Marburg Virus Disease in Sub-Saharan Africa: A Review of Currently Available Comprehensive Genomic Data up to 2024
Simple Summary
Abstract
1. Introduction
2. Search Strategy and Selection of Research Articles
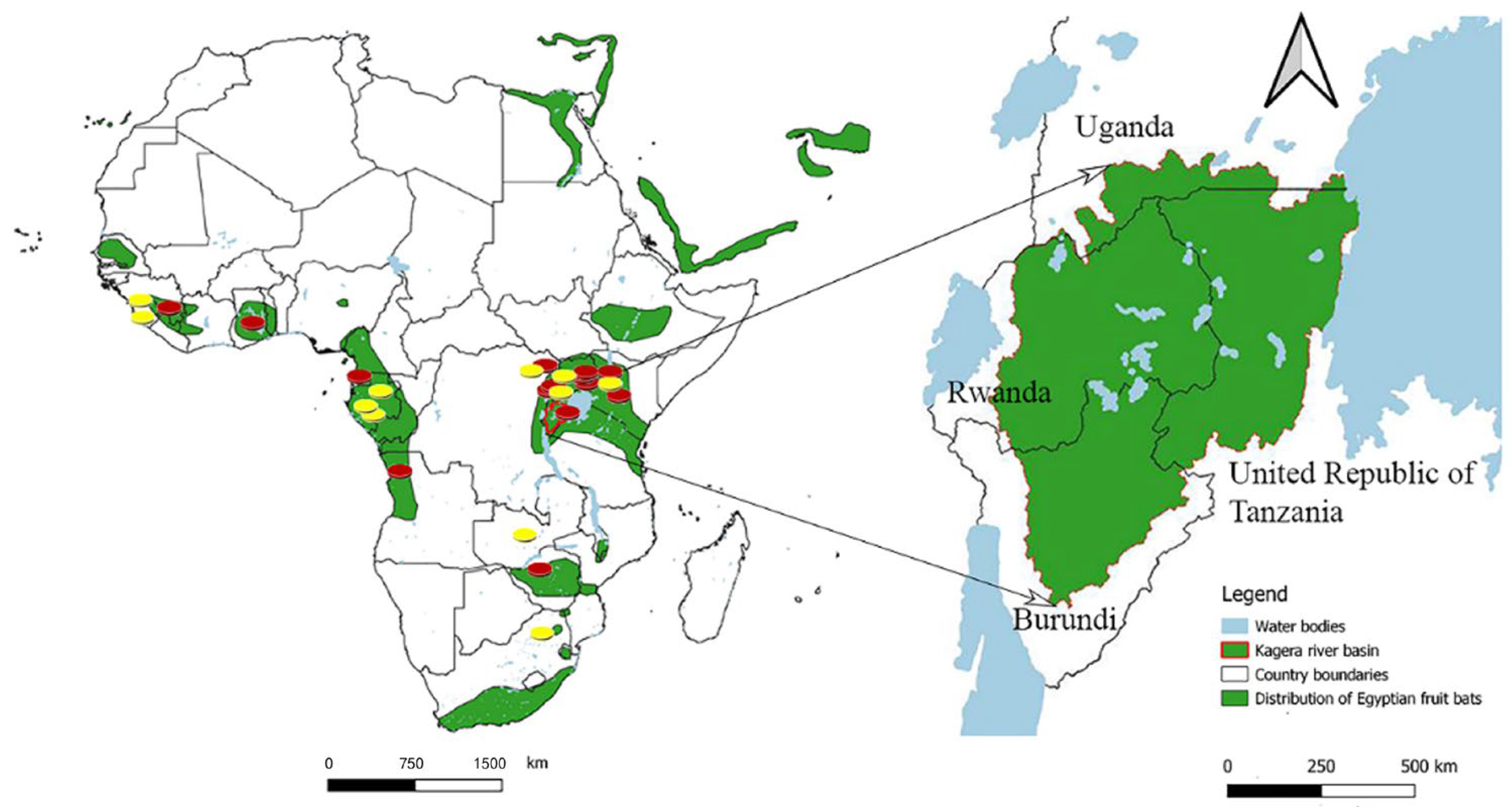
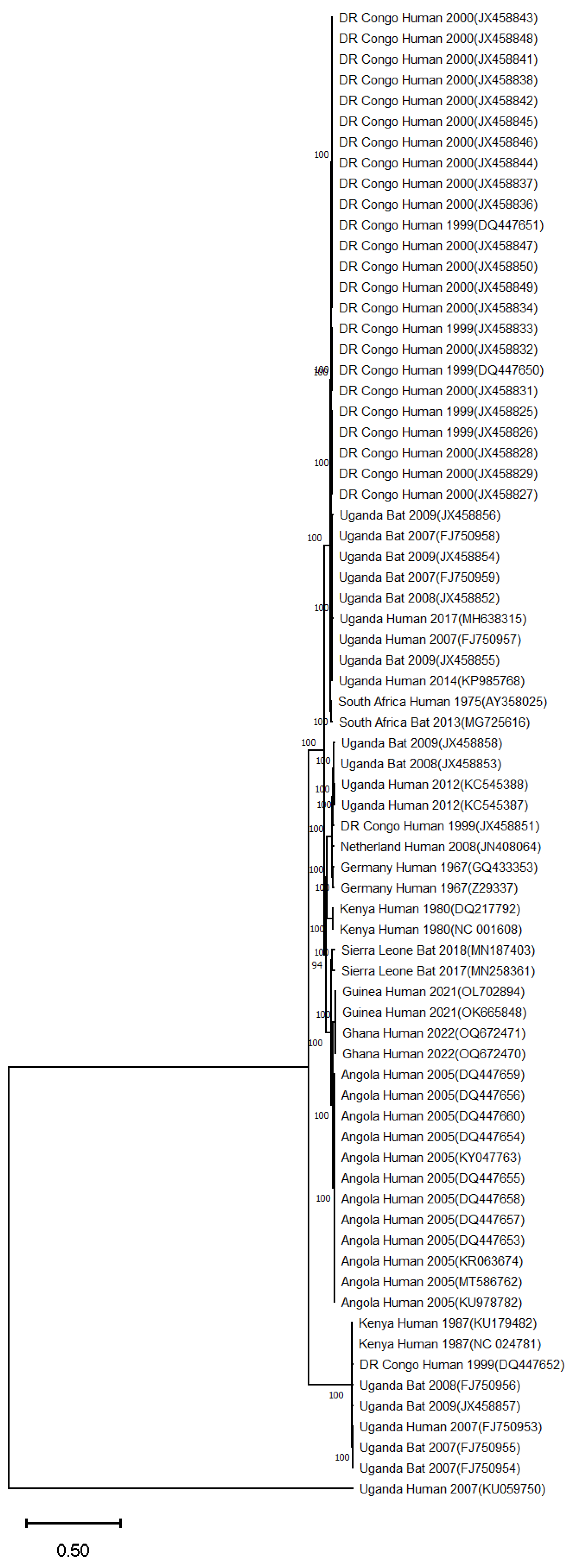
3. Marburg Virus Disease Epidemics and Associated Genomic Data in Sub-Saharan Africa
3.1. South Africa
3.2. Kenya
3.3. Democratic Republic of the Congo
3.4. Angola
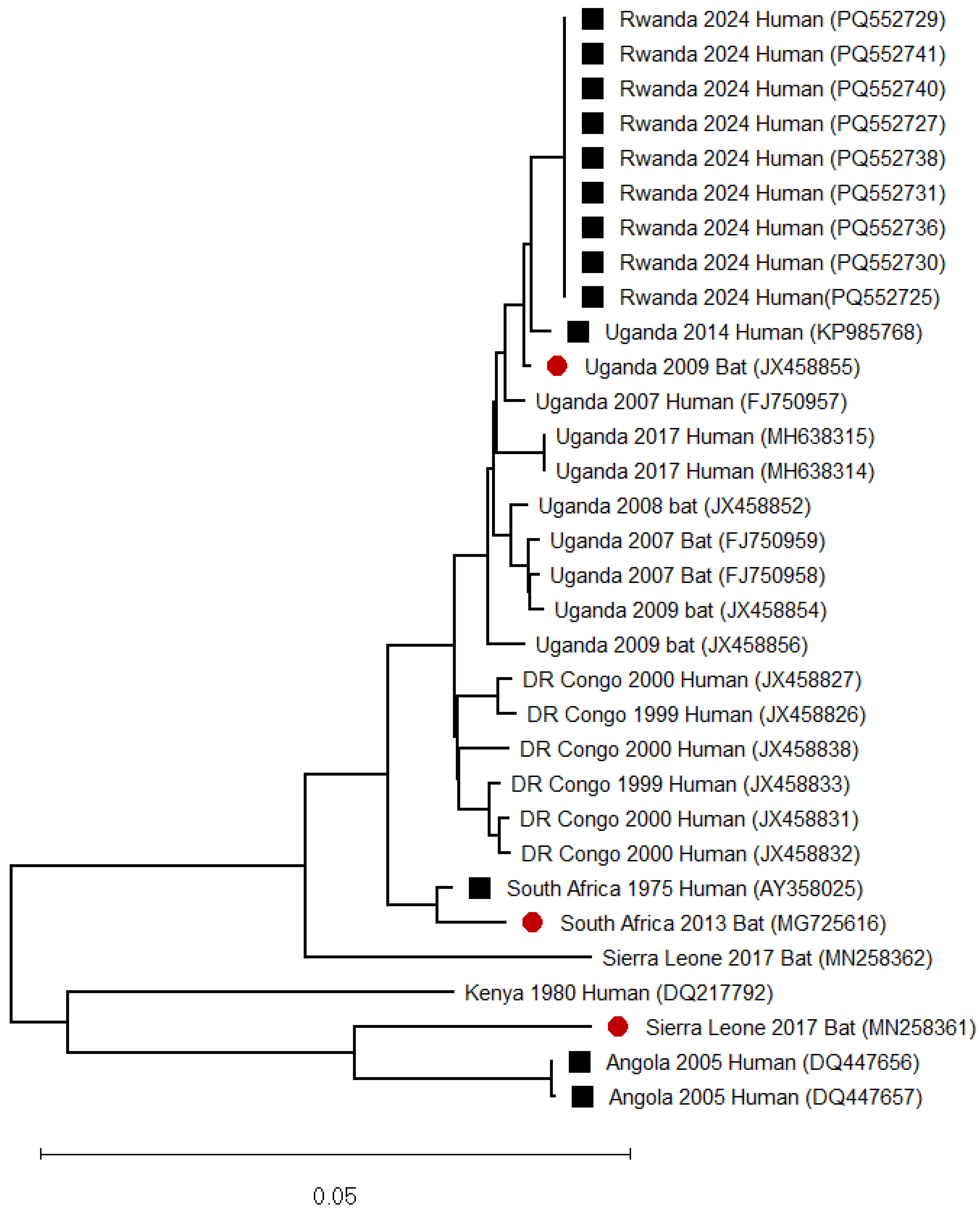
3.5. Uganda
3.6. Guinea
3.7. Ghana
3.8. Equatorial Guinea
3.9. Tanzania
3.10. Rwanda
4. Detection of Marburg Virus in Bats with No Reported Human Outbreak of Marburg Virus Disease
4.1. Gabon
4.2. Zambia
4.3. Sierra Leone
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biedenkopf, N.; Bukreyev, A.; Chandran, K.; Di Paola, N.; Formenty, P.B.H.; Griffiths, A.; Hume, A.J.; Mühlberger, E.; Netesov, S.V.; Palacios, G.; et al. Renaming of Genera Ebolavirus and Marburgvirus to Orthoebolavirus and Orthomarburgvirus, Respectively, and Introduction of Binomial Species Names within Family Filoviridae. Arch. Virol. 2023, 168, 220. [Google Scholar] [CrossRef]
- Kiley, M.P.; Bowen, E.T.; Eddy, G.A.; Isaäcson, M.; Johnson, K.M.; McCormick, J.B.; Murphy, F.A.; Pattyn, S.R.; Peters, D.; Prozesky, O.W.; et al. Filoviridae: A Taxonomic Home for Marburg and Ebola Viruses? Intervirology 1982, 18, 24–32. [Google Scholar] [CrossRef]
- Srivastava, D.; Kutikuppala, L.V.S.; Shanker, P.; Sahoo, R.N.; Pattnaik, G.; Dash, R.; Kandi, V.; Ansari, A.; Mishra, S.; Desai, D.N.; et al. The Neglected Continuously Emerging Marburg Virus Disease in Africa: A Global Public Health Threat. Health Sci. Rep. 2023, 6, e1661. [Google Scholar] [CrossRef] [PubMed]
- Brauburger, K.; Hume, A.J.; Mühlberger, E.; Olejnik, J. Forty-Five Years of Marburg Virus Research. Viruses 2012, 4, 1878–1927. [Google Scholar] [CrossRef] [PubMed]
- Luby, J.P.; Sanders, C.V. Green Monkey Disease (“Marburg Virus” Disease): A New Zoonosis. Ann. Intern. Med. 1969, 71, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Mmbaga, V.; Mrema, G.; Ngenzi, D.; Magoge, W.; Mwakapasa, E.; Jacob, F.; Matimba, H.; Beyanga, M.; Samweli, A.; Kiremeji, M.; et al. Epidemiological Description of Marburg Virus Disease Outbreak in Kagera Region, Northwestern Tanzania. PLoS ONE 2024, 19, e0309762. [Google Scholar] [CrossRef]
