Zoonotic Diseases in Sub-Saharan Africa: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
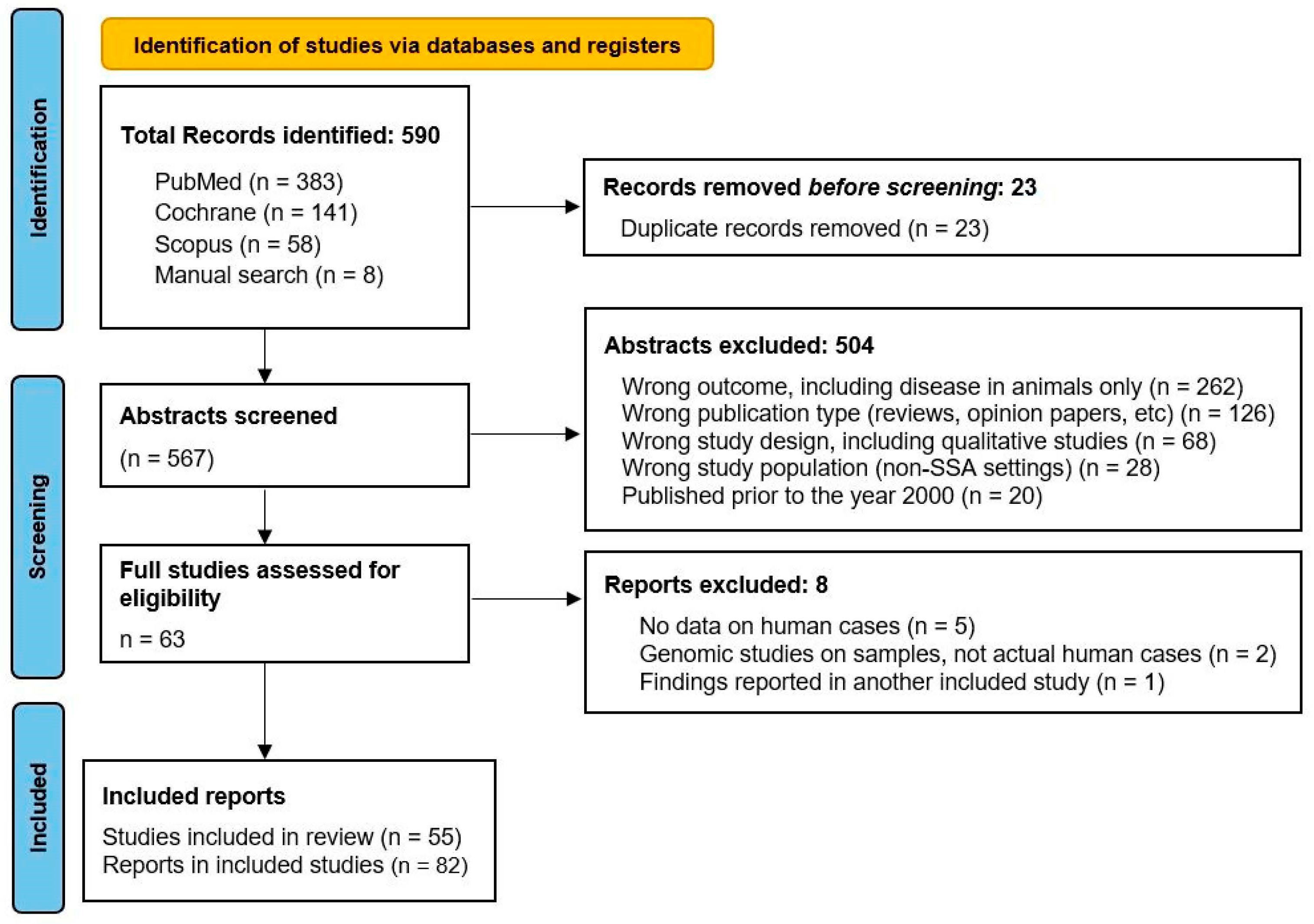
2.2. Screening and Data Extraction
2.3. Definition of Terms
2.4. Data Analysis
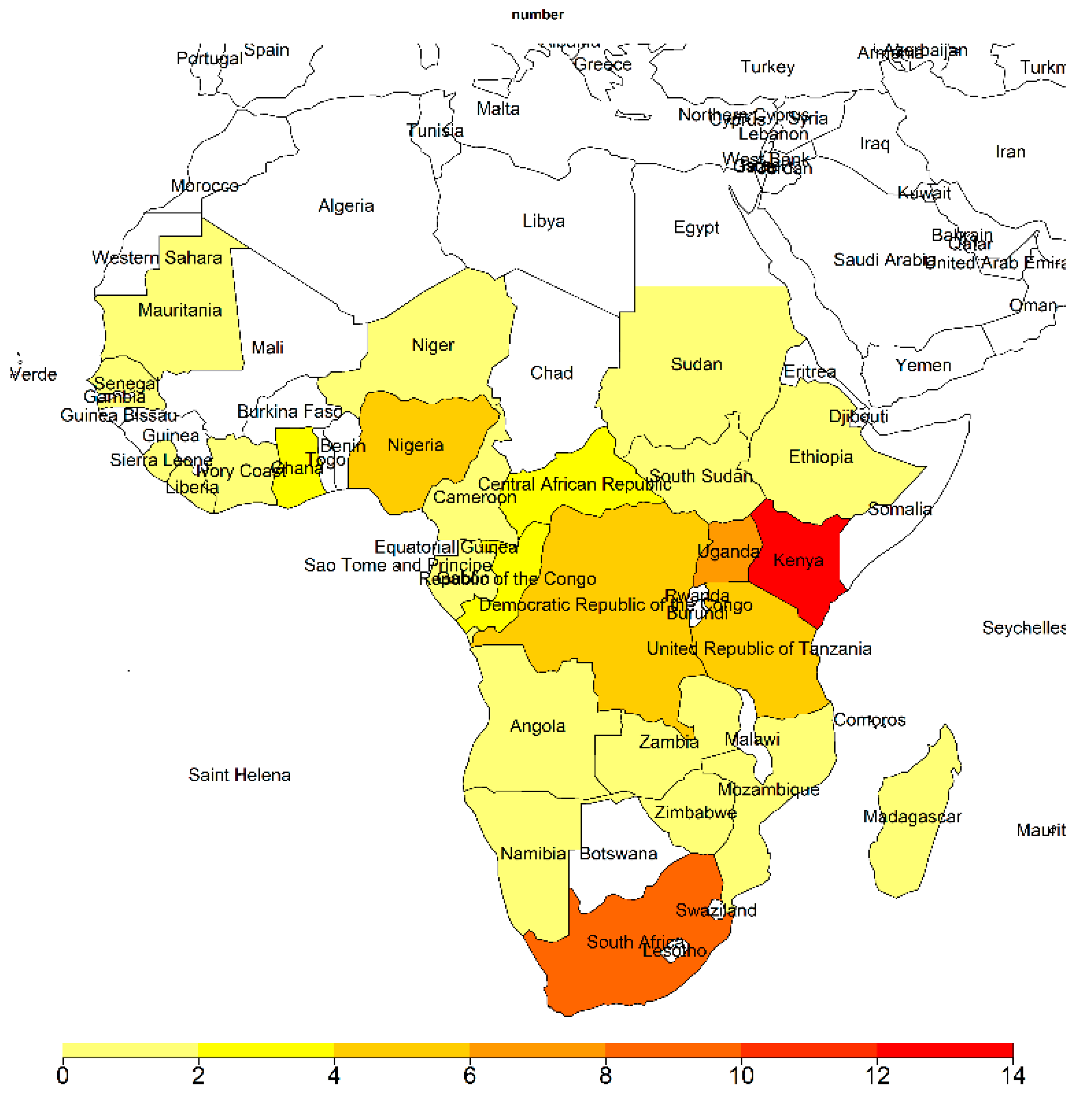
3. Results
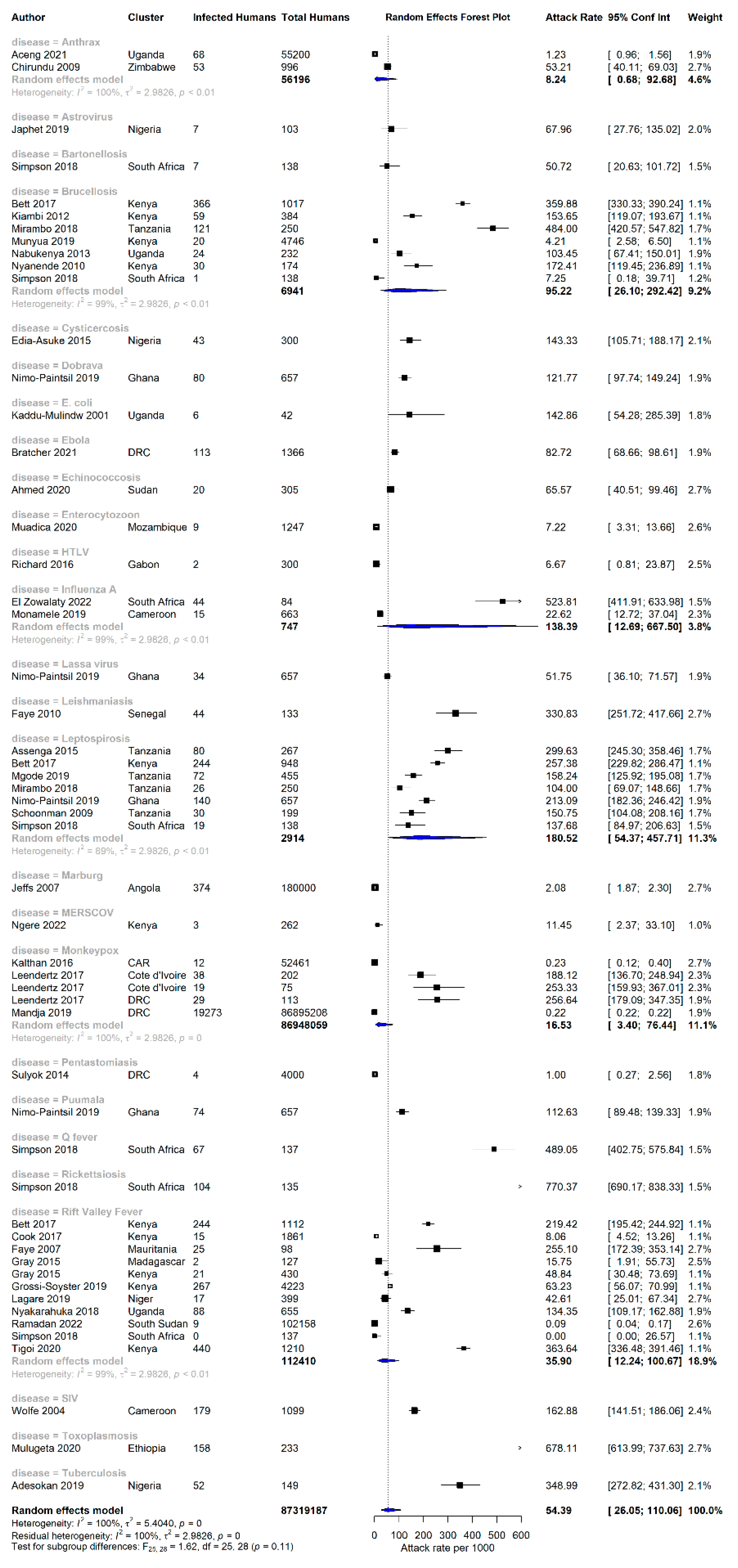
3.1. Attack Rate of Zoonotic Diseases in Sub-Saharan Africa
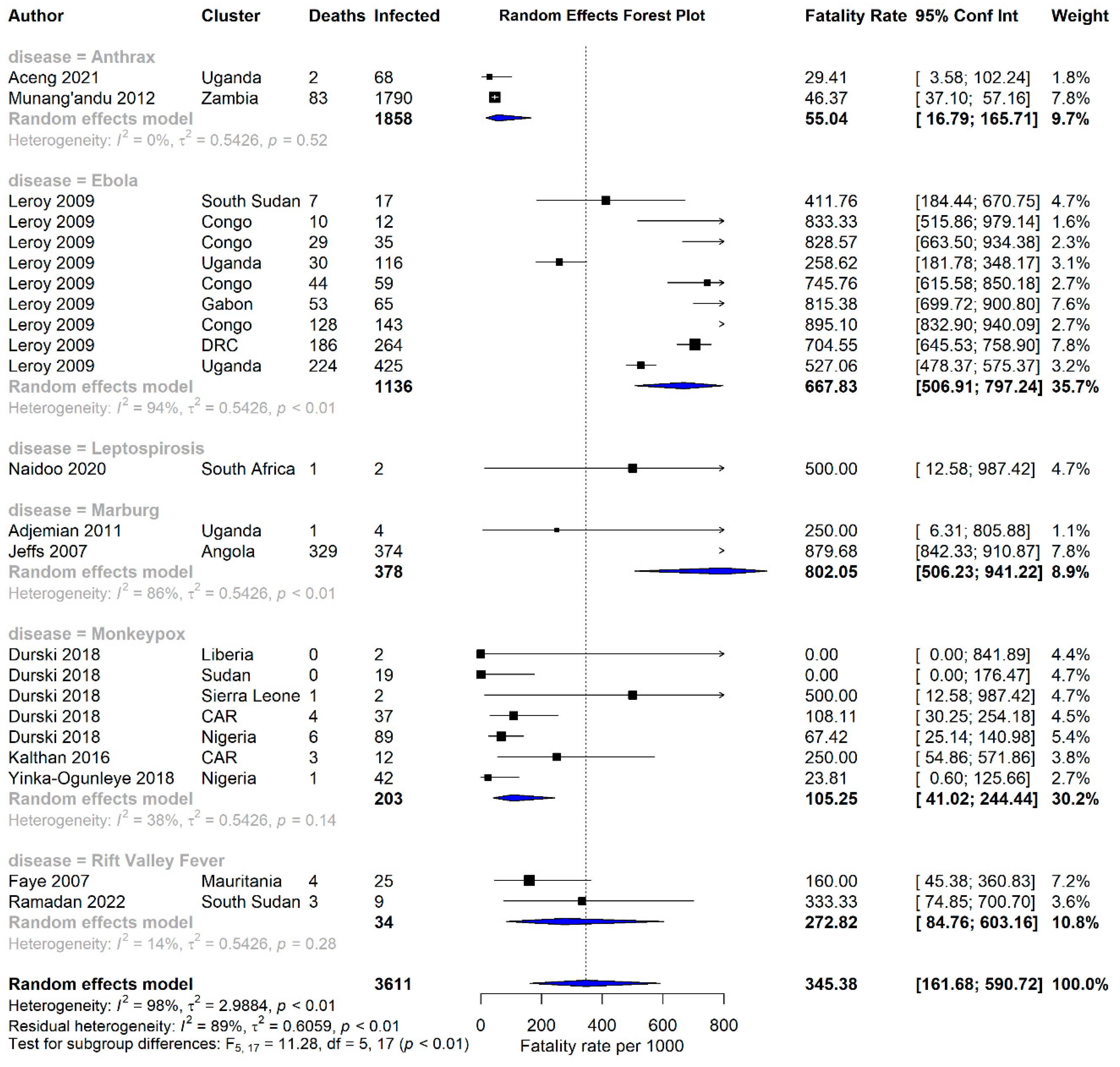
3.2. Case Fatality Rates of Zoonotic Diseases in Africa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balehegn, M.; Kebreab, E.; Tolera, A.; Hunt, S.; Erickson, P.; Crane, T.A.; Adesogan, A.T. Livestock sustainability research in Africa with a focus on the environment. Anim. Front. 2021, 11, 47–56. [Google Scholar] [CrossRef]
- Wang, L.-F.; Crameri, G. Emerging zoonotic viral diseases. Rev. Sci. Tech. 2014, 33, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Schaechter, M. Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Sharma, A.K.; Dhasmana, N.; Arora, G. Bridging the Gap: Exploring the Connection between Animal and Human Health. Zoonotic Dis. 2023, 3, 176–178. [Google Scholar] [CrossRef]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Smith, D.W. COVID-19: A novel zoonotic disease caused by a coronavirus from China: What we know and what we don’t. Microbiol. Aust. 2020, 41, 45–50. [Google Scholar] [CrossRef]
- Kolahchi, Z.; De Domenico, M.; Uddin, L.Q.; Cauda, V.; Grossmann, I.; Lacasa, L.; Grancini, G.; Mahmoudi, M.; Rezaei, N. COVID-19 and Its Global Economic Impact. Adv. Exp. Med. Biol. 2021, 1318, 825–837. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. Monkeypox 2022 outbreak: An update. Trop. Med. Int. Health 2022, 27, 604–605. [Google Scholar] [CrossRef]
- Cho, C.T.; Wenner, H.A. Monkeypox virus. Bacteriol. Rev. 1973, 37, 1–18. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- Moyo, E.; Mhango, M.; Moyo, P.; Dzinamarira, T.; Chitungo, I.; Murewanhema, G. Emerging infectious disease outbreaks in Sub-Saharan Africa: Learning from the past and present to be better prepared for future outbreaks. Front. Public Health 2023, 11. Available online: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1049986 (accessed on 30 June 2023). [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Aceng, F.L.; Ario, A.R.; Alitubeera, P.H.; Neckyon, M.M.; Kadobera, D.; Sekamatte, M.; Okethwangu, D.; Bulage, L.; Harris, J.R.; Nguma, W.; et al. Cutaneous anthrax associated with handling carcasses of animals that died suddenly of unknown cause: Arua District, Uganda, January 2015–August 2017. PLoS Negl. Trop. Dis. 2021, 15, e0009645. [Google Scholar] [CrossRef]
- Adesokan, H.K.; Akinseye, V.O.; Streicher, E.M.; Van Helden, P.; Warren, R.M.; Cadmus, S.I. Reverse zoonotic tuberculosis transmission from an emerging Uganda I strain between pastoralists and cattle in South-Eastern Nigeria. BMC Vet. Res. 2019, 15, 437. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Farnon, E.C.; Tschioko, F.; Wamala, J.F.; Byaruhanga, E.; Bwire, G.S.; Kansiime, E.; Kagirita, A.; Ahimbisibwe, S.; Katunguka, F.; et al. Outbreak of Marburg Hemorrhagic Fever among Miners in Kamwenge and Ibanda Districts, Uganda, 2007. J. Infect. Dis. 2011, 204, S796–S799. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Abdalla, S.S.; Adam, I.A.; Grobusch, M.P.; Aradaib, I.E. Prevalence of cystic echinococcosis and associated risk factors among humans in Khartoum State, Central Sudan. Int. Health 2021, 13, 327–333. [Google Scholar] [CrossRef]
