Comparative Analysis of Endophytic Curtobacterium Species Reveals Commonalities and Adaptations
Abstract
1. Introduction
2. Methods
2.1. Bacterial Growth Media
2.2. Isolation of Bacterial Endophytes
2.3. Visualization of Bacteria
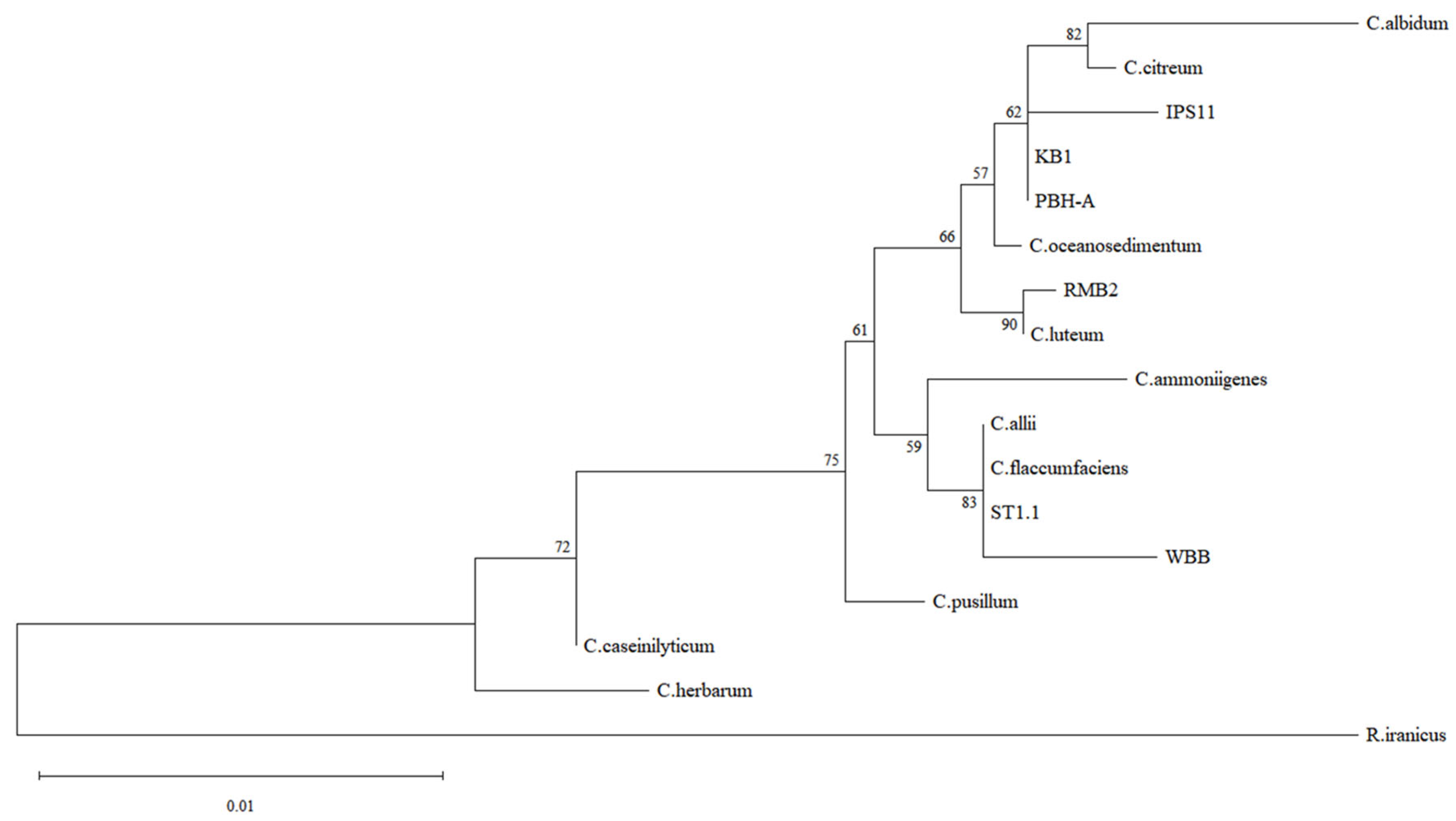
2.4. Molecular Identification and Phylogenetic Analysis of Bacterial Isolates
2.5. Detection of Bacterial Pigments
2.6. Metabolic Tests of Bacterial Isolates
2.7. Stress Tolerance of Bacterial Isolates
2.8. Statistical Tests
3. Results
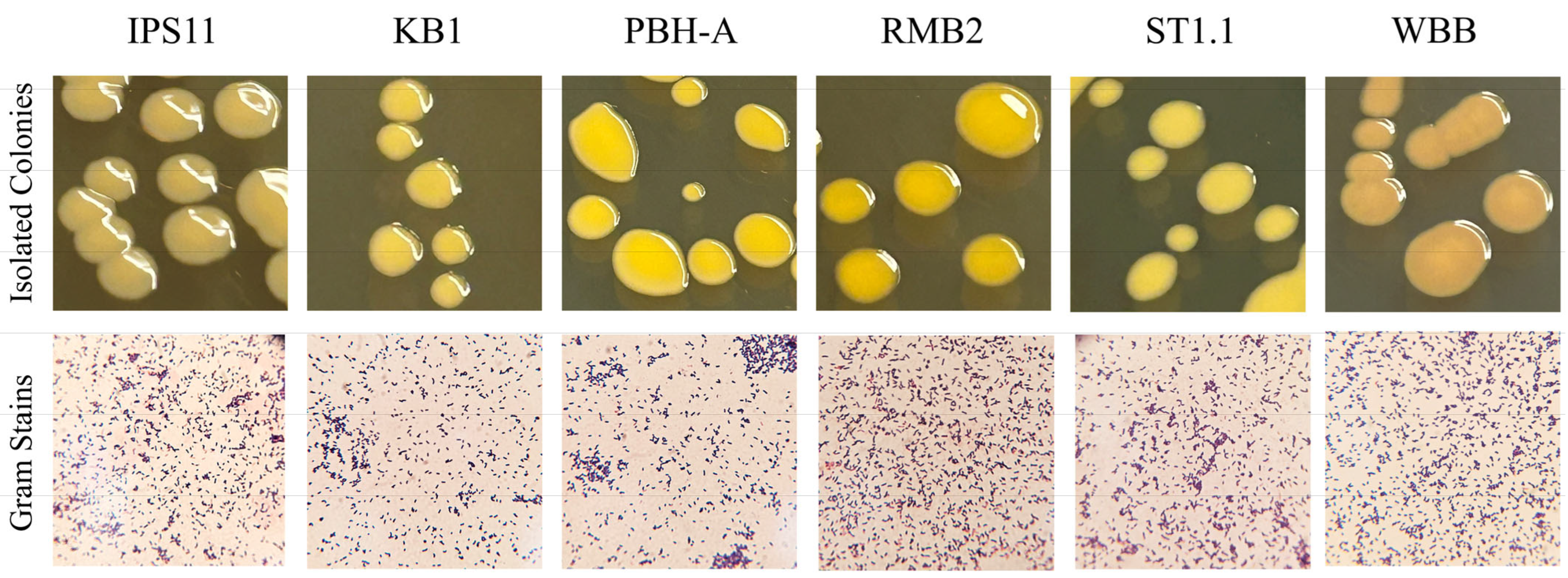
3.1. Curtobacterium Species Isolated from Various Sources
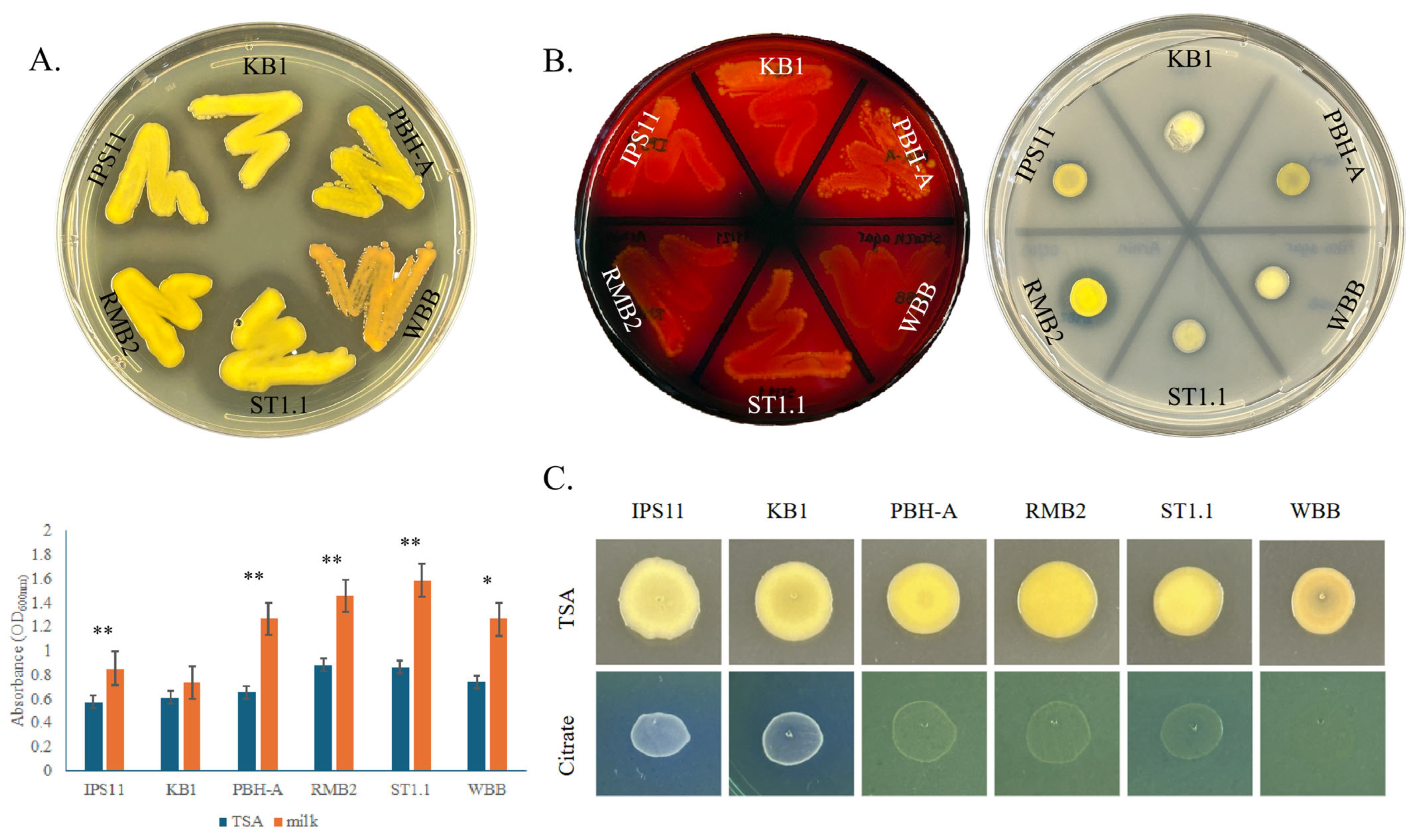
3.2. Pigmentation in Curtobacterium Species
3.3. All Curtobacterium Isolates Could Digest Starch, Casein and Insoluble Phosphate, but Differed in Their Ability to Utilize Citrate
3.4. All Curtobacterium Isolates Could Ferment Fructose, Sucrose and Glucose, but Some Isolates Developed Specialized Sugar Fermentation Capacity
3.5. All Curtobacterium Isolates Are Psychrotolerant, with the Exception of One Isolate That Is Thermotolerant
4. Discussion
4.1. Curtobacterium Species as Plant Endophytes
4.2. Morphological Features of Curtobacterium Species
4.3. Nutritional Preferences of Curtobacterium Species
4.4. Temperature Adaptations of Curtobacterium Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | Extracellular Polysaccharide |
| IPS11 | Indian Pipe Stem 1 |
| OD | Optical Density |
| PCR | Polymerase Chain Reaction |
| TSA(B) | Tryptic Soy Agar (Broth) |
References
- Saddler, G.S.; Guimarāes, P.M. Curtobacterium. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 1–14. [Google Scholar] [CrossRef]
- Evseev, P.; Lukianova, A.; Tarakanov, R.; Tokmakova, A.; Shneider, M.; Ignatov, A.; Miroshnikov, K. Curtobacterium spp. and Curtobacterium flaccumfaciens: Phylogeny, Genomics-Based Taxonomy, Pathogenicity, and Diagnostics. Curr. Issues Mol. Biol. 2022, 44, 889–927. [Google Scholar] [CrossRef]
- Chase, A.B.; Arevalo, P.; Polz, M.F.; Berlemont, R.; Martiny, J.B. Evidence for Ecological Flexibility in the Cosmopolitan Genus Curtobacterium. Front. Microbiol. 2016, 7, 1874. [Google Scholar] [CrossRef] [PubMed]
- Osdaghi, E.; Taghavi, S.M.; Calamai, S.; Biancalani, C.; Cerboneschi, M.; Tegli, S.; Harveson, R.M. Phenotypic and Molecular-Phylogenetic Analysis Provide Novel Insights into the Diversity of Curtobacterium flaccumfaciens. Phytopathology 2018, 108, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Khanal, M.; Bhatta, B.P.; Malla, S. Isolation and Characterization of Bacteria Associated with Onion and First Report of Onion Diseases Caused by Five Bacterial Pathogens in Texas, U.S.A. Plant Dis. 2023, 107, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Sturz, A.V.; Christie, B.R.; Matheson, B.G.; Nowak, J. Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. Biol. Fertil. Soils 1997, 25, 13–19. [Google Scholar] [CrossRef]