- Sibomana, O.; Kubwimana, E. First-ever Marburg Virus Disease Outbreak in Equatorial Guinea and Tanzania: An Imminent Crisis in West and East Africa. Immun. Inflamm. Dis. 2023, 11, e980. [Google Scholar] [CrossRef]
- Butera, Y.; Mutesa, L.; Parker, E.; Muvunyi, R.; Umumararungu, E.; Ayitewala, A.; Musabyimana, J.P.; Olono, A.; Sesonga, P.; Ogunsanya, O.; et al. Genomic and Transmission Dynamics of the 2024 Marburg Virus Outbreak in Rwanda. Nat. Med. 2024, 31, 422–426. [Google Scholar] [CrossRef]
- Amman, B.R.; Carroll, S.A.; Reed, Z.D.; Sealy, T.K.; Balinandi, S.; Swanepoel, R.; Kemp, A.; Erickson, B.R.; Comer, J.A.; Campbell, S.; et al. Seasonal Pulses of Marburg Virus Circulation in Juvenile Rousettus aegyptiacus Bats Coincide with Periods of Increased Risk of Human Infection. PLoS Pathog. 2012, 8, e1002877. [Google Scholar] [CrossRef]
- Amman, B.R.; Bird, B.H.; Bakarr, I.A.; Bangura, J.; Schuh, A.J.; Johnny, J.; Sealy, T.K.; Conteh, I.; Koroma, A.H.; Foday, I.; et al. Isolation of Angola-like Marburg Virus from Egyptian Rousette Bats from West Africa. Nat. Commun. 2020, 11, 510. [Google Scholar] [CrossRef]
- Kuzmin, I.V.; Niezgoda, M.; Franka, R.; Agwanda, B.; Markotter, W.; Breiman, R.F.; Shieh, W.-J.; Zaki, S.R.; Rupprecht, C.E. Marburg Virus in Fruit Bat, Kenya. Emerg. Infect. Dis. 2010, 16, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Pawęska, J.T.; Jansen van Vuren, P.; Kemp, A.; Storm, N.; Grobbelaar, A.A.; Wiley, M.R.; Palacios, G.; Markotter, W. Marburg Virus Infection in Egyptian Rousette Bats, South Africa, 2013–20141. Emerg. Infect. Dis. 2018, 24, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Towner, J.S.; Amman, B.R.; Sealy, T.K.; Carroll, S.A.R.; Comer, J.A.; Kemp, A.; Swanepoel, R.; Paddock, C.D.; Balinandi, S.; Khristova, M.L.; et al. Isolation of Genetically Diverse Marburg Viruses from Egyptian Fruit Bats. PLoS Pathog. 2009, 5, e1000536. [Google Scholar] [CrossRef]