- Ari, M.D.; Guracha, A.; Fadeel, M.A.; Njuguna, C.; Njenga, M.K.; Kalani, R.; Abdi, H.; Warfu, O.; Omballa, V.; Tetteh, C.; et al. Challenges of establishing the correct diagnosis of outbreaks of acute febrile illnesses in Africa: The case of a likely Brucella outbreak among nomadic pastoralists, northeast Kenya, March–July 2005. Am. J. Trop. Med. Hyg. 2011, 85, 909–912. [Google Scholar] [CrossRef]
- Assenga, J.A.; Matemba, L.E.; Muller, S.K.; Mhamphi, G.G.; Kazwala, R.R. Predominant Leptospiral Serogroups Circulating among Humans, Livestock and Wildlife in Katavi-Rukwa Ecosystem, Tanzania. PLoS Negl. Trop. Dis. 2015, 9, e0003607. [Google Scholar] [CrossRef]
- Bardonnet, K.; Piarroux, R.; Dia, L.; Schneegans, F.; Beurdeley, A.; Godot, V.; Vuitton, D.A. Combined eco-epidemiological and molecular biology approaches to assess Echinococcus granulosus transmission to humans in Mauritania: Occurrence of the ‘camel’ strain and human cystic echinococcosis. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Besombes, C.; Gonofio, E.; Konamna, X.; Selekon, B.; Gessain, A.; Berthet, N.; Manuguerra, J.C.; Fontanet, A.; Nakouné, E. Intrafamily Transmission of Monkeypox Virus, Central African Republic, 2018. Emerg. Infect. Dis. 2019, 25, 1602–1604. [Google Scholar] [CrossRef]
- Bett, B.; Said, M.Y.; Sang, R.; Bukachi, S.; Wanyoike, S.; Kifugo, S.C.; Otieno, F.; Ontiri, E.; Njeru, I.; Lindahl, J.; et al. Effects of flood irrigation on the risk of selected zoonotic pathogens in an arid and semi-arid area in the eastern Kenya. PLoS ONE 2017, 12, e0172626. [Google Scholar] [CrossRef]
- Bratcher, A.; Hoff, N.A.; Doshi, R.H.; Gadoth, A.; Halbrook, M.; Mukadi, P.; Musene, K.; Ilunga-Kebela, B.; Spencer, D.A.; Bramble, M.S.; et al. Zoonotic risk factors associated with seroprevalence of Ebola virus GP antibodies in the absence of diagnosed Ebola virus disease in the Democratic Republic of Congo. PLoS Negl. Trop. Dis. 2021, 15, e0009566. [Google Scholar] [CrossRef] [PubMed]
- Chirundu, D.; Chihanga, S.; Chimusoro, A.; Chirenda, J.; Apollo, T.; Tshimanga, M. Behavioural factors associated with cutaneous anthrax in Musadzi area of Gokwe North, Zimbabwe. Cent. Afr. J. Med. 2009, 55, 50–54. [Google Scholar] [CrossRef]
- Cook, E.A.J.; Grossi-Soyster, E.N.; De Glanville, W.A.; Thomas, L.F.; Kariuki, S.; Bronsvoort, B.M.D.C.; Wamae, C.N.; LaBeaud, A.D.; Fevre, E.M. The sero-epidemiology of Rift Valley fever in people in the Lake Victoria Basin of western Kenya. PLoS Negl. Trop. Dis. 2017, 11, e0005731. [Google Scholar] [CrossRef] [PubMed]
- Durski, K.N.; McCollum, A.M.; Nakazawa, Y.; Petersen, B.W.; Reynolds, M.G.; Briand, S.; Djingarey, M.H.; Olson, V.; Damon, I.K.; Khalakdina, A. Emergence of monkeypox in West Africa and Central Africa, 1970–2017. Wkly. Epidemiol. Rec. 2018, 93, 125–132. [Google Scholar] [CrossRef]
- Edia-Asuke, A.U.; Inabo, H.I.; Mukaratirwa, S.; Umoh, V.J.; Whong, C.M.; Asuke, S.; Ella, E.E. Seroprevalence of human cysticercosis and its associated risk factors among humans in areas of Kaduna metropolis, Nigeria. J. Infect. Dev. Ctries 2015, 9, 799–805. [Google Scholar] [CrossRef][Green Version]
