Modeling the Transmission of ESBL and AmpC-Producing Escherichia coli in Denmark: A Compartmental and Source Attribution Approach
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
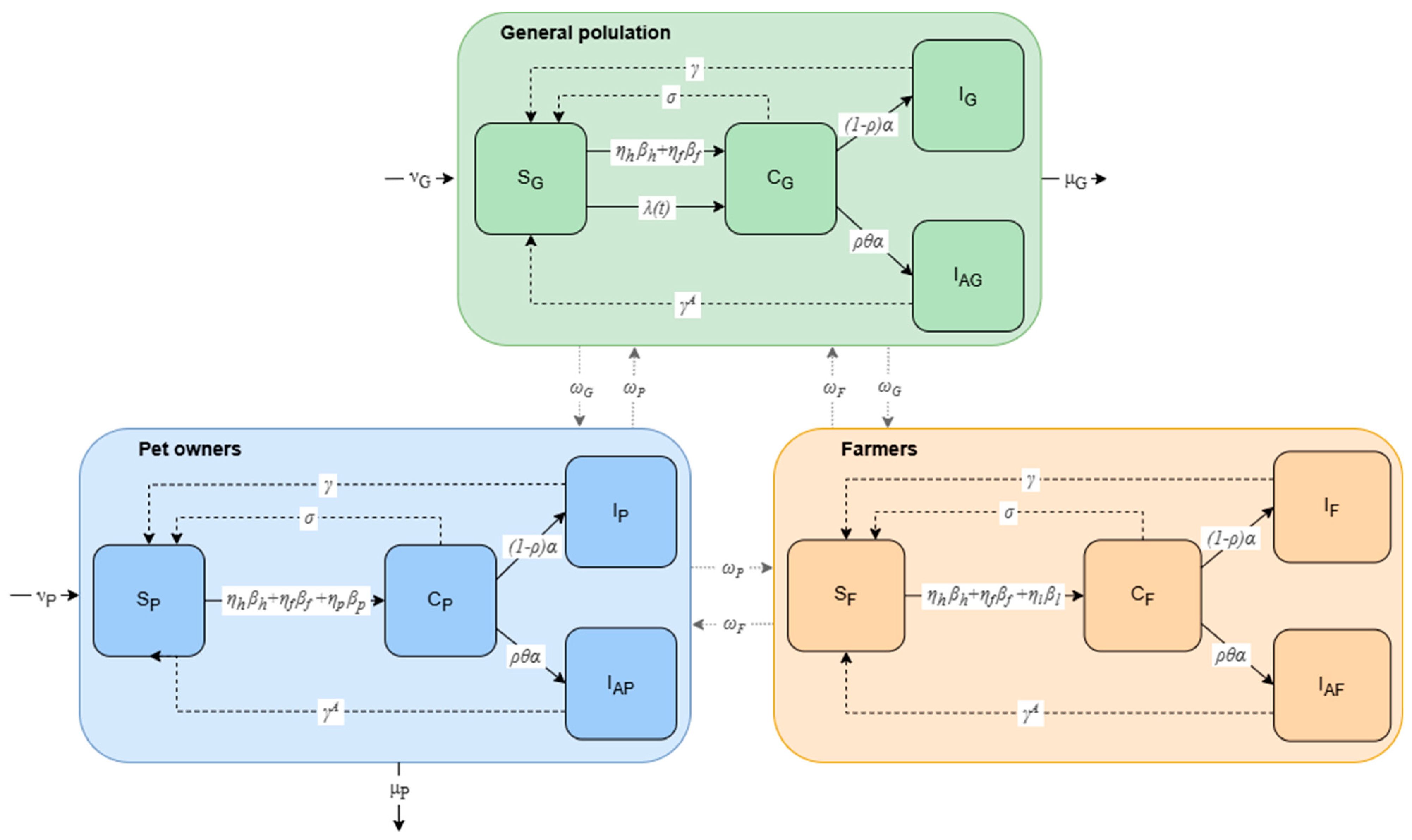
2.1. Compartmental Model of the General Population, Farmers, and Pet Owners
| Parameter | Description | Value | Source |
|---|---|---|---|
| Total size of the Danish population at the beginning of the model period | 5.97 × 106 | [34] | |
| Size of the population in the general population at the beginning of the model period | 3.92 × 106 | ||
| Size of the population that is farmers (broilers, cattle, or pigs) | 2.49 × 104 | [35] | |
| Size of the population that has pets (cat, dog, or horse) at the beginning of the model period | 2.02 × 106 | [36] | |
| Human-to-human transmission | 2.51 × 10−3/day | Estimated | |
| Total transmission rate for food | 4.08 × 10−3/day | Table S1 [16,37,38,39,40,41] | |
| Total transmission rate for pets (cat, dog, and horse) | 6.27 × 10−2/day | Table S1 [36,42,43,44] | |
| Total transmission rate from livestock | 2.03 × 10−2/day | Table S1 [16,35,41,45] | |
| Rate of colonized travelers | 2.64 × 10−5/day | Table S1 [18,46,47,48] | |
| Probability of exposure to food | 1.89 × 10−2/day | Estimated | |
| Probability of exposure to pets | 1.83 × 10−3/day | Estimated | |
| Probability of exposure to livestock | 8.13 × 10−2/day | Estimated | |
| Rate of infection | 6.91 × 10−4/day | Table S1 | |
| Rate of antibiotic exposure | 2.43 × 10−5/day | Table S1 | |