- Amman, B.R.; Nyakarahuka, L.; McElroy, A.K.; Dodd, K.A.; Sealy, T.K.; Schuh, A.J.; Shoemaker, T.R.; Balinandi, S.; Atimnedi, P.; Kaboyo, W.; et al. Marburgvirus Resurgence in Kitaka Mine Bat Population after Extermination Attempts, Uganda. Emerg. Infect. Dis. 2014, 20, 1761–1764. [Google Scholar] [CrossRef]
- Knust, B.; Schafer, I.J.; Wamala, J.; Nyakarahuka, L.; Okot, C.; Shoemaker, T.; Dodd, K.; Gibbons, A.; Balinandi, S.; Tumusiime, A.; et al. Multidistrict Outbreak of Marburg Virus Disease-Uganda, 2012. J. Infect. Dis. 2015, 212 (Suppl. S2), S119–S128. [Google Scholar] [CrossRef]
- Ramírez-Fráncel, L.A.; García-Herrera, L.V.; Losada-Prado, S.; Reinoso-Flórez, G.; Sánchez-Hernández, A.; Estrada-Villegas, S.; Lim, B.K.; Guevara, G. Bats and Their Vital Ecosystem Services: A Global Review. Integr. Zool. 2022, 17, 2–23. [Google Scholar] [CrossRef]
- Bukreyev, A.A.; Volchkov, V.E.; Blinov, V.M.; Dryga, S.A.; Netesov, S.V. The Complete Nucleotide Sequence of the Popp (1967) Strain of Marburg Virus: A Comparison with the Musoke (1980) Strain. Arch. Virol. 1995, 140, 1589–1600. [Google Scholar] [CrossRef]
- Feldmann, H.; Mühlberger, E.; Randolf, A.; Will, C.; Kiley, M.P.; Sanchez, A.; Klenk, H.D. Marburg Virus, a Filovirus: Messenger RNAs, Gene Order, and Regulatory Elements of the Replication Cycle. Virus Res. 1992, 24, 1–19. [Google Scholar] [CrossRef]
- Changula, K.; Kajihara, M.; Muramatsu, S.; Hiraoka, K.; Yamaguchi, T.; Yago, Y.; Kato, D.; Miyamoto, H.; Mori-Kajihara, A.; Shigeno, A.; et al. Development of an Immunochromatography Assay to Detect Marburg Virus and Ravn Virus. Viruses 2023, 15, 2349. [Google Scholar] [CrossRef]
- Towner, J.S.; Khristova, M.L.; Sealy, T.K.; Vincent, M.J.; Erickson, B.R.; Bawiec, D.A.; Hartman, A.L.; Comer, J.A.; Zaki, S.R.; Ströher, U.; et al. Marburgvirus Genomics and Association with a Large Hemorrhagic Fever Outbreak in Angola. J. Virol. 2006, 80, 6497–6516. [Google Scholar] [CrossRef]
- Hamer, M.J.; Houser, K.V.; Hofstetter, A.R.; Ortega-Villa, A.M.; Lee, C.; Preston, A.; Augustine, B.; Andrews, C.; Yamshchikov, G.V.; Hickman, S.; et al. Safety, Tolerability, and Immunogenicity of the Chimpanzee Adenovirus Type 3-Vectored Marburg Virus (cAd3-Marburg) Vaccine in Healthy Adults in the USA: A First-in-Human, Phase 