Perception and Risk Factors Associated with Tuberculosis in the Manyara Region, Tanzania
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
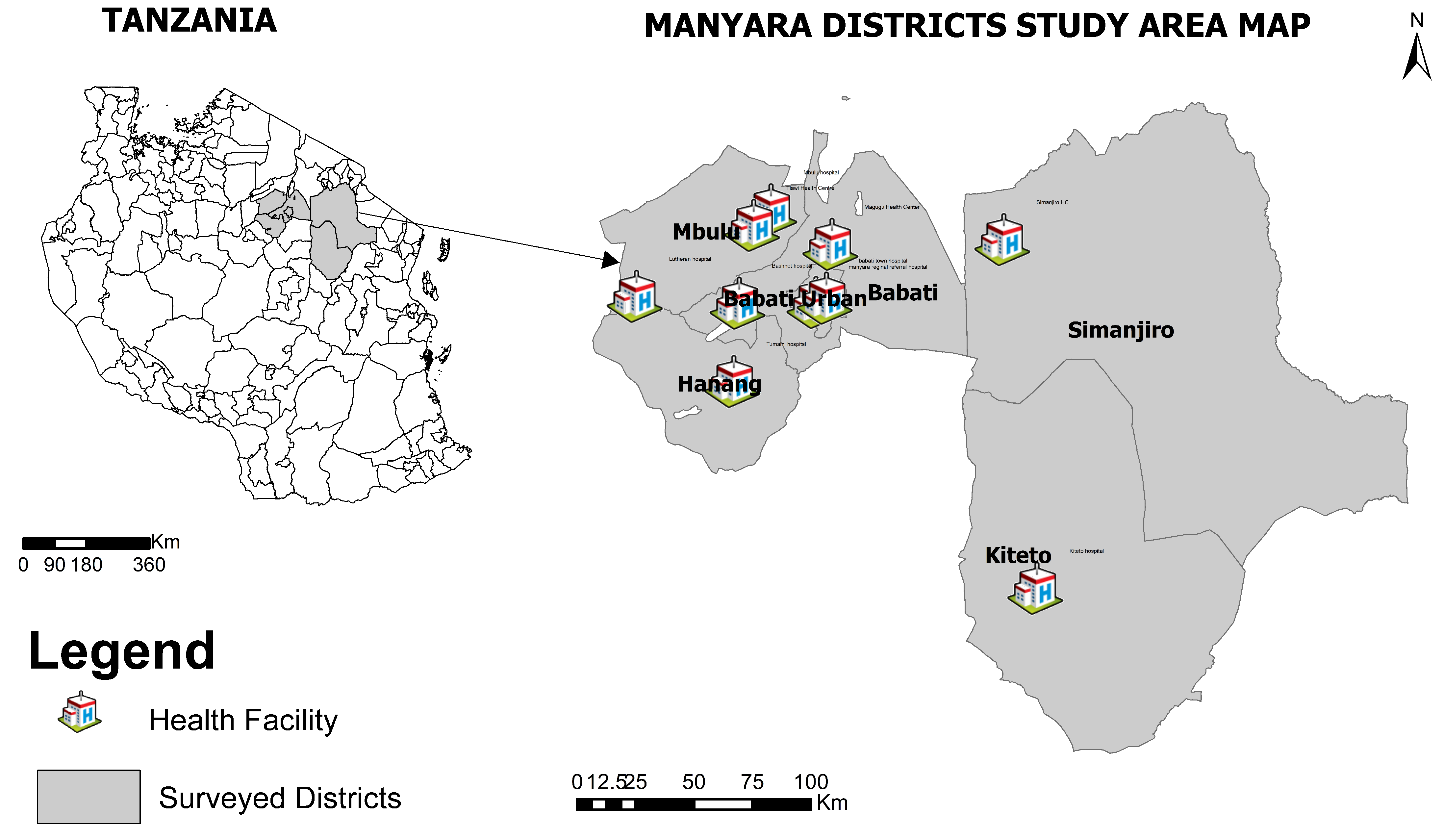
2.1. Study Design and Participant Recruitment
2.2. Sample Size
2.3. Specimen Collection
2.4. Questionnaire Data Collection
2.5. Laboratory Analysis
2.5.1. Detection of Samples That Are Positive for Mycobacteria Culture
2.5.2. Confirmatory Test for Positive Culture Identification
2.5.3. MTBC Speciation with PCR
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics of the Studied Population
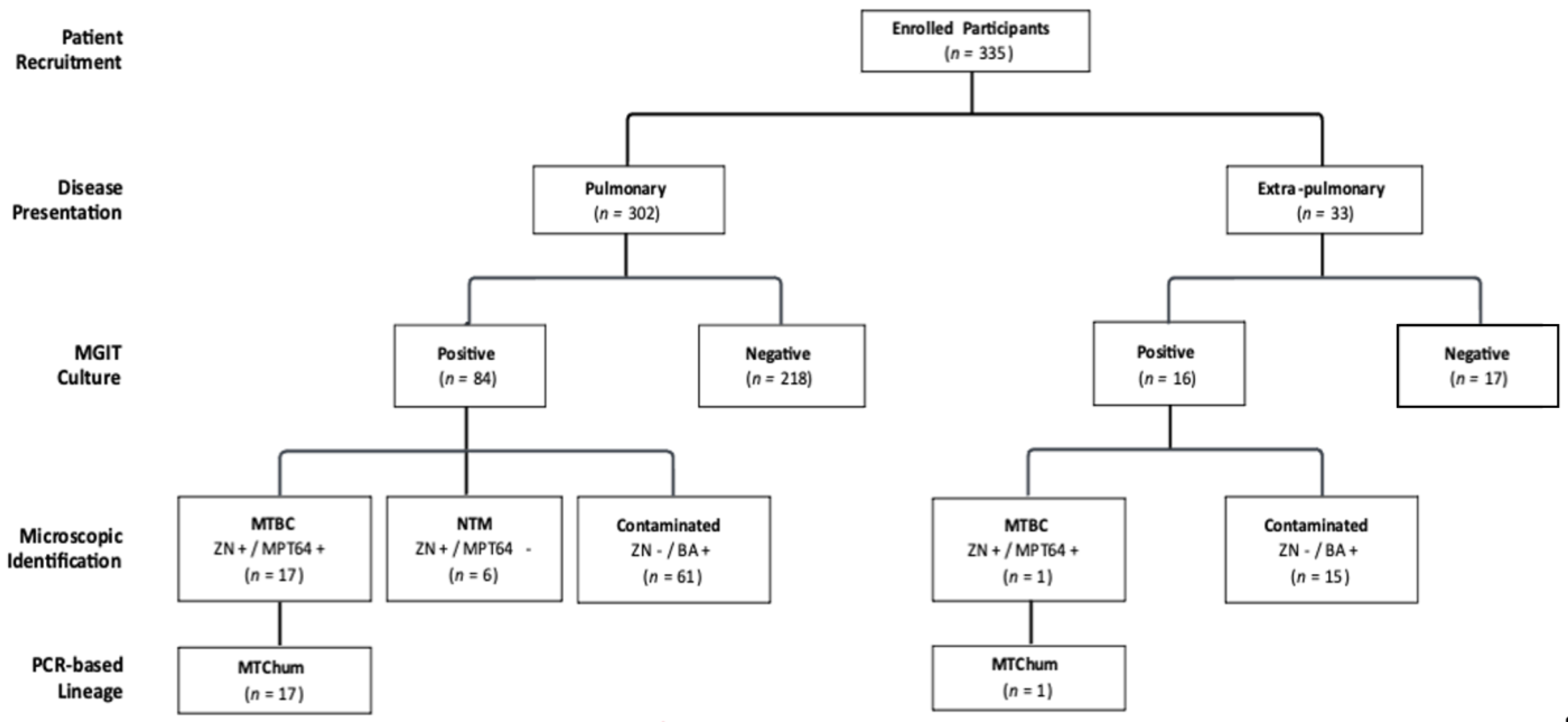
3.2. Recovery and Identification of Mycobacterium Species from the Target Population
3.3. TB Knowledge and Level of Awareness
3.4. Practices That Increase the Risk of Zoonotic TB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Risk Factor Group | General Description |
|---|---|
| General | Sex, age, marital status, level of education, occupation, tribe, and residence, |
| Animal management | Herd type, herd size, husbandry system, animal type and breed, feeding practices, and body condition of animals. |
| Animal ownership and caretaking | Reason, sources of cattle, number and type of cattle, other livestock, reasons and rate of exploitation of herds, size of household (involved in animal business). |
| Housing of animals (especially at nights), Contact with animal (human–animal interactions) | Type and degree (duration)of interaction with cattle, contact with other livestock, consumption of unpasteurized milk or milk products, eat raw meat, keeping other animals, abattoirs, cattle markets, vaccination campaign, communal dips. |