| θ | Increased risk of infection while on antibiotics | 3.7 | [20] |
| Rate of infected individuals initiating antibiotic therapy | 9.00 × 10−2/day | Assumed | |
| Recovery rate | 2.60 × 10−3/day | [49] | |
| Recovery rate in case of antibiotic usage | 4.76 × 10−2/day | Assumed | |
| Decolonization rate | 1.75 × 10−2/day | Estimated | |
| , , | Disease transmission between groups | - | Supplementary S1 |
| , | Birth rates | 1.04 × 102/day 5.34 × 101/day | [50] |
| , | Natural death rates | 2.22 × 10−5/day 1.14 × 10−5/day | [51] |
2.2. Source Attribution Model Based on Resistance Genes
3. Results
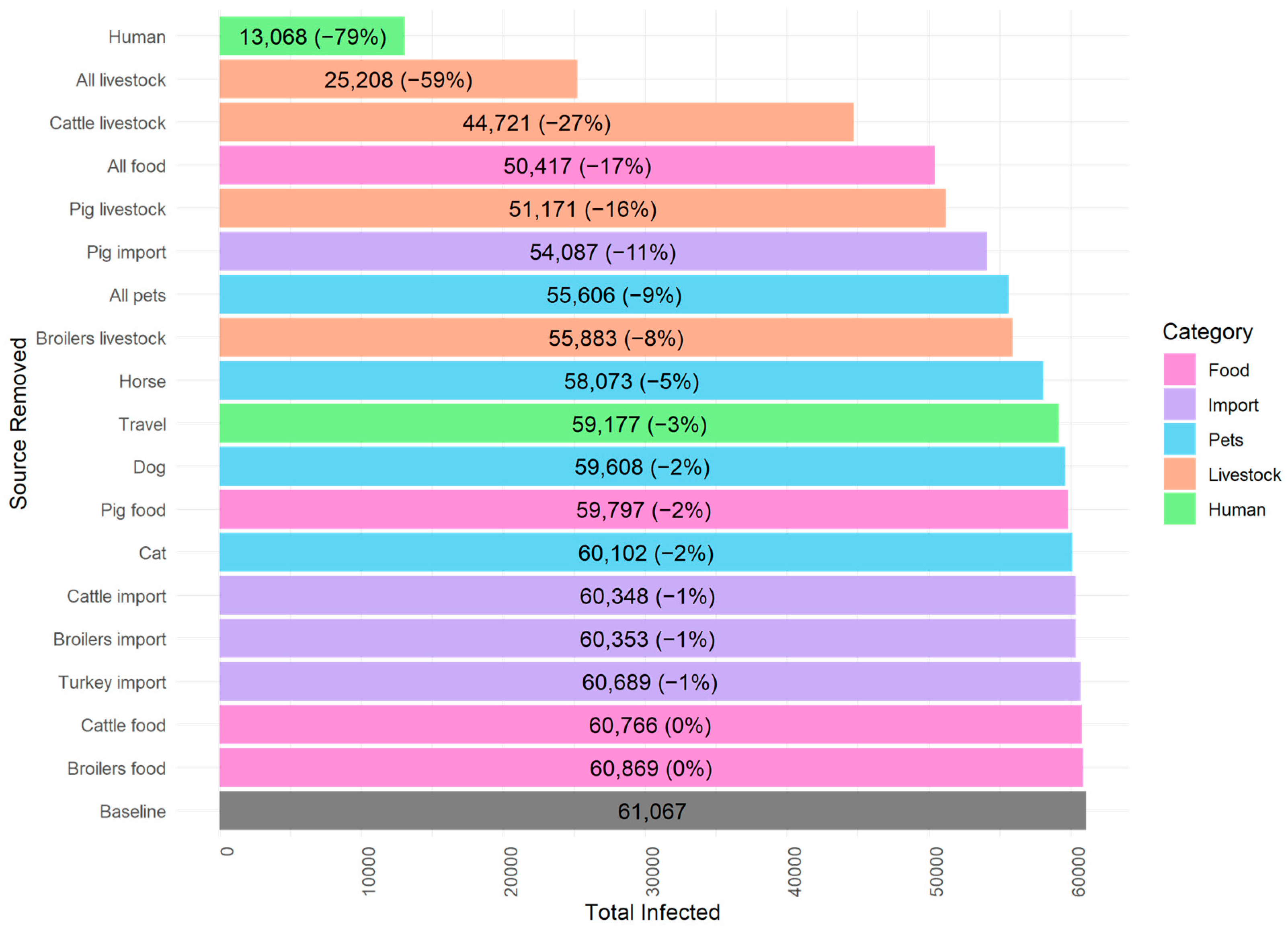
3.1. Compartmental Model
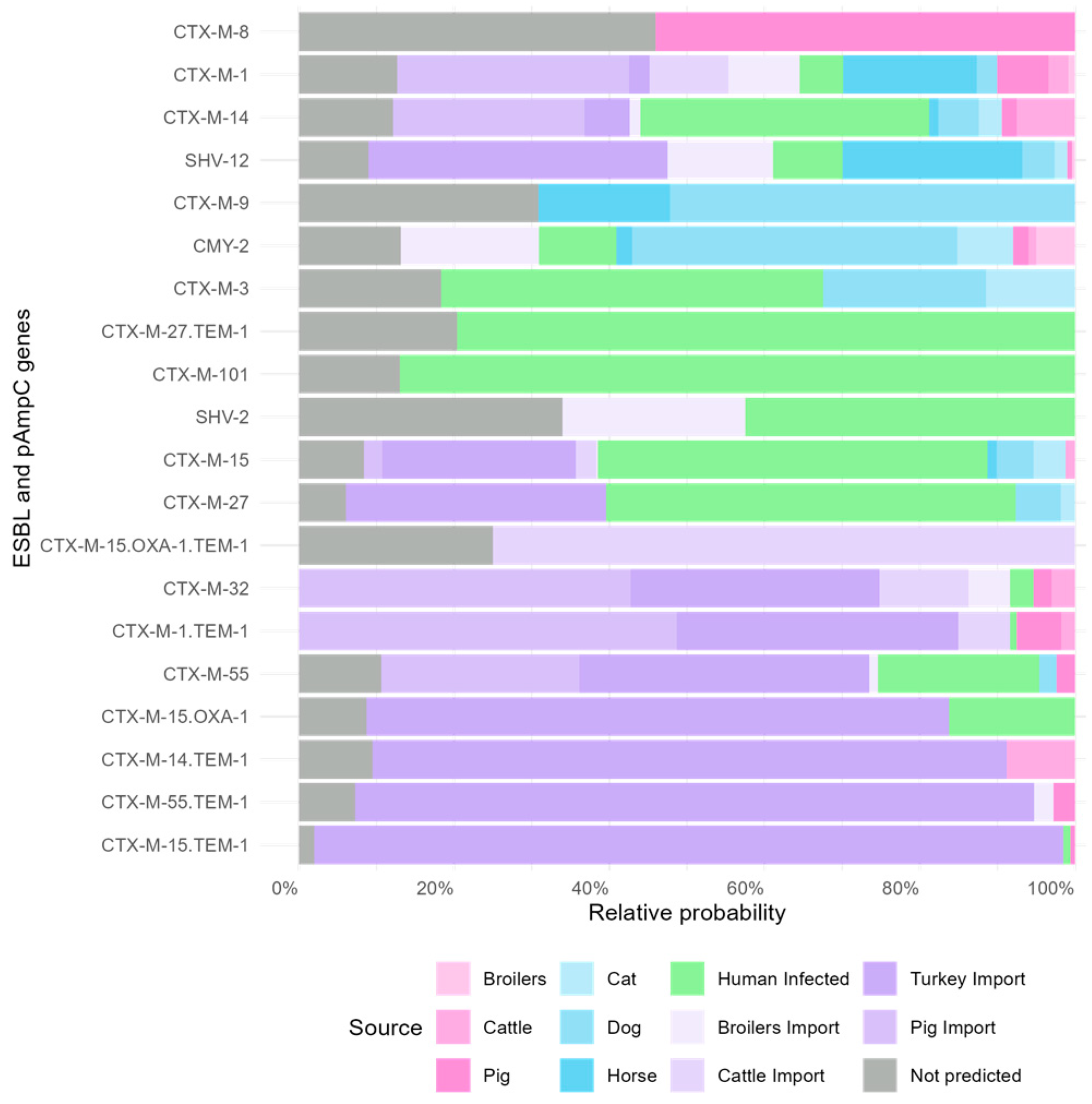
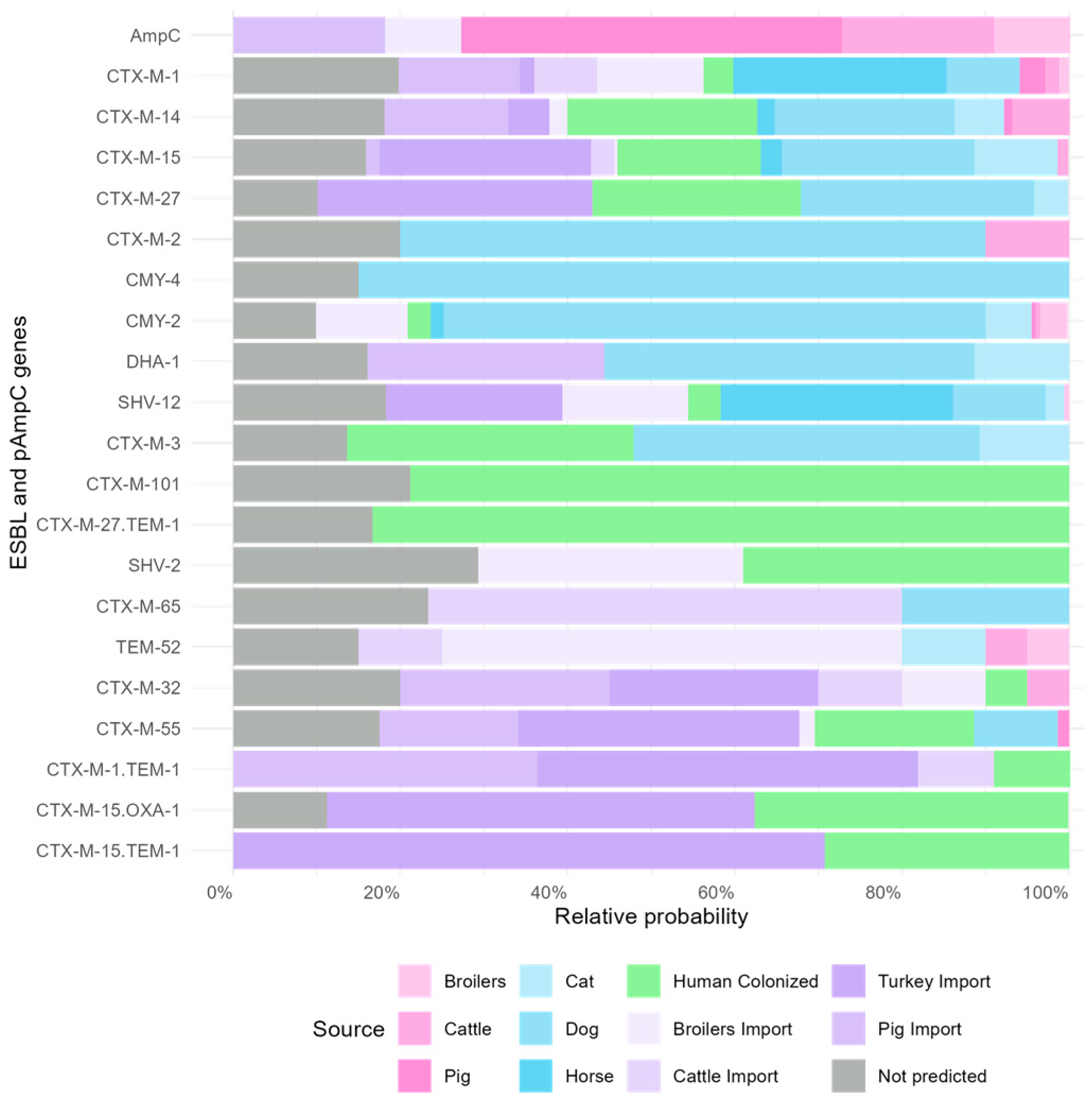
3.2. Gene-Specific Source Attribution Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.; Shunji Ikuta, K.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 619–655. [Google Scholar] [CrossRef]
- Pitout, J.D.; Laupland, K.B. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: An Emerging Public-Health Concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bezabih, Y.M.; Sabiiti, W.; Alamneh, E.; Bezabih, A.; Peterson, G.M.; Bezabhe, W.M.; Roujeinikova, A. The Global Prevalence and Trend of Human Intestinal Carriage of ESBL-Producing Escherichia Coli in the Community. J. Antimicrob. Chemother. 2021, 76, 22–29. [Google Scholar] [CrossRef]
- Food, E.; Authority, S.; Amore, G.; Beloeil, P.-A.; Fierro, R.G.; Guerra, B.; Papanikolaou, A.; Rizzi, V.; Stoicescu, A.-V. Manual for Reporting 2021 Antimicrobial Resistance Data within the Framework of Directive 2003/99/EC and Decision 2020/1729/EU. EFSA Support. Publ. 2021, 18, 6652E. [Google Scholar] [CrossRef]
