Extended-Spectrum Beta-Lactamases (ESBLs) Gene Mutations in Kuwait: How Much Do We Know? Not Much!
Abstract
1. Brief History of Antibiotics
2. Antibiotic Resistance
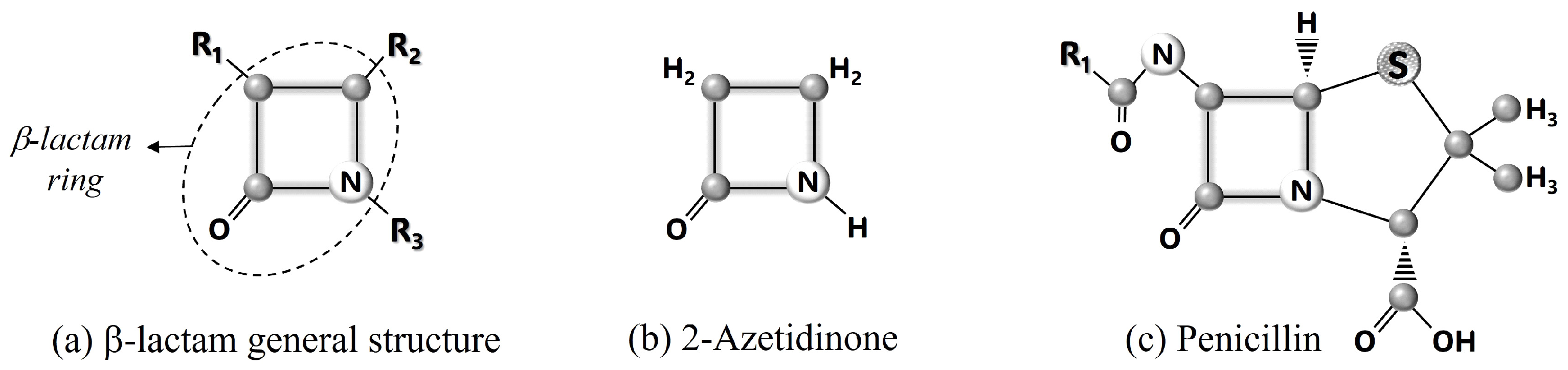
3. β-Lactam Molecules
4. How Do β-Lactam Antibiotics Work?
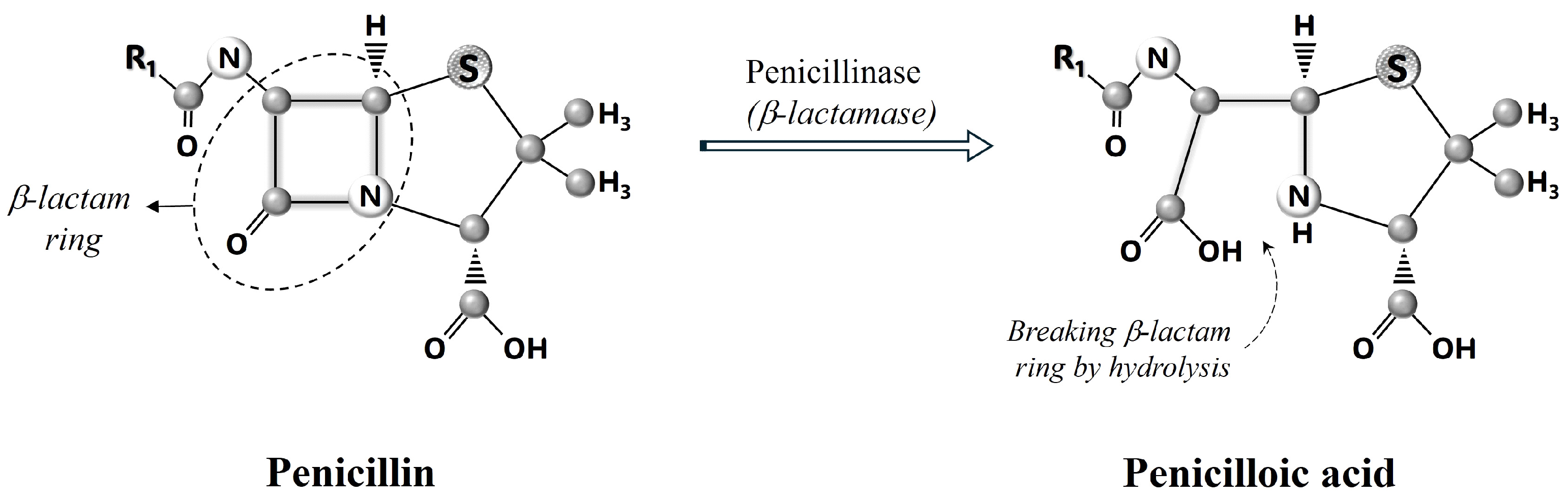
5. ESBLs
6. Classification of ESBLs
7. Genetics of ESBLs
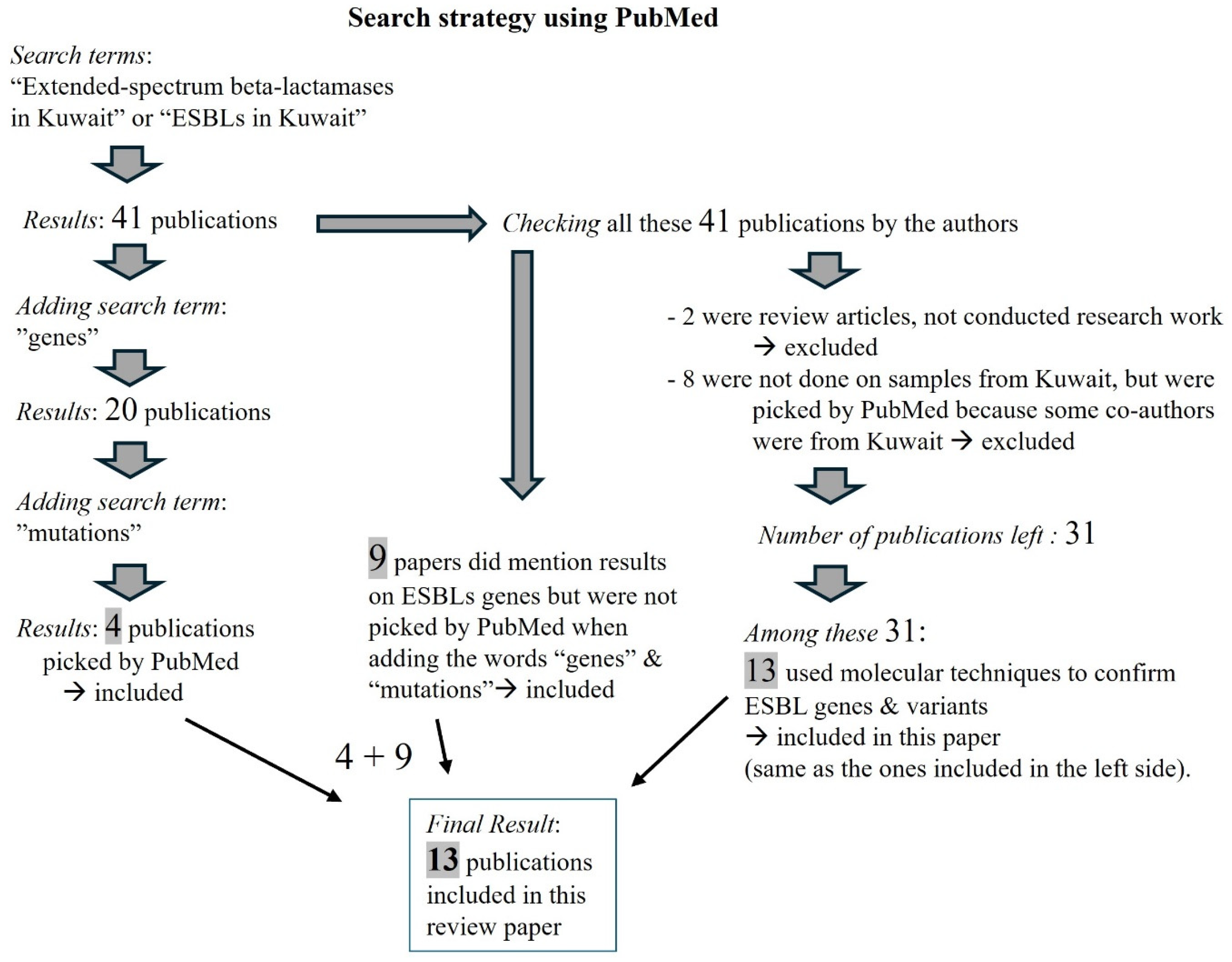
8. ESBLs Mutations in Kuwait
Search Strategy
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobanovska, M.; Pilla, G. Penicillin’s Discovery and Antibiotic Resistance: Lessons for the Future? Yale J. Biol. Med. 2017, 90, 135–145. [Google Scholar] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.R.; Halls, G.; Hu, Y. Novel classes of antibiotics or more of the same? Br. J. Pharmacol. 2011, 163, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Muraoka, A.; Bedenbaugh, M.; Childress, B.; Pernot, L.; Wiencek, M.; Peterson, Y.K. The Chemical Relationship Among Beta-Lactam Antibiotics and Potential Impacts on Reactivity and Decomposition. Front. Microbiol. 2022, 13, 807955. [Google Scholar] [CrossRef]
- Abraham, E.P.; Chain, E. An enzyme from bacteria able to destroy penicillin. 1940. Rev. Infect. Dis. 1988, 10, 677–678. [Google Scholar]
- Rammelkamp, T. Resistance of Staphylococcus aureus to the action of penicillin. Exp. Biol. Med. 1942, 51, 386–389. [Google Scholar] [CrossRef]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Dever, L.A.; Dermody, T.S. Mechanisms of bacterial resistance to antibiotics. Arch. Intern. Med. 1991, 151, 886–895. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, P629–P655. [Google Scholar] [CrossRef]
