ATP synthase subunit gamma | A0A023ENY5 | 32,711 |
| Uncharacterized protein (Aedes albopictus) | A0A182GDW0 | 29,466 |
| ADP/ATP translocase | A0A023EP24 | 32,904 |
| Proteasome subunit alpha type | A0A023ENX8 | 28,843 |
| Regulator of microtubule dynamics protein 1 | A0A182GHN2 | 26,069 |
| Electron transfer flavoprotein subunit beta | A0A023ENM9 | 27,466 |
| N-acetyltransferase domain-containing protein | A0A023EKP0 | 27,121 |
| Proteasome subunit alpha type | A0A023EL07 | 27,671 |
| Putative 11beta-hydroxysteroid dehydrogenase type 1 | A0A023ENR7 | 27,211 |
| Uncharacterized protein (Aedes albopictus) | A0A182H2F8 | 26,847 |
| Uncharacterized protein (Aedes albopictus) | A0A182G1S0 | 27,190 |
| Culex quinquefasciatus ~68–70 kDa band | ||
| H(+)-transporting two-sector ATPase | A0A8D8DSN3 | 68,188 |
| Transmembrane protease serine 9 | A0A8D8JS99 | 70,089 |
| Heat shock protein 70 B2 | B0X501 | 69,919 |
| Culex quinquefasciatus ~30–36 kDa band | ||
| Regucalcin | A0A8D8BZB5 | 33,670 |
| Long-form salivary protein D7L2 | B0X6Z1 | 36,051 |
| Tropomyosin-2 | A0A8D8A960 | 35,025 |
| Probable elongation factor 1-beta | A0A8D8KQV6 | 31,741 |
| Enoyl-CoA hydratase ECHA12 | B0W6D4 | 33,912 |
| Malate dehydrogenase | A0A1Q3FI32 | 35,186 |
| ADP/ATP translocase | B0WFA5 | 32,972 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwinn, A.R.; Harris, S.; Jacobs, Z.; de Verges, J.; Jameson, S.B.; Wesson, D.M.; Michaels, S.R.; Caillouët, K.A.; Londoño-Renteria, B. Immune Responses Against West Nile Virus and Mosquito Salivary Proteins in Wild Birds from St. Tammany Parish, Louisiana. Zoonotic Dis. 2025, 5, 11. https://doi.org/10.3390/zoonoticdis5020011
Schwinn AR, Harris S, Jacobs Z, de Verges J, Jameson SB, Wesson DM, Michaels SR, Caillouët KA, Londoño-Renteria B. Immune Responses Against West Nile Virus and Mosquito Salivary Proteins in Wild Birds from St. Tammany Parish, Louisiana. Zoonotic Diseases. 2025; 5(2):11. https://doi.org/10.3390/zoonoticdis5020011
Chicago/Turabian StyleSchwinn, Alyssa R., Sara Harris, Zoe Jacobs, Jane de Verges, Samuel B. Jameson, Dawn M. Wesson, Sarah R. Michaels, Kevin A. Caillouët, and Berlin Londoño-Renteria. 2025. "Immune Responses Against West Nile Virus and Mosquito Salivary Proteins in Wild Birds from St. Tammany Parish, Louisiana" Zoonotic Diseases 5, no. 2: 11. https://doi.org/10.3390/zoonoticdis5020011
APA StyleSchwinn, A. R., Harris, S., Jacobs, Z., de Verges, J., Jameson, S. B., Wesson, D. M., Michaels, S. R., Caillouët, K. A., & Londoño-Renteria, B. (2025). Immune Responses Against West Nile Virus and Mosquito Salivary Proteins in Wild Birds from St. Tammany Parish, Louisiana. Zoonotic Diseases, 5(2), 11. https://doi.org/10.3390/zoonoticdis5020011