- El Zowalaty, M.E.; Abdelgadir, A.; Borkenhagen, L.K.; Ducatez, M.F.; Bailey, E.S.; Gray, G.C. Influenza A viruses are likely highly prevalent in South African swine farms. Transbounding Emerg. Dis. 2022, 69, 2373–2383. [Google Scholar] [CrossRef]
- Faye, O.; Diallo, M.; Diop, D.; Bezeid, O.E.; Bâ, H.; Niang, M.; Dia, I.; Mohamed, S.A.O.; Ndiaye, K.; Diallo, D.; et al. Rift Valley Fever Outbreak with East-Central African Virus Lineage in Mauritania, 2003. Emerg. Infect. Dis. 2007, 13, 1016–1023. [Google Scholar] [CrossRef]
- Faye, B.; Bañuls, A.L.; Bucheton, B.; Dione, M.M.; Bassanganam, O.; Hide, M.; Dereure, J.; Choisy, M.; Ndiaye, J.L.; Konate, O.; et al. Canine visceral leishmaniasis caused by Leishmania infantum in Senegal: Risk of emergence in humans? Microbes Infect. 2010, 12, 1219–1225. [Google Scholar] [CrossRef]
- Gray, G.C.; Anderson, B.D.; LaBeaud, A.D.; Heraud, J.-M.; Fèvre, E.M.; Andriamandimby, S.F.; Cook, E.A.; Dahir, S.; De Glanville, W.A.; Heil, G.L.; et al. Seroepidemiological Study of Interepidemic Rift Valley Fever Virus Infection Among Persons with Intense Ruminant Exposure in Madagascar and Kenya. Am. J. Trop. Med. Hyg. 2015, 93, 1364–1370. [Google Scholar] [CrossRef]
- Grossi-Soyster, E.N.; Lee, J.; King, C.H.; LaBeaud, A.D. The influence of raw milk exposures on Rift Valley fever virus transmission. PLoS Negl. Trop. Dis. 2019, 13, e0007258. [Google Scholar] [CrossRef]
- Hikufe, E.H.; Freuling, C.M.; Athingo, R.; Shilongo, A.; Ndevaetela, E.-E.; Helao, M.; Shiindi, M.; Hassel, R.; Bishi, A.; Khaiseb, S.; et al. Ecology and epidemiology of rabies in humans, domestic animals and wildlife in Namibia, 2011–2017. PLoS Negl. Trop. Dis. 2019, 13, e0007355. [Google Scholar] [CrossRef]
- Jansen Van Vuren, P.; Kgaladi, J.; Patharoo, V.; Ohaebosim, P.; Msimang, V.; Nyokong, B.; Paweska, J.T. Human Cases of Rift Valley Fever in South Africa, 2018. Vector-Borne Zoonotic Dis. 2018, 18, 713–715. [Google Scholar] [CrossRef]
- Japhet, M.O.; Famurewa, O.; Adesina, O.A.; Opaleye, O.O.; Wang, B.; Höhne, M.; Bock, C.T.; Marques, A.M.; Niendorf, S. Viral gastroenteritis among children of 0–5 years in Nigeria: Characterization of the first Nigerian aichivirus, recombinant noroviruses and detection of a zoonotic astrovirus. J. Clin. Virol. 2019, 111, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, B.; Roddy, P.; Weatherill, D.; De La Rosa, O.; Dorion, C.; Iscla, M.; Grovas, I.; Palma, P.P.; Villa, L.; Bernal, O.; et al. The Médecins Sans Frontières Intervention in the Marburg Hemorrhagic Fever Epidemic, Uige, Angola, 2005. I. Lessons Learned in the Hospital. J. Infect. Dis. 2007, 196, S154–S161. [Google Scholar] [CrossRef] [PubMed]
- Kaddu-Mulindwa, D. Occurrence of Shiga toxin-producing Escherichia coli in fecal samples from children with diarrhea and from healthy zebu cattle in Uganda. Int. J. Food Microbiol. 2001, 66, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kalthan, E.; Dondo-Fongbia, J.P.; Yambele, S.; Dieu-Creer, L.R.; Zepio, R.; Pamatika, C.M. Epidémie de 12 cas de maladie à virus monkeypox dans le district de Bangassou en République Centrafricaine en décembre 2015. Bull. Soc. Pathol. Exot. 2016, 109, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kiambi, S.G. Prevalence and Factors Associated with Brucellosis among Febrile Patients Attending Ijara District Hospital, Kenya. Master’s Thesis, Jomo Kenyatta University of Agriculture and Technology, Juja, Kenya, 2012. Available online: http://ir.jkuat.ac.ke/bitstream/handle/123456789/1415/Kiambi,%20Stella%20Gaichugi%20%E2%80%93Msc%20Applied%20Epidemiology%20-2012.pdf (accessed on 15 March 2023).