- Jonas, O.B.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.G.; Marquez, P.V. Drug-Resistant Infections: A Threat to Our Economic Future (Vol. 2): Final Report (English). HNP/Agriculture Global Antimicrobial Resistance Initiative Washington, D.C.: World Bank Group. Available online: http://documents.worldbank.org/curated/en/323311493396993758/final-report (accessed on 1 August 2024).
- Hodgkin, D.C. The X-ray analysis of the structure of penicillin. Adv. Sci. 1949, 6, 85–89. [Google Scholar] [PubMed]
- Abraham, E.P.; Chain, E.; Fletcher, C.M.; Gardner, A.D.; Heatley, N.G.; Jennings, M.A. Further observations on penicillin. Lancet 1941, 238, 177–189. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. beta-lactams and beta-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.; Kwon, Y.; Kim, Y.; Park, H.; Kwak, K.; Lee, H.; Lee, J.H.; Jang, K.-M.; Kim, D.; et al. Structural Insights for β-Lactam Antibiotics. Biomol. Ther. 2023, 31, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Amador, P.; Prudêncio, C. β-Lactams chemical structure, mode of action and mechanisms of resistance. Rev. Med. Microbiol. 2013, 24, 7–17. [Google Scholar] [CrossRef]
- Bush, K.; Jacoby, G.A. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Drawz, S.M.; Bonomo, R.A. Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef]