- DUNLEAVY, J.M. Curtobacterium plantarum sp. nov. Is Ubiquitous in Plant Leaves and Is Seed Transmitted in Soybean and Corn†. Int. J. Syst. Evol. Microbiol. 1989, 39, 240–249. [Google Scholar] [CrossRef]
- Munir, S.; Li, Y.; He, P.; Huang, M.; He, P.; He, P.; Cui, W.; Wu, Y.; He, Y. Core endophyte communities of different citrus varieties from citrus growing regions in China. Sci. Rep. 2020, 10, 3648. [Google Scholar] [CrossRef]
- Behrendt, U.; Ulrich, A.; Schumann, P.; Naumann, D.; Suzuki, K.I. Diversity of grass-associated Microbacteriaceae isolated from the phyllosphere and litter layer after mulching the sward; polyphasic characterization of Subtercola pratensis sp. nov., Curtobacterium herbarum sp. nov. and Plantibacter flavus gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 1441–1454. [Google Scholar] [CrossRef]
- Saranya, K.; Sundaramanickam, A.; Manupoori, S.; Kanth, S.V. Screening of multi-faceted phosphate-solubilising bacterium from seagrass meadow and their plant growth promotion under saline stress condition. Microbiol. Res. 2022, 261, 127080. [Google Scholar] [CrossRef]
- Patel, M.; Patel, K.; Al-Keridis, L.A.; Alshammari, N.; Badraoui, R.; Elasbali, A.M.; Al-Soud, W.A.; Hassan, M.I.; Yadav, D.K.; Adnan, M. Cadmium-Tolerant Plant Growth-Promoting Bacteria Curtobacterium oceanosedimentum Improves Growth Attributes and Strengthens Antioxidant System in Chili (Capsicum frutescens). Sustainability 2022, 14, 4335. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Magalhaes, K.T.; Lorenzetii, E.R.; Souza, T.P.; Schwan, R.F. A multiphasic approach for the identification of endophytic bacterial in strawberry fruit and their potential for plant growth promotion. Microb. Ecol. 2012, 63, 405–417. [Google Scholar] [CrossRef]
- Vimal, S.R.; Patel, V.K.; Singh, J.S. Plant growth promoting Curtobacterium albidum strain SRV4: An agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indic. 2019, 105, 553–562. [Google Scholar] [CrossRef]
- Funke, G.; Aravena-Roman, M.; Frodl, R. First description of Curtobacterium spp. isolated from human clinical specimens. J. Clin. Microbiol. 2005, 43, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhao, Y.; Shen, W.; Han, D.; Yang, M. Seed-to-Seed: Plant Core Vertically Transmitted Microbiota. J. Agric. Food Chem. 2023, 71, 19255–19264. [Google Scholar] [CrossRef] [PubMed]
- Fagorzi, C.; Mengoni, A. Endophytes: Improving Plant Performance. Microorganisms 2022, 10, 1777. [Google Scholar] [CrossRef]
- Chesneau, G.; Torres-Cortes, G.; Briand, M.; Darrasse, A.; Preveaux, A.; Marais, C.; Jacques, M.-A.; Shade, A.; Barret, M. Temporal dynamics of bacterial communities during seed development and maturation. FEMS Microbiol. Ecol. 2020, 96, fiaa190. [Google Scholar] [CrossRef]