- Lagare, A.; Fall, G.; Ibrahim, A.; Ousmane, S.; Sadio, B.; Abdoulaye, M.; Alhassane, A.; Mahaman, A.E.; Issaka, B.; Sidikou, F.; et al. First occurrence of Rift Valley fever outbreak in Niger, 2016. Vet. Med. Sci. 2019, 5, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Leendertz, S.; Stern, D.; Theophil, D.; Anoh, E.; Mossoun, A.; Schubert, G.; Wiersma, L.; Akoua-Koffi, C.; Couacy-Hymann, E.; Muyembe-Tamfum, J.J.; et al. A Cross-Sectional Serosurvey of Anti-Orthopoxvirus Antibodies in Central and Western Africa. Viruses 2017, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Epelboin, A.; Mondonge, V.; Pourrut, X.; Gonzalez, J.-P.; Muyembe-Tamfum, J.-J.; Formenty, P. Human Ebola Outbreak Resulting from Direct Exposure to Fruit Bats in Luebo, Democratic Republic of Congo, 2007. Vector-Borne Zoonotic Dis. 2009, 9, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Mandja, B.-A.M.; Brembilla, A.; Handschumacher, P.; Bompangue, D.; Gonzalez, J.-P.; Muyembe, J.-J.; Mauny, F. Temporal and Spatial Dynamics of Monkeypox in Democratic Republic of Congo, 2000–2015. EcoHealth 2019, 16, 476–487. [Google Scholar] [CrossRef]
- Mgode, G.F.; Japhary, M.M.; Mhamphi, G.G.; Kiwelu, I.; Athaide, I.; Machang’u, R.S. Leptospirosis in sugarcane plantation and fishing communities in Kagera northwestern Tanzania. PLoS Negl. Trop. Dis. 2019, 13, e0007225. [Google Scholar] [CrossRef] [PubMed]
- Mirambo, M.M.; Mgode, G.F.; Malima, Z.O.; John, M.; Mngumi, E.B.; Mhamphi, G.G.; Mshana, S.E. Seropositivity of Brucella spp. and Leptospira spp. antibodies among abattoir workers and meat vendors in the city of Mwanza, Tanzania: A call for one health approach control strategies. PLoS Negl. Trop. Dis. 2018, 12, e0006600. [Google Scholar] [CrossRef] [PubMed]
- Monamele, C.G.; Y., P.; Karlsson, E.A.; Vernet, M.-A.; Wade, A.; Okomo, M.-C.A.; Abah, A.S.A.; Yann, S.; Etoundi, G.A.M.; Mohamadou, N.R.; et al. Evidence of exposure and human seroconversion during an outbreak of avian influenza A(H5N1) among poultry in Cameroon. Emerg. Microbes Infect. 2019, 8, 186–196. [Google Scholar] [CrossRef]
- Muadica, A.S.; Messa, A.E.; Dashti, A.; Balasegaram, S.; Santin, M.; Manjate, F.; Chirinda, P.; Garrine, M.; Vubil, D.; Acácio, S.; et al. First identification of genotypes of Enterocytozoon bieneusi (Microsporidia) among symptomatic and asymptomatic children in Mozambique. PLoS Negl. Trop. Dis. 2020, 14, e0008419. [Google Scholar] [CrossRef]
- Mulugeta, S.; Munshea, A.; Nibret, E. Seroprevalence of Anti–Toxoplasma gondii Antibodies and Associated Factors among Pregnant Women Attending Antenatal Care at Debre Markos Referral Hospital, Northwest Ethiopia. Infect. Dis 2020, 13, 117863372094887. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Banda, F.; Siamudaala, V.M.; Munyeme, M.; Kasanga, C.J.; Hamududu, B. The effect of seasonal variation on anthrax epidemiology in the upper Zambezi floodplain of western Zambia. J. Vet. Sci. 2012, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Munyua, P.; Osoro, E.; Hunsperger, E.; Ngere, I.; Muturi, M.; Mwatondo, A.; Marwanga, D.; Ngere, P.; Tiller, R.; Onyango, C.O.; et al. High incidence of human brucellosis in a rural Pastoralist community in Kenya, 2015. PLoS Neglected Trop. Dis. 2021, 15, e0009049. [Google Scholar] [CrossRef] [PubMed]