| Contacts of owned cattle with other cattle (animal–animal interactions) | Use of same bulls for breeding (group bull), contact with other livestock, transhumance, communal grazing, cattle market, going to or coming from cattle market, vaccination centers, drinking spots, communal dips. |
| Awareness and recognition of human TB | Previous contact/exposure to TB cases/knowledge, mode of transmission (milk, meat, aerosols), humans affected by bovine TB |
| Clinical signs in humans and detection | Clinical symptoms including fever, cough with expectoration, chills, night sweats, chest pain, abdominal pain, body ache, weight loss, loss of appetite? Detection by acid fast bacilli (AFB) smear and culture of appropriate sputum samples and chest X-ray |
| Awareness and recognition of TB in animals | Previous contact/knowledge, Veterinary service, know bovine TB is zoonotic, mode of transmission (milk, meat, aerosol), cattle be affected by human TB |
| Vaccination programs The request of veterinary services | Vaccines? Routine vaccination? Reasons for veterinary attention, sick animals, average number of veterinary visits per year. |
| Clinical signs in animals and bovine TB detection | Low productivity, weak, emaciated or diseased? Diagnostic methods? Screening frequency, testing service/agency, awareness and implementation of bovine TB control law, action after test (if positive result), acceptance of routine testing, payment for bovine TB test. |
| Parameters | Concentration | Volume |
|---|---|---|
| Forward Primer | 100 µM | 20 µL |
| Reverse Primer | 100 µM | 20l µL |
| Probe | 100 µM | 10 µL |
| Sterile water | 50l µL | |