- Bulgari, D.; Minio, A.; Casati, P.; Quaglino, F.; Delledonne, M.; Bianco, P.A. Curtobacterium sp. Genome Sequencing Underlines Plant Growth Promotion-Related Traits. Genome Announc. 2014, 2, e00592-14. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Li, X.P.; Du, Y.; Xu, B.T.; Zhang, X.L. Responses of soil microbial communities in mulberry rhizophere to intercropping and nitrogen application. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2019, 30, 1983–1992. [Google Scholar] [CrossRef]
- Burgdorf, R.J.; Laing, M.D.; Morris, C.D.; Jamal-Ally, S.F. A procedure to evaluate the efficiency of surface sterilization methods in culture-independent fungal endophyte studies. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2014, 45, 977–983. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Chen, H.H.; Zhao, G.Z.; Zhu, W.Y.; Jiang, C.L.; Xu, L.H.; Li, W.J. Isolation, diversity, and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl. Environ. Microbiol. 2009, 75, 6176–6186. [Google Scholar] [CrossRef]
- Moyes, R.B.; Reynolds, J.; Breakwell, D.P. Differential Staining of Bacteria: Gram Stain. Curr. Protoc. Microbiol. 2009, 15, A.3C.1–A.3C.8. [Google Scholar] [CrossRef]
- Suzuki, M.T.; Giovannoni, S.J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 1996, 62, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.D.; Poulsen, L.K.; Stahl, D.A. Monitoring the enrichment and isolation of sulfate-reducing bacteria by using oligonucleotide hybridization probes designed from environmentally derived 16S rRNA sequences. Appl. Environ. Microbiol. 1993, 59, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.M.; O’Sullivan, O.; Higgins, D.G.; Notredame, C. M-Coffee: Combining multiple sequence alignment methods with T-Coffee. Nucleic Acids Res. 2006, 34, 1692–1699. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.R.; Mahawar, H.; Bajpai, A.; Dubey, G.; Parmar, R.; Atoliya, N.; Devi, M.H.; Singh, A.B.; Jain, D.; Patra, A.; et al. Methylotroph bacteria and cellular metabolite carotenoid alleviate ultraviolet radiation-driven abiotic stress in plants. Front. Microbiol. 2022, 13, 899268. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiology 1948, 17, 362–370. [Google Scholar]
- Wan, Y.; Du, Y.; Miyakoshi, T. Enzymatic catalysis of 2,6-dimethoxyphenol by laccases and products characterization in organic solutions. Sci. China Ser. B Chem. 2008, 51, 669–676. [Google Scholar] [CrossRef]
- Neifar, M.; Chouchane, H.; Mahjoubi, M.; Jaouani, A.; Cherif, A. Pseudomonas extremorientalis BU118: A new salt-tolerant laccase-secreting bacterium with biotechnological potential in textile azo dye decolourization. 3 Biotech 2016, 6, 107. [Google Scholar] [CrossRef]
- Schillaci, M.; Raio, A. Pseudomonas and Curtobacterium Strains from Olive Rhizosphere Characterized and Evaluated for Plant Growth Promoting Traits. Plants 2022, 11, 2245. [Google Scholar] [CrossRef] [PubMed]
- Chacon, F.I.; Sineli, P.E.; Mansilla, F.I.; Pereyra, M.M.; Diaz, M.A.; Volentini, S.I.; Poehlein, A.; Meinhardt, F.; Daniel, R.; Dib, J.R. Native Cultivable Bacteria from the Blueberry Microbiome as Novel Potential Biocontrol Agents. Microorganisms 2022, 10, 969. [Google Scholar] [CrossRef]