- Nabukenya, I.; Kaddu-Mulindwa, D.; Nasinyama, G.W. Survey of Brucellainfection and malaria among Abattoir workers in Kampala and Mbarara Districts, Uganda. BMC Public Health 2013, 13, 901. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Moseley, M.; McCarthy, K.; Chingonzoh, R.; Lawrence, C.; Setshedi, G.M.; Frean, J.; Rossouw, J. Fatal Rodentborne Leptospirosis in Prison Inmates, South Africa, 2015. Emerg. Infect. Dis. 2020, 26, 1033–1035. [Google Scholar] [CrossRef]
- Nanyende, D.W. Estimation of the Prevalence of Brucellosis in Humans and Livestock in Northern Turkana District, Kenya. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2010. Available online: http://erepository.uonbi.ac.ke/bitstream/handle/11295/19177/Nanyende_Estimation%20of%20the%20prevalence%20of%20brucellosis%20in%20humans%20and%20Livestock%20in%20northern%20Turkana%20district%2C%20Kenya.pdf (accessed on 17 September 2023).
- Ngere, I.; Hunsperger, E.A.; Tong, S.; Oyugi, J.; Jaoko, W.; Harcourt, J.L.; Thornburg, N.J.; Oyas, H.; Muturi, M.; Osoro, E.M.; et al. Outbreak of Middle East Respiratory Syndrome Coronavirus in Camels and Probable Spillover Infection to Humans in Kenya. Viruses 2022, 14, 1743. [Google Scholar] [CrossRef]
- Nimo-Paintsil, S.C.; Fichet-Calvet, E.; Borremans, B.; Letizia, A.G.; Mohareb, E.; Bonney, J.H.K.; Obiri-Danso, K.; Ampofo, W.K.; Schoepp, R.J.; Kronmann, K.C. Rodent-borne infections in rural Ghanaian farming communities. PLoS ONE 2019, 14, e0215224. [Google Scholar] [CrossRef]
- Nyakarahuka, L.; De St Maurice, A.; Purpura, L.; Ervin, E.; Balinandi, S.; Tumusiime, A.; Kyondo, J.; Mulei, S.; Tusiime, P.; Lutwama, J.; et al. Prevalence and risk factors of Rift Valley fever in humans and animals from Kabale district in Southwestern Uganda, 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006412. [Google Scholar] [CrossRef]
- Pembi, E.; Awang, S.; Salaudeen, S.O.; Agaba, I.A.; Omoleke, S. First confirmed case of Monkeypox in Adamawa State, Nigeria: A clinico-epidemiological case report. Pan Afr. Med. J. 2022, 42. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Nakao, R.; Hang’ombe, B.M.; Sato, K.; Kajihara, M.; Kanchela, S.; Changula, K.; Eto, Y.; Ndebe, J.; Sasaki, M.; et al. Human Borreliosis Caused by a New World Relapsing Fever Borrelia–like Organism in the Old World. Clin. Infect. Dis. 2019, 69, 107–112. [Google Scholar] [CrossRef]
- Ramadan, O.P.C.; Berta, K.K.; Wamala, J.F.; Maleghemi, S.; Rumunu, J.; Ryan, C.; Ladu, A.I.; Joseph, J.L.K.; Abenego, A.A.; Ndenzako, F.; et al. Analysis of the 2017–2018 Rift valley fever outbreak in Yirol East County, South Sudan: A one health perspective. Pan Afr. Med. J. 2022, 42, 5. [Google Scholar] [CrossRef]
- Richard, L.; Mouinga-Ondémé, A.; Betsem, E.; Filippone, C.; Nerrienet, E.; Kazanji, M.; Gessain, A. Zoonotic Transmission of Two New Strains of Human T-lymphotropic Virus Type 4 in Hunters Bitten by a Gorilla in Central Africa. Clin. Infect. Dis. 2016, 63, 800–803. [Google Scholar] [CrossRef]