| Total | 40× | 100l µL |
| Component | Final Concentration | Volume per Reaction |
|---|---|---|
| Prime Time Gene Expression Master | 1× | 10 µL |
| Mix (2×) with ROX dye | 1× | 0.5 µL |
| prime Time qPCR Assay 1 (40×) | 1× | 0.5 µL |
| prime Time qPCR Assay 2 (40×) | 1× | 0.5 µL |
| prime Time qPCR Assay 3 (40×) | 1× | 0.5 µL |
| DNA template (with internal control) | 1 µL | |
| Nuclease free water | 7.5 µL | |
| Total | 20 µL |
| Step | Temp | Time | Cycles |
|---|---|---|---|
| Polymerase activation | 95 °C | 3 min | 1 |
| Denaturation | 95 °C | 15 s | 40 |
| Annealing/Extension | 63 °C | 1 min |
| Assay | Primer | Primer Sequence | Tm |
|---|---|---|---|
| IS1081 | IS1081_F | GGCTGCTCTCGACGTTCATC | 58.2 |
| IS1081_R | CGCTGATTGGACCGCTCAT | 58 | |
| IS1081_P | CTGAAGCCGACGCCCTGTGC | 63.8 | |
| MTCAni | MTCAni_F | GGTTTCTCTTCAACGTCTTGCT | 55.4 |
| MTCAni_R | CCGTCCCACGGCTTTGG | 59.6 | |
| MTCAni_P | CGGCTGTGCGATCTTCACCGTGAA | 63.5 | |
| MTCHum | MTCHum_F | CGGTGTTTCTCATGCACGTCTC | 58.3 |
| MTCHum_R | CGTCGCCTTGATCATCGAAAT | 55.5 | |
| MTCHum_P | TTACCACGCTGACCCACACCGT | 63.1 | |
| Mbov | Mbov_F | AGCCGTAGTCGTGCAGAA | 56.4 |
| Mbov_R | CCCGTAGCGTTACTGAGAAATTG | 55.7 | |
| Mbov_P | CAACACTCTTGGAGTGGCCTACAACG | 61.3 | |
| McapRT | Mcap_F | ACCGTGCGGATCTTG | 52.9 |
| Mcap_R | CATGGAGATCACCCGT | 52 | |
| Mcap_P | TATCGGGTACACAAAGACGA | 56 | |
| Morg | Morg_F | ATTGTCGCGCCGAGACTG | 58.2 |
| Morg_R | GTACCATCTTGGCCGAGCTG | 58.2 | |
| Morg_P | CGTCCTCGGCTGACCC | 58.6 |
References
- World Health Organization. Global Tuberculosis Report 2022. Available online: https://reliefweb.int/report/world/global-tuberculosis-report-2022 (accessed on 15 May 2023).