1, Open-Label, Dose-Escalation Trial. Lancet 2023, 401, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Azim, K.F.; Begum, A.; Khan, N.A.; Shammi, T.S.; Imran, A.S.; Chowdhury, I.M.; Urme, S.R.A. Vaccinomics Strategy for Developing a Unique Multi-Epitope Monovalent Vaccine against Marburg Marburgvirus. Infect. Genet. Evol. 2019, 70, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Manno, D. Developing a Vaccine against Marburg Virus Disease. Lancet 2023, 401, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.A.; Towner, J.S.; Sealy, T.K.; McMullan, L.K.; Khristova, M.L.; Burt, F.J.; Swanepoel, R.; Rollin, P.E.; Nichol, S.T. Molecular Evolution of Viruses of the Family Filoviridae Based on 97 Whole-Genome Sequences. J. Virol. 2013, 87, 2608–2616. [Google Scholar] [CrossRef]
- Zehender, G.; Sorrentino, C.; Veo, C.; Fiaschi, L.; Gioffrè, S.; Ebranati, E.; Tanzi, E.; Ciccozzi, M.; Lai, A.; Galli, M. Distribution of Marburg Virus in Africa: An Evolutionary Approach. Infect. Genet. Evol. 2016, 44, 8–16. [Google Scholar] [CrossRef]
- Bausch, D.G.; Nichol, S.T.; Muyembe-Tamfum, J.J.; Borchert, M.; Rollin, P.E.; Sleurs, H.; Campbell, P.; Tshioko, F.K.; Roth, C.; Colebunders, R.; et al. Marburg Hemorrhagic Fever Associated with Multiple Genetic Lineages of Virus. N. Engl. J. Med. 2006, 355, 909–919. [Google Scholar] [CrossRef]
- Colebunders, R.; Tshomba, A.; Van Kerkhove, M.D.; Bausch, D.G.; Campbell, P.; Libande, M.; Pirard, P.; Tshioko, F.; Mardel, S.; Mulangu, S.; et al. Marburg Hemorrhagic Fever in Durba and Watsa, Democratic Republic of the Congo: Clinical Documentation, Features of Illness, and Treatment. J. Infect. Dis. 2007, 196, S148–S153. [Google Scholar] [CrossRef]
- Johnson, E.D.; Johnson, B.K.; Silverstein, D.; Tukei, P.; Geisbert, T.W.; Sanchez, A.N.; Jahrling, P.B. Characterization of a New Marburg Virus Isolated from a 1987 Fatal Case in Kenya. Arch. Virol. Suppl. 1996, 11, 101–114. [Google Scholar] [CrossRef]
- Adjemian, J.; Farnon, E.C.; Tschioko, F.; Wamala, J.F.; Byaruhanga, E.; Bwire, G.S.; Kansiime, E.; Kagirita, A.; Ahimbisibwe, S.; Katunguka, F.; et al. Outbreak of Marburg Hemorrhagic Fever Among Miners in Kamwenge and Ibanda Districts, Uganda, 2007. J. Infect. Dis. 2011, 204, S796–S799. [Google Scholar] [CrossRef]