- Schoonman, L.; Swai, E.S. Risk factors associated with the seroprevalence of leptospirosis, amongst at-risk groups in and around Tanga city, Tanzania. Ann. Trop. Med. Parasitol. 2009, 103, 711–718. [Google Scholar] [CrossRef]
- Simpson, G.J.G.; Quan, V.; Frean, J.; Knobel, D.L.; Rossouw, J.; Weyer, J.; Marcotty, T.; Godfroid, J.; Blumberg, L.H. Prevalence of Selected Zoonotic Diseases and Risk Factors at a Human-Wildlife-Livestock Interface in Mpumalanga Province, South Africa. Vector-Borne Zoonotic Dis. 2018, 18, 303–310. [Google Scholar] [CrossRef]
- Sulyok, M.; Rózsa, L.; Bodó, I.; Tappe, D.; Hardi, R. Ocular Pentastomiasis in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2014, 8, e3041. [Google Scholar] [CrossRef]
- Tappe, D.; Sulyok, M.; Rózsa, L.; Muntau, B.; Haeupler, A.; Bodó, I.; Hardi, R. Molecular Diagnosis of Abdominal Armillifer grandis Pentastomiasis in the Democratic Republic of Congo. J. Clin. Microbiol. 2015, 53, 2362–2364. [Google Scholar] [CrossRef]
- Teklu, G.G.; Hailu, T.G.; Eshetu, G.R. High Incidence of Human Rabies Exposure in Northwestern Tigray, Ethiopia: A Four-Year Retrospective Study. PLoS Negl. Trop. Dis. 2017, 11, e0005271. [Google Scholar] [CrossRef]
- Tigoi, C.; Sang, R.; Chepkorir, E.; Orindi, B.; Arum, S.O.; Mulwa, F.; Mosomtai, G.; Limbaso, S.; Hassan, O.A.; Irura, Z.; et al. High risk for human exposure to Rift Valley fever virus in communities living along livestock movement routes: A cross-sectional survey in Kenya. PLoS Negl. Trop. Dis. 2020, 14, e0007979. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, N.D.; Switzer, W.M.; Carr, J.K.; Bhullar, V.B.; Shanmugam, V.; Tamoufe, U.; Prosser, A.T.; Torimiro, J.N.; Wright, A.; Mpoudi-Ngole, E.; et al. Naturally acquired simian retrovirus infections in central African hunters. Lancet 2004, 363, 932–937. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Ogoina, D.; Aworabhi, N.; Eteng, W.; Badaru, S.; Mohammed, A.; Agenyi, J.; Etebu, E.N.; Numbere, T.W.; et al. Reemergence of Human Monkeypox in Nigeria, 2017. Emerg. Infect. Dis. 2018, 24, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Paige, S.B.; Frost, S.D.W.; Gibson, M.A.; Jones, J.H.; Shankar, A.; Switzer, W.M.; Ting, N.; Goldberg, T.L. Beyond bushmeat: Animal contact, injury, and zoonotic disease risk in Western Uganda. EcoHealth 2014, 11, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Pigott, D.M.; Golding, N.; Mylne, A.; Huang, Z.; Henry, A.J.; Weiss, D.J.; Brady, O.J.; Kraemer, M.U.; Smith, D.L.; Moyes, C.L.; et al. Mapping the zoonotic niche of Ebola virus disease in Africa. eLife 2014, 3, e04395. [Google Scholar] [CrossRef]
- Bwire, G.; Ario, A.R.; Eyu, P.; Ocom, F.; Wamala, J.F.; Kusi, K.A.; Ndeketa, L.; Jambo, K.C.; Wanyenze, R.K.; Talisuna, A.O. The COVID-19 pandemic in the African continent. BMC Med. 2022, 20, 167. [Google Scholar] [CrossRef] [PubMed]
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primers 2017, 3, 17091. [Google Scholar] [CrossRef] [PubMed]