- Dorado-García, A.; Smid, J.H.; Van Pelt, W.; Bonten, M.J.M.; Fluit, A.C.; Van Den Bunt, G.; Wagenaar, J.A.; Hordijk, J.; Dierikx, C.M.; Veldman, K.T.; et al. Molecular Relatedness of ESBL/AmpC-Producing Escherichia Coli from Humans, Animals, Food and the Environment: A Pooled Analysis. J. Antimicrob. Chemother. 2018, 73, 339–347. [Google Scholar] [CrossRef]
- Perestrelo, S.; Amaro, A.; Brouwer, M.S.M.; Clemente, L.; Ribeiro Duarte, A.S.; Kaesbohrer, A.; Karpíšková, R.; Lopez-Chavarrias, V.; Morris, D.; Prendergast, D.; et al. Building an International One Health Strain Level Database to Characterise the Epidemiology of AMR Threats: ESBL—AmpC Producing E. coli as An Example—Challenges and Perspectives. Antibiotics 2023, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fèvre, E.M.; Woolhouse, M.E.J.; Van Bunnik, B.A.D. Are Food Animals Responsible for Transfer of Antimicrobial-Resistant Escherichia Coli or Their Resistance Determinants to Human Populations? A Systematic Review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Wee, B.A.; Muloi, D.M.; van Bunnik, B.A.D. Quantifying the Transmission of Antimicrobial Resistance at the Human and Livestock Interface with Genomics. Clin. Microbiol. Infect. 2020, 26, 1612–1616. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Dorado-García, A.; van Duijkeren, E.; van den Bunt, G.; Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.; Schmitt, H.; Hald, T.; Evers, E.G.; et al. Attributable Sources of Community-Acquired Carriage of Escherichia Coli Containing β-Lactam Antibiotic Resistance Genes: A Population-Based Modelling Study. Lancet Planet. Health 2019, 3, e357–e369. [Google Scholar] [CrossRef]
- Perestrelo, S.; Carreira, G.C.; Valentin, L.; Fischer, J.; Pfeifer, Y.; Werner, G.; Schmiedel, J.; Falgenhauer, L.; Imirzalioglu, C.; Chakraborty, T.; et al. Comparison of Approaches for Source Attribution of ESBL-Producing Escherichia Coli in Germany. PLoS ONE 2022, 17, e0271317. [Google Scholar] [CrossRef]
- de Freitas Costa, E.; Hagenaars, T.J.; Dame-Korevaar, A.; Brouwer, M.S.M.; de Vos, C.J. Multidirectional Dynamic Model for the Spread of Extended-Spectrum-β-Lactamase-Producing Escherichia Coli in the Netherlands. Microb. Risk Anal. 2022, 22, 100230. [Google Scholar] [CrossRef]
- Evers, E.G.; Pielaat, A.; Smid, J.H.; Van Duijkeren, E.; Vennemann, F.B.C.; Wijnands, L.M.; Chardon, J.E. Comparative Exposure Assessment of ESBL-Producing Escherichia Coli through Meat Consumption. PLoS ONE 2017, 12, e0169589. [Google Scholar] [CrossRef] [PubMed]
- Hammerum, A.M.; Heuer, O.E.; Emborg, H.D.; Bagger-Skjøt, L.; Jensen, V.F.; Rogues, A.M.; Skov, R.L.; Agersø, Y.; Brandt, C.T.; Seyfarth, A.M.; et al. Danish Integrated Antimicrobial Resistance Monitoring and Research Program. Emerg. Infect. Dis. 2007, 13, 1633. [Google Scholar] [CrossRef]
- DANMAP 2023—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; DTU: Lyngby, Denmark; Statens Serum Institute: Copenhagen, Denmark, 2023.
