Are We Missing Brucella spp. in Portugal? The First Nationwide Systematic Review, Meta-Analysis, and Retrospective Serological Study of Brucella canis (2013–2025)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection, Sampling, and Diagnostic Procedures
2.2. Systematic Review and Meta-Analysis
2.3. Eligibility Criteria
- Total sample size;
- Number of positive cases (or prevalence with 95% CI);
- Clear indication of location (Mainland Portugal and the Insular Autonomous Regions (Azores and Madeira);
- Year or study period (2000 to 2025).
- Case reports, editorials, or opinion articles;
- Experimental infection studies or vaccine trials;
- Studies lacking extractable epidemiological data or not clearly related to Brucella spp.;
- Duplicate data from the same population.
2.4. Data Extraction
- Study characteristics, including the name(s) of the author(s), year of publication, geographical region (e.g., mainland Portugal, Azores), species studied, and the classification of the host (e.g., domestic animals, livestock, wildlife, or humans);
- Sample size, defined as the total number of individuals tested, and the number of positive cases identified;
- Diagnostic method employed in the study (e.g., Rapid Slide Agglutination Test [RSAT], Indirect Fluorescent Antibody Test [IFAT], Enzyme-Linked Immunosorbent Assay [ELISA], among others);
- Prevalence estimates, expressed as a percentage (%), accompanied by the corresponding 95% confidence intervals (95% CI).
2.5. Statistical Analysis
3. Results
3.1. Seroepidemiological Study of Brucella canis in Portugal Between 2013–2025
3.1.1. General Seropositivity, Geographical Distribution, and Seasons
3.1.2. Breed
3.1.3. Sex
3.1.4. Age
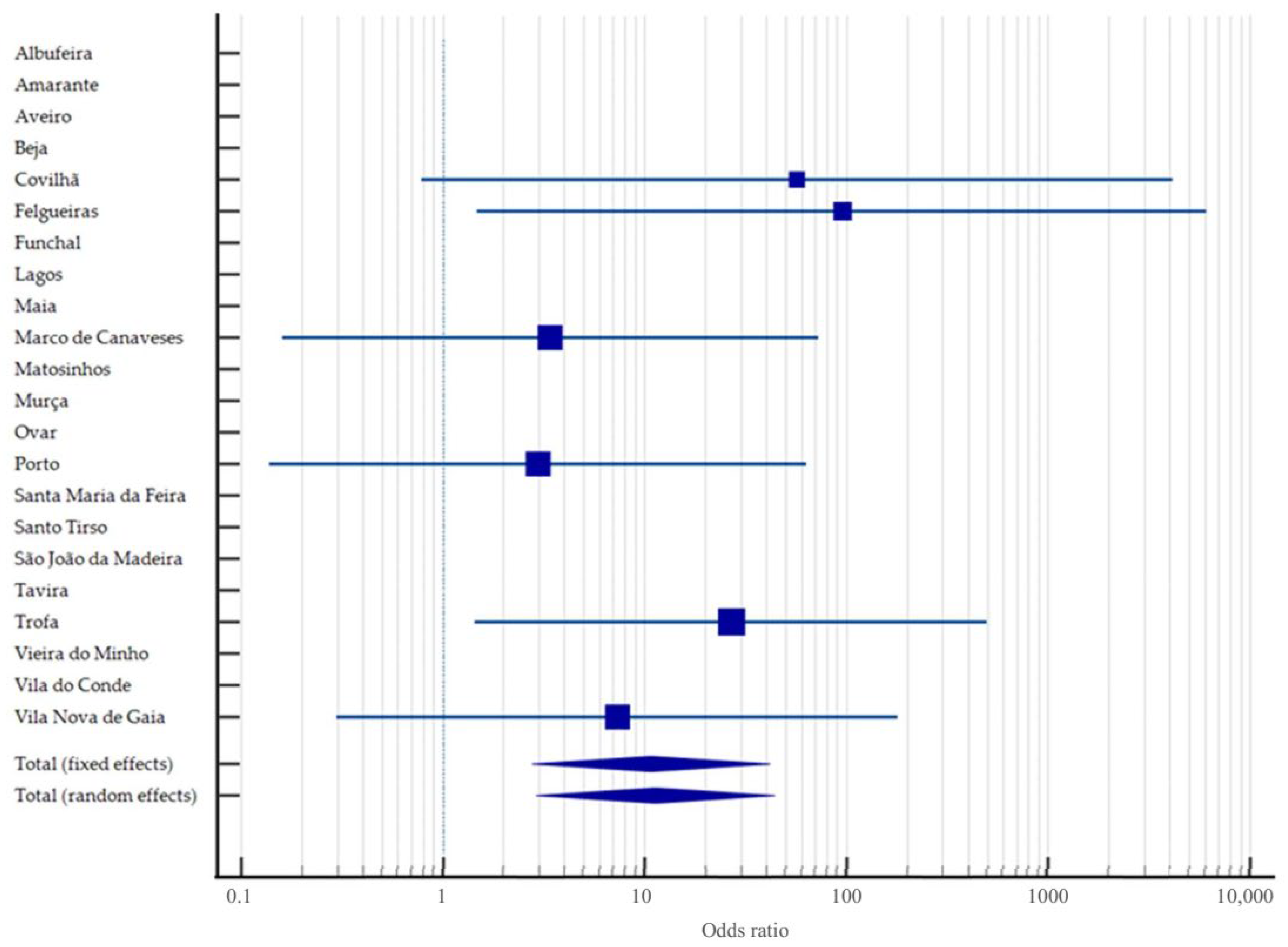
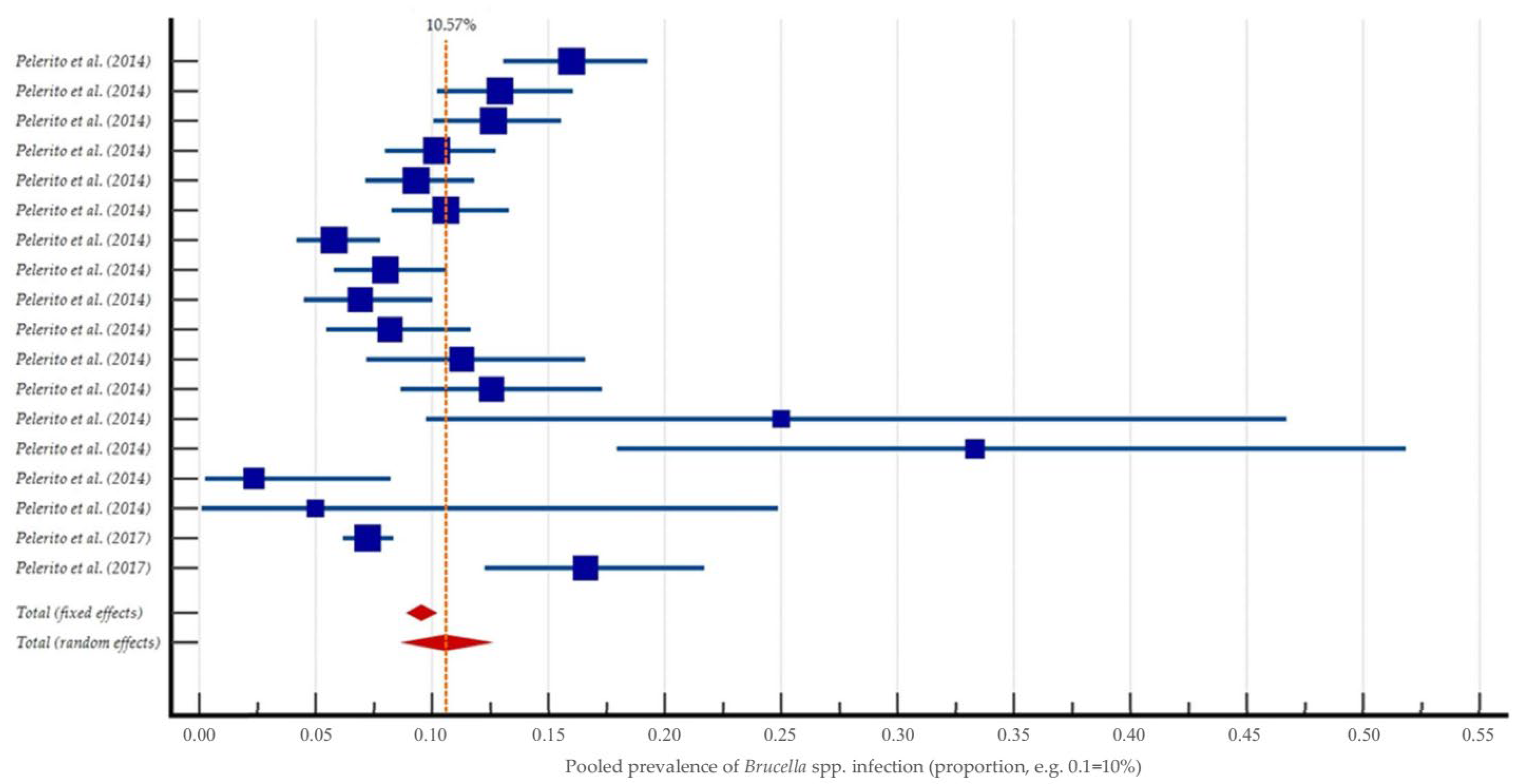
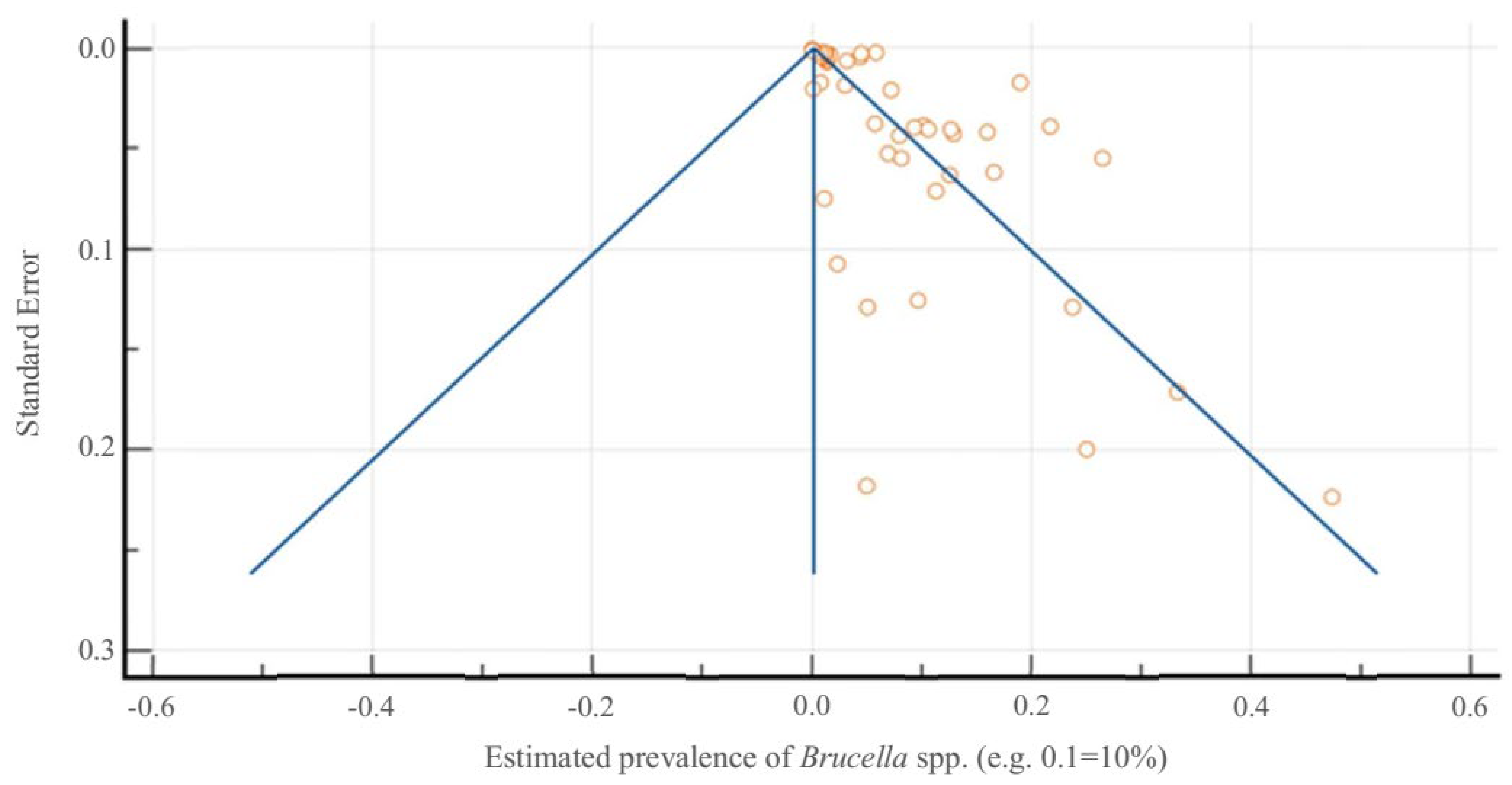
3.2. Systematic Review and Meta-Analysis on Brucella spp. in Portugal
4. Discussion
4.1. Contextualisation of Canine Brucellosis and Zoonotic Relevance
4.2. Critical Analysis of Study Findings
4.3. Contextualising National Findings Within the European Landscape
4.4. Zoonotic Risk and One Health Implications
- Mandatory notification of confirmed canine brucellosis cases to veterinary authorities, with cross-reporting mechanisms to alert public health bodies in cases of human exposure.