- Elton, L.; Haider, N.; Kock, R.; Thomason, M.J.; Tembo, J.; Arruda, L.B.; Ntoumi, F.; Zumla, A.; McHugh, T.D.; PANDORA-ID-NET Consortium. Zoonotic disease preparedness in sub-Saharan African countries. One Health Outlook 2021, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Xu, B.; Gutierrez, B.; Mekaru, S.; Sewalk, K.; Goodwin, L.; Loskill, A.; Cohn, E.L.; Hswen, Y.; Hill, S.C.; Cobo, M.M.; et al. Epidemiological data from the COVID-19 outbreak, real-time case information. Sci. Data 2020, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Aldhaeefi, M.; Rungkitwattanakul, D.; Unonu, J.; Franklin, C.-J.; Lyons, J.; Hager, K.; Daftary, M.N. The 2022 human monkeypox outbreak: Clinical review and management guidance. Am. J. Health Syst. Pharm. 2023, 80, 44–52. [Google Scholar] [CrossRef]
- Tabassum, S.; Naeem, F.; Azhar, M.; Naeem, A.; Oduoye, M.O.; Dave, T. Rift Valley fever virus outbreak in Mauritania yet again in 2022: No room for complacency. Health Sci. Rep. 2023, 6, e1278. [Google Scholar] [CrossRef]
- Kadanali, A.; Karagoz, G. An overview of Ebola virus disease. North. Clin. Istanb. 2015, 2, 81–86. [Google Scholar] [CrossRef]
- Ata, I.; Riaz, N.; Mallhi, T.H. Monkeypox: A Rising Health Concern in Pakistan and Wake-Up Call for the Government. Disaster Med. Public Health Prep. 2023, 17, e376. [Google Scholar] [CrossRef]
- Kilpatrick, P.A.M.; Randolph, P.S.E. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012, 380, 1946. [Google Scholar] [CrossRef]
- Maeda, K. Globalization and zoonosis. Nihon Rinsho 2016, 74, 1948–1955. [Google Scholar] [PubMed]
- Thacker, S.B.; Parrish, R.G.; Trowbridge, F.L. A method for evaluating systems of epidemiological surveillance. World Health Stat. Q. 1988, 41, 11–18. [Google Scholar]
- Zhou, S.; Liu, B.; Han, Y.; Wang, Y.; Chen, L.; Wu, Z.; Yang, J. ZOVER: The database of zoonotic and vector-borne viruses. Nucleic Acids Res. 2022, 50, D943–D949. [Google Scholar] [CrossRef]
- Dafale, N.A.; Srivastava, S.; Purohit, H.J. Zoonosis: An Emerging Link to Antibiotic Resistance Under “One Health Approach”. Indian J Microbiol. 2020, 60, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rohr, J.; Cui, R.; Xin, Y.; Han, L.; Yang, X.; Gu, S.; Du, Y.; Liang, J.; Wang, X. Biological invasions facilitate zoonotic disease emergences. Nat. Commun. 2022, 13, 1762. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.J.; Washburne, A.D.; Faust, C.L.; Pulliam, J.R.C.; Mordecai, E.A.; Lloyd-Smith, J.O.; Plowright, R.K. Dynamic and integrative approaches to understanding pathogen spillover. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190014. [Google Scholar] [CrossRef]
- Borremans, B.; Faust, C.; Manlove, K.R.; Sokolow, S.H.; Lloyd-Smith, J.O. Cross-species pathogen spillover across ecosystem boundaries: Mechanisms and theory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180344. [Google Scholar] [CrossRef]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Vora, N.M.; Hannah, L.; Walzer, C.; Vale, M.M.; Lieberman, S.; Emerson, A.; Jennings, J.; Alders, R.; Bonds, M.H.; Evans, J.; et al. Interventions to Reduce Risk for Pathogen Spillover and Early Disease Spread to Prevent Outbreaks, Epidemics, and Pandemics. Emerg. Infect. Dis. J. 2023, 29. [Google Scholar] [CrossRef]
- FAO; WOAH; UNEP; WHO. Quadripartite Memorandum of Understanding (MoU) Signed for a New Era of One Health Collaboration. 2022. Available online: https://www.fao.org/3/cb9403en/cb9403en.pdf (accessed on 17 September 2023).