- Gouda, H.N.; Charlson, F.; Sorsdahl, K.; Ahmadzada, S.; Ferrari, A.J.; Erskine, H.; Leung, J.; Santamauro, D.; Lund, C.; Aminde, L.N.; et al. Burden of Non-Communicable Diseases in Sub-Saharan Africa, 1990–2017: Results from the Global Burden of Disease Study 2017. Lancet Glob. Health 2019, 7, e1375–e1387. [Google Scholar] [CrossRef]
- Moyo, E.; Mhango, M.; Moyo, P.; Dzinamarira, T.; Chitungo, I.; Murewanhema, G. Emerging Infectious Disease Outbreaks in Sub-Saharan Africa: Learning from the Past and Present to Be Better Prepared for Future Outbreaks. Front. Public Health 2023, 11, 1049986. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Rodés-Guirao, L.; Roser, M. Peak Global Population and Other Key Findings from the 2024 UN World Population Prospects; Our World Data: Oxford, UK, 2024. [Google Scholar]
- Bonney, J.K.; Adu, B.; Sanders, T.; Pratt, D.; Adams, P.; Asante, I.A.; Bonney, E.Y.; Agbodzi, B.; Kumordjie, S.; Faye, M.; et al. Marburg Virus Disease in Ghana. N. Engl. J. Med. 2023, 388, 2393–2394. [Google Scholar] [CrossRef] [PubMed]
- Koundouno, F.R.; Kafetzopoulou, L.E.; Faye, M.; Renevey, A.; Soropogui, B.; Ifono, K.; Nelson, E.V.; Kamano, A.A.; Tolno, C.; Annibaldis, G.; et al. Detection of Marburg Virus Disease in Guinea. N. Engl. J. Med. 2022, 386, 2528–2530. [Google Scholar] [CrossRef]
- Ahmad, A.; Ashraf, S.; Komai, S. Are Developing Countries Prepared to Face Ebola-like Outbreaks? Virol. Sin. 2015, 30, 234–237. [Google Scholar] [CrossRef]
- Swanepoel, R.; Smit, S.B.; Rollin, P.E.; Formenty, P.; Leman, P.A.; Kemp, A.; Burt, F.J.; Grobbelaar, A.A.; Croft, J.; Bausch, D.G.; et al. Studies of Reservoir Hosts for Marburg Virus. Emerg. Infect. Dis. 2007, 13, 1847–1851. [Google Scholar] [CrossRef]
- Gear, J.S.; Cassel, G.A.; Gear, A.J.; Trappler, B.; Clausen, L.; Meyers, A.M.; Kew, M.C.; Bothwell, T.H.; Sher, R.; Miller, G.B.; et al. Outbreake of Marburg Virus Disease in Johannesburg. Br. Med. J. 1975, 4, 489–493. [Google Scholar] [CrossRef]
- Maganga, G.D.; Bourgarel, M.; Ella, G.E.; Drexler, J.F.; Gonzalez, J.-P.; Drosten, C.; Leroy, E.M. Is Marburg Virus Enzootic in Gabon? J. Infect. Dis. 2011, 204 (Suppl. S3), S800–S803. [Google Scholar] [CrossRef]