- Bush, K. Classification for β-lactamases: Historical perspectives. Expert. Rev. Anti Infect. Ther. 2023, 21, 513–522. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Ambler, R.P. The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980, 289, 321–331. [Google Scholar] [PubMed]
- Bush, K.; Jacoby, G.; Medeiros, A. A functional classification scheme for b-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 1995, 39, 1211–1233. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Fisher, J.F. Epidemiological expansion, structural studies, and clinical challenges of new b-lactamases from gram-negative bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Turton, J.F.; Livermore, D.M. Multi resistant gram negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Datta, N.; Kontomichalou, P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature 1965, 208, 239–241. [Google Scholar] [CrossRef]
- Pitton, J.S. Mechanisms of bacterial resistance to antibiotics. Ergeb. Physiol. 1972, 65, 15–93. [Google Scholar]
- Jacoby, G.A.; Bush, K. Amino Acid Sequences for TEM, SHV and OXA Extended-Spectrum and Inhibitor Resistant β-Lactamases: Lahey Clinic. 1997. Available online: https://www.ncbi.nlm.nih.gov/pathogens/refgene/# (accessed on 24 February 2023).
- NCBI National Center for Biotechnology Information. Pathogen Detection Reference Gene Catalog. Available online: https://www.ncbi.nlm.nih.gov/pathogens/refgene/# (accessed on 24 February 2023).