- Kooner, A.; Soby, S. Draft Genome Sequence of Curtobacterium sp. Strain MWU13-2055, Isolated from a Wild Cranberry Fruit Surface in Massachusetts, USA. Microbiol. Resour. Announc. 2022, 11, e0056522. [Google Scholar] [CrossRef]
- Lorenzini, M.; Zapparoli, G. Epiphytic bacteria from withered grapes and their antagonistic effects on grape-rotting fungi. Int. J. Food Microbiol. 2020, 319, 108505. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, W.J.; Buyer, J.S. Culturable bacterial microflora associated with nectarine fruit and their potential for control of brown rot. Can. J. Microbiol. 2010, 56, 480–486. [Google Scholar] [CrossRef]
- Vega, F.E.; Pava-Ripoll, M.; Posada, F.; Buyer, J.S. Endophytic bacteria in Coffea arabica L. J. Basic Microbiol. 2005, 45, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Steven, B.; Zeng, Q. Complete Genome Sequences of Curtobacterium, Pantoea, Erwinia, and Two Pseudomonas sp. Strains, Isolated from Apple Flower Stigmas from Connecticut, USA. Microbiol. Resour. Announc. 2021, 10, e00154-21. [Google Scholar] [CrossRef]
- Harveson, R.M.; Schwartz, H.F.; Urrea, C.A.; Yonts, C.D. Bacterial Wilt of Dry-Edible Beans in the Central High Plains of the U.S.: Past, Present, and Future. Plant Dis. 2015, 99, 1665–1677. [Google Scholar] [CrossRef]
- Procopio, R.E.; Araujo, W.L.; Maccheroni, W., Jr.; Azevedo, J.L. Characterization of an endophytic bacterial community associated with Eucalyptus spp. Genet. Mol. Res. GMR 2009, 8, 1408–1422. [Google Scholar] [CrossRef]
- Magnani, G.S.; Didonet, C.M.; Cruz, L.M.; Picheth, C.F.; Pedrosa, F.O.; Souza, E.M. Diversity of endophytic bacteria in Brazilian sugarcane. Genet. Mol. Res. GMR 2010, 9, 250–258. [Google Scholar] [CrossRef]
- Sun, T.; Yang, Y.; Duan, K.; Liao, Y.; Zhang, Z.; Guan, Z.; Chen, S.; Fang, W.; Chen, F.; Zhao, S. Biodiversity of Endophytic Microbes in Diverse Tea Chrysanthemum Cultivars and Their Potential Promoting Effects on Plant Growth and Quality. Biology 2023, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Kizheva, Y.; Pandova, M.; Dimitrova, M.; Gladicheva, Y.; Garkova, M.; Pirnareva, D.; Donchev, D.; Moncheva, P.; Hristova, P. First Report of Curtobacterium flaccumfaciens in Bulgaria. Pathogens 2024, 13, 483. [Google Scholar] [CrossRef]
- Freeman, J.; Firrincieli, A.; Baker, D.; Doty, S. Curtobacterium salicis sp. nov., isolated from willow tree stems in Washington state. Antonie Leeuwenhoek 2024, 117, 62. [Google Scholar] [CrossRef]
- Perez, M.L.; Collavino, M.M.; Sansberro, P.A.; Mroginski, L.A.; Galdeano, E. Diversity of endophytic fungal and bacterial communities in Ilex paraguariensis grown under field conditions. World J. Microbiol. Biotechnol. 2016, 32, 61. [Google Scholar] [CrossRef] [PubMed]