- Pawęska, J.T.; Storm, N.; Markotter, W.; Di Paola, N.; Wiley, M.R.; Palacios, G.; Jansen van Vuren, P. Shedding of Marburg Virus in Naturally Infected Egyptian Rousette Bats, South Africa, 2017. Emerg. Infect. Dis. 2020, 26, 3051–3055. [Google Scholar] [CrossRef]
- Smith, D.H.; Isaacson, M.; Johnson, K.M.; Bagshawe, A.; Johnson, B.K.; Swanapoel, R.; Killey, M.; Siongok, T.; Keruga, W.K. Marburg-Virus disease in Kenya. Lancet 1982, 319, 816–820. [Google Scholar] [CrossRef]
- Bausch, D.G.; Borchert, M.; Grein, T.; Roth, C.; Swanepoel, R.; Libande, M.L.; Talarmin, A.; Bertherat, E.; Muyembe-Tamfum, J.-J.; Tugume, B.; et al. Risk Factors for Marburg Hemorrhagic Fever, Democratic Republic of the Congo. Emerg. Infect. Dis. 2003, 9, 1531–1537. [Google Scholar] [CrossRef]
- Ligon, B.L. Outbreak of Marburg Hemorrhagic Fever in Angola: A Review of the History of the Disease and Its Biological Aspects. Semin. Pediatr. Infect. Dis. 2005, 16, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Smetana, J.; Chlíbek, R.; Vacková, M. Outbreak of Marburg hemorrhagic fever in Angola. Epidemiol. Mikrobiol. Imunol. 2006, 55, 63–67. [Google Scholar]
- Stille, W.; Böhle, E.; Helm, E.; van Rey, W.; Siede, W. On an infectious disease transmitted by Cercopithecus aethiops. (“Green monkey disease”). Dtsch. Med. Wochenschr. 1968, 93, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Nyakarahuka, L.; Ojwang, J.; Tumusiime, A.; Balinandi, S.; Whitmer, S.; Kyazze, S.; Kasozi, S.; Wetaka, M.; Makumbi, I.; Dahlke, M.; et al. Isolated Case of Marburg Virus Disease, Kampala, Uganda, 2014. Emerg. Infect. Dis. 2017, 23, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Imported Case of Marburg Hemorrhagic Fever—Colorado, 2008. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 1377–1381. [Google Scholar]
- Timen, A.; Koopmans, M.P.G.; Vossen, A.C.T.M.; van Doornum, G.J.J.; Günther, S.; van den Berkmortel, F.; Verduin, K.M.; Dittrich, S.; Emmerich, P.; Osterhaus, A.D.M.E.; et al. Response to Imported Case of Marburg Hemorrhagic Fever, the Netherlands. Emerg. Infect. Dis. 2009, 15, 1171–1175. [Google Scholar] [CrossRef]