| Zoonotic Disease (Countries) | Number of Reports | Studies Conducted in Outbreak Setting | Total Number of Human Infections Reported | Total Number of Human Deaths Reported | Total Number of Animal Infections | Animal Reservoirs Identified | Identified Risk Factors |
|---|---|---|---|---|---|---|---|
| Anthrax (Uganda, Zambia, Zimbabwe) | 3 [18,28,53] | 3 (100%) | 1911 | 85 | Cattle | Handling raw meat/cattle carcasses | |
| Astrovirus (Nigeria) | 1 [39] | 0 (0.0%) | 7 | ||||
| Bartonellosis (South Africa) | 1 [66] | 0 (0.0%) | 7 | ||||
| Borreliosis (Zambia) | 1 [62] | 0 (0.0%) | 1 | 64 | |||
| Brucellosis (Kenya, South Africa, Tanzania, Uganda) | 8 [22,26,43,49,54,55,57,66] | 1 (12.5%) | 630 | 74 | Cattle | Handling raw meat | |
| Cysticercosis (Nigeria) | 1 [31] | 0 (0.0%) | 43 | Pigs | |||
| Dobrava (Ghana) | 1 [59] | 0 (0.0%) | 80 | Rodents | |||
| Escherichia. coli (Uganda) | 1 [41] | 0 (0.0%) | 6 | 45 | Cattle | ||
| Ebola (Congo, DRC, Gabon, South Sudan, Uganda) | 10 [27,46] | 9 (90.0%) | 1249 | 711 | Bats, gorillas, monkeys | Contact with rodents | |
| Echinococcosis (Mauritania, Sudan) | 2 [21,24] | 0 (0.0%) | 23 | 22 | Dogs, camels | ||
| Enterocytozoon (Mozambique) | 1 [51] | 0 (0.0%) | 9 | ||||
| HTLV (Gabon) | 1 [64] | 0 (0.0%) | 2 | 2 | Gorillas | ||
| Influenza A (Cameroon, South Africa) | 2 [32,50] | 1 (50.0%) | 59 | 64 | Poultry, pig | ||
| Lassa virus (Ghana) | 1 [59] | 0 (0.0%) | 34 | 2 | Rodents | ||
| Leishmaniasis (Senegal) | 1 [34] | 0 (0.0%) | 44 | 57 | Dogs | ||
| Leptospirosis (Ghana, Kenya, South Africa, Tanzania) | 9 [22,23,26,48,49,56,59,65,66] | 1 (11.1%) | 616 | 1 | 347 | Cattle, rats, rodents | Milking, exposure to contaminated water |
| Marburg (Angola, Uganda) | 2 [20,40] | 2 (100%) | 378 | 330 | 31 | Bats | |
| MERSCOV (Kenya) | 1 [58] | 1 (100%) | 3 | 124 | Camel | ||
| Monkeypox (CAR, Cote d’Ivoire, DRC, Liberia, Nigeria, Sierra Leone, Sudan) | 13 [25,30,42,45,47,61,72] | 10 (76.9%) | 19,767 | 15 | Unknown (Monkeys?) | Contact with live or dead animals | |
| Pentastomiasis (DRC) | 2 [67,68] | 0 (0.0%) | 6 | Snakes | Cooking/eating snake meat | ||
| Puumala (Ghana) | 1 [59] | 0 (0.0%) | 74 | Rodents | |||
| Q fever (South Africa) | 1 [66] | 0 (0.0%) | 67 | Cattle | |||
| Rabies (Ethiopia, Namibia) | 2 [37,69] | 0 (0.0%) | 2293 | 1907 | Kudus, jackals, dogs, cattle, cats, elands, goats | Animal bites | |
| Rickettsiosis (South Africa) | 1 [66] | 0 (0.0%) | 104 | Exposure to dogs? | |||
| Rift Valley Fever (Kenya, Madagascar, Mauritania, Niger, South Africa, South Sudan, Uganda) | 12 [26,29,33,35,36,38,44,60,63,66,70] | 3 (25.0%) | 1132 | 40 | 191 | Cattle, livestock, cow | Milking, handling raw meat, animal birthing/slaughtering |
| SIV (Cameroon) | 1 [71] | 0 (0.0%) | 179 | Exposure to fresh primate blood | |||
| Toxoplasmosis (Ethiopia) | 1 [52] | 0 (0.0%) | 158 | Cats | Exposure to cats | ||
| Tuberculosis (Nigeria) | 1 [19] | 0 (0.0%) | 52 | 2 | Cattle | ||
| OVERALL | 82 | 31 (37.8%) | 28,934 | 1182 | 2932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ateudjieu, J.; Siewe Fodjo, J.N.; Ambomatei, C.; Tchio-Nighie, K.H.; Zoung Kanyi Bissek, A.-C. Zoonotic Diseases in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Zoonotic Dis. 2023, 3, 251-265. https://doi.org/10.3390/zoonoticdis3040021
Ateudjieu J, Siewe Fodjo JN, Ambomatei C, Tchio-Nighie KH, Zoung Kanyi Bissek A-C. Zoonotic Diseases in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Zoonotic Diseases. 2023; 3(4):251-265. https://doi.org/10.3390/zoonoticdis3040021
Chicago/Turabian StyleAteudjieu, Jérôme, Joseph Nelson Siewe Fodjo, Calson Ambomatei, Ketina Hirma Tchio-Nighie, and Anne-Cecile Zoung Kanyi Bissek. 2023. "Zoonotic Diseases in Sub-Saharan Africa: A Systematic Review and Meta-Analysis" Zoonotic Diseases 3, no. 4: 251-265. https://doi.org/10.3390/zoonoticdis3040021
APA StyleAteudjieu, J., Siewe Fodjo, J. N., Ambomatei, C., Tchio-Nighie, K. H., & Zoung Kanyi Bissek, A.-C. (2023). Zoonotic Diseases in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Zoonotic Diseases, 3(4), 251-265. https://doi.org/10.3390/zoonoticdis3040021