- Gagne-Bourgue, F.; Aliferis, K.A.; Seguin, P.; Rani, M.; Samson, R.; Jabaji, S. Isolation and characterization of indigenous endophytic bacteria associated with leaves of switchgrass (Panicum virgatum L.) cultivars. J. Appl. Microbiol. 2013, 114, 836–853. [Google Scholar] [CrossRef] [PubMed]
- Passera, A.; Compant, S.; Casati, P.; Maturo, M.G.; Battelli, G.; Quaglino, F.; Antonielli, L.; Salerno, D.; Brasca, M.; Toffolatti, S.L.; et al. Not Just a Pathogen? Description of a Plant-Beneficial Pseudomonas syringae Strain. Front. Microbiol. 2019, 10, 1409. [Google Scholar] [CrossRef]
- Osdaghi, E.; Young, A.J.; Harveson, R.M. Bacterial wilt of dry beans caused by Curtobacterium flaccumfaciens pv. flaccumfaciens: A new threat from an old enemy. Mol. Plant Pathol. 2020, 21, 605–621. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Mohammadi, M.; Burbank, L.; Roper, M.C. Biological role of pigment production for the bacterial phytopathogen Pantoea stewartii subsp. stewartii. Appl. Environ. Microbiol. 2012, 78, 6859–6865. [Google Scholar] [CrossRef]
- Ungureanu, C.; Ferdeș, M. Evaluation of Antioxidant and Antimicrobial Activities of Torularhodin. Adv. Sci. Lett. 2012, 18, 50–53. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M. What Do We Know about Antimicrobial Activity of Astaxanthin and Fucoxanthin? Mar. Drugs 2021, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Biller, S.J.; Lundeen, R.A. Prochlorococcus extracellular vesicles: Molecular composition and adsorption to diverse microbes. Environ. Microbiol. 2022, 24, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.C.; Lang, J.M.; Darling, A.E.; Eisen, J.A.; Coil, D.A. Draft Genome Sequence of Curtobacterium flaccumfaciens Strain UCD-AKU (Phylum Actinobacteria). Genome Announc. 2013, 1, e00244-13. [Google Scholar] [CrossRef] [PubMed]
- Preda, M.; Mihai, M.M. Phenotypic and genotypic virulence features of staphylococcal strains isolated from difficult-to-treat skin and soft tissue infections. PLoS ONE 2021, 16, e0246478. [Google Scholar] [CrossRef]
- Feng, G.-D.; Li, J.; Yang, S.; Zhang, J.; Zhu, H. Curtobacterium caseinilyticum sp. nov., Curtobacterium subtropicum sp. nov. and Curtobacterium citri sp. nov., isolated from citrus phyllosphere. Int. J. Syst. Evol. Microbiol. 2023, 73, 006152. [Google Scholar] [CrossRef]
- Krimi, Z.; Ziouche, C.; Tafifet, L.; Djellout, H.; Mohamed-Mahmoud, F.; Raio, A. Euphorbia helioscopia a Putative Plant Reservoir of Pathogenic Curtobacterium flaccumfaciens. Curr. Microbiol. 2023, 80, 154. [Google Scholar] [CrossRef]
- Chen, Y.F.; Yin, Y.N.; Zhang, X.M.; Guo, J.H. Curtobacterium flaccumfaciens pv. beticola, A New Pathovar of Pathogens in Sugar Beet. Plant Dis. 2007, 91, 677–684. [Google Scholar] [CrossRef]
- Gonzalez, A.J.; Tello, J.C.; Rodicio, M.R. Bacterial Wilt of Beans (Phaseolus vulgaris) Caused by Curtobacterium flaccumfaciens in Southeastern Spain. Plant Dis. 2005, 89, 1361. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Weigend, M.; Mustafa, A.; Ensikat, H.J. Calcium phosphate in plant trichomes: The overlooked biomineral. Planta 2018, 247, 277–285. [Google Scholar] [CrossRef]