- Carvalho, A.C.; Barbosa, A.V.; Arais, L.R.; Ribeiro, P.F.; Carneiro, V.C.; Cerqueira, A.M.F. Resistance Patterns, ESBL Genes, and Genetic Relatedness of Escherichia Coli from Dogs and Owners. Braz. J. Microbiol. 2016, 47, 150–158. [Google Scholar] [CrossRef] [PubMed]
- DANMAP 2022—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; DTU: Lyngby, Denmark; Statens Serum Institute: Copenhagen, Denmark, 2022.
- Arcilla, M.S.; van Hattem, J.M.; Haverkate, M.R.; Bootsma, M.C.J.; van Genderen, P.J.J.; Goorhuis, A.; Grobusch, M.P.; Lashof, A.M.O.; Molhoek, N.; Schultsz, C.; et al. Import and Spread of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae by International Travellers (COMBAT Study): A Prospective, Multicentre Cohort Study. Lancet Infect. Dis. 2017, 17, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Molina, D.; Berglund, F.; Blaak, H.; Flach, C.F.; Kemper, M.; Marutescu, L.; Gradisteanu, G.P.; Popa, M.; Spießberger, B.; Wengenroth, L.; et al. International Travel as a Risk Factor for Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia Coli in a Large Sample of European Individuals—The AWARE Study. Int. J. Environ. Res. Public Health 2022, 19, 4758. [Google Scholar] [CrossRef]
- Tängdén, T.; Cars, O.; Melhus, Å.; Löwdin, E. Foreign Travel Is a Major Risk Factor for Colonization with Escherichia Coli Producing CTX-M-Type Extended-Spectrum β-Lactamases: A Prospective Study with Swedish Volunteers. Antimicrob. Agents Chemother. 2010, 54, 3564–3568. [Google Scholar] [CrossRef]
- Reese, S.; Knepper, B.; Price, C.; Young, H. Extended Spectrum β-Lactamase-Producing Escherichia Coli in the Community: Risk Factor Analysis. Open Forum Infect. Dis. 2015, 2, 1807. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; López-Cerero, L.; Navarro, M.D.; de Alba, P.D.; Pascual, A. Faecal Carriage of Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli: Prevalence, Risk Factors and Molecular Epidemiology. J. Antimicrob. Chemother. 2008, 62, 1142–1149. [Google Scholar] [CrossRef]
- Knight, G.M.; Costelloe, C.; Deeny, S.R.; Moore, L.S.P.; Hopkins, S.; Johnson, A.P.; Robotham, J.V.; Holmes, A.H. Quantifying Where Human Acquisition of Antibiotic Resistance Occurs: A Mathematical Modelling Study. BMC Med. 2018, 16, 137. [Google Scholar] [CrossRef]
- Ny, S.; Löfmark, S.; Börjesson, S.; Englund, S.; Ringman, M.; Bergström, J.; Nauclér, P.; Giske, C.G.; Byfors, S. Community Carriage of ESBL-Producing Escherichia Coli Is Associated with Strains of Low Pathogenicity: A Swedish Nationwide Study. J. Antimicrob. Chemother. 2017, 72, 582–588. [Google Scholar] [CrossRef]
- Raffelsberger, N.; Buczek, D.J.; Svendsen, K.; Småbrekke, L.; Pöntinen, A.K.; Löhr, I.H.; Andreassen, L.L.E.; Simonsen, G.S.; Sundsfjord, A.; Gravningen, K.; et al. Community Carriage of ESBL-Producing Escherichia coli and Klebsiella pneumoniae: A Cross-Sectional Study of Risk Factors and Comparative Genomics of Carriage and Clinical Isolates. mSphere 2023, 8, e0002523. [Google Scholar] [CrossRef]
- Dahms, C.; Hubner, N.O.; Kossow, A.; Mellmann, A.; Dittmann, K.; Kramer, A. Occurrence of ESBL-Producing Escherichia Coli in Livestock and Farm Workers in Mecklenburg-Western Pomerania, Germany. PLoS ONE 2015, 10, e0143326. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, W.; Bonten, M.J.M.; Bos, M.E.H.; van Marm, S.; Scharringa, J.; Wagenaar, J.A.; Heederik, D.J.J. Carriage of Extended-Spectrum β-Lactamases in Pig Farmers Is Associated with Occurrence in Pigs. Clin. Microbiol. Infect. 2015, 21, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Hammerum, A.M.; Larsen, J.; Andersen, V.D.; Lester, C.H.; Skytte, T.S.S.; Hansen, F.; Olsen, S.S.; Mordhorst, H.; Skov, R.L.; Aarestrup, F.M.; et al. Characterization of Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia coli Obtained from Danish Pigs, Pig Farmers and Their Families from Farms with High or No Consumption of Third- or Fourth-Generation Cephalosporins. J. Antimicrob. Chemother. 2014, 69, 2650–2657. [Google Scholar] [CrossRef]
- Huijbers, P.M.C.; Graat, E.A.M.; Haenen, A.P.J.; van Santen, M.G.; van Essen-Zandbergen, A.; Mevius, D.J.; van Duijkeren, E.; van Hoek, A.H.A.M. Extended-Spectrum and AmpC β-Lactamase-Producing Escherichia Coli in Broilers and People Living and/or Working on Broiler Farms: Prevalence, Risk Factors and Molecular Characteristics. J. Antimicrob. Chemother. 2014, 69, 2669–2675. [Google Scholar] [CrossRef]