- Dashti, A.A.; Jadaon, M.M.; Amyes, S.G. Retrospective study of an outbreak in a Kuwaiti hospital of multidrug-resistant Klebsiella pneumoniae possessing the new SHV-112 extended-spectrum beta-lactamase. J. Chemother. 2010, 22, 335–338. [Google Scholar] [CrossRef]
- Dashti, A.A.; Vali, L.; Jadaon, M.M.; El-Shazly, S. The emergence of a multidrug-resistant Escherichia coli isolate harboring a novel SHV-122 enzyme is a serious threat for hospitalised patients. In Proceedings of the 16th HSC Poster Conference 2011, Jabriyah, Kuwait, 3–5 May 2011. [Google Scholar]
- Dashti, A.A.; Jadaon, M.M.; Gomaa, H.H.; Noronha, B.; Udo, E.E. Transmission of a Klebsiella pneumoniae clone harbouring genes for CTX-M-15-like and SHV-112 enzymes in a neonatal intensive care unit of a Kuwaiti hospital. J. Med. Microbiol. 2010, 59 Pt 6, 687–692. [Google Scholar] [CrossRef]
- West, P.W. Extended-spectrum beta-lactamase-producing Klebsiella spp. Br. J. Biomed. Sci. 2000, 57, 226–233. [Google Scholar]
- Alajmi, R.Z.; Alfouzan, W.A.; Mustafa, A.S. The Prevalence of Multidrug-Resistant Enterobacteriaceae among Neonates in Kuwait. Diagnostics 2023, 13, 1505. [Google Scholar] [CrossRef]
- Zowawi, H.M.; Balkhy, H.H.; Walsh, T.R.; Paterson, D.L. β-Lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin. Microbiol. Rev. 2013, 26, 361–380. [Google Scholar] [CrossRef]
- Dashti, A.A.; West, P.W. Extended-spectrum beta-lactamase-producing Escherichia coli isolated in the Al-Amiri Hospital in 2003 and compared with isolates from the Farwania hospital outbreak in 1994-96 in Kuwait. J. Chemother. 2007, 19, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V.; Poirel, L.; Rotimi, V.; Soussy, C.J.; Nordmann, P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 2007, 60, 394–397. [Google Scholar] [CrossRef]
- Rotimi, V.O.; Jamal, W.; Pal, T.; Sovenned, A.; Albert, M.J. Emergence of CTX-M-15 type extended-spectrum beta-lactamase-producing Salmonella spp. in Kuwait and the United Arab Emirates. J. Med. Microbiol. 2008, 57 Pt 7, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Al Sweih, N.; Salama, M.F.; Jamal, W.; Al Hashem, G.; Rotimi, V.O. An outbreak of CTX-M-15-producing Klebsiella pneumoniae isolates in an intensive care unit of a teaching hospital in Kuwait. Indian. J. Med. Microbiol. 2011, 29, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Al Hashem, G.; Al Sweih, N.; Jamal, W.; Rotimi, V.O. Sequence analysis of bla(CTX-M) genes carried by clinically significant Escherichia coli isolates in Kuwait hospitals. Med. Princ. Pract. 2011, 20, 213–219. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Rotimi, V.O.; Al Hubail, M.; Gasiorowski, E.; Al Sweih, N.; Nordmann, P.; Poirel, L. Wide dissemination of GES-type carbapenemases in Acinetobacter baumannii isolates in Kuwait. Antimicrob. Agents Chemother. 2013, 57, 183–188. [Google Scholar] [CrossRef]