- Targeted screening of high-risk canine populations, including imported dogs, breeding stock, and residents of shelters or kennels, especially those from or linked to regions with known outbreaks.
- Clinical awareness campaigns aimed at physicians and veterinarians to promote early recognition of zoonotic risk and atypical brucellosis presentations.
- Serological and molecular monitoring of at-risk professionals (e.g., veterinary staff, laboratory workers), as adopted in occupational health protocols for other Brucella species.
- Public education to inform dog owners about the potential zoonotic nature of B. canis, particularly in households with vulnerable individuals.
- Outbreak investigation protocols: Unusual clusters of seropositive dogs, such as the 2018 outbreak in Trofa, should trigger immediate veterinary investigation, with notification of public health authorities in case of potential human exposure. Similarly, any cluster of human brucellosis cases should prompt inquiry into possible canine sources.
4.5. Future Recommendations
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| DGAV | Direção-Geral de Alimentação e Veterinária |
| IFAT | Indirect Fluorescent Antibody Test |
| IgG | Immunoglobulin G |
| MOOSE | Meta-analysis of Observational Studies in Epidemiology |
| NA | Not Applicable |
| NUTS2 | Nomenclature of Territorial Units for Statistics Level 2 |
| ORs | Odds Ratios |
| PCR | Polymerase Chain Reaction |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| qPCR | Quantitative Polymerase Chain Reaction |
| RCAAP | Portuguese Scientific Open Access Repository |
| RSAT | Rapid Slide Agglutination Test |
| SAS | Statistical Analysis System |
| SD | Standard Deviation |
| WOAH | World Organisation for Animal Health |
References
- Qureshi, K.A.; Parvez, A.; Fahmy, N.A.; Abdel Hady, B.H.; Kumar, S.; Ganguly, A.; Atiya, A.; Elhassan, G.O.; Alfadly, S.O.; Parkkila, S.; et al. Brucellosis: Epidemiology, pathogenesis, diagnosis and treatment—A comprehensive review. Ann. Med. 2023, 55, 2295398. [Google Scholar] [CrossRef]
- Jiao, H.; Zhou, Z.; Li, B.; Xiao, Y.; Li, M.; Zeng, H.; Guo, X.; Gu, G. The mechanism of facultative intracellular parasitism of Brucella. Int. J. Mol. Sci. 2021, 22, 3673. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Chambel, L.; Corrêa de Sá, M.I. VNTR-Based Typing of B. Melitensis Isolate; Sociedade Portuguesa de Microbiologia: Lisbon, Portugal, 2013; pp. 1–5. [Google Scholar]
- Seleem, M.N.; Boyle, S.M.; Sriranganathan, N. Brucellosis: A re-emerging zoonosis. Vet. Microbiol. 2010, 140, 392–398. [Google Scholar] [CrossRef]
- Moreno, E. Retrospective and prospective perspectives on zoonotic brucellosis. Front. Microbiol. 2014, 5, 213. [Google Scholar] [CrossRef]
- Khairullah, A.; Kurniawan, S.; Puspitasari, Y.; Aryaloka, S.; Silaen, O.; Yanestria, S.; Widodo, A.; Moses, I.; Effendi, M.; Afnani, D.; et al. Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impacts. Open Vet. J. 2024, 14, 1081. [Google Scholar] [CrossRef]
- Pires, H.; Cardoso, L.; Lopes, A.P.; Fontes, M.d.C.; Santos-Silva, S.; Matos, M.; Pintado, C.; Roque, N.; Fonseca, L.F.; Morgado, I.; et al. Hunting for answers: Assessing Brucella spp. seroprevalence and risks in red deer and wild boar in central Portugal. Pathogens 2024, 13, 242. [Google Scholar] [CrossRef]
- Ruano, Z.M.; Mateus, T.L.; Chorense, A.; Santos-Silva, S.; Vieira-Pinto, M. Seroprevalence study of brucellosis in wild boar hunted for private consumption in northeast Portugal. Vet. Res. Commun. 2024, 48, 1859–1865. [Google Scholar] [CrossRef]
- Ruano, Z.M.; Mateus, T.L.; Vieira-Pinto, M. An insight into brucellosis in wild boar and domestic pigs in Europe: A systematic review. J. Infect. Public Health 2025, 18, 102691. [Google Scholar] [CrossRef]
- Godfroid, J. Brucellosis in Livestock and Wildlife: Zoonotic diseases without pandemic potential in need of innovative One Health approaches. Arch. Public Health 2017, 75, 34. [Google Scholar] [CrossRef]
- Mallappa, A.; Kuralayanapalya Puttahonnappa, S.; Shome, R.; Patil, S.S.; Amachawadi, R.G.; Mohan, K.S.K.; Venkatesh, S.P.; Ramesh, V.; Sekar, Y.S.; Thippeswamy, H.; et al. Systematic review, meta-analysis, and pan-genome analytics predict the surging of Brucella melitensis by China and India-specific strains, elucidating the demand for enhanced preparedness. J. Infect. Public Health 2025, 18, 102693. [Google Scholar] [CrossRef]
- Naseri, Z.; Alikhani, M.Y.; Hashemi, S.H.; Kamarehei, F.; Arabestani, M.R. Prevalence of the most common virulence-associated genes among Brucella melitensis isolates from human blood cultures in Hamadan province, west of Iran. Iran. J. Med. Sci. 2016, 41, 422–429. [Google Scholar] [PubMed]
- Papaparaskevas, J.; Procopiou, A.; Routsias, J.; Vrioni, G.; Tsakris, A. Detection of virulence-associated genes among Brucella melitensis and Brucella abortus clinical isolates in Greece, 2001–2022. Pathogens 2023, 12, 1274. [Google Scholar] [CrossRef]
- Halah, A.; Tahreer, S.; Zainab, H.; Bahaa, A.; Sawsan, H. Molecular identification of intracellular survival related Brucella melitensis virulence factors. Biomedicine 2022, 42, 761–765. [Google Scholar] [CrossRef]
- Ayoub, H.; Kumar, M.S.; Mehta, R.; Sethuraj, S.E.; Thomas, P.; Dhanze, H.; Dubey, M.; Salih, H.M.; Chandrashekaraiah, G.B.; Cull, C.A.; et al. Genomic insights into Brucella melitensis in India: Stability of ST8 and the role of virulence genes in regional adaptations. Microbiol. Spectr. 2025, 13, e02647-24. [Google Scholar] [CrossRef]
- Ullah, I.; Naz, S.; Khattak, U.S.; Saeed, M.; Akbar, N.; Rauf, S. Molecular prevalence, phylogenetic analysis, and PCR-based detection of Brucella melitensis in humans and cattle in southern Khyber Pakhtunkhwa, Pakistan. Comp. Immunol. Microbiol. Infect. Dis. 2024, 115, 102262. [Google Scholar] [CrossRef]
- Hamdy, M.E.R.; Zaki, H.M. Detection of virulence-associated genes in Brucella melitensis biovar 3, the prevalent field strain in different animal species in Egypt. Open Vet. J. 2018, 8, 112. [Google Scholar] [CrossRef]
- Ramadan, E.S.; Mousa, W.S.; Gafer, J.A.; Elbaz, H.T.; Abdeen, E.; Hussien, H. Substantial virulence genes among Brucella melitensis field strains isolated from cattle in Egypt. Pak. J. Biol. Sci. 2019, 22, 239–246. [Google Scholar] [CrossRef]