- Pereira, S.I.; Castro, P.M. Diversity and characterization of culturable bacterial endophytes from Zea mays and their potential as plant growth-promoting agents in metal-degraded soils. Environ. Sci. Pollut. Res. Int. 2014, 21, 14110–14123. [Google Scholar] [CrossRef] [PubMed]
- Diez-Mendez, A.; Rivas, R. Improvement of saffron production using Curtobacterium herbarum as a bioinoculant under greenhouse conditions. AIMS Microbiol. 2017, 3, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Kirui, C.K.; Njeru, E.M.; Runo, S. Diversity and Phosphate Solubilization Efficiency of Phosphate Solubilizing Bacteria Isolated from Semi-Arid Agroecosystems of Eastern Kenya. Microbiol. Insights 2022, 15, 11786361221088991. [Google Scholar] [CrossRef]
- Kandel, S.L.; Firrincieli, A.; Joubert, P.M.; Okubara, P.A.; Leston, N.D.; McGeorge, K.M.; Mugnozza, G.S.; Harfouche, A.; Kim, S.H.; Doty, S.L. An In vitro Study of Bio-Control and Plant Growth Promotion Potential of Salicaceae Endophytes. Front. Microbiol. 2017, 8, 386. [Google Scholar] [CrossRef]
- Verma, S.K.; White, J.F. Indigenous endophytic seed bacteria promote seedling development and defend against fungal disease in browntop millet (Urochloa ramosa L.). J. Appl. Microbiol. 2018, 124, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Concórdio-Reis, P.; Pereira, J.R.; Torres, C.A.V.; Sevrin, C.; Grandfils, C.; Freitas, F. Effect of mono- and dipotassium phosphate concentration on extracellular polysaccharide production by the bacterium Enterobacter A47. Process Biochem. 2018, 75, 16–21. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, Y.J.; Kim, H.B.; Kim, S.Y.; Yi, T.H.; Yang, D.C. Curtobacterium ginsengisoli sp. nov., isolated from soil of a ginseng field. Int. J. Syst. Evol. Microbiol. 2008, 58, 2393–2397. [Google Scholar] [CrossRef]
- Vincente, A.R.; Manganaris, G.A.; Ortiz, C.M.; Sozzi, G.O.; Crisosto, C.H. Chapter 5—Nutritional Quality of Fruits and Vegetables. In Postharvest Handling, 3rd ed.; Florkowski, W.J., Shewfelt, R.L., Brueckner, B., Prussia, S.E., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 69–122. [Google Scholar]
- Aizawa, T.; Ve, N.B.; Kimoto, K.I.; Iwabuchi, N.; Sumida, H.; Hasegawa, I.; Sasaki, S.; Tamura, T.; Kudo, T.; Suzuki, K.I.; et al. Curtobacterium ammoniigenes sp. nov., an ammonia-producing bacterium isolated from plants inhabiting acidic swamps in actual acid sulfate soil areas of Vietnam. Int. J. Syst. Evol. Microbiol. 2007, 57, 1447–1452. [Google Scholar] [CrossRef]
- Tokmakova, A.D.; Tarakanov, R.I.; Lukianova, A.A.; Evseev, P.V.; Dorofeeva, L.V.; Ignatov, A.N.; Dzhalilov, F.S.; Subbotin, S.A.; Miroshnikov, K.A. Phytopathogenic Curtobacterium flaccumfaciens Strains Circulating on Leguminous Plants, Alternative Hosts and Weeds in Russia. Plants 2024, 13, 667. [Google Scholar] [CrossRef]