- Vali, L.; Dashti, A.A.; Jadaon, M.M.; El-Shazly, S. The emergence of plasmid mediated quinolone resistance qnrA2 in extended spectrum β-lactamase producing Klebsiella pneumoniae in the Middle East. Daru 2015, 23, 34. [Google Scholar] [CrossRef]
- Al-Sweih, N.; Jamal, W.; Mokaddas, E.; Habashy, N.; Kurdi, A.; Mohamed, N. Evaluation of the in vitro activity of ceftaroline, ceftazidime/avibactam and comparator antimicrobial agents against clinical isolates from paediatric patients in Kuwait: ATLAS data 2012-19. JAC Antimicrob Resist. 2021, 3, dlab159. [Google Scholar] [CrossRef]
- Moghnia, O.H.; Al-Sweih, N.A. Whole Genome Sequence Analysis of Multidrug Resistant Escherichia coli and Klebsiella pneumoniae Strains in Kuwait. Microorganisms 2022, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.; Sierra, R.; Raro, O.H.F.; Aires-de-Sousa, M.; Andrey, D.O.; Nordmann, P. Plasmid-mediated fosfomycin resistance in Escherichia coli isolates of worldwide origin. J. Glob. Antimicrob. Resist. 2023, 35, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Redha, M.A.; Al Sweih, N.; Albert, M.J. Multidrug-Resistant and Extensively Drug-Resistant Escherichia coli in Sewage in Kuwait: Their Implications. Microorganisms 2023, 11, 2610. [Google Scholar] [CrossRef]
- Raphael, E.; Glymour, M.M.; Chambers, H.F. Trends in prevalence of extended-spectrum beta-lactamase-producing Escherichia coli isolated from patients with community- and healthcare-associated bacteriuria: Results from 2014 to 2020 in an urban safety-net healthcare system. Antimicrob. Resist. Infect. Control 2021, 10, 118. [Google Scholar] [CrossRef]
- Denisuik, A.J.; Karlowsky, J.A.; Adam, H.J.; Baxter, M.R.; Lagacé-Wiens, P.R.S.; Mulvey, M.R.; Hoban, D.J.; Zhanel, G.G. Canadian Antimicrobial Resistance Alliance (CARA) and CANWARD. Dramatic rise in the proportion of ESBL-producing Escherichia coli and Klebsiella pneumoniae among clinical isolates identified in Canadian hospital laboratories from 2007 to 2016. J. Antimicrob. Chemother. 2019, 74 (Suppl. 4), iv64–iv71. [Google Scholar] [CrossRef] [PubMed]
- Coque, T.M.; Baquero, F.; Cantón, R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Eurosurveillance 2008, 13, 19044. [Google Scholar] [CrossRef]