- Akinyemi, K.O.; Fakorede, C.O.; Amisu, K.O.; Wareth, G. Human and animal brucellosis in Nigeria: A systemic review and meta-analysis in the last twenty-one Years (2001–2021). Vet. Sci. 2022, 9, 384. [Google Scholar] [CrossRef]
- Simpson, G.; Thompson, P.N.; Saegerman, C.; Marcotty, T.; Letesson, J.-J.; de Bolle, X.; Godfroid, J. Brucellosis in wildlife in Africa: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 5960. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Song, J.; Li, G.; Cai, W.; Zong, S.; Li, Z.; Liu, W.; Hu, S.; Bu, Z. A novel small RNA Bmsr1 enhances virulence in Brucella melitensis M28. Vet. Microbiol. 2018, 223, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hashemifar, I.; Yadegar, A.; Jazi, F.M.; Amirmozafari, N. Molecular prevalence of putative virulence-associated genes in Brucella melitensis and Brucella abortus isolates from human and livestock specimens in Iran. Microb. Pathog. 2017, 105, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.K.; Sehrawat, A.; Tiwari, R.; Prasad, M.; Gulati, B.; Shabbir, M.Z.; Chhabra, R.; Karthik, K.; Patel, S.K.; Pathak, M.; et al. Bovine brucellosis—A comprehensive review. Vet. Q. 2021, 41, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Nejad, R.; Krecek, R.C.; Khalaf, O.H.; Hailat, N.; Arenas-Gamboa, A.M. Brucellosis in the middle east: Current situation and a pathway forward. PLoS Negl. Trop. Dis. 2020, 14, e0008071. [Google Scholar] [CrossRef]
- Williams, C.; Swisher, S.; Miller, N.; Pinn-Woodcock, T.; Austin, C.; Hsiao, S.; Arenas-Gamboa, A.M.; Tiller, R.; Thacker, T.; Taetzsch, S.; et al. Human exposures to Brucella canis from a pregnant dog during an international flight: Public health risks, diagnostic challenges and future considerations. Zoonoses Public Health 2024, 71, 629–641. [Google Scholar] [CrossRef]
- Ma, R.; Li, C.; Gao, A.; Jiang, N.; Feng, X.; Li, J.; Hu, W. Evidence-practice gap analysis in the role of tick in brucellosis transmission: A scoping review. Infect. Dis. Poverty 2024, 13, 3. [Google Scholar] [CrossRef]
- El-Sayed, A.; Awad, W. Brucellosis: Evolution and expected comeback. Int. J. Vet. Sci. Med. 2018, 6, S31–S35. [Google Scholar] [CrossRef]
- Djokic, V.; Freddi, L.; de Massis, F.; Lahti, E.; van den Esker, M.H.; Whatmore, A.; Haughey, A.; Ferreira, A.C.; Garofolo, G.; Melzer, F.; et al. The emergence of Brucella canis as a public health threat in Europe: What we know and what we need to learn. Emerg. Microbes Infect. 2023, 12, 2249126. [Google Scholar] [CrossRef]
- Pinn-Woodcock, T.; Frye, E.; Guarino, C.; Franklin-Guild, R.; Newman, A.P.; Bennett, J.; Goodrich, E.L. A One-Health review on brucellosis in the United States. J. Am. Vet. Med. Assoc. 2023, 261, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Galarce, N.; Escobar, B.; Martínez, E.; Alvarado, N.; Peralta, G.; Dettleff, P.; Dorner, J.; Martínez, V.; Borie, C. Prevalence and genomic characterization of Brucella canis strains isolated from kennels, household, and stray dogs in Chile. Animals 2020, 10, 2073. [Google Scholar] [CrossRef]
- Rasool, A.; Kannan, P.; Thulasiraman, S. A comprehensive review of Brucella canis: Zoonotic risks and preventive strategies. Indian. J. Anim. Reprod. 2023, 44, 8–13. [Google Scholar] [CrossRef]
- Lali, K.; Dhar, P.; Chahota, R.; Verma, S.; Sharma, M. Brucella canis infection in dogs—A neglected zoonosis. Indian. J. Comp. Microbiol. Immunol. Infect. Dis. 2021, 42, 213–225. [Google Scholar] [CrossRef]
- Mendes, A.; Gomes, B.; Sousa, L.; Moreira, H.; Rosa, I.; Marques, S.; Machado, E.; Cruz Alves, G.; Neto, M. Brucellosis: A rapid risk assessment by a regional outbreak team and its coordinated response with the directorate-general for food and veterinary, North region of Portugal, 2019. Zoonoses Public Health 2020, 67, 587–590. [Google Scholar] [CrossRef]
- Caetano, M.C.; Afonso, F.; Ribeiro, R.; Fonseca, A.P.; Abernethy, D.A.; Boinas, F. Control of bovine brucellosis from persistently infected holdings using RB51 vaccination with test-and-slaughter: A comparative case report from a high incidence area in Portugal. Transbound. Emerg. Dis. 2014, 63, e39–e47. [Google Scholar] [CrossRef]
- Fernandes, M.O.L. Brucelose dos Pequenos Ruminantes: Estudo de Focos na Área Administrativa da Divisão de Intervenção Veterinária de Vila Real. Master’s Thesis, Universidade Técnica de Lisboa, Faculdade de Medicina Veterinária, Lisbon, Portugal, 2012. [Google Scholar]
- Díez, J.G.; Coelho, A.C. An Evaluation of cattle farmers’ knowledge of bovine brucellosis in northeast Portugal. J. Infect. Public Health 2013, 6, 363–369. [Google Scholar] [CrossRef]
- Petrie, A.; Watson, P. Statistics for Veterinary and Animal Science, 3rd ed.; Wiley-Blackwell: Oxford, UK, 2013; ISBN 978-0-470-67075-0. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.M.; Pinto, M.d.L.; García Díez, J.; Coelho, A.C. Impact of B. melitensis rev-1 vaccination on brucellosis prevalence. Turk. J. Vet. Anim. Sci. 2015, 39, 261–270. [Google Scholar] [CrossRef]
- Direção-Geral de Alimentação e Veterinária. Sanidade Animal: Resumo de Atividades 2016–2021; Direção-Geral de Alimentação e Veterinária: Lisbon, Portugal, 2022. [Google Scholar]
- Cavaco, S.; Grilo, M.L.; Dias, R.; Nunes, M.; Pascoal, P.; Pereira, M.; Fogaça, C.; Costa, A.B.; Pardal, S.; Ferreira, A.C. Brucella ceti in common dolphins (Delphinus delphis) in Portugal—Characterization of first isolates. Animals 2025, 15, 374. [Google Scholar] [CrossRef]
- Pelerito, A.; Cordeiro, R.; Matos, R.; Santos, M.A.; Soeiro, S.; Núncio, S. Brucelose Humana: Análise Retrospetiva de Casos Clínicos Suspeitos de Infeção Entre 2002–2013; Instituto Nacional de Saúde Doutor Ricardo Jorge: Lisbon, Portugal, 2014. [Google Scholar]
- Pelerito, A.; Cordeiro, R.; Matos, R.; Santos, M.A.; Soeiro, S.; Santos, J.; Manita, C.; Rio, C.; Santo, M.; Paixão, E.; et al. Human brucellosis in Portugal—Retrospective analysis of suspected clinical cases of infection from 2009 to 2016. PLoS ONE 2017, 12, e0179667. [Google Scholar] [CrossRef]
- Buhmann, G.; Paul, F.; Herbst, W.; Melzer, F.; Wolf, G.; Hartmann, K.; Fischer, A. Canine brucellosis: Insights into the epidemiologic situation in Europe. Front. Vet. Sci. 2019, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. HAIRS Risk Assessment: Brucella canis; UK Government: London, UK, 2023. Available online: https://www.gov.uk/government/publications/hairs-risk-assessment-brucella-canis (accessed on 22 June 2025).