- Schmithausen, R.M.; Schulze-Geisthoevel, S.V.; Stemmer, F.; El-Jade, M.; Reif, M.; Hack, S.; Meilaender, A.; Montabauer, G.; Fimmers, R.; Parcina, M.; et al. Analysis of Transmission of MRSA and ESBL-E among Pigs and Farm Personnel. PLoS ONE 2015, 10, e0138173. [Google Scholar] [CrossRef] [PubMed]
- Dazio, V.; Nigg, A.; Schmidt, J.S.; Brilhante, M.; Campos-Madueno, E.I.; Mauri, N.; Kuster, S.P.; Brawand, S.G.; Willi, B.; Endimiani, A.; et al. Duration of Carriage of Multidrug-Resistant Bacteria in Dogs and Cats in Veterinary Care and Co-Carriage with Their Owners. One Health 2021, 13, 100322. [Google Scholar] [CrossRef]
- Menezes, J.; Frosini, S.M.; Belas, A.; Marques, C.; da Silva, J.M.; Amaral, A.J.; Loeffler, A.; Pomba, C. Longitudinal Study of ESBL/AmpC-Producing Enterobacterales Strains Sharing between Cohabiting Healthy Companion Animals and Humans in Portugal and in the United Kingdom. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1011–1024. [Google Scholar] [CrossRef]
- Van Den Bunt, G.; Fluit, A.C.; Spaninks, M.P.; Timmerman, A.J.; Geurts, Y.; Kant, A.; Scharringa, J.; Mevius, D.; Wagenaar, J.A.; Bonten, M.J.M.; et al. Faecal Carriage, Risk Factors, Acquisition and Persistence of ESBL-Producing Enterobacteriaceae in Dogs and Cats and Co-Carriage with Humans Belonging to the Same Household. J. Antimicrob. Chemother. 2020, 75, 342. [Google Scholar] [CrossRef]
- Wu, J.; Dhingra, R.; Gambhir, M.; Remais, J.V. Sensitivity Analysis of Infectious Disease Models: Methods, Advances and Their Application. J. R. Soc. Interface 2013, 10, 20121018. [Google Scholar] [CrossRef] [PubMed]
- Statistics Denmark. Population Figures. Available online: https://www.dst.dk/en/Statistik/emner/borgere/befolkning/befolkningstal (accessed on 31 May 2024).
- Employed by Industry (DB07) and Sex—StatBank Denmark—Data and Statistics. Available online: https://www.statistikbanken.dk/statbank5a/SelectVarVal/Define.asp?Maintable=RAS309&PLanguage=1 (accessed on 8 October 2024).
- Lund, T.B.; Sandøe, P. Resultater Fra Undersøgelse Af Danskernes Hold Af Og Tilknytning Til Kaele-Og Hobbydyr Gennemført i 2021; Institut for Fødevare- og Ressourceøkonomi, Københavns Universitet: Copenhagen, Denmark, 2021. [Google Scholar]
- Slaughterings and Production of Poultry by Category and Unit—StatBank Denmark—Data and Statistics. Available online: https://www.statistikbanken.dk/ANI61 (accessed on 9 October 2024).
- Danish Agriculture & Food Council. Statistic 2022 Beef and Veal-in Danish. 2023. Available online: https://agricultureandfood.dk/statistics/annual-statistics/ (accessed on 9 October 2024).
- Danish Agriculture & Food Council. Statistics 2023 Pigmeat. 2024. Available online: https://agricultureandfood.dk/statistics/annual-statistics/ (accessed on 9 October 2024).
- Danish Agriculture & Food Council. Annual Statistics 2023 for Danish Poultry Production. 2023. Available online: https://agricultureandfood.dk/statistics/annual-statistics/ (accessed on 9 October 2024).
- DANMAP 2021—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; DTU: Lyngby, Denmark; Statens Serum Institute: Copenhagen, Denmark, 2021.
- Zogg, A.L.; Simmen, S.; Zurfluh, K.; Stephan, R.; Schmitt, S.N.; Nüesch-Inderbinen, M. High Prevalence of Extended-Spectrum β-Lactamase Producing Enterobacteriaceae among Clinical Isolates from Cats and Dogs Admitted to a Veterinary Hospital in Switzerland. Front. Vet. Sci. 2018, 5, 27. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Hald, T. Deliverable-D-JRPFBZ-DISCOVER-1-WP4.4 AMR Source AĴribution Results by Region/Country for Each Applied Method. 2022. Available online: https://zenodo.org/records/7303549 (accessed on 9 October 2024).
- Walther, B.; Klein, K.S.; Barton, A.K.; Semmler, T.; Huber, C.; Wolf, S.A.; Tedin, K.; Merle, R.; Mitrach, F.; Guenther, S.; et al. Extended-Spectrum Beta-Lactamase (ESBL)—Producing Escherichia Coli and Acinetobacter Baumannii among Horses Entering a Veterinary Teaching Hospital: The Contemporary “Trojan Horse”. PLoS ONE 2018, 13, e0191873. [Google Scholar] [CrossRef] [PubMed]
- Farms with Livestock by Region, Unit and Type-StatBank Denmark-Data and Statistics. Available online: https://www.statistikbanken.dk/statbank5a/default.asp?w=1920 (accessed on 9 October 2024).