- Marshall, R.J. Food and nutritional analysis|Dairy Products. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 312–319. [Google Scholar]
- Cortez, M.A.S.; Duarte, M.C.K.H.; de Melo, L.R.B. Chapter 1—Relevant factors for raw milk quality for dairy foods manufacture. In Dairy Foods; Cruz, A.G.d., Ranadheera, C.S., Nazzaro, F., Mortazavian, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 1–17. [Google Scholar]
- Kuddus, M.; Ramteke, P.W. A cold-active extracellular metalloprotease from Curtobacterium luteum (MTCC 7529): Enzyme production and characterization. J. Gen. Appl. Microbiol. 2008, 54, 385–392. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Hou, L.; Xu, G.; Guan, F.; Zhang, W.; Luo, H.; Wu, N.; Yao, B.; Zhang, C.; et al. The seed endophytic microbe Microbacterium testaceum M15 enhances the cold tolerance and growth of rice (Oryza sativa L.). Microbiol. Res. 2024, 289, 127908. [Google Scholar] [CrossRef] [PubMed]
- Licciardello, G.; Doppler, M.; Sicher, C.; Bueschl, C.; Ruso, D.; Schuhmacher, R.; Perazzolli, M. Metabolic changes in tomato plants caused by psychrotolerant Antarctic endophytic bacteria might be implicated in cold stress mitigation. Physiol. Plant. 2024, 176, e14352. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Hurtado-Bautista, E.; Pérez Sánchez, L.F.; Islas-Robles, A.; Santoyo, G.; Olmedo-Alvarez, G. Phenotypic plasticity and evolution of thermal tolerance in bacteria from temperate and hot spring environments. PeerJ 2021, 9, e11734. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gong, H.; Xu, Y.; Cai, C.; Hua, Y.; Li, L.; Dai, L.; Dai, X. The transition temperature (42 °C) from mesophilic to thermophilic micro-organisms enhances biomethane potential of corn stover. Sci. Total Environ. 2021, 759, 143549. [Google Scholar] [CrossRef] [PubMed]


| Isolate | Source | Contig Size (bp) | Best Match in NCBI BLAST | Query Cover % | % Identity | Match Accession |
|---|---|---|---|---|---|---|
| IPS11 | Monotropa uniflora (Indian pipe) stem | 1380 | Curtobacterium sp. | 100 | 99.13 | MH043942.1 |
| KB1 | Rambutan fruit | 1382 | C. oceanosedimentum | 100 | 99.49 | OL413667.1 |
| PBH-A | Hydrangea petal | 1393 | Curtobacterium sp. | 100 | 99.64 | MK704290.1 |
| RMB2 | Rambutan fruit | 1372 | C.luteum | 100 | 99.05 | MW052578.1 |
| ST1.1 | Steak tomato fruit | 1396 | C. flaccumfaciens | 100 | 99.71 | DQ015978.1 |
| WBB | Roughleaf dogwood berry fruit | 1399 | Curtobacterium sp. | 100 | 99.64 | MN989052.1 |
| Code | Probable ID | Color | Form | Margin | Elevation | Surface |
|---|---|---|---|---|---|---|
| IPS11 | Curtobacterium sp. | yellow | circular | entire | raised | smooth, glistening |
| KB1 | C. oceanosedimentum | yellow | circular | entire | raised | smooth, glistening |
| PBH-A | Curtobacterium sp. | yellow | ovoid | entire | raised | smooth, glistening |
| RMB2 | C. luteum | yellow | ovoid | entire | raised | smooth, glistening |