- Lucero, N.E.; Escobar, G.I.; Ayala, S.M.; Lopez, G. Sensitivity and specificity of an indirect enzyme-linked immunoassay for the diagnosis of Brucella canis infection in dogs. J. Med. Microbiol. 2002, 51, 656–660. [Google Scholar] [CrossRef]
- Ayala, S.M.; Hasan, D.B.; Celestino, C.A.; Escobar, G.I.; Zhao, D.M.; Lucero, N.E. Validation of a simple universal iELISA for the diagnosis of human brucellosis. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- California Department of Public Health. CDPH IDB Guidance for Managing Select Communicable Diseases: Canine Brucellosis (Brucellosis in Dogs). Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/ (accessed on 22 June 2025).
- Weese, J.S.; Hrinivich, K.; Anderson, M.E.C. Brucella canis in commercial dog breeding kennels, Ontario, Canada. Emerg. Infect. Dis. 2020, 26, 3079–3080. [Google Scholar] [CrossRef]
- Keid, L.B.; Chiebao, D.P.; Batinga, M.C.A.; Faita, T.; Diniz, J.A.; Oliveira, T.M.F.d.S.; Ferreira, H.L.; Soares, R.M. Brucella canis infection in dogs from commercial breeding kennels in Brazil. Transbound. Emerg. Dis. 2017, 64, 691–697. [Google Scholar] [CrossRef]
- Graham, H.; van der Most, M.; Kampfraath, A.A.; Visser, V.; Dinkla, A.; Harders, F.; Ruuls, R.; van Essen-Zandbergen, A.; van den Esker, M.H.; van der Heide, R.; et al. Transmission of Brucella canis in a canine kennel following introduction of an infected dog. Vet. Microbiol. 2024, 296, 110183. [Google Scholar] [CrossRef]
- Houlton, J. A survey of gundog lameness and injuries in Great Britain in the shooting seasons 2005/2006 and 2006/2007. VCOT 2008, 21, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Brenøe, U.T.; Larsgard, A.G.; Johannessen, K.-R.; Uldal, S.H. Estimates of genetic parameters for hunting performance traits in three breeds of gun hunting dogs in Norway. Appl. Anim. Behav. Sci. 2002, 77, 209–215. [Google Scholar] [CrossRef]
- Zink, C.; Schlehr, M.R. Working dog structure: Evaluation and relationship to function. Front. Vet. Sci. 2020, 7, 559055. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.-M.; Jiang, H.-Y.; Jiang, Q.; Zhang, Y.; Yu, J.; Li, L.-M.; Wang, Q.; Li, T.; Xiang, W.; Chen, C.; et al. Prevalence of Brucella in dogs in China: A systematic review and meta-analysis—Epidemiological analysis of canine brucellosis. Front. Vet. Sci. 2025, 11, 1515405. [Google Scholar] [CrossRef]
- Costa, A.C.T.R.B.; Ferreira, A.C.R.; Costa, D.A.C.; Colocho, R.A.B.; Lopes, J.; Souza, K.C.d.; Alvez, S.M.R.; Pinho, G.Z.d.; Silva, Y.D.d.; Brito, G.F.d.; et al. Prospective study of leptospirosis and brucellosis in dogs from a public shelter in the municipality of Lavras, Minas Gerais State, Brazil. Ciência Rural 2025, 55, e20230552. [Google Scholar] [CrossRef]
- Hamdy, M.E.R.; Abdel-Haleem, M.H.; Dawod, R.E.; Ismail, R.I.; Hazem, S.S.; Fahmy, H.A.; Abdel-Hamid, N.H. First seroprevalence and molecular identification report of Brucella canis among dogs in Greater Cairo region and Damietta Governorate of Egypt. Vet. World 2023, 16, 229–238. [Google Scholar] [CrossRef]
- Cheong, S. Canine Brucellosis. Available online: https://www.vet.cornell.edu/departments-centers-and-institutes/riney-canine-health-center/health-topics/canine-health-information/canine-brucellosis (accessed on 22 June 2025).