- Schuh, A.J.; Amman, B.R.; Jones, M.E.B.; Sealy, T.K.; Uebelhoer, L.S.; Spengler, J.R.; Martin, B.E.; Coleman-McCray, J.A.D.; Nichol, S.T.; Towner, J.S. Modelling Filovirus Maintenance in Nature by Experimental Transmission of Marburg Virus between Egyptian Rousette Bats. Nat. Commun. 2017, 8, 14446. [Google Scholar] [CrossRef]
- Makenov, M.T.; Boumbaly, S.; Tolno, F.R.; Sacko, N.; N’Fatoma, L.T.; Mansare, O.; Kolie, B.; Stukolova, O.A.; Morozkin, E.S.; Kholodilov, I.S.; et al. Marburg Virus in Egyptian Rousettus Bats in Guinea: Investigation of Marburg Virus Outbreak Origin in 2021. PLoS Negl. Trop. Dis. 2023, 17, e0011279. [Google Scholar] [CrossRef]
- Uwishema, O. First Marburg Virus Outbreak in Rwanda: Urgent Actions Needed. Lancet 2024, 404, 1639. [Google Scholar] [CrossRef]
- Pourrut, X.; Souris, M.; Towner, J.S.; Rollin, P.E.; Nichol, S.T.; Gonzalez, J.-P.; Leroy, E. Large Serological Survey Showing Cocirculation of Ebola and Marburg Viruses in Gabonese Bat Populations, and a High Seroprevalence of Both Viruses in Rousettus Aegyptiacus. BMC Infect. Dis. 2009, 9, 159. [Google Scholar] [CrossRef]
- Towner, J.S.; Pourrut, X.; Albariño, C.G.; Nkogue, C.N.; Bird, B.H.; Grard, G.; Ksiazek, T.G.; Gonzalez, J.-P.; Nichol, S.T.; Leroy, E.M. Marburg Virus Infection Detected in a Common African Bat. PLoS ONE 2007, 2, e764. [Google Scholar] [CrossRef] [PubMed]
- Changula, K.; Kajihara, M.; Mori-Kajihara, A.; Eto, Y.; Miyamoto, H.; Yoshida, R.; Shigeno, A.; Hang’ombe, B.; Qiu, Y.; Mwizabi, D.; et al. Seroprevalence of Filovirus Infection of Rousettus aegyptiacus Bats in Zambia. J. Infect. Dis. 2018, 218, S312–S317. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, M.; Hang’ombe, B.M.; Changula, K.; Harima, H.; Isono, M.; Okuya, K.; Yoshida, R.; Mori-Kajihara, A.; Eto, Y.; Orba, Y.; et al. Marburgvirus in Egyptian Fruit Bats, Zambia. Emerg. Infect. Dis. 2019, 25, 1577–1580. [Google Scholar] [CrossRef] [PubMed]
| Institute | Country | Platform | Reference |
|---|---|---|---|
| National Institute for Communicable Diseases | South Africa | Three Illumina Nextseq 2000 sequencers, one Nextseq 1000 sequencer, and a Pacbio Sequel | [36] |