| ST1.1 | C. flaccumfaciens | yellow | ovoid | entire | raised | smooth, glistening |
| WBB | Curtobacterium sp. | orange | circular | entire | raised | smooth, glistening |
| Isolate | Probable ID | Catalase | Oxidase | Amylase | Caseinase | Phosphatase | Citrate | Urease | Gelatinase | Laccase |
|---|---|---|---|---|---|---|---|---|---|---|
| IPS11 | Curtobacterium sp. | + | - | + | + | + | + | - | - | - |
| KB1 | C. oceanosedimentum | + | - | + | + | + | + | - | - | - |
| PBH-A | Curtobacterium sp. | + | - | + | + | + | - | - | - | - |
| RMB2 | C. luteum | + | - | + | + | + | - | - | - | - |
| ST1.1 | C. flaccumfaciens | + | - | + | + | + | - | - | - | - |
| WBB | Curtobacterium sp. | + | - | + | + | + | nd | - | - | - |
| Isolate | Probable ID | Ara | Fru | Gal | Glu | Lac | Mal | Man | Sor | Suc | Tre |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IPS11 | Curtobacterium sp. | + | + | + | + | - | + | + | - | + | - |
| KB1 | C. oceanosedimentum | + | + | + | + | - | - | - | - | + | - |
| PBH-A | Curtobacterium sp. | + | + | + | + | - | - | - | - | + | - |
| RMB2 | C. luteum | + | + | + | + | - | + | - | - | + | - |
| ST1.1 | C. flaccumfaciens | + | + | + | + | - | - | - | - | + | - |
| WBB | Curtobacterium sp. | + | + | + | + | - | - | - | - | + | - |
| Isolate | Probable ID | Salt Tolerance | Temperature Tolerance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5% | 7.5% | 10% | 2 °C | 6 °C | 25 °C | 37 °C | 42 °C | ||
| IPS11 | Curtobacterium sp. | + | + | - | - | + | + | + | - |
| KB1 | C. oceanosedimentum | + | + | - | - | + | + | + | - |
| PBH-A | Curtobacterium sp. | + | + | - | - | + | + | + | - |
| RMB2 | C. luteum | + | + | - | - | - | + | + | + |
| ST1.1 | C. flaccumfaciens | + | + | - | - | + | + | + | - |
| WBB | Curtobacterium sp. | + | + | - | - | + | + | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arhin, A.; Wiegand, S.; Foriska, I.; Brown, K.; Crayne, K.; Stroscio, K.; Mohan, R. Comparative Analysis of Endophytic Curtobacterium Species Reveals Commonalities and Adaptations. Bacteria 2025, 4, 25. https://doi.org/10.3390/bacteria4020025
Arhin A, Wiegand S, Foriska I, Brown K, Crayne K, Stroscio K, Mohan R. Comparative Analysis of Endophytic Curtobacterium Species Reveals Commonalities and Adaptations. Bacteria. 2025; 4(2):25. https://doi.org/10.3390/bacteria4020025
Chicago/Turabian StyleArhin, Annabel, Sydney Wiegand, Isabella Foriska, Kiersten Brown, Kylee Crayne, Kaitlyn Stroscio, and Rajinikanth Mohan. 2025. "Comparative Analysis of Endophytic Curtobacterium Species Reveals Commonalities and Adaptations" Bacteria 4, no. 2: 25. https://doi.org/10.3390/bacteria4020025
APA StyleArhin, A., Wiegand, S., Foriska, I., Brown, K., Crayne, K., Stroscio, K., & Mohan, R. (2025). Comparative Analysis of Endophytic Curtobacterium Species Reveals Commonalities and Adaptations. Bacteria, 4(2), 25. https://doi.org/10.3390/bacteria4020025