- Huard, R.C.; Fabre, M.; de Haas, P.; Lazzarini, L.C.O.; van Soolingen, D.; Cousins, D.; Ho, J.L. Novel genetic polymorphisms that further delineate the phylogeny of the Mycobacterium tuberculosis complex. J. Bacteriol. 2006, 188, 4271–4287. [Google Scholar] [CrossRef]
- World Health Organization. A Situational Analysis of Programmatic Management of TB Preventive Treatment in the WHO South-East Asia Region. Available online: https://iris.who.int/handle/10665/337381 (accessed on 12 May 2023).
- De la Rua-Domenech, R. Human Mycobacterium bovis infection in the United Kingdom: Incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis 2006, 86, 77–109. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Dürr, S.; Alonso, S.; Hattendorf, J.; Laisse, C.J.; Parsons, S.D.; Van Helden, P.D.; Zinsstag, J. Zoonotic Mycobacterium bovis–induced tuberculosis in humans. Emerg. Infect. Dis. 2013, 19, 899. [Google Scholar] [CrossRef]
- Sichewo, P.R.; Vander Kelen, C.; Thys, S.; Michel, A.L. Risk practices for bovine tuberculosis transmission to cattle and livestock farming communities living at wildlife-livestock-human interface in northern KwaZulu Natal, South Africa. PLoS Neglected Trop. Dis. 2020, 14, e0007618. [Google Scholar] [CrossRef]
- Etchechoury, I.; Valencia, G.E.; Morcillo, N.; Sequeira, M.; Imperiale, B.; López, M.; Caimi, K.; Zumárraga, M.; Cataldi, A.; Romano, M. Molecular typing of Mycobacterium bovis isolates in Argentina: First description of a Person-to-Person transmission case. Zoonoses Public Health 2010, 57, 375–381. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tuberculosis Profile 2017. Available online: https://apps.who.int/iris/handle/10665/259366 (accessed on 18 May 2023).
- National Tuberculosis and Leprosy Programme. Final_Joint_External_Program_Review_Report_March-Converted.pdf. Available online: https://ntlp.go.tz/tuberculosis/tb-prevalence-in-tanzania/ (accessed on 28 May 2022).
- World Health Organization. Global Tuberculosis Control, Global, Region and Country-Specific for Key Indicators. Available online: http://www.who.int/tb/data (accessed on 25 June 2023).
- Mfinanga, S.G.; Mørkve, O.; Kazwala, R.; Cleaveland, S.; Sharp, J.; Shirima, G.; Nilsen, R. The role of livestock keeping in tuberculosis trends in Arusha, Tanzania. Int. J. Tuberc. Lung Dis. 2003, 7, 695–704. [Google Scholar]
- Jain, A. Extra pulmonary tuberculosis: A diagnostic dilemma. Indian J. Clin. Biochem. 2011, 26, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Mkombozi, F.; Kazwala, R.; Lupindu, A. Prevalence and Risk factors of Bovine Tuberculosis in smallholder dairy cattle in Babati town council of Manyara region, Tanzania. Tanzan. Vet. J. 2014, 29, 30–38. [Google Scholar]
- United Republic of Tanzania. National Sample Census of Agriculture 2002/2003. Regional Report: Manyara. Available online: https://www.kilimo.go.tz/uploads/statistics/Manyara_Report.pdf (accessed on 15 December 2022).
- Arya, R.; Antonisamy, B.; Kumar, S. Sample size estimation in prevalence studies. Indian J. Pediatr. 2012, 79, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.; Tripathi, P.C.; Nema, S.; Shrivastava, A.K.; Dwiwedi, K.; Dhanvijay, A.K. Modified Petroff’s method: An excellent simplified decontamination technique in comparison with Petroff’s method. Int. J. Recent Trends Sci. Technol. 2014, 10, 461–464. [Google Scholar]
- Patil, P.S.; Chandi, D.H.; Rangaiahagari, A. Utility of GeneXpert MTB/RIF in the Rapid Diagnosis of Extra Pulmonary Tuberculosis. Indian J. Respir. Care 2023, 12, 46–48. [Google Scholar] [CrossRef]
- Siddiqi, S.H.; Rüsch-Gerdes, S. For BACTEC™ MGIT 960™ TB System. In Mycobacteria Growth Indicator Tube (MGIT) Culture and Drug Susceptibility Demonstration Projects; Foundation for innovative new Diagnostic (FinD): Borstel, Germany, 2006. [Google Scholar]
- Stewart, L.D.; McCallan, L.; McNair, J.; McGoldrick, A.; Morris, R.; Moyen, J.-L.; Ferré, L.D.J.; Romero, B.; Alonso, E.; Parsons, S.D. Multilaboratory evaluation of a novel lateral flow immunochromatographic assay for confirming isolation of Mycobacterium bovis from veterinary diagnostic specimens. J. Clin. Microbiol. 2017, 55, 3411–3425. [Google Scholar] [CrossRef]
- Amaro, A.; Duarte, E.; Amado, A.; Ferronha, H.; Botelho, A. Comparison of three DNA extraction methods for Mycobacterium bovis, Mycobacterium tuberculosis and Mycobacterium avium subsp. avium. Lett. Appl. Microbiol. 2008, 47, 8–11. [Google Scholar] [CrossRef]
- Halse, T.A.; Escuyer, V.E.; Musser, K.A. Evaluation of a single-tube multiplex real-time PCR for differentiation of members of the Mycobacterium tuberculosis complex in clinical specimens. J. Clin. Microbiol. 2011, 49, 2562–2567. [Google Scholar] [CrossRef]
- Ismail, A.; Josephat, P. Knowledge and perception on tuberculosis transmission in Tanzania: Multinomial logistic regression analysis of secondary data. Tanzan. J. Health Res. 2014, 16, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Maja, T.F.; Maposa, D. An investigation of risk factors associated with tuberculosis transmission in South Africa using logistic regression model. Infect. Dis. Rep. 2022, 14, 609–620. [Google Scholar] [CrossRef]
- Kazaura, M.; Kamazima, S.R. Knowledge, attitudes and practices on tuberculosis infection prevention and associated factors among rural and urban adults in northeast Tanzania: A cross-sectional study. PLoS Glob. Public Health 2021, 1, e0000104. [Google Scholar] [CrossRef]
- Mushtaq, M.U.; Shahid, U.; Abdullah, H.M.; Saeed, A.; Omer, F.; Shad, M.A.; Siddiqui, A.M.; Akram, J. Urban-rural inequities in knowledge, attitudes and practices regarding tuberculosis in two districts of Pakistan’s Punjab province. Int. J. Equity Health 2011, 10, 8. [Google Scholar] [CrossRef]
- Katale, B.Z.; Mbugi, E.V.; Karimuribo, E.D.; Keyyu, J.D.; Kendall, S.; Kibiki, G.S.; Godfrey-Faussett, P.; Michel, A.L.; Kazwala, R.R.; Van Helden, P. Prevalence and risk factors for infection of bovine tuberculosis in indigenous cattle in the Serengeti ecosystem, Tanzania. BMC Vet. Res. 2013, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Kilale, A.M.; Ngadaya, E.; Kagaruki, G.B.; Lema, Y.L.; Muhumuza, J.; Ngowi, B.J.; Mfinanga, S.G.; Hinderaker, S.G. Experienced and perceived risks of mycobacterial diseases: A cross sectional study among agropastoral communities in northern Tanzania. PLoS ONE 2015, 10, e0130180. [Google Scholar] [CrossRef]