- Akhtardanesh, B.; Mohammadi, E.; Sadr, S.; Askari, A.; Tavakoli, Z.M.; Ahmadi, R.; Nazemian, S.; Rashidi, H.; Aghamiri, M.; Golchin, M.; et al. Molecular and serological investigation of Brucella species in kennel and farm dogs in Iran. Acta Trop. 2025, 262, 107521. [Google Scholar] [CrossRef]
- Sato, S.; Nabeshima, K.; Kabeya, H.; Maruyama, S. Seroepidemiological survey of Brucella canis infection in dogs in Japan. Jpn. J. Vet. Res. 2020, 68, 129–132. [Google Scholar]
- Santos, R.L.; Souza, T.D.; Mol, J.P.S.; Eckstein, C.; Paíxão, T.A. Canine brucellosis: An update. Front. Vet. Sci. 2021, 8, 594291. [Google Scholar] [CrossRef]
- Mol, J.P.S.; Guedes, A.C.B.; Eckstein, C.; Quintal, A.P.N.; Souza, T.D.; Mathias, L.A.; Haddad, J.P.A.; Paixão, T.A.; Santos, R.L. Diagnosis of canine brucellosis: Comparison of various serologic tests and PCR. J. Vet. Diagn. Investig. 2020, 32, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Gonçalves, S.C.; Alves, M.; Borges, P.; Ferreira, A.C. O8-6 insights into the seroprevalence of Brucella canis infection in dogs in Portugal. In Proceedings of the Brucellosis 2022 International Research Conference—74th Brucellosis Research Conference, Giulianova-Teramo, Italy, 16–19 September 2022; Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise “G. Caporale”: Giulianova-Teramo, Italy, 2022. [Google Scholar]
- Wright, I.; Whitfield, V.; Hanaghan, R.; Upjohn, M.; Boyden, P. Analysis of exotic pathogens found in a large group of imported dogs following an animal welfare investigation. Vet. Rec. 2023, 193, e2996. [Google Scholar] [CrossRef] [PubMed]
- Gavaudan, S.; Garofolo, G.; Lomolino, R.; D’Alterio, N.; Santucci, U.; De Massis, F.; Sacchini, F.; Petrini, A.; Crotti, S.; Tittarelli, M.; et al. First isolation of Brucella canis from a breeding kennel in Italy. Vet. Ital. 2021, 57, 3. [Google Scholar]
- Holst, B.S.; Löfqvist, K.; Ernholm, L.; Eld, K.; Cedersmyg, M.; Hallgren, G. The first case of Brucella canis in Sweden: Background, case report and recommendations from a northern European perspective. Acta Vet. Scand. 2012, 54, 18. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.A. Imported rescue dogs: Lack of research impedes evidence-based advice to ensure the welfare of individual dogs. Vet. Rec. 2020, 186, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Laverde, A.-J.; Restrepo-Botero, D.; Hernández-Pulido, D.; Rodríguez-Bautista, J.L.; Sandoval, I.-S. Seroprevalencia de Brucella canis en perros de un refugio para animales de compañía en Bogotá, Colombia. Biomédica 2021, 41, 260–270. [Google Scholar] [CrossRef]
- Jasrotia, N.; Gulagi, N.; Patra, M.; Kujur, A. Current status of canine brucellosis in India. Indian. J. Anim. Reprod. 2021, 42, 1–5. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Cardoso, R.; Dias, I.T.; Mariano, I.; Belo, A.; Preto, I.R.; Manteigas, A.; Fonseca, A.P.; De Sá, M.I.C. Evaluation of a modified rose bengal test and an indirect enzyme-linked immunosorbent assay for the diagnosis of Brucella melitensis infection in sheep. Vet. Res. 2003, 34, 297–305. [Google Scholar] [CrossRef]
- Pelerito, A.; Nunes, A.; Grilo, T.; Isidro, J.; Silva, C.; Ferreira, A.C.; Valdezate, S.; Núncio, M.S.; Georgi, E.; Gomes, J.P. Genetic characterization of Brucella spp.: Whole genome sequencing-based approach for the determination of multiple locus variable number tandem repeat profiles. Front. Microbiol. 2021, 12, 740068. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Tenreiro, R.; de Sá, M.I.C.; Dias, R. Evolution and genome specialization of Brucella suis biovar 2 iberian lineages. BMC Genomics 2017, 18, 726. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Corrêa de Sá, M.I.; Dias, R.; Tenreiro, R. MLVA-16 typing of Brucella suis biovar 2 strains circulating in Europe. Vet. Microbiol. 2017, 210, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Dias, R.; de Sá, M.I.C.; Tenreiro, R. Whole-genome mapping reveals a large chromosomal inversion on iberian Brucella suis biovar 2 strains. Vet. Microbiol. 2016, 192, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Almendra, C.; Cardoso, R.; Pereira, M.S.; Beja-Pereira, A.; Luikart, G.; Corrêa de Sá, M.I. Development and evaluation of a selective medium for Brucella suis. Res. Vet. Sci. 2012, 93, 565–567. [Google Scholar] [CrossRef]
- Almendra, C.; Silva, T.L.; Beja-Pereira, A.; Ferreira, A.C.; Ferrão-Beck, L.; de Sá, M.I.C.; Bricker, B.J.; Luikart, G. “HOOF-Print” genotyping and haplotype inference discriminates among Brucella spp. isolates from a small spatial scale. Infect. Genet. Evol. 2009, 9, 104–107. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Chambel, L.; Tenreiro, T.; Cardoso, R.; Flor, L.; Dias, I.T.; Pacheco, T.; Garin-Bastuji, B.; Le Flèche, P.; Vergnaud, G.; et al. MLVA16 typing of portuguese human and animal Brucella melitensis and Brucella abortus isolates. PLoS ONE 2012, 7, e42514. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, M.M.; de la Cuesta Zuluaga, J.J.; Garcia-Montoya, G.M.; Dabral, N.; Alzate, J.F.; Vemulapalli, R.; Olivera-Angel, M. Diagnosis of human and canine Brucella canis infection: Development and evaluation of indirect enzyme-linked immunosorbent assays using recombinant Brucella proteins. Heliyon 2020, 6, e04393. [Google Scholar] [CrossRef]