- Bezabih, Y.M.; Bezabih, A.; Dion, M.; Batard, E.; Teka, S.; Obole, A.; Dessalegn, N.; Enyew, A.; Roujeinikova, A.; Alamneh, E.; et al. Comparison of the global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli between healthcare and community settings: A systematic review and meta-analysis. JAC Antimicrob Resist. 2022, 4, dlac048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
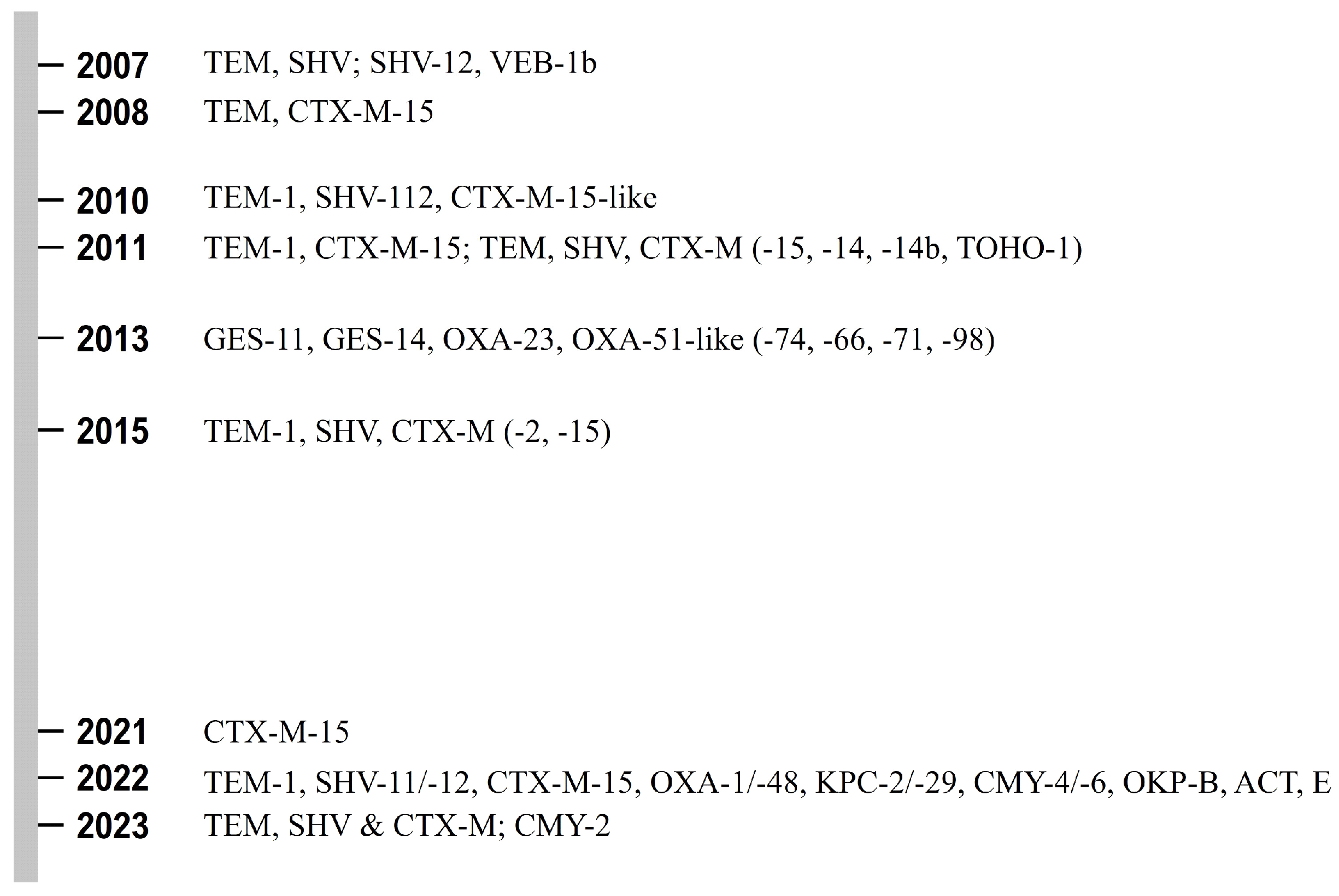
| Years of Testing | Bacteria | Hospital in Kuwait | ESBL Genes Reported (Percentage Not Shown) | Reference (Publication Year) |
|---|---|---|---|---|
| 2003 1994 to 1996 | E. coli | Al-Amiri Al-Farwania | TEM, SHV (general) | [37] (2007) |
| 2002 to 2004 | E. cloacae, C. freundii | Mubarak Al-Kabir | SHV-12, VEB-1b | [38] (2007) |
| 2003 to 2006 | Salmonella spp. | Mubarak Al-Kabir Infectious Diseases H. | TEM, CTX-M-15 | [39] (2008) |
| 2005 to 2006 | K. pneumoniae | Al-Jahra | TEM-1, SHV-112, CTX-M-15-like | [33] (2010) |
| 2010 | K. pneumoniae | Al-Amiri | SHV-112 | [31] (2010) |
| 2008 | K. pneumoniae | Mubarak Al-Kabeer | TEM-1, CTX-M-15 | [40] (2011) |
| 2008 | E. coli | Mubarak Al-Kabeer Al-Amiri Al-Sabah Al-Adan Al-Jahra Ibn Sina Al-Farwaniya Maternity | TEM, SHV (general), CTX-M (−15, −14, −14b, TOHO-1) | [41] (2011) |
| 2007 to 2008 | A. baumannii | Mubarak Al-Kabeer Al-Sabah Al-Adan Al-Jahra Al-Babtain Al- Razi | GES-11, GES-14, OXA-23 OXA-51-like (−74, −66, −71, −98) | [42] (2013) |
| Non-specified | K. pneumoniae | Al-Amiri Al-Adan, Al-Ahmadi (Kuwait Oil Company H.) | TEM-1, SHV, CTX-M (−2, −15) | [43] (2015) |
| 2012 to 2019 | E. coli, K. pneumoniae | 3 non-specified hospitals | CTX-M-15 | [44] (2021) |
| 2020 to 2021 | E. coli, K. pneumoniae | Mubarak Al-Kabeer Ibn Sina Al-Babtain | TEM-1, SHV-11/-12, CTX-M-15, OXA-1/-48, KPC-2/-29, CMY-4/-6, OKP-B, ACT, EC | [45] (2022) |
| 2020 | Enterobacteriaceae spp. (mostly E. coli, Klebsiella spp., Enterobacter spp.) | Al-Farwaniya | TEM, SHV, CTX-M (general) | [35] (2023) |
| 2017 to 2022 | E. coli | Non-specified | CMY-2 | [46] (2023) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dashti, A.A.; Jadaon, M.M. Extended-Spectrum Beta-Lactamases (ESBLs) Gene Mutations in Kuwait: How Much Do We Know? Not Much! Bacteria 2025, 4, 22. https://doi.org/10.3390/bacteria4020022
Dashti AA, Jadaon MM. Extended-Spectrum Beta-Lactamases (ESBLs) Gene Mutations in Kuwait: How Much Do We Know? Not Much! Bacteria. 2025; 4(2):22. https://doi.org/10.3390/bacteria4020022
Chicago/Turabian StyleDashti, Ali A., and Mehrez M. Jadaon. 2025. "Extended-Spectrum Beta-Lactamases (ESBLs) Gene Mutations in Kuwait: How Much Do We Know? Not Much!" Bacteria 4, no. 2: 22. https://doi.org/10.3390/bacteria4020022
APA StyleDashti, A. A., & Jadaon, M. M. (2025). Extended-Spectrum Beta-Lactamