Simple Summary
Diseases passed from animals to humans are a big concern for public health, often making people sick or even causing death. These illnesses can also affect people’s ability to have children, especially in men. Yet, we don’t know much about how these diseases impact men’s ability to have kids. This review looks at common animal-related diseases from bacteria and how they might affect men’s fertility. Even though there’s not a lot of human research in this area, studies on animals suggest these diseases could harm men’s ability to have children by causing inflammation and messing up the body’s natural defenses, leading to harmful substances being produced. It’s important to do more research to understand how these diseases affect men’s fertility so we can find ways to prevent or treat them, which would help keep people healthy and able to have families.
Abstract
Bacterial zoonotic diseases hold significant public health importance due to their substantial contribution to human morbidity and mortality. These infections have been implicated in reducing the fertility rate among couples of reproductive age. Despite the increasing prevalence of infertility and sub-fertility in men, there has been limited investigation into the possible effects of bacterial zoonotic infections on the male reproductive system. The purpose of this review is to describe common bacterial zoonotic diseases and their effects on human reproduction in order to unveil the hidden roles these infections could play in male factor infertility. While there is a dearth of information on this subject from human studies, available evidence from experimental animals suggests that bacterial zoonotic diseases impair male reproductive functions and structures primarily through the activation of the inflammatory response and distortion of the antioxidant system, resulting in the generation of oxidative species. In light of the limited research on bacterial zoonotic diseases and their role in male reproduction, efforts must be directed towards the subject to unravel the underlying pathological mechanisms and reduce the incidence among the human populace, either through preventive or curative measures.
1. Introduction
Infertility poses a significant reproductive health challenge affecting couples worldwide, with an estimated 10–15% of couples within the reproductive age (typically between 15 and 49 years) experiencing difficulties in conceiving [1]. The etiology of infertility is multifactorial, involving both male and female factors that contribute to the overall burden of this condition. Female factors include ovulatory disorders, fallopian tube abnormalities, and endometriosis, while male factors encompass abnormalities in the production, function, and transport of sperm cells.
Infections, including zoonotic diseases, are a noteworthy cause of male-factor infertility among couples of reproductive age [2]. Bacterial infection-induced tissue damage and inflammation may lead to male infertility by adversely affecting both spermatogenesis and the synthesis of testosterone [3]. Previous observations have highlighted that viral zoonotic diseases have the potential to cause significant disruptions in male reproductive function [4].
Zoonotic diseases present substantial public health risks responsible for over half of all human diseases [5,6]. Bacterial zoonotic diseases exhibit considerable diversity, yet studies estimating the burden of each disease in different regions of the world are inadequate. Due to the paucity of burden of disease estimates from various regions, it is usually described based on a global scale [7]. Globally, these diseases have been responsible for approximately 60–75% of recent disease outbreaks [8,9]. However, a systematic study addressing bacterial zoonosis in Africa reported prevalence rates of up to 40% for brucellosis, 24% for leptospirosis, and 28% for Q fever [8]. Notably, zoonotic diseases like Ebola, COVID-19, Lassa fever, Rift Valley fever, and anthrax have triggered significant outbreaks, impacting the lives of millions.
The transmission of diseases from animals to humans results from intricate interactions among animals, humans, and pathogens within shared environments. Several factors facilitate pathogen spread, including close interactions between animals and humans, intensive animal farming practices, consumption of contaminated food, bites from infected vectors, and the misuse of antibiotics, which is exacerbated by the development of antimicrobial resistance [10].
Bacterial zoonotic diseases exhibit considerable diversity, with multiple types of bacteria potentially being transmitted simultaneously. For instance, a bite from a dog or cat may result in the transmission of several bacteria, including Pasteurella, Campylobacter, Capnocytophaga, Bordetella bronchiseptica, and Staphylococcus intermedius, which constitute the oral and nasopharyngeal flora of these animals [11]. Exposure to various Gram-positive and Gram-negative bacteria increases the risk of morbidity and mortality, potentially causing damage to multiple organs in humans. In general, infections have been recognized as a possible contributing factor to male reproductive dysfunction and infertility, with Gram-negative and Gram-positive bacteria collectively responsible for approximately 15% of cases of male primary infertility [12].
It is worth noting that bacteria can inflict damage on the structure and function of sperm cells through processes such as DNA fragmentation [13], impairment of the acrosome reaction [14,15], and peroxidation of the cell membrane [16]. Bacteria such as Pasteurella multocida and Salmonella typhymurium induce detrimental effects indirectly by secreting toxins such as porins and lipopolysaccharides which induces inflammation and oxidative stress and consequently destroy sperm DNA through apoptosis [17], while others, such as mycoplasmas, directly attach to sperm cells causing hyperactivation and impaired acrosome reaction [18], subsequently triggering oxidative stress, inflammation, and apoptosis [19].
Due to human interactions with animals, there is potential for the transmission of bacteria from animals to humans, resulting in significant morbidity and posing a risk to male reproductive health. This review focuses specifically on examining certain bacterial zoonotic diseases with public health significance and the potential for transmission from animals to humans, exploring their effect on male reproductive function. To compile information for this review, an extensive literature search was conducted. Peer-reviewed articles from PubMed and Google Scholar were used as primary references, with a search timeframe extending up to December 2023. The search strategy involved using keywords related to male reproductive health, such as ‘spermatozoa’, ‘semen analysis’, ‘testes’, ‘male reproduction’, ‘male reproductive dysfunction’, and ‘testicular dysfunction’. These keywords were paired with terms for each of the selected bacterial zoonotic diseases. The included studies encompassed research on male reproductive function in both experimental animals and human subjects, along with relevant case reports. This comprehensive approach aims to provide a thorough understanding of the relationship between bacterial zoonotic diseases and male reproductive dysfunction.
2. Physiology of Male Reproductive System
The male reproductive function is a complex process that relies on the coordinated actions of various organs and systems within the body. The primary role of the male reproductive system is to accomplish several key functions, including androgen production, support for spermatogenesis, and facilitating the transport of spermatozoa into the female reproductive tract for fertilization [20]. This intricate process is regulated by a combination of endocrine hormones produced in the hypothalamus (Gonadotropin-releasing hormone) and anterior pituitary glands (Follicle-stimulating hormone (FSH), Luteinizing hormone (LH)), as well as locally within the testes (testosterone) and adrenal glands (testosterone in small amounts). These hormones play crucial roles in governing the processes of spermatogenesis and sperm transport, with testosterone and Follicle-Stimulating Hormone (FSH) working together to ensure optimal testicular growth and sperm production.
The testes, a vital organ of the male reproductive system, bear the responsibility for testosterone and sperm production. Three main types of functioning cells within the testes are Leydig cells, germ cells, and Sertoli cells. Germ cells, situated within the interior of the seminiferous tubules, undergo differentiation and multiple rounds of cell division to ultimately produce mature spermatozoa. Leydig cells, situated near the seminiferous tubules in the testicular interstitium, have the primary function of producing testosterone, influencing many tissues by attaching to intracellular receptors and regulating protein expression [21]. On the other hand, Sertoli cells, found on the outermost part of the seminiferous tubules, play an essential role in sperm production [22]. These cells establish the blood–testis barrier, containing the germinal cells within the seminiferous tubules and aiding in their growth [23]. Furthermore, Sertoli cells are linked together by tight connections and interact intimately with primitive spermatogonia [24], distinguished by their larger size and less visible nuclei compared to germ cells.
Years of research have uncovered many intricate methods by which spermatogonial stem cells convert into highly specialized, motile spermatozoa. While testosterone and FSH have long been known to influence spermatogenesis, a variety of paracrine factors, hormones, tightly controlled protein expression programs, non-coding RNA species, and epigenetic genome alterations work together to promote spermatogenesis [25].
Spermatogenesis occurs in cycles, with each cycle taking approximately two months to complete [26]. However, not all seminiferous tubules undergo spermatogenesis simultaneously. The initiation of spermatogenesis begins when diploid spermatogonia divide through mitosis, resulting in the formation of primary spermatocytes, which undergo meiosis I, and leading to the production of haploid spermatocytes. These haploid spermatocytes then undergo another process of meiosis to yield haploid spermatids [27].
In the seminiferous tubules, the initial stages of spermatocytes, often referred to as primitive spermatocytes, are primarily located around the periphery of the tubules [28]. As they progress toward the lumen of the tubules, they continue to mature. Spermatids undergo cytoplasmic reduction as part of their development, ultimately transforming into spermatozoa [29]. These spermatozoa, initially immotile, are discharged into the tubules and subsequently migrate to the epididymis, where they further mature [30].
It takes around twelve days for spermatozoa to develop and acquire motility inside the epididymis [19]. Following maturation, they are retained in the epididymis tail until ejaculation. The epididymis is connected to the vas deferens, serving as an outlet for the mature sperm to be discharged [31]. Mature spermatozoa typically exhibit a distinct structure consisting of three main parts: the head, mid-piece, and tail [32]. The head of the spermatozoa is typically capped with the acrosome, a structure filled with lysosomes [33]. Additionally, the head contains the sperm’s nucleus and only a small amount of cytoplasm. The flagellum or tail of the spermatozoa is responsible for propulsion and receives energy from the numerous mitochondria located in the midpiece [32].
3. Hypothalamic–Pituitary–Gonadal Axis in Male Reproductive Function Control
The hypothalamic–pituitary–gonadal axis plays a critical role in male sexual maturation, spermatogenesis, and the development of secondary sexual traits [26]. The hypothalamus stimulates the anterior pituitary gland by releasing Gonadotropin-Releasing Hormone (GnRH) into the hypothalamo–hypophyseal portal system [34]. GnRH is secreted in a pulsatile manner by the kisspeptin–neurokinin–dynorphin neuronal network in the hypothalamus [35]. In turn, GnRH acts on gonadotrophs in the anterior pituitary through a G protein receptor, causing the production of FSH and Luteinizing Hormone (LH) by increasing intracellular calcium levels through the activation of inositol 1, 4, 5-triphosphate (IP3) [19]. It is noteworthy that the secretion of GnRH is inhibited by hormones like testosterone, estrogen, estradiol, and prolactin.
FSH and LH interact with membrane receptors found in the Leydig and Sertoli cells. Both LH and FSH activate G protein receptors, resulting in an increase in cellular cAMP levels [36]. This activation stimulates Leydig cells to convert cholesterol into testosterone. LH specifically promotes the activity of desmolase, the enzyme responsible for converting cholesterol into pregnenolone, a precursor to other weaker androgens [37]. Subsequently, androstenedione is converted to testosterone through the action of the enzyme 17-beta-hydroxysteroid dehydrogenase [38].
The secretion of LH and FSH is reduced through a negative feedback action of testosterone on the hypothalamus and anterior pituitary [39]. Furthermore, testosterone affects Sertoli cells, found on the outermost layer of the testicular seminiferous tubules. FSH and testosterone stimulate Sertoli cells to produce androgen-binding protein (ABP), which is necessary for delivering testosterone to germ cells during sperm production [40]. FSH induces the release of inhibin B and Mullerian-inhibiting substance (MIS), thereby increasing sperm production [41]. Inhibin acts as a negative feedback mechanism through which Sertoli cells regulate the hypothalamic–pituitary system, resulting in a lower FSH secretion [40].
In the pre-pubertal stage, androgen and gonadotropin levels in the body remain low and constant [42]. However, during puberty, the hypothalamus releases GnRH in a pulsatile manner, occurring approximately every one to two hours [43]. This pulsatile release pattern helps maintain appropriate levels of FSH, LH, and plasma testosterone, collectively regulating each other to preserve hormonal balance. As men reach their third decade of life, testosterone levels tend to decline naturally [42].
While the majority of testosterone production in men occurs in the Leydig cells of the testes, the adrenal cortex also contributes to androgen production [44]. Similar to the hypothalamic–pituitary–gonadal axis, the adrenal glands are under the regulatory control of the hypothalamus and anterior pituitary, forming the hypothalamic–pituitary–adrenal axis. In this process, the hypothalamus releases corticotropin-releasing hormone (CRH), stimulating the anterior pituitary to secrete adrenocorticotropic hormone (ACTH). ACTH, in turn, triggers the enzyme desmolase to convert cholesterol into pregnenolone within the adrenal glands, a process analogous to testosterone synthesis in the testes [45]. Specifically, the zona reticularis of the adrenal cortex generates weak androgens like DHEA and androstenedione, which can be further converted to testosterone or estradiol in peripheral tissues [26].
4. Pathogenesis of Bacterial Zoonotic Diseases
Pathogenesis describes the events involving the transmission of pathogens from animals to humans, encompassing entry, replication, spread, and the establishment of infection in target organs of the human body [46]. It generally begins with exposure to pathogens, their spread and adherence to the portal of entry, subsequent spread and invasion of target organs, and multiplication in target organs with the concomitant action of host immune cells, ultimately culminating in the morbidity, and sometimes mortality, of the host (human) (Table 1).
Close contact between humans and infected animals, such as pets, livestock, and wildlife, can lead to the transmission of bacterial zoonotic diseases to people. Animal bites and scratches are the primary means through which zoonotic bacterial diseases spread from animals to humans [47]. Besides direct contact with animal saliva, blood, urine, and feces, especially by veterinarians, humans can also contract bacterial zoonotic diseases through contact with contaminated water or soil or by ingesting undercooked, unwashed, or unprocessed food products contaminated by infected animals. For instance, Campylobacter can be transmitted through ingesting raw milk and undercooked poultry [48].
After entry into the host, pathogens adhere, spread, and replicate within the host. The replication of a pathogen within the human host depends on various factors, including the presence of specific receptors in cells and organs, existing tissue damage, the host’s immune responses, and other defensive mechanisms [47]. Eventually, the infection could result in various outcomes, such as complete eradication by the host immune response, persistence of the pathogen in a dormant state facilitating transmission to another host, or a combination of these outcomes.
The virulence of bacterial zoonotic diseases is usually mediated by several factors determined by DNA strands in a chromosome, plasmids, bacteriophages, or some other units of the bacteria. Some bacteria adhere to the host’s epithelium with specialized structures called pili. Others invade human host cells by directly entering into the cell, some produce outer capsules to prevent them from being phagocytosed by host immune cells (such as pneumococcus), while others produce toxins (endotoxins and exotoxins). Additionally, some bacteria produce siderophores that scavenge host iron stores, affecting most nucleated cells [47].
The pathogenesis of bacterial zoonotic diseases is complex, involving multiple stages, from initial exposure to bacteria to the development of clinical symptoms. The table below outlines the general pathogenesis of bacterial zoonotic diseases (Table 1). Also, Figure 1 shows a schematic description of the pathogenesis of bacterial zoonotic diseases leading to death or recovery of infected human (Figure 1).
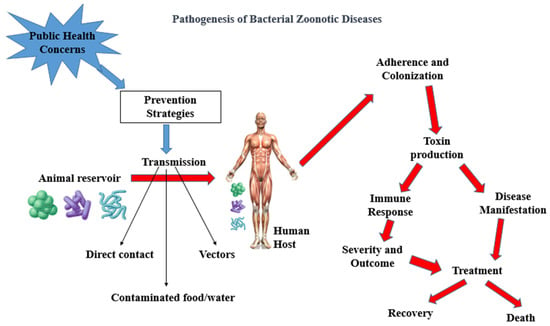
Figure 1.
Schematics of pathogenesis of bacterial zoonotic diseases. Adherence and Colonization: represents bacteria attaching and multiplying within the human body. Immune Response: depicts the body’s immune cells responding to the infection; Disease Manifestation: shows how toxins lead to the development of symptoms; Toxin Production: illustrates the production of toxins by bacteria based on [49,50].

Table 1.
Stages of pathogenesis of bacterial zoonotic diseases (own compilation based on [49,50]).
Table 1.
Stages of pathogenesis of bacterial zoonotic diseases (own compilation based on [49,50]).
| Stage | Description |
|---|---|
| Exposure | Humans are exposed to the bacteria through direct contact with infected animals, consumption of contaminated food or water, or contact with contaminated soil or surfaces. |
| Adherence | The bacteria adhere to and colonize the host’s mucosal surfaces, such as the respiratory or gastrointestinal tract. |
| Invasion | The bacteria invade host cells or tissues, using a variety of mechanisms such as secretion of virulence factors, inducing host cell uptake, or direct penetration. |
| Multiplication | The bacteria multiply rapidly in host tissues, often leading to tissue damage and inflammation. |
| Spread | The bacteria may spread to other tissues or organs through the bloodstream or lymphatic system. |
| Clinical symptoms | The host develops clinical symptoms, which can range from mild to severe and may include fever, chills, diarrhea, respiratory symptoms, or systemic illness. |
It is important to note that the specific pathogenesis can vary depending on the bacterial species involved and the mode of transmission. For instance, leptospires enter the body through mucous membranes or compromised skin, such as through cuts or abrasions, or exposure to water. After this, they spread quickly in the blood. Intact leptospiral cells and various leptospiral proteins adhere to host tissues and components. Many leptospiral proteins interact with multiple host components. Pathogenic leptospira can withstand phagocytosis by macrophages and neutrophils in the presence of specific antibodies. The major virulence characteristics of leptospires include receptor-mediated endocytosis, release of lipopolysaccharide (LPS), motility, and the ability to acquire iron in vivo [51]. Understanding the pathogenesis of bacterial zoonotic diseases is critical for developing effective prevention and control strategies.
5. Bacterial Zoonosis and Their Effect on Male Reproduction
The exposure of reproductive cells to infectious agents has been identified as a key factor contributing to male infertility or subfertility. Bacterial zoonoses, in particular, can exert various potential impacts on male reproductive function. For instance, certain bacterial infections like Brucella or Pasteurella have been observed to induce inflammation or damage to the testes and other reproductive organs, consequently leading to infertility or other reproductive health issues [52,53]. This review specifically concentrates on bacterial zoonotic diseases and their potential effects on male reproductive function, encompassing studies conducted on both humans and experimental animals.
5.1. Leptospirosis on Male Reproductive Function
Leptospirosis is a zoonotic disease primarily caused by the spirochetal bacteria known as Leptospira, exhibiting a global distribution and an increasing prevalence [54]. Leptospirosis comprises 64 genomic species, with 38 of them identified as pathogenic [55].
The transmission of the disease to humans typically occurs environmentally, often through contact with water or damp soil contaminated with leptospires. However, direct contact with body fluids such as urine or blood from an infected animal can also lead to transmission [56].
While there is a paucity of studies directly correlating leptospirosis to male infertility in human subjects, numerous investigations have explored the susceptibility of male reproductive organs to leptospirosis using animal models, such as bulls. Previous reports indicate that approximately 20 to 40 percent of bovine bulls may exhibit abnormalities in sperm viability, resulting in infertility or subfertility [57]. Factors contributing to low fertility in bovine bulls include testicular degeneration, delayed sexual maturity, testicular hypoplasia, abnormal spermiogenesis, sexual immaturity, and infectious agents. Bovine bulls have been reported to shed leptospirosis in their semen, suggesting the potential sexual transmission of leptospires among these animals [58]. Leptospira interrogans and Leptospira kirschneri have been isolated from the male reproductive organs of wild boars, further supporting the possibility of the sexual transmission of leptospires among animals [59].
The detection of bacteria in semen can be accomplished through the microscopic agglutination test (MAT) or through molecular diagnosis methods. Notably, active infection by leptospires in bovines can occur without detectable anti-leptospira agglutinins in the semen. However, periodic shedding of the pathogen in the semen of bulls has been observed [57]. The microscopic agglutination test is deemed non-specific for detecting pathogens, and seminal plasma agglutination may not be a reliable method for diagnosis, suggesting that leptospires might have limited tropism for the genital tract of bovine bulls [58]. Molecular tests such as polymerase chain reaction (PCR) offer a prompt and early diagnosis of leptospirosis [58]. PCR can detect leptospirosis in blood samples within one week of symptom presentation and can also be employed with tissue samples, urine samples, and cerebrospinal fluid samples [60,61].
In summary, studies on animal models, particularly bulls, have provided insights into the presence of leptospires in the male genital tract, highlighting a gap in understanding the potential effects of leptospirosis on the human male reproductive system.
5.2. Anthrax on Male Reproductive Function
The causative organism for Anthrax is Bacillus anthracis. Anthrax pathogens can be transmitted through direct contact with animals and/or animal products such as wool, hides, or hair from infected livestock [62]. Handling contaminated animal materials without proper protection can result in spore entry and subsequent infection. Spores of Bacillus anthracis can be inhaled, causing inhalation anthrax or pulmonary anthrax, and can also be transmitted through the consumption of contaminated meat from infected animals [63].
Anthrax antigens are now introduced into the human body mainly through vaccination due to its potential use as a biological weapon [64]. However, there is no clear study suggesting the long-term impact of anthrax vaccination on general male reproductive function. It has been discovered that exposure to the anthrax vaccine by males undergoing assisted reproduction does not negatively impact semen parameters, fertilization rates, embryo quality, or clinical pregnancy rates [65]. In contrast, a study reported that after vaccinating stallions against anthrax, there was a reduction in sperm quality [66]. It was also noted that the anthrax vaccine had a strong effect on sperm quality after cryopreservation, making it unsuitable for assisted reproduction [65]. Evidence from these studies shows conflicting results regarding the effect of anthrax on sperm parameters. Further studies are required to delineate the role of anthrax infection or vaccination in male fertility.
5.3. Brucellosis on Male Reproduction
Brucellosis, primarily caused by bacteria of the genus Brucella, includes several species that can infect humans, namely, Brucella abortus, Brucella melitensis, Brucella suis, and Brucella canis [67]. Transmission to humans occurs through direct contact with infected animals, consumption of unpasteurized dairy products, inhalation of aerosols, and undercooked food. Unpasteurized milk consumption is the most common cause of human brucellosis, accounting for rates ranging from 33.9 to 100% [68]. While person-to-person transmission is rare, it can occur through sexual contact, blood transfusion, vertical transmission from mothers to offspring, tissue transplantation, and contact with contaminated materials [68,69,70].
The early detection and prevention of brucellosis involve key tests for Brucella antibodies, such as the milk ring test and enzyme-linked immunoassay (ELISA). Real-time PCR and biosensors are now utilized for rapid organism detection, reducing the risk of human contamination in laboratories [71].
Chronic brucellosis has the potential to affect reproductive organs. In females, it increases the risk of abortion, especially in endemic regions [72]. Males with brucellosis may experience sperm abnormalities, orchitis, epididymitis, testicular atrophy, and infertility [73].
Brucellosis induces a widespread inflammatory response affecting various organs, particularly the reproductive system. Epididymo-orchitis is a common genitourinary complication, necessitating scrotal investigation for infertility, especially in endemic areas [74]. In cases of acute scrotal pain and fever with recent travel to endemic areas, Brucellosis epididymo-orchitis should be considered [75].
Chronic brucellosis may impair sexual potency; a study of men aged 20–45 treated for chronic brucellosis reported 68% with impaired sexual potency, some exhibiting physical organ dysfunction [76]. This suggests a correlation between brucellosis and male reproductive dysfunction. Animal studies have indicated a harmful effect of Brucella on reproductive tissues and cells through oxidative stress mechanisms. In rats infected with Brucella melitensis, abnormal spermatozoa were observed, associated with the generation of reactive oxygen species (ROS) [52]. Elevated ROS levels are linked to impaired sperm function and quantity [77]. Approximately 5.7% of humans with brucellosis develop epididymo-orchitis [78]. Chronic brucellosis has also been linked to testicular inflammation and subsequent tumors [53].
In summary, limited studies have reported on the effect of Brucella on male reproduction. Evidence suggests that Brucellosis may lead to orchitis, epididymo-orchitis, impaired sexual potency, reproductive organ dysfunction, and abnormal sperm parameters, implying a potential risk of future infertility or subfertility following Brucella infection.
5.4. Pasteurella Multocida and Male Reproduction
Pasteurella multocida, a predominant pathogenic species within the Pasteurella genus, is commonly responsible for infections in humans [79]. The transmission of this bacterium primarily occurs through animal bites, respiratory droplets, and direct contact with secretions from infected animals [80].
Experimental studies conducted on bucks have revealed that Pasteurella multocida infection adversely affects their genital structures, leading to conditions like orchitis and reduced semen quality [81]. Another study on rams reported that P. multocida infection resulted in structural impairments in the scrotum, testicular atrophy, and the development of epididymal granulomas characterized by fibrosis. Additionally, testes exhibited areas of sperm stasis with micro-calcifications [82]. These findings suggest that Pasteurella multocida infection has the potential to disrupt the integrity of male genital structures, potentially leading to infertility later in life.
Regarding the impact of Pasteurella multocida on human reproductive structures, a single study assessing the effect of porins and lipopolysaccharides from Salmonella enterica and Pasteurella multocida indicated that toxins from these bacteria increased the level of naturally occurring apoptosis in human spermatozoa [83]. However, this study did not explore the impact of Pasteurella multocida alone, highlighting the need for further research to elucidate its role in human spermatozoa apoptosis.
It is essential to recognize that additional research is imperative to determine whether the effects of P. multocida on male reproductive structures are reversible. This understanding is crucial for assessing the long-term implications of such infections on male fertility and reproductive health.
5.5. Bartonellosis and Male Reproduction
Bartonellosis, commonly transmitted through cat scratches and also known as Cat-scratch disease, is caused by the bacterial agent Bartonella henselae. Despite a scarcity of studies connecting Bartonellosis to male reproductive dysfunction, a case report by Trefois and colleagues highlighted symptoms of autonomic dysfunction, including erectile dysfunction, in a man infected with Bartonella henselae. The patient showed improvements after three weeks of treatment with tetracyclines [84]. This case report suggests that Bartonellosis may lead to regional lymphadenopathy and erectile dysfunction [84]. Further exploration is necessary to understand the potential impact of this infection on various aspects of male reproduction if left untreated.
5.6. Yersiniosis and Male Reproduction
Yersiniosis is commonly transmitted through contaminated food, particularly undercooked meat such as pork. Yersinia enterocolitica, along with other species like Yersinia pestis and Yersinia pseudotuberculosis, is responsible for causing human diseases [85]. Yersinia enterocolitica affects the gastrointestinal tract and can also invade lymphoid tissues, employing various mechanisms to evade the host immune system [85].
Currently, there is no study that directly links infection with Yersinia enterocolitica to male reproductive dysfunction. However, an experimental study reported significant histological changes in the gonads of mice infected with Yersinia pseudotuberculosis, raising the possibility of causing male reproductive dysfunction [86]. Further research is necessary to explore the potential impact of Yersinia enterocolitica on male reproductive health.
5.7. Q Fever and Male Reproduction
Coxiella burnetii, the causative agent of Q fever, is commonly contracted from ticks, cats, goats, sheep, and cattle [87,88]. The animal-to-human transmission of Q fever occurs when individuals inhale dust contaminated with animal feces, urine, and birth products [87]. Although limited, several studies have indicated that C. burnetii can be shed from the semen of experimental animals such as mice and bulls, with its presence detectable through ELISA tests and immunofluorescence assays [89,90]. C. burnetii has also been detected in semen samples for artificial insemination using PCR [91]. Reports of the sexual transmission of the bacterium to female mice, with the detection of C. burnetii antibodies in the blood, spleen, and amniotic fluids of female mice, suggest a potential for the sexual transmission of the bacteria that should be considered in epidemiological investigations of the disease [92].
It is worth noting a documented case of the sexual transmission of Q fever detected 15 days after coitus, with evidence of pathogen persistence in semen for an additional 23 months [92]. The persistence of C. burnetii in semen was detected using a highly sensitive PCR capable of identifying less than five Coxiella cells, while the detection of infection in the wife was performed through serological tests for antibodies to C. burnetii [93]. In addition to semen, C. burnetii can be shed through various other routes, including the placenta, birth fluids, vaginal mucus, milk, feces, and urine [94]. Consequently, further investigations are urgently needed to assess the impact of the persistence of this pathogen on overall semen function and viability, given its potential implications for both animal and human reproductive health.
5.8. Staphylococcus and Male Reproduction
Staphylococcus bacteria encompass various strains, and while not all are recognized as pathogenic to humans, some can indeed cause diseases. For instance, Staphylococcus pseudintermedius, originally associated with animals, is increasingly being acknowledged as a pathogen in humans. Blondeau et al. (2021) recently reported the first documented case of this bacterium causing a urinary tract infection in a male patient who reportedly contracted the infection from a family dog [95].
Moreover, investigations into bacteria present in semen samples from bulls have revealed the presence of several Staphylococcus species, including Staphylococcus aureus, haemolyticus, epidermidis, cohnii, and kloosii. Importantly, the presence of these bacteria in semen has been found to have a discernible impact on sperm motility and semen composition [96]. Specifically, in vitro studies have demonstrated that Staphylococcus aureus can lead to a significant reduction in sperm motility and result in ultrastructural abnormalities in sperm morphology [97].
These findings underscore the significance of considering Staphylococcus species, even those originally associated with animals, as potential contributors to male reproductive dysfunction. Further research is warranted to elucidate the mechanisms through which Staphylococcus bacteria affect sperm function and reproductive health in both animals and humans.
5.9. Tuberculosis and Male Reproduction
Tuberculosis, caused by Mycobacterium bovis and Mycobacterium tuberculosis, represents a highly pathogenic disease that can affect various animals and pose a source of infection to humans [98]. Both species of Mycobacterium have the capacity to infect humans and can persist in the body in a latent or active state, depending on the host’s immune response. The presence of tuberculosis in the human body, whether latent or active, poses a significant threat to male fertility.
The literature contains an extensive documentation of the impact of tuberculosis on the reproductive tracts of both males and females, with the potential for irreversible damage if not promptly detected [99]. Tuberculosis affecting the male genitals has been found to have a substantial impact on fertility, resulting in alterations in sperm quality among male patients diagnosed with genital tuberculosis [100]. Furthermore, male genital tuberculosis can affect the epididymis, leading to conditions such as tuberculous epididymitis and obstructive infertility [101]. Chatterjee et al. (2020) reported elevated DNA fragmentation indices and abnormal sperm function test results in patients with latent tuberculosis, indicating its adverse effects on male reproductive function [102].
Moreover, studies have provided evidence that tuberculosis can affect the male reproductive tract even when it is not localized to the genital tract. Among patients with kidney tuberculosis, approximately 75% exhibited oligoasthenozoospermia, a condition characterized by a low sperm count and reduced sperm motility [103]. The use of anti-TB medications has also been associated with impaired sexual function [101]. In summary, tuberculosis in any form can be a significant contributor to male infertility, particularly when diagnosis is delayed. Many patients with tuberculous infertility ultimately require assisted reproductive techniques [104]. This underscores the importance of early detection and intervention to mitigate the impact of tuberculosis on male reproductive health.
5.10. Campylobacteriosis and Male Reproduction
Campylobacteriosis is an infectious disease caused by bacteria belonging to the Campylobacter genus, with the two primary species implicated in food-borne infections in humans being Campylobacter jejuni and C. coli [105]. This disease is chiefly transmitted through the consumption of contaminated food, contact with infected animals, person-to-person transmission, and environmental contamination.
While research exploring the impact of Campylobacter on the male reproductive system remains limited, a few studies on experimental animals have provided valuable insights. These investigations suggest that Campylobacter has the potential to disrupt sperm quality and exhibit sexual transmission in poultry. In chickens, Campylobacter has been observed to attach to various segments of spermatozoa, contributing to male-to-female transmission of the bacterium [106]. Additionally, Campylobacter fetus, a specific species within the Campylobacter genus, has been found to bind irreversibly to bull spermatozoa, adversely affecting sperm quality. It is postulated that this adherence may serve as the primary cause of sperm alterations and represents a crucial step in pathogenesis and venereal transmission [107]. Furthermore, ram spermatozoa exposed to Campylobacter exhibited a high prevalence of morphological damage, with bacteria attaching to the tail and acrosome regions of the sperm. Infected sperm cells displayed decreased motility, an increased incidence of early acrosome reaction, and chromatin damage, collectively indicating a detrimental effect on ram sperm quality [108].
In humans, a notable case report by Sanagawa and colleagues documented testicular pain in a 51-year-old man with Campylobacter jejuni enterocolitis [109]. Campylobacter infections in the genital tract typically arise as a consequence of systemic infection, rather than originating as primary genital infections. The bacteria can enter the genital tract either through the bloodstream or by spreading from other infected areas. This observation suggests a potential interplay between the gastrointestinal tract and the testes in the context of Campylobacter infection. Subsequent research involving larger cohorts is warranted to ascertain whether such testicular pain is associated with significant testicular damage or has broader implications for reproductive function.
While research on the effects of Campylobacter on the male reproductive system is limited, existing evidence from animal studies and clinical cases underscores the need for further investigation. Understanding the potential consequences of Campylobacter infections on male reproductive health is crucial, especially given the bacterium’s prevalence and its ability to impact various organ systems.
5.11. Escherichia coli (Shiga (Vero) Toxin-Producing (E. coli) (STEC)) and Male Reproduction
Escherichia coli, commonly found in the intestines of humans and other warm-blooded animals, exhibits species variations, with certain strains serving as primary etiological agents responsible for enteritis and various extra-intestinal disorders. Of particular concern is the subset of E. coli known as Shiga (Vero) toxin-producing E. coli (STEC/VTEC), which has the capacity to induce severe illnesses in humans when transmitted through the food chain from its animal reservoirs [110]. STEC encompasses a diverse group of E. coli strains that produce Shiga toxins, including, but not limited to, E. coli O26, O111, O103, O145, and O104 and E. coli O157:H7. Notably, E. coli O157:H7 is recognized for its role in causing outbreaks of hemorrhagic colitis and hemolytic uremic syndrome (HUS) [111]. These strains are considered zoonotic due to their potential transmission between animals, particularly cattle and sheep, and humans [112].
A study that investigated the impact of enterotoxigenic and verotoxigenic E. coli on boar sperm quality revealed that verotoxigenic E. coli significantly reduced sperm viability and motility, with these effects becoming apparent as early as 24 h after incubation [113]. Presently, the scientific literature lacks extensive evidence indicating a direct influence of Shiga toxin-producing E. coli (STEC) on male reproductive function in humans. The majority of research pertaining to STEC has centered on its gastrointestinal and renal effects, particularly its association with hemolytic uremic syndrome (HUS), a severe condition characterized by kidney damage. However, it is crucial to acknowledge that STEC infections, when severe, can lead to systemic complications, including multi-organ damage. While STEC is primarily recognized for its gastrointestinal and renal effects, its potential influence on the male reproductive function remains an area that requires further investigation and study within the scientific community.
5.12. Listeriosis and Male Reproduction
Listeriosis represents an infectious disease brought about by the bacterium Listeria monocytogenes [114]. This ubiquitous bacterium is found in the environment and can contaminate various food sources, thereby leading to infections in humans. Transmission can occur through the consumption of contaminated foods, such as raw or undercooked meat, as well as through vertical transmission from mothers to unborn children and person-to-person contact.
Studies addressing the impact of Listeria monocytogenes on male reproduction are limited, yet several notable observations have been made. In a study involving the inoculation of Listeria monocytogenes into the left testes of guinea pigs, researchers reported that the bacterium did not spread to the contralateral testes or other organs. However, they did observe pathological changes in the contralateral testes, manifesting as orchitis, potentially attributable to an autoimmune response [115]. In a study involving West African dwarf bucks infected with Listeria monocytogenes, the bacterium was found to cause a significant decrease in sperm volume, motility, and morphology [116].
Among asymptomatic infertile couples, the presence of L. monocytogenes was not found to have a significant effect on semen parameters. However, it was noted that the accurate detection of this bacterium in semen samples can be achieved through polymerase chain reaction (PCR) [117]. In a case involving Listeria monocytogenes infection following a liver transplant, the patient exhibited epididymitis and orchitis as a consequence of immunosuppression. This observation underscores the potential deleterious impact of Listeria monocytogenes on male reproductive organs, particularly among individuals with compromised immune systems [118].
In summary, while studies examining the effects of Listeria monocytogenes on male reproductive health are limited, existing research suggests that this bacterium can indeed exert adverse effects on sperm quality and male reproductive organs, especially in cases of compromised immunity or specific experimental conditions. Further investigations are warranted to comprehensively understand the implications of Listeria monocytogenes infection on male reproductive function. The table below shows a summary of the effect of bacterial zoonotic diseases on male reproduction (Table 2).

Table 2.
Effect of bacterial zoonotic diseases on male reproductive structure and function.
6. Bacterial Zoonotic Infections on Male Reproductive Function: Identified Mechanisms
The influence of bacterial zoonotic diseases on the male reproductive system is a multi-faceted phenomenon, contingent upon several factors, including the specific pathogen, its virulence, the host immune status, and the overall health of the affected individual. While bacterial zoonotic diseases are primarily recognized for their impact on other organ systems, it is important to acknowledge that severe infections or systemic repercussions can potentially exert indirect effects on male reproductive function.
Several key mechanisms have been identified in this review, elucidating how bacterial zoonotic infections can impact male reproduction. These mechanisms encompass inflammatory responses localized within reproductive structures such as the testes and epididymis, which can, in turn, influence hormonal regulation. Also, immune cell infiltration and the induction of oxidative stress play pivotal roles in mediating the effects of these zoonotic pathogens on male reproductive health.
Notably, Listeriosis, a bacterial zoonotic disease, has been documented as capable of eliciting testicular damage through auto-immune-related mechanisms. This underscores the intricate interplay between bacterial zoonotic infections and male reproductive function, emphasizing the need for further research and understanding of these complex interactions.
7. Future Perspectives
This review underscores a notable research gap regarding the impact of bacterial zoonotic diseases on male reproductive processes. While it is well-established that infections can contribute to infertility, the primary focus has been on understanding the effects of infections on female reproductive health, with comparatively less attention devoted to their impact on the male reproductive tract. There is a critical need for extensive investigations to fully elucidate the role of bacterial zoonotic infections, including latent ones, in male reproductive dysfunction.
Many of the bacteria discussed in this review have zoonotic origins and can be acquired through contact with animals. Additionally, some can be transmitted via sexual contact. Importantly, some zoonotic bacterial infections may remain asymptomatic for extended periods, during which time they continue to inflict damage upon male reproductive structures. Therefore, the utilization of polymerase chain reaction and other advanced technologies for the early detection of these microbial agents in semen, even in the absence of a systemic manifestation, is a promising approach.
8. Conclusions
This review discusses some major bacterial zoonotic diseases and explores available studies pointing to their possible effects on male reproduction. It is noted in this review that the effect of these infections on the human male reproductive system has not been well explored. However, studies on experimental animals and some in vitro studies have shown some effects on male reproduction. Evidence supports that some bacterial zoonotic infections show harmful effects on male reproductive function, while a few studies show contrasting or indifferent reports. This exploratory review contributes to knowledge by demonstrating the possible contribution of bacterial zoonotic diseases to the rising male-factor infertility and male reproductive dysfunction.
Author Contributions
Conceptualization, A.F.A. and L.O.O.; validation, A.F.A., W.J.A. and B.S.O.; writing—original draft preparation, L.O.O.; writing—review and editing, L.O.A., A.N.O., V.A., B.G.A., M.C.O., B.S.O. and W.J.A.; supervision, A.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. This manuscript didn’t involve humans or animals studies.
Informed Consent Statement
Not applicable. This manuscript didn’t involve involving humans.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deshpande, P.S.; Gupta, A.S. Causes and prevalence of factors causing infertility in a public health facility. J. Hum. Reprod. Sci. 2019, 12, 287–293. [Google Scholar] [CrossRef]
- Henkel, R. Long-term consequences of sexually transmitted infections on men’s sexual function: A systematic review. Arab J. Urol. 2021, 19, 411–418. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Yao, Y.; Li, J.; Deng, S. Bacterial infections affect male fertility: A focus on the oxidative stress-autophagy axis. Front. Cell Dev. Biol. 2021, 9, e1005523. [Google Scholar] [CrossRef]
- Okeleji, O.L.; Ajayi, L.O.; Odeyemi, A.N.; Amos, V.; Ajayi, H.O.; Akinyemi, A.O.; Nzekwe, C.S.; Adeyemi, J.W.; Ajayi, A.F. Viral zoonotic diseases of public health importance and their effect on male reproduction. Zoonotic Dis. 2022, 2, 291–300. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic diseases: Etiology, impact, and control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- World Health Organization. Asia Pacific Strategy for Emerging Diseases; WHO Regional Office for the Western Pacific: Manila, PL, USA, 2010; Available online: https://iris.wpro.who.int/bitstream/handle/10665.1/7819/9789290615040_eng.pdf (accessed on 23 August 2023).
- Bari, C.D.; Venkateswaran, N.; Fastl, C.; Gabriël, S.; Grace, D.; Havelaar, A.H.; Devleesschauwer, B. The global burden of neglected zoonotic diseases: Current state of evidence. One Health 2023, 17, e100595. [Google Scholar] [CrossRef]
- Asante, J.; Noreddin, A.; El Zowalaty, M.E. Systematic review of important bacterial zoonoses in Africa in the last decade in light of the ‘One Health’ concept. Pathogens 2019, 8, 50. [Google Scholar] [CrossRef]
- Haider, N.; Rothman-Ostrow, P.; Osman, A.Y.; Arruda Liã, B.; Macfarlane-Berry, L.; Elton, L.; Kock, R.A. COVID-19—Zoonosis or emerging infectious disease? Front. Public Health 2020, 8, 763. [Google Scholar] [CrossRef]
- Cantas, L.; Suer, K. Review: The important bacterial zoonoses in “One Health” concept. Front. Public Health 2014, 2, 144. [Google Scholar] [CrossRef]
- Ghasemzadeh, I.; Namazi, S.H. Review of bacterial and viral zoonotic infections transmitted by dogs. J. Med. Life 2015, 8, 1–5, PMID: 28316698PMCID: PMC5319273. [Google Scholar]
- Schaeffer, A.J. Aetiopathology and pathogenesis of urogenital infections. Andrologia 1998, 30, 3–6. [Google Scholar] [CrossRef]
- Sasikumar, S.; Dakshayani, D.; Sarasa, D. An investigation of DNA fragmentation and morphological changes caused by bacteria and fungi in human spermatozoa. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 84–96. [Google Scholar] [CrossRef]
- Elmulla, K.; Köhn, F.; Dandal, M.; Beheiry, A.H.E.; Schiefer, H.; Weidner, W.; Schill, W. In vitro effect of Escherichia coli on human sperm acrosome reaction. Arch. Androl. 1996, 37, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Thaper, D.; Vander, H.; Prabha, V. Pseudomonas aeruginosa: A risk factor for fertility in male mice. Reprod. Biol. 2018, 18, 450–455. [Google Scholar] [CrossRef]
- Oghbaei, H.; Yeganeh Rastgar Rezaei, S.; Nikanfar, S.; Zarezadeh, R.; Sadegi, M.; Latifi, Z.; Nouri, M.; Fattahi, A.; Ahmadi, Y.; Bleisinger, N. Effects of bacteria on male fertility: Spermatogenesis and sperm function. Life Sci. 2020, 256, 117891. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Pacey, A.; Eley, A. Chlamydia trachomatis-induced death of human spermatozoa is caused primarily by lipopolysaccharide. J. Med. Microbiol. 2003, 52, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.I.; Scott, B.L. Sperm motility, morphology, hyperactivation, and ionophore-induced acrosome reactions after overnight incubation with mycoplasmas. Fertil. Steril. 1994, 61, 341–348. [Google Scholar] [CrossRef]
- Gurung, P.; Yetiskul, E.; Jialal, I. Physiology, Male Reproductive System. StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538429/ (accessed on 15 January 2024).
- Al-Agha, O.M.; Axiotis, C.A. An in-depth look at Leydig cell tumor of the testis. Arch. Pathol. Lab. Med. 2007, 131, 311–317. [Google Scholar] [CrossRef]
- O’Donnell, L.; Stanton, P.; de Kretser, D.M. Endocrinology of the Male Reproductive system and Spermatogenesis. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279031/ (accessed on 17 August 2023).
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.A. Male reproductive system. In Pathologic Basis of Veterinary Disease; Elsevier: Philadelphia, PA, USA, 2017; pp. 1194–1222.e1. [Google Scholar] [CrossRef]
- Wong, W.J.; Khan, Y.S. Histology, Sertoli Cell. StatPearls. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560631/ (accessed on 18 August 2023).
- Houda, A.; Nyaz, S.; Mohamed Sobhy, B.; Hussein Bosilah, A.; Romeo, M.; Peter Michael, J.; Mohamad Eid, H. Seminiferous Tubules and Spermatogenesis; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Mawhinney, M.; Mariotti, A. Physiology, pathology and pharmacology of the male reproductive system. Periodontol. 2000 2013, 61, 232–251. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A. Spermatogenesis: An overview. In Sperm Chromatin; Zini, A., Agarwal, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Suede, S.H.; Malik, A.; Sapra, A. Histology, Spermatogenesis. StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553142/ (accessed on 18 August 2023).
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Hafez, E.S.E. Spermatozoa and seminal plasma. In Reproduction in Farm Animals; Hafez, B.E., Hafez, S., Eds.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology 2017, 5, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, D. The functional anatomy of the human spermatozoon: Relating ultrastructure and function. Mol. Hum. Reprod. 2018, 24, 567–592. [Google Scholar] [CrossRef]
- Khawar, M.; Gao, H.; Li, W. Mechanism of acrosome biogenesis in mammals. Front. Cell Dev. Biol. 2019, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Veldhuis, J.D. Hypothalamo-pituitary unit, testis, and male accessory organs. In Yen and Jaffe’s Reproductive Endocrinology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–300.e8. [Google Scholar] [CrossRef]
- Marques, P.; Skorupskaite, K.; Rozario, K.S.; Anderson, R.A.; George, J.T. Physiology of GnRH and Gonadotropin Secretion; Endotext. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279070/ (accessed on 18 August 2023).
- Sadiq, N.M.; Tadi, P. Physiology, Pituitary Hormones. StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557556/ (accessed on 18 August 2023).
- Dorfman, R.I.; Menon, K.M.J.; Sharma, D.C.; Joshi, S.; Forchielli, E. Steroid hormone biosynthesis in rat, rabbit and capuchin testis. Ciba Found. Colloq. Endocrinol. 1967, 16, 91. [Google Scholar] [CrossRef]
- Mindnich, R.; Haller, F.; Halbach, F.; Moeller, G.; Angelis, M.H.D.; Adamski, J. Androgen metabolism via 17β-hydroxysteroid dehydrogenase type 3 in mammalian and non-mammalian vertebrates: Comparison of the human and the zebrafish enzyme. J. Mol. Endocrinol. 2005, 35, 305–316. [Google Scholar] [CrossRef]
- Nedresky, D.; Singh, G. Physiology, Luteinizing Hormone. StatPearls. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539692/ (accessed on 18 August 2023).
- Hinson, J.P. Hormonal control of reproduction part i. In The Endocrine System; Churchill Livingstone: London, UK, 2010; pp. 87–98. [Google Scholar] [CrossRef]
- Eldar-Geva, T.; Liberty, G.; Chertin, B.; Farkas, A.; Margalioth, E.J.; Spitz, I.M. Relationships between FSH, inhibin b, anti-mullerian hormone, and testosterone during long-term treatment with the GnRH-agonist histrelin in patients with prostate cancer. Eur. J. Endocrinol. 2010, 162, 177–181. [Google Scholar] [CrossRef]
- Nassar, G.N.; Leslie, S.W. Physiology, Testosterone. StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526128/ (accessed on 15 January 2024).
- Jones, R.E. Endocrinology, brain, and pituitary gland. Hum. Reprod. Biology. 2014, 3–22. [Google Scholar] [CrossRef]
- Turcu, A.; Smith, J.M.; Auchus, R.; Rainey, W.E. Adrenal androgens and androgen precursors—Definition, synthesis, regulation and physiologic actions. Compr. Physiol. 2014, 4, 1369–1381. [Google Scholar] [CrossRef]
- Johnson, L.R.; Ruhmann-Wennhold, A.; Nelson, D.H. The in vivo effect of ACTH on utilization of reducing energy for pregnenolone synthesis by adrenal mitochondria. Ann. N. Y. Acad. Sci. 1973, 212, 307–318. [Google Scholar] [CrossRef]
- Robert, E.S. Pathogenesis and virulence of zoonotic infections in humans. In The Emergence of Zoonotic Diseases: Understanding the Impact on Animal and Human Health: Workshop Summary; National Academies Press: Washington, DC, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK98094/ (accessed on 8 August 2023).
- Morwal, H.; Bacterial, S.S. zoonosis—A public health importance. J. Dairy Vet. Anim. Res. 2017, 5, 56–59. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Food Safety. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 15 January 2024).
- Peterson, J.W. Bacterial Pathogenesis. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 7. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8526/ (accessed on 15 January 2024).
- Ribet, D.; Cossart, P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015, 17, 173–183. [Google Scholar] [CrossRef]
- Adler, B. Pathogenesis of leptospirosis: Cellular and molecular aspects. Vet. Microbiol. 2014, 172, 353–358. [Google Scholar] [CrossRef]
- Al-Khafaji Alwan, M.J. Effects of oxidative stress on the male reproductive tract of mice infected with Brucella melitensis. World J. Pharm. Res. 2017, 6, 186–199. [Google Scholar] [CrossRef]
- Bapir, R.; Ahmed, S.F.; Tahir, S.H.; Salih, A.M.; Kakamad, F.H.; Ahmed, G.S.; Ali, R.K.; Ahmed, S.M.; Sidiq, S.H. Brucella orchitis presenting as a testicular mass mimicking a testicular tumor: A rare case report. Afr. J. Urol. 2023, 29, 5. [Google Scholar] [CrossRef]
- Haake, D.A.; Levett, P.N. Leptospirosis in humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar] [CrossRef]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Mohd Khalid, M.K.; Amran, F.; Masuzawa, T.; et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Neglected Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef]
- Kastelic, J.P.; Thundathil, J.C. Breeding soundness evaluation and semen analysis for predicting bull fertility. Reprod. Domest. Anim. 2008, 43, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; Bonfanti, L.; Natale, A.; Comin, A.; Ferronato, A.; La Greca, E.; Patregnani, T.; Lucchese, L.; Maragon, S. Application of an integrated outbreak management plan for the control of leptospirosis in dairy cattle herds. Epidemiol. Infect. 2014, 142, 1172–1181. [Google Scholar] [CrossRef]
- Magajevski, F.S.; Girio, R.J.S.; Mathias, L.A.; Myashiro, S.; Genovez, M.E.; Scarcelli, E.P. Detection of Leptospira spp. in the semen and urine of bulls serologically reactive to Leptospira interrogans serovar Hardjo. Braz. J. Microbiol. 2005, 36, 43–47. [Google Scholar] [CrossRef]
- Vijayachari, P.; Sugunan, A.P.; Shriram, A.N. Leptospirosis: An emerging global public health problem. J. Biosci. 2008, 33, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; de Vries, S.G.; Ahmed, A.; Visser, B.J.; Nagel, I.M.; Spijker, R.; Grobusch, M.P.; Hartskeerl, R.A.; Goris, M.G.; Leeflang, M.M. Nucleic acid and antigen detection tests for leptospirosis. Cochrane Database Syst. Rev. 2019, 8, CD011871. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Leptospirosis: Fact Sheet for Clinicians. 2022. Available online: https://www.cdc.gov/leptospirosis/pdf/fs-leptospirosis-clinicians-eng-508.pdf (accessed on 18 August 2022).
- Ngetich, W. Review of Anthrax: A Disease of Animals and Humans. Int. J. Agric. Environ. Biores. 2019, 4, 123–134. [Google Scholar]
- Goel, A.K. Anthrax: A disease of biowarfare and public health importance. World J. Clin. Cases 2015, 3, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Scorpio, A.; Blank, T.E.; Day, W.A.; Chabot, D.J. Biological weapons. Cell. Mol. Life Sci. 2006, 63, 2237–2248. [Google Scholar] [CrossRef]
- Catherino, W.H.; Levi, A.; Kao, T.C.; Leondires, M.P.; McKeeby, J.; Segars, J.H. Anthrax vaccine does not affect semen parameters, embryo quality, or pregnancy outcome in couples with a vaccinated male military service member. Fertil. Steril. 2005, 83, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Naumenkova, V.A.; Kalinova, A.V. Changes in Parameters of Fresh and Deconserved Sperm of Stallions after Their Vaccination against Anthrax. Russ. Agric. Sci. 2019, 45, 589–592. [Google Scholar] [CrossRef]
- Nardi Júnior, G.; Megid, J.; Mathias, L.A.; Paulin, L.; Vicente, A.F.; Cortez, A.; Ribeiro, M.G. Performance of microbiological, serological, molecular, and modified seminal plasma methods in the diagnosis of Brucella abortus in semen and serum of bovine bulls. Biology 2017, 48, 6–9. [Google Scholar] [CrossRef]
- Meltzer, E.; Sidi, Y.; Smolen, G.; Banai, M.; Bardenstein, S.; Schwartz, E. Sexually Transmitted Brucellosis in Humans. Clin. Infect. Dis. 2010, 51, e12–e15. [Google Scholar] [CrossRef]
- Tuon, F.F.; Gondolfo, R.B.; Cerchiari, N. Human-to-human transmission of Brucella—A systematic review. Trop. Med. Int. Health 2017, 22, 539–546. [Google Scholar] [CrossRef]
- Alsaif, M.; Dabelah, K.; Girim, H.; Featherstone, R.; Robinson, J.L. Congenital Brucellosis: A Systematic Review of the Literature. Vector-Borne Zoonotic Dis. 2018, 18, 393–403. [Google Scholar] [CrossRef]
- Islam, M.S.; Islam, M.A.; Rahman, M.M.; Islam, K.; Islam, M.M.; Kamal, M.M.; Islam, M.N. Presence of brucella spp. in milk and dairy products: A comprehensive review and its perspectives. J. Food Qual. 2023, 2023, 2932883. [Google Scholar] [CrossRef]
- Baud, D.; Greub, G. Intracellular bacteria and adverse pregnancy outcomes. Clin. Microbiol. Infect. 2017, 17, 1312–1322. [Google Scholar] [CrossRef]
- Megid, J.; Mathias, L.A.; Robles, C. Clinical manifestations of Brucellosis in domestic animals and humans. Open Vet. Sci. J. 2010, 4, 119–126. [Google Scholar] [CrossRef]
- Akınci, E.; Hürrem, B.; Mustafa, A.Ç.; Ayşe, E.; Selim, S.E.; İpek, Z.; Neriman, B.; Ali, A.; Gülüşan, E. A complication of brucellosis: Epididymoorchitis. Int. J. Infect. Dis. 2006, 10, 171–177. [Google Scholar] [CrossRef]
- Ip, C.C.K.; Tumali, K.; Hoh, I.M.; Arunasalam, A. Acute epididymo-orchitis from brucellosis melitensis in Australia. BMJ Case Rep. CP 2019, 12, e230007. [Google Scholar] [CrossRef]
- Kassur, B.; Dziubek, Z. Andrologic studies and sexual potency in chronic human brucellosis. Infection 1980, 8, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Colagar, H.A.; Karimi, F.; Jorsaraei, S.G. Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoastheno- teratospermic men. Iran. Red Crescent Med. J. 2013, 15, 780–785. [Google Scholar] [CrossRef]
- Karaköse, A.; Yuksel, M.B.; Aydoğdu, O.; Hamidi, A.A. Epididymoorchitis as the first finding in patients with brucellosis. Adv. Urol. 2013, 2013, 765023. [Google Scholar] [CrossRef] [PubMed]
- Piorunek, M.; Brajer-Luftmann, B.; Walkowiak, J. Pasteurella Multocida Infection in Humans. Pathogens 2023, 12, 1210. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Hug, M. Pasteurella Multocida; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557629/ (accessed on 15 January 2024).
- Rehab, E.M.; Hend, M.M.; Amal, S.E. Pathological Studies on Infertility in Bucks as a Sign to Some Bacterial Infection. Int. J. Curr. Res. Biosci. Plant Biol. 2018, 5, 7–33. [Google Scholar] [CrossRef]
- García-Pastor, L.; Blasco, J.M.; Barberán, M. Pasteurellosis as a cause of genital lesions in rams. A descriptive study. Small Rumin. Res. 2009, 87, 111–115. [Google Scholar] [CrossRef]
- Gorga, F.; Galdiero, M.; Buommino, E.; Galdiero, E. Porins and lipopolysaccharide induce apoptosis in human spermatozoa. Clin. Diagn. Lab. Immunol. 2001, 8, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Trefois, Q.; Marot, J.C.; Yildiz, H.; Wieers, G. Fever, bone pain and erectile dysfunction. Where Is Cat? BMJ Case Rep. 2017, 2017, bcr2017221397. [Google Scholar] [CrossRef] [PubMed]
- Mary, D.B. Yersinia enterocolitica. In Reference Module in Food Science; Elsevier: Philadelphia, PA, USA, 2016; Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780081005965009963 (accessed on 15 January 2024).
- Elena, T.; Victoria, N.; Veselin, P. Annuaire de L’universite de Sofia “st. Kliment Ohridski” Faculte de Biologie Livre 1—Zoologie Tome; University Publishing House “St. Kliment Ohridski”: Sofia, Bulgaria, 2011; p. 99. [Google Scholar]
- Maurin, M.; Raoult, D. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef] [PubMed]
- Kopecny, L.; Katrina, L.B.; Amanda, S.; Jacqueline, M.N. Investigating Coxiella burnetiid infection in a breeding cattery at the centre of a Q fever outbreak. J. Feline Med. Surg. 2013, 15, 1037–1045. [Google Scholar] [CrossRef]
- Kruszewska, D.; Tylewska-Wierzbanowska, S.K. Coxiella burnetii penetration into the reproductive system of male mice, promoting sexual transmission of infection. ASM J. Infect. Immun. 1993, 61, 4188–4195. [Google Scholar] [CrossRef]
- Tylewska-Wierzbanowska, S.; Kruszewska, D. Detection of Coxiella burnetii bacteria in urine and semen with dot-ELISA and IFA as diagnostic methods in rapid recognition of Q fever. Serodiagn. Immunother. Infect. Dis. 1993, 5, 220–223. [Google Scholar] [CrossRef]
- Yatsentyuk, S.P.; Lazareva, E.A.; Gorbacheva, N.S.; Krasnikova, M.S.; Kozlova, A.D. PCR Detection of Coxiella burnetii from bull semen samples used for artificial insemination. Russ. J. Agric. Socio-Econ. Sci. 2019, 8, 664. [Google Scholar] [CrossRef]
- Kruszewska, D.; Tylewska-Wierzbanowska, S. Isolation of Coxiella burnetii from bull semen. Res. Vet. Sci. 1997, 62, 299–300. [Google Scholar] [CrossRef]
- Milazzo, A.; Hall, R.; Storm, P.A.; Harris, R.J.; Winslow, W.; Marmion, B.P. Sexually transmitted Q fever. Clin. Infect. Dis. 2001, 33, 399–402. [Google Scholar] [CrossRef]
- Guatteo, R.; Beaudeau, F.; Berri, M.; Rodolakis, A.; Joly, A.; Rodolakis, A.; Seegers, H. Shedding routes of Coxiella burnetii in dairy cows: Implications for detection and control. Vet. Res. 2006, 37, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, L.D.; Deutscher, M.; Rubin, J.E.; Deneer, H.; Kanthan, R.; Sanche, S.; Blondeau, J.M. Urinary tract infection in a human male patient with Staphylococcus pseudintermedius transmission from the family dog. J. Chemother. 2022, 34, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Ďuračka, M.; Kováčik, A.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Bacteria may deteriorate progressive motility of bovine spermatozoa and biochemical parameters of seminal plasma. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 844–847. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Song, J.; Liu, H.; Bi, W.; Dong, G.; Zhou, T. Characteristic and mechanism of immobilization effect of Staphylococcus aureus on human spermatozoa. Microb. Pathog. 2018, 119, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Une, Y.; Mori, T. Tuberculosis as a zoonosis from a veterinary perspective. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Malik, S. Genital Tuberculosis and its Impact on Male and Female Infertility. US Endocrinol. 2020, 16, 97–103. [Google Scholar] [CrossRef]
- Tzvetkov, D.; Tzvetkova, P. Tuberculosis of Male Genital System—Myth or Reality in 21st Century. Arch. Androl. 2006, 52, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kulchavenya, E.; Osadchiy, A.; Khomyakov, V. Tuberculosis as a Reason for Male and Female Sexual Dysfunction (Review). Ann. Infect. Dis. 2017, 1, 101. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharya, S.M.; Bagchi, B.; Chaudhuri, A.R.; Datta, A. Latent Genital Tuberculosis in Male—A Possible Cause of Reproductive Failure. J. Reprod. Med. Gynecol. Obstet. 2020, 5, 057. [Google Scholar] [CrossRef]
- Sole-Balcells, F.; Jimenez-Cruz, F.; de Cabezon, J.S.; Rosello, A.S. Tuberculosis and infertility in men. Eus. Urol. 1977, 3, 129–131. [Google Scholar] [CrossRef]
- Kumar, R. Reproductive tract tuberculosis and male infertility. Indian J. Urol. 2008, 24, 392–395. [Google Scholar] [CrossRef]
- Humphrey, T.; O’Brien, S.; Madsen, M. Campylobacters as zoonotic pathogens: A food production perspective. Int. J. Food Microbiol. 2007, 117, 237–257. [Google Scholar] [CrossRef]
- Vizzier-Thaxton, Y.; Cox, N.A.; Richardson, L.J.; Buhr, R.J.; McDaniel, C.D.; Cosby, D.E.; Wilson, J.L.; Bourassa, D.V.; Ard, M.B. Apparent attachment of Campylobacter and Salmonella to broiler breeder rooster spermatozoa. Poult. Sci. 2016, 85, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Cagnoli, C.; Chiapparrone, M.L.; Cacciato, C.S.; Rodríguez, M.G.; Aller, J.F.; Catena, M.C. Effects of Campylobacter fetus on bull sperm quality. Microb. Pathog. 2020, 149, 104486. [Google Scholar] [CrossRef] [PubMed]
- Bar, T.Z.; Yehuda, R.; Hacham, T.; Krupnik, S.; Bartoov, B. Influence of Campylobacter fetus subsp. fetus on ram sperm cell quality. J. Med. Microbiol. 2008, 57, 1405–1410. [Google Scholar] [CrossRef]
- Sanagawa, M.; Kenzaka, T.; Kato, S.; Yamaoka, I.; Fujimoto, S. Campylobacter jejuni enterocolitis presenting with testicular pain: A case report. World J. Clin. Cases 2020, 8, 3280–3283. [Google Scholar] [CrossRef]
- Wasteson, Y. Zoonotic Escherichia coli. Acta Vet. Scand. Suppl. 2001, 95, 79–84. [Google Scholar] [CrossRef]
- Alharbi, M.G.; Al-Hindi, R.R.; Esmael, A.; Alotibi, I.A.; Azhari, S.A.; Alseghayer, M.S.; Teklemariam, A.D. The “big six”: Hidden emerging foodborne bacterial pathogens. Trop. Med. Infect. Dis. 2022, 7, 356. [Google Scholar] [CrossRef]
- Beutin, L.; Krause, G.; Zimmermann, S.; Kaulfuss, S.; Gleier, K. Characterization of Shiga toxin-producing Escherichia coli strains isolated from human patients in Germany over a 3-year period. J. Clin. Microbiol. 2004, 42, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Bussalleu, E.Y.M.; Sepúlveda, L.; Torner, E.; Pinart, E.; Bonet, S. Effects of different concentrations of enterotoxigenic and verotoxigenic E. coli on boar sperm quality. Anim. Reprod. Sci. 2011, 127, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Fsihi, H.; Steffen, P.; Cossart, P. Listeria monocytogenes. In Principles of Bacterial Pathogenesis; Groisman, E.A., Ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 751–803. ISBN 9780123042200. [Google Scholar]
- Sanui, H.; Yoshida, S.; Himeno, K.; Nomoto, K. Experimental allergic orchitis induced by unilateral intratesticular bacterial infection in guinea-pigs. Immunology 1983, 49, 45–51. [Google Scholar]
- Oyeyemi, M.O.; Ayinmode, A.B.; Adetunji, V.O.; Akin-Taiwo, M.A. The semen characteristics of West African Dwarf bucks infected with Listeria monocytogenes. Bull. Anim. Health Prod. Afr. 2008, 56, 307–313. [Google Scholar] [CrossRef]
- Tohidpour, M.; Shahhosseiny, M.; Mehrabian, S.; Saremi, A. Prevalence of Chlamydia trachomatis and Listeria monocytogenes in Infertile Men and the Effect on Semen Parameters. Jundishapur J. Microbiol. 2020, 13, e97780. [Google Scholar] [CrossRef]
- von Schnakenburg, C.; Hinrichs, B.; Fuchs, J.; Kardorff, R. Post-transplant epididymitis and orchitis following Listeria monocytogenes septicaemia. Pediatr. Transplant. 2000, 4, 156–158. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).