- Number of Trips by Purpose of Visit, Means of Transport, Duration and Destination-StatBank Denmark-Data and Statistics. Available online: https://www.statistikbanken.dk/FF3 (accessed on 9 October 2024).
- Espenhain, L.; Jørgensen, S.B.; Leegaard, T.M.; Lelek, M.M.; Hänsgen, S.H.; Nakstad, B.; Sunde, M.; Steinbakk, M. Travel to Asia Is a Strong Predictor for Carriage of Cephalosporin Resistant E. coli and Klebsiella spp. but Does Not Explain Everything; Prevalence Study at a Norwegian Hospital 2014–2016. Antimicrob. Resist. Infect. Control 2018, 7, 146. [Google Scholar] [CrossRef]
- Holidays Abroad by Destination and Duration-StatBank Denmark-Data and Statistics Available online:. Available online: https://www.statistikbanken.dk/statbank5a/SelectVarVal/Define.asp?Maintable=FF1&PLanguage=1 (accessed on 9 October 2024).
- Haverkate, M.R.; Platteel, T.N.; Fluit, A.C.; Cohen Stuart, J.W.; Leverstein-van Hall, M.A.; Thijsen, S.F.T.; Scharringa, J.; Kloosterman, R.C.; Bonten, M.J.M.; Bootsma, M.C.J. Quantifying Within-Household Transmission of Extended-Spectrum β-Lactamase-Producing Bacteria. Clin. Microbiol. Infect. 2017, 23, 46.e1–46.e7. [Google Scholar] [CrossRef]
- Statistics Denmark. Births. Available online: https://www.dst.dk/en/Statistik/emner/borgere/befolkning/foedsler (accessed on 1 October 2024).
- Statistics Denmark. Life Expectancy. Available online: https://www.dst.dk/en/Statistik/emner/borgere/befolkning/middellevetid (accessed on 1 October 2024).
- Rebelo, A.R.; Ibfelt, T.; Bortolaia, V.; Leekitcharoenphon, P.; Hansen, D.S.; Nielsen, H.L.; Ellermann-Eriksen, S.; Kemp, M.; Røder, B.L.; Frimodt-Møller, N.; et al. One Day in Denmark: Nationwide Point-Prevalence Survey of Human Bacterial Isolates and Comparison of Classical and Whole-Genome Sequence-Based Species Identification Methods. PLoS ONE 2022, 17, e0261999. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.R.; Bortolaia, V.; Leekitcharoenphon, P.; Hansen, D.S.; Nielsen, H.L.; Ellermann-Eriksen, S.; Kemp, M.; Røder, B.L.; Frimodt-Møller, N.; Søndergaard, T.S.; et al. One Day in Denmark: Whole-Genome Sequence-Based Analysis of Escherichia Coli Isolates from Clinical Settings. J. Antimicrob. Chemother. 2025, dkaf028. [Google Scholar] [CrossRef] [PubMed]
- Swedres-Svarm. Sales of Antibiotics and Occurrence of Resistance in Sweden; Public Health Agency of Sweden and National Veterinary Institute: Solna, Sweden, 2022; ISSN 2001-7901. [Google Scholar]
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Hald, T.; Vose, D.; Wegener, H.C.; Koupeev, T. A Bayesian Approach to Quantify the Contribution of Animal-Food Sources to Human Sahnonellosis. Risk Anal. 2004, 24, 255–269. [Google Scholar] [CrossRef]
- Little, C.L.; Pires, S.M.; Gillespie, I.A.; Grant, K.; Nichols, G.L. Attribution of Human Listeria Monocytogenes Infections in England and Wales to Ready-to-Eat Food Sources Placed on the Market: Adaptation of the Hald Salmonella Source Attribution Model. Foodborne Pathog. Dis. 2010, 7, 749–756. [Google Scholar] [CrossRef]
- Mullner, P.; Jones, G.; Noble, A.; Spencer, S.E.F.; Hathaway, S.; French, N.P. Source Attribution of Food-Borne Zoonoses in New Zealand: A Modified Hald Model. Risk Anal. 2009, 29, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M. Bayesian Graphical Models Using MCMC [R Package Rjags Version 4-16]. CRAN: Contributed Packages. 2024. Available online: https://cran.r-project.org/web/packages/rjags/index.html (accessed on 9 October 2024).