- Shirima, G.; Kazwala, R.; Kambarage, D. Prevalence of bovine tuberculosis in cattle in different farming systems in the eastern zone of Tanzania. Prev. Vet. Med. 2003, 57, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Belay, M.; Ameni, G.; Bjune, G.; Couvin, D.; Rastogi, N.; Abebe, F. Strain diversity of Mycobacterium tuberculosis isolates from pulmonary tuberculosis patients in Afar pastoral region of Ethiopia. BioMed Res. Int. 2014, 2014, 238532. [Google Scholar] [CrossRef] [PubMed]
- Kazwala, R.; Daborn, C.; Sharp, J.; Kambarage, D.; Jiwa, S.; Mbembati, N. Isolation of Mycobacterium bovis from human cases of cervical adenitis in Tanzania: A cause for concern? Int. J. Tuberc. Lung Dis. 2001, 5, 87–91. [Google Scholar]
- Agada, C.; Adesokan, H.; Igwe, D.; Cadmus, S. Mycobacterium africanum and nontuberculous mycobacteria from fresh milk of pastoral cattle and soft cheese in Oyo State--implications for public health. Afr. J. Med. Med. Sci. 2014, 43, 13–20. [Google Scholar] [PubMed]
- Katale, B.Z.; Mbugi, E.V.; Botha, L.; Keyyu, J.D.; Kendall, S.; Dockrell, H.M.; Michel, A.L.; Kazwala, R.R.; Rweyemamu, M.M.; Van Helden, P. Species diversity of non-tuberculous mycobacteria isolated from humans, livestock and wildlife in the Serengeti ecosystem, Tanzania. BMC Infect. Dis. 2014, 14, 616. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wang, G.; Chen, S.; Yu, X.; Wang, X.; Zhao, L.; Ma, Y.; Dong, L.; Huang, H. Pulmonary tuberculosis caused by Mycobacterium bovis in China. Sci. Rep. 2015, 5, 8538. [Google Scholar] [CrossRef]
- Vågene, Å.J.; Honap, T.P.; Harkins, K.M.; Rosenberg, M.S.; Giffin, K.; Cárdenas-Arroyo, F.; Leguizamón, L.P.; Arnett, J.; Buikstra, J.E.; Herbig, A. Geographically dispersed zoonotic tuberculosis in pre-contact South American human populations. Nat. Commun. 2022, 13, 1195. [Google Scholar] [CrossRef]
- Roulette, C.J.; Njau, E.-F.A.; Quinlan, M.B.; Quinlan, R.J.; Call, D.R. Medicinal foods and beverages among Maasai agro-pastoralists in northern Tanzania. J. Ethnopharmacol. 2018, 216, 191–202. [Google Scholar] [CrossRef]
- Kazwala, R.; Kambarage, D.; Daborn, C.; Nyange, J.; Jiwa, S.; Sharp, J. Risk factors associated with the occurrence of bovine tuberculosis in cattle in the Southern Highlands of Tanzania. Vet. Res. Commun. 2001, 25, 609–614. [Google Scholar] [CrossRef]
- Mfinanga, S.; Morkve, O.; Kazwala, R.; Cleaveland, S.; Sharp, M.; Kunda, J.; Nilsen, R. Mycobacterial adenitis: Role of Mycobacterium bovis, non-tuberculous mycobacteria, HIV infection, and risk factors in Arusha, Tanzania. East Afr. Med. J. 2004, 81, 171–178. [Google Scholar] [CrossRef]
- Ameni, G.; Amenu, K.; Tibbo, M. Bovine tuberculosis: Prevalence and risk factor assessment in cattle and cattle owners in Wuchale-Jida district, Central Ethiopia. J. Appl. Res. Vet. Med. 2003, 1, 17–26. [Google Scholar]
| Variable | Category | n | % |
|---|---|---|---|
| Sex | female | 169 | 50.5 |
| male | 166 | 49.6 | |
| 0–18 | 16 | 4.8 | |
| 19–54 | 190 | 56.7 | |
| >55 | 129 | 38.5 | |
| Babati | 81 | 24.2 | |
| Hanang | 73 | 21.8 | |
| Districts | Kiteto | 7 | 2.1 |
| Mbulu | 155 | 46.3 | |
| Simanjiro | 2 | 0.6 | |
| Other | 17 | 5.1 | |
| Education | No education | 92 | 27.5 |
| Primary | 178 | 53.1 | |
| Secondary | 65 | 19.4 | |
| Professional | livestock attendants | 203 | 60.6 |
| Housewives | 37 | 11.0 | |