- Ahmed-Bentley, J.; Roman, S.; Mirzanejad, Y.; Fraser, E.; Hoang, L.; Young, E.J.; Morshed, M.; Deans, G. Laboratory exposures from an unsuspected case of human infection with Brucella canis. Emerg. Infect. Dis. 2021, 27, 2489–2491. [Google Scholar] [CrossRef]
- Kolwijck, E.; Lutgens, S.P.M.; Visser, V.X.N.; van Apeldoorn, M.J.; Graham, H.; Koets, A.P.; Schrauwen, M.M.W.P.; Reubsaet, F.A.G.; Broens, E.M.; Kortbeek, L.M. First case of human Brucella canis infection in the Netherlands. Clin. Infect. Dis. 2022, 75, 2250–2252. [Google Scholar] [CrossRef]
- Dentinger, C.M.; Jacob, K.; Lee, L.V.; Mendez, H.A.; Chotikanatis, K.; McDonough, P.L.; Chico, D.M.; De, B.K.; Tiller, R.V.; Traxler, R.M.; et al. Human Brucella canis infection and subsequent laboratory exposures associated with a puppy, New York city, 2012. Zoonoses Public Health 2015, 62, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Weese, H.E. Brucellosis in humans caused by Brucella canis: A scoping review. Can. Vet. J. 2025, 66, 327–334. [Google Scholar] [PubMed]
- Nomura, A.; Imaoka, K.; Imanishi, H.; Shimizu, H.; Nagura, F.; Maeda, K.; Tomino, T.; Fujita, Y.; Kimura, M.; Stein, G.H. Human Brucella canis infections diagnosed by blood culture. Emerg. Infect. Dis. 2010, 16, 1183–1185. [Google Scholar] [CrossRef]
- LeCuyer, T.E.; Franklin-Guild, R.; Guarino, C.; Fox, A.; Maddock, K.; Barber, R.; Baum, D.H.; Bustamante, F.; Daniels, J.; de Avila, D.M.; et al. Performance characteristics of three Brucella canis serological assays in the United States. Front. Vet. Sci. 2025, 12, 1556965. [Google Scholar] [CrossRef]


| Titre | n | % | 95% CI (%) |
|---|---|---|---|
| Negative | 101 | 76.52 | 68.60–82.93 |
| 1:50 (low positive) | 6 | 4.55 | 2.10–9.56 |
| 1:100 (moderate positive) | 9 | 6.82 | 3.10–11.50 |
| 1:200 (moderate positive) | 8 | 6.06 | 3.10–11.50 |
| 1:400 (high positive) | 7 | 5.30 | 2.60–10.54 |
| 1:800 (high positive) | 1 | 0.76 | 0.13–4.20 |
| Total | 132 | 100 | - |
| Regions (NUTS 2) | n of Dogs Tested (%) | % of Seropositive Dogs a (n) | 95% CI (%) |
|---|---|---|---|
| North | 109 (72.48) | 27.52 (30) | 20.01–36.56 |
| Centre | 6 (83.33) | 16.67 (1) | 3.01–56.35 |
| Alentejo | 2 (100) | 0.00 (0) | - |
| Algarve | 11 (100) | 0.00 (0) | - |
| Autonomous Region of Madeira | 4 (100) | 0.00 (0) | - |
| Region (NUTS 2) | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | Average (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| North | 9.7% | 74.2% | 9.7% | 0.0% | 0.0% | 0.0% | 0.0% | 3.2% | 0.0% | 7.4% |
| National Average (%) | 6.5% | 37.1% | 3.2% | 0.0% | 0.0% | 0.0% | 0.0% | 3.2% | 0.0% | 1.6% |
| Titre | Summer n (%) | Winter n (%) | Spring n (%) | Autumn n (%) | Total n (%) |
|---|---|---|---|---|---|
| Negative | 29 (82.9%) | 25 (96.2%) | 23 (57.5%) | 24 (77.4%) | 101 (76.5%) |
| 1:50 | 3 (8.6%) | 1 (3.8%) | 1 (2.5%) | 1 (3.2%) | 6 (4.5%) |
| 1:100 | 2 (5.7%) | 0 (0.0%) | 2 (5.0%) | 5 (16.1%) | 9 (6.8%) |
| 1:200 | 1 (2.9%) | 0 (0.0%) | 6 (15.0%) | 1 (3.2%) | 8 (6.1%) |
| 1:400 | 0 (0.0%) | 0 (0.0%) | 7 (17.5%) | 0 (0.0%) | 7 (5.3%) |
| 1:800 | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) | 0 (0.0%) | 1 (0.8%) |
| Total | 35 (100%) | 26 (100%) | 40 (100%) | 31 (100%) | 132 (100%) |
| Ref. | Study | Study Period | Host | Brucella Species | Sample Size (n) | Positivity (%) | 95% CI | Weight (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Fixed | Random | ||||||||
| [39] | Coelho et al. (2015) | 2001 | Goat | B. melitensis | 41,825 | 4.36 | 4.16–4.56 | 0.68 | 2.41 |
| [39] | Coelho et al. (2015) | 2001 | Sheep | B. melitensis | 175,666 | 5.84 | 5.73–5.95 | 2.86 | 2.41 |
| [39] | Coelho et al. (2015) | 2002 | Goat | B. melitensis | 23,162 | 3.23 | 3.00–3.46 | 0.38 | 2.41 |
| [39] | Coelho et al. (2015) | 2002 | Sheep | B. melitensis | 110,275 | 4.45 | 4.33–4.58 | 1.79 | 2.41 |
| [42] | Pelerito et al. (2014) | 2002 | Human | Brucella spp. | 569 | 15.99 | 13.08–19.27 | 0.01 | 2.15 |
| [39] | Coelho et al. (2015) | 2003 | Goat | B. melitensis | 21,784 | 1.40 | 1.24–1.56 | 0.35 | 2.41 |
| [39] | Coelho et al. (2015) | 2003 | Sheep | B. melitensis | 93,331 | 1.64 | 1.56–1.72 | 1.52 | 2.41 |
| [42] | Pelerito et al. (2014) | 2003 | Human | Brucella spp. | 541 | 12.94 | 10.23–16.06 | 0.01 | 2.14 |
| [34] | Caetano et al. (2014) | 2004 | Bovine | B. abortus | 3400 | 19.00 | 17.69–20.36 | 0.06 | 2.36 |
| [39] | Coelho et al. (2015) | 2004 | Goat | B. melitensis | 28,985 | 1.37 | 1.24–1.51 | 0.47 | 2.41 |
| [39] | Coelho et al. (2015) | 2004 | Sheep | B. melitensis | 121,005 | 1.33 | 1.26–1.39 | 1.97 | 2.41 |
| [42] | Pelerito et al. (2014) | 2004 | Human | Brucella spp. | 601 | 12.65 | 10.09–15.57 | 0.01 | 2.16 |
| [39] | Coelho et al. (2015) | 2005 | Goat | B. melitensis | 43,143 | 1.26 | 1.16–1.37 | 0.70 | 2.41 |
| [39] | Coelho et al. (2015) | 2005 | Sheep | B. melitensis | 197,667 | 1.19 | 1.14–1.24 | 3.22 | 2.41 |
| [42] | Pelerito et al. (2014) | 2005 | Human | Brucella spp. | 667 | 10.20 | 8.00–12.75 | 0.01 | 2.18 |
| [39] | Coelho et al. (2015) | 2006 | Goat | B. melitensis | 47,697 | 1.02 | 0.93–1.11 | 0.78 | 2.41 |