| Institut Pasteur de Dakar | Senegal | Illumina NovaSeq 6000 sequencer; Nanopore MinION | [34] |
| Centers for Disease Control and Prevention (CDC) field lab and CDC, Atlanta, GA | USA | ABI BigDye 3.1 dye chemistry and ABI 3730XL automated DNA sequencers; Ion Torrent; Illumina; and 454 | [20,37] |
| Franceville Centre International de Recherches Médicales de Franceville | Gabon | Big Dye Terminator Cycle sequencing; Illumina NovaSeq 6000 | [38] |
| Laboratoire des Fièvres Hémorragiques Virales de la Guinée’ | Guinea | Nanopore MinION | [34] |
| Country | Year | No. Cases | No. Deaths | Case Fatality Rate |
|---|---|---|---|---|
| Germany and Yugoslavia | 1967 | 31 | 7 | 23 |
| South Africa | 1975 | 3 | 1 | 33 |
| Kenya | 1980 | 2 | 1 | 50 |
| Kenya | 1987 | 1 | 1 | 100 |
| Russia | 1990 | 1 | 1 | 100 |
| DR Congo | 1998–2000 | 154 | 128 | 83 |
| Angola | 2004–2005 | 252 | 227 | 90 |
| Uganda | 2007 | 4 | 1 | 25 |
| USA | 2008 | 1 | 0 | - |
| Uganda | 2012 | 26 | 15 | 57.7 |
| Uganda | 2014 | 1 | 1 | 100 |
| Uganda | 2017 | 4 | 3 | 75 |
| Guinea | 2021 | 1 | 1 | 100 |
| Ghana | 2022 | 3 | 2 | 66.7 |
| Equatorial Guinea | 2023 | 40 | 35 | 87.5 |
| Tanzania | 2023 | 9 | 6 | 66.7 |
| Rwanda | 2024 | 66 | 15 | 23.1 |
| Tanzania * | 2025 | 9 | 8 | 89% |
| Total | 608 | 453 | 74.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinimi, E. Marburg Virus Disease in Sub-Saharan Africa: A Review of Currently Available Comprehensive Genomic Data up to 2024. Zoonotic Dis. 2025, 5, 6. https://doi.org/10.3390/zoonoticdis5010006
Kinimi E. Marburg Virus Disease in Sub-Saharan Africa: A Review of Currently Available Comprehensive Genomic Data up to 2024. Zoonotic Diseases. 2025; 5(1):6. https://doi.org/10.3390/zoonoticdis5010006
Chicago/Turabian StyleKinimi, Edson. 2025. "Marburg Virus Disease in Sub-Saharan Africa: A Review of Currently Available Comprehensive Genomic Data up to 2024" Zoonotic Diseases 5, no. 1: 6. https://doi.org/10.3390/zoonoticdis5010006
APA StyleKinimi, E. (2025). Marburg Virus Disease in Sub-Saharan Africa: A Review of Currently Available Comprehensive Genomic Data up to 2024. Zoonotic Diseases, 5(1), 6. https://doi.org/10.3390/zoonoticdis5010006