| Merchant | 47 | 14.0 | |
| peasant | 22 | 6.6 | |
| students | 13 | 3.9 | |
| office work | 1 | 0.3 | |
| driver | 1 | 0.3 | |
| unemployed | 9 | 2.7 | |
| wildlife workers | 1 | 0.3 | |
| craft | 1 | 0.3 |
| Category | n | % | |
|---|---|---|---|
| Human TB awareness | Yes | 229 | 68.4 |
| No | 106 | 31.6 | |
| Awareness of Human TB symptoms | None | 106 | 31.6 |
| Poor | 82 | 24.5 | |
| Average | 103 | 30.8 | |
| Good | 44 | 13.1 | |
| Awareness of Bovine TB | Yes | 59 | 17.6 |
| No | 276 | 82.4 | |
| Awareness of Bovine TB symptoms | None | 286 | 85.4 |
| Good | 49 | 14.6 | |
| Awareness of Zoonotic TB | Yes | 16 | 4.8 |
| No | 319 | 95.2 | |
| Awareness of Zoonotic TB transmission | None | 321 | 95.8 |
| Good | 14 | 4.2 |
| Exposure Variables | Total Sample | Positive Cases | Negative Cases | OR (95%CI) | p-Value | |
|---|---|---|---|---|---|---|
| Category | (n = 335) n (%) | (n = 18) n (%) | (n = 317) n (%) | |||
| Raw meat | yes | 122 (36.4) | 5 (27.8) | 117 (36.9) | 0.66 (0.21–1.79) | 0.436 |
| no | 213 (63.6) | 13 (72.2) | 200 (63.1) | |||
| Raw dairy product | Yes | 110 (32.8) | 4 (22.2) | 106 (33.4) | 0.57 (0.18–1.77) | 0.33 |
| No | 225 (67.2) | 14 (77.8) | 211 (66.5) | |||
| Soup with blood | yes | 116 (34.6) | 6 (33.3) | 110 (34.7) | 0.94 (0.32–2.49) | 0.9 |
| No | 219 (65.4) | 12 (66.7) | 207 (65.3) | |||
| Blood mixed with milk | yes | 40 (11.9) | 2 (11.1) | 38 (11.9) | 0.92 (0.14–3.39) | 0.91 |
| no | 295 (88.1) | 16 (88.9) | 279 (88.0) | |||
| Consumed aborted animal | Yes | 19 (5.7) | 2 (11.1) | 17 (5.4) | 2.21 (0.33–8.64) | 0.32 |
| No | 316 (94.3) | 16 (88.9) | 300 (94.6) | |||
| Share the roof with cattle | Yes | 110 (32.8) | 6 (33.3) | 104 (32.8) | 1.02 (0.35–2.72) | 0.96 |
| No | 225 (67.2) | 12 (66.7) | 213 (67.2) | |||
| Handled aborted products | Yes | 51 (15.2) | 5 (27.8) | 46 (14.5) | 1.81 (0.70–6.33) | 0.28 |
| No | 284 (84.8) | 13 (72.2) | 217 (85.5) | |||
| Family member with TB | Yes | 25 (7.5) | 0 (0) | 25 (7.9) | 0 (0–NaN) | 0.41 |
| No | 310 (92.5) | 18 (100) | 292 (92.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masanga, P.; Paul, S.; Mbelele, P.; Daud, P.; Liyoyo, A.; Munuo, L.; Lyimo, S.; Lyimo, B.; Srinivasan, S.; Cattadori, I.; et al. Perception and Risk Factors Associated with Tuberculosis in the Manyara Region, Tanzania. Zoonotic Dis. 2023, 3, 266-278. https://doi.org/10.3390/zoonoticdis3040022
Masanga P, Paul S, Mbelele P, Daud P, Liyoyo A, Munuo L, Lyimo S, Lyimo B, Srinivasan S, Cattadori I, et al. Perception and Risk Factors Associated with Tuberculosis in the Manyara Region, Tanzania. Zoonotic Diseases. 2023; 3(4):266-278. https://doi.org/10.3390/zoonoticdis3040022
Chicago/Turabian StyleMasanga, Prudence, Sarapia Paul, Peter Mbelele, Peter Daud, Alphonce Liyoyo, Lidia Munuo, Samson Lyimo, Beatus Lyimo, Sreenidhi Srinivasan, Isabella Cattadori, and et al. 2023. "Perception and Risk Factors Associated with Tuberculosis in the Manyara Region, Tanzania" Zoonotic Diseases 3, no. 4: 266-278. https://doi.org/10.3390/zoonoticdis3040022
APA StyleMasanga, P., Paul, S., Mbelele, P., Daud, P., Liyoyo, A., Munuo, L., Lyimo, S., Lyimo, B., Srinivasan, S., Cattadori, I., Katani, R., Kapur, V., Mpagama, S., & Buza, J. (2023). Perception and Risk Factors Associated with Tuberculosis in the Manyara Region, Tanzania. Zoonotic Diseases, 3(4), 266-278. https://doi.org/10.3390/zoonoticdis3040022