| [39] | Coelho et al. (2015) | 2006 | Sheep | B. melitensis | 206,628 | 0.77 | 0.74–0.81 | 3.36 | 2.41 |
| [42] | Pelerito et al. (2014) | 2006 | Human | Brucella spp. | 633 | 9.32 | 7.17–11.86 | 0.01 | 2.17 |
| [39] | Coelho et al. (2015) | 2007 | Goat | B. melitensis | 51,298 | 0.75 | 0.68–0.83 | 0.83 | 2.41 |
| [39] | Coelho et al. (2015) | 2007 | Sheep | B. melitensis | 226,799 | 0.38 | 0.35–0.40 | 3.69 | 2.41 |
| [42] | Pelerito et al. (2014) | 2007 | Human | Brucella spp. | 613 | 10.60 | 8.28–13.32 | 0.01 | 2.17 |
| [42] | Pelerito et al. (2014) | 2008 | Human | Brucella spp. | 707 | 5.80 | 4.19–7.79 | 0.01 | 2.20 |
| [34] | Caetano et al. (2014) | 2009 | Bovine | B. abortus | 2930 | 3.00 | 2.42–3.69 | 0.05 | 2.36 |
| [34] | Caetano et al. (2014) | 2009 | Bovine | B. abortus | 3324 | 0.81 | 0.54–1.18 | 0.05 | 2.36 |
| [34] | Caetano et al. (2014) | 2009 | Bovine | B. abortus | 2332 | 0.09 | 0.01–0.31 | 0.04 | 2.34 |
| [34] | Caetano et al. (2014) | 2009 | Bovine | B. abortus | 177 | 1.13 | 0.14–4.02 | 0.00 | 1.73 |
| [42] | Pelerito et al. (2014) | 2009 | Human | Brucella spp. | 526 | 7.99 | 5.82–10.64 | 0.01 | 2.13 |
| [42] | Pelerito et al. (2014) | 2010 | Human | Brucella spp. | 361 | 6.93 | 4.53–10.05 | 0.01 | 2.02 |
| [42] | Pelerito et al. (2014) | 2010 | Human | Brucella spp. | 24 | 25.00 | 9.77–46.71 | 0.0004 | 0.64 |
| [42] | Pelerito et al. (2014) | 2011 | Human | Brucella spp. | 330 | 8.18 | 5.46–11.68 | 0.01 | 1.99 |
| [42] | Pelerito et al. (2014) | 2011 | Human | Brucella spp. | 33 | 33.33 | 17.96–51.83 | 0.001 | 0.79 |
| [42] | Pelerito et al. (2014) | 2012 | Human | Brucella spp. | 195 | 11.28 | 7.21–16.58 | 0.003 | 1.78 |
| [42] | Pelerito et al. (2014) | 2012 | Human | Brucella spp. | 247 | 12.55 | 8.69–17.34 | 0.004 | 1.88 |
| [42] | Pelerito et al. (2014) | 2012 | Human | Brucella spp. | 85 | 2.35 | 0.29–8.24 | 0.001 | 1.33 |
| [42] | Pelerito et al. (2014) | 2013 | Human | Brucella spp. | 20 | 5.00 | 0.13–24.87 | 0.0003 | 0.56 |
| [40] | DGAV (2022) | 2016 | Bovine | B. abortus | 849,252 | 0.04 | 0.03–0.04 | 13.82 | 2.41 |
| [40] | DGAV (2022) | 2017 | Bovine | B. abortus | 820,044 | 0.04 | 0.04–0.05 | 13.34 | 2.41 |
| [40] | DGAV (2022) | 2018 | Bovine | B. abortus | 817,721 | 0.03 | 0.03–0.04 | 13.30 | 2.41 |
| [40] | DGAV (2022) | 2019 | Bovine | B. abortus | 811,945 | 0.05 | 0.05–0.06 | 13.21 | 2.41 |
| [40] | DGAV (2022) | 2020 | Bovine | B. abortus | 737,093 | 0.03 | 0.02–0.03 | 11.99 | 2.41 |
| [40] | DGAV (2022) | 2020 | Bovine | B. abortus | 698,559 | 0.02 | 0.02–0.03 | 11.37 | 2.41 |
| [43] | Pelerito et al. (2017) | 2009–2016 | Human | B. melitensis | 2313 | 7.22 | 6.20–8.35 | 0.04 | 2.34 |
| [43] | Pelerito et al. (2017) | 2009–2016 | Human | B. melitensis | 259 | 16.60 | 12.28–21.70 | 0.004 | 1.90 |
| [28] | Djokic et al. (2023) | 2013–2014 | Dog | B. canis | 62 | 9.68 | 3.64–19.88 | 0.001 | 1.14 |
| [7] | Pires et al. (2024) | 2016–2023 | Wild Boar | Brucella spp. | 650 | 21.69 | 18.58–25.06 | 0.01 | 2.18 |
| [28] | Djokic et al. (2023) | 2018–2019 | Dog | B. canis | 19 | 47.37 | 24.45–71.14 | 0.0003 | 0.54 |
| [8] | Ruano et al. (2024) | 2022–2023 | Wild Boar | B. melitensis | 332 | 26.51 | 21.84–31.60 | 0.01 | 1.99 |
| [41] | Cavaco et al. (2025) | 2022–2024 | Dolphin | B. ceti | 59 | 5.09 | 1.06–14.15 | 0.001 | 1.11 |
| [41] | Cavaco et al. (2025) | 2022–2024 | Dolphin | B. ceti | 59 | 23.73 | 13.62–36.60 | 0.001 | 1.11 |
| Total (fixed effects) | 6,145,947 | 0.20 | 0.19–0.20 | 100.00 | 100.00 | ||||
| Total (random effects) b | 6,145,947 | 4.49 a | 3.77–5.27 | 100.00 | 100.00 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, R.; de Carvalho, H.L.; Ferreira, A.C.; Garcês, A.; Fernandes, C.; Silva, A.R.; Lopes, A.P.; Cardoso, L.; Duarte, E.L.; Coelho, A.C. Are We Missing Brucella spp. in Portugal? The First Nationwide Systematic Review, Meta-Analysis, and Retrospective Serological Study of Brucella canis (2013–2025). Zoonotic Dis. 2025, 5, 26. https://doi.org/10.3390/zoonoticdis5040026
Lopes R, de Carvalho HL, Ferreira AC, Garcês A, Fernandes C, Silva AR, Lopes AP, Cardoso L, Duarte EL, Coelho AC. Are We Missing Brucella spp. in Portugal? The First Nationwide Systematic Review, Meta-Analysis, and Retrospective Serological Study of Brucella canis (2013–2025). Zoonotic Diseases. 2025; 5(4):26. https://doi.org/10.3390/zoonoticdis5040026
Chicago/Turabian StyleLopes, Ricardo, Hugo Lima de Carvalho, Ana Cristina Ferreira, Andreia Garcês, Cátia Fernandes, Ana Rita Silva, Ana Patrícia Lopes, Luís Cardoso, Elsa Leclerc Duarte, and Ana Cláudia Coelho. 2025. "Are We Missing Brucella spp. in Portugal? The First Nationwide Systematic Review, Meta-Analysis, and Retrospective Serological Study of Brucella canis (2013–2025)" Zoonotic Diseases 5, no. 4: 26. https://doi.org/10.3390/zoonoticdis5040026
APA StyleLopes, R., de Carvalho, H. L., Ferreira, A. C., Garcês, A., Fernandes, C., Silva, A. R., Lopes, A. P., Cardoso, L., Duarte, E. L., & Coelho, A. C. (2025). Are We Missing Brucella spp. in Portugal? The First Nationwide Systematic Review, Meta-Analysis, and Retrospective Serological Study of Brucella canis (2013–2025). Zoonotic Diseases, 5(4), 26. https://doi.org/10.3390/zoonoticdis5040026