- Day, M.J.; Hopkins, K.L.; Wareham, D.W.; Toleman, M.A.; Elviss, N.; Randall, L.; Teale, C.; Cleary, P.; Wiuff, C.; Doumith, M.; et al. Extended-Spectrum β-Lactamase-Producing Escherichia Coli in Human-Derived and Foodchain-Derived Samples from England, Wales, and Scotland: An Epidemiological Surveillance and Typing Study. Lancet Infect. Dis. 2019, 19, 1325–1335. [Google Scholar] [CrossRef]
- Kuenzli, E.; Jaeger, V.K.; Frei, R.; Neumayr, A.; DeCrom, S.; Haller, S.; Blum, J.; Widmer, A.F.; Furrer, H.; Battegay, M.; et al. High Colonization Rates of Extended-Spectrum β-Lactamase (ESBL)-Producing Escherichia Coli in Swiss Travellers to South Asia—A Prospective Observational Multicentre Cohort Study Looking at Epidemiology, Microbiology and Risk Factors. BMC Infect. Dis. 2014, 14, 528. [Google Scholar] [CrossRef] [PubMed]
- de Been, M.; Lanza, V.F.; de Toro, M.; Scharringa, J.; Dohmen, W.; Du, Y.; Hu, J.; Lei, Y.; Li, N.; Tooming-Klunderud, A.; et al. Dissemination of Cephalosporin Resistance Genes between Escherichia Coli Strains from Farm Animals and Humans by Specific Plasmid Lineages. PLoS Genet. 2014, 10, e1004776. [Google Scholar] [CrossRef] [PubMed]
- Aziminia, N.; Hadjipavlou, M.; Philippou, Y.; Pandian, S.S.; Malde, S.; Hammadeh, M.Y. Vaccines for the Prevention of Recurrent Urinary Tract Infections: A Systematic Review. BJU Int. 2019, 123, 753–768. [Google Scholar] [CrossRef]
- Kawamura, K.; Nagano, N.; Suzuki, M.; Wachino, J.; Kimura, K.; Arakawa, Y. ESBL-Producing Escherichia coli and Its Rapid Rise among Healthy People. Food Saf. 2017, 5, 122. [Google Scholar] [CrossRef]
| Colonized | Infected | Infected on Antibiotics | |
|---|---|---|---|
| General population | 925,015 | 37,365 | 1198 |
| Pet owners | 544,307 | 21,098 | 705 |
| Farmers | 17,369 | 678 | 23 |
| Total | 1,486,691 | 59,141 | 1926 |
| Source | Mean (95% CI) | Median | SD | Percent |
|---|---|---|---|---|
| Turkey import | 95.3 (52.7–145.4) | 93.8 | 23.7 | 33.3 |
| Human Infected | 85.6 (33.2–138.1) | 85.7 | 27.3 | 29.9 |
| Pig import | 19.8 (4.4–43.5) | 18.3 | 10.3 | 6.9 |
| Dog | 17.5 (1.2–47.8) | 15.1 | 12.3 | 6.1 |
| Cat | 9.2 (0.2–35.1) | 6.2 | 9.5 | 3.2 |
| Horse | 7.4 (0.3–22.1) | 6 | 6 | 2.6 |
| Cattle | 7.3 (0.7–22.5) | 5.8 | 5.8 | 2.6 |
| Cattle import | 6.5 (1.2–17.1) | 5.6 | 4.2 | 2.3 |
| Broilers import | 6.2 (0.2–19.3) | 4.9 | 5.2 | 2.2 |
| Pig | 4.8 (1.4–10.3) | 4.4 | 2.3 | 1.7 |
| Broilers | 0.7 (0–2.2) | 0.5 | 0.6 | 0.2 |
| Not predicted | 25.8 | 9.0 |
| Source | Mean (95% CI) | Median | SD | Percent |
|---|---|---|---|---|
| Dog | 262.9 (50.5–566.3) | 244.8 | 137.1 | 20.3 |
| Turkey import | 242.0 (11.4–587.4) | 220.6 | 161.6 | 18.7 |
| Human colonized | 206.6 (48.1–448.4) | 191.6 | 104.9 | 16.0 |
| Cat | 106.5 (2.5–380.1) | 74.2 | 102.4 | 8.2 |
| Pig import | 58.0 (1.9–183.2) | 45.2 | 49.5 | 4.5 |
| Horse | 41.4 (1.3–130.7) | 32.5 | 35.1 | 3.2 |
| Cattle import | 38.5 (1.2–98.1) | 21.4 | 25.8 | 2.2 |
| Broilers import | 24.2 (1.0–75.5) | 19.4 | 20.1 | 1.9 |
| Cattle | 21.7 (0.8–73.8) | 16.2 | 19.7 | 1.7 |
| Pig | 5.1 (0.2–15.6) | 4.1 | 4.2 | 0.4 |
| Broilers | 2.1 (0.1–6.7) | 1.6 | 1.8 | 0.2 |
| Not predicted | 295.7 | 22.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinch, M.L.; Duarte, A.S.R.; Apenteng, O.O.; Hald, T. Modeling the Transmission of ESBL and AmpC-Producing Escherichia coli in Denmark: A Compartmental and Source Attribution Approach. Zoonotic Dis. 2025, 5, 7. https://doi.org/10.3390/zoonoticdis5010007
Brinch ML, Duarte ASR, Apenteng OO, Hald T. Modeling the Transmission of ESBL and AmpC-Producing Escherichia coli in Denmark: A Compartmental and Source Attribution Approach. Zoonotic Diseases. 2025; 5(1):7. https://doi.org/10.3390/zoonoticdis5010007
Chicago/Turabian StyleBrinch, Maja Lykke, Ana Sofia Ribeiro Duarte, Ofosuhene O. Apenteng, and Tine Hald. 2025. "Modeling the Transmission of ESBL and AmpC-Producing Escherichia coli in Denmark: A Compartmental and Source Attribution Approach" Zoonotic Diseases 5, no. 1: 7. https://doi.org/10.3390/zoonoticdis5010007
APA StyleBrinch, M. L., Duarte, A. S. R., Apenteng, O. O., & Hald, T. (2025). Modeling the Transmission of ESBL and AmpC-Producing Escherichia coli in Denmark: A Compartmental and Source Attribution Approach. Zoonotic Diseases, 5(1), 7. https://doi.org/10.3390/zoonoticdis5010007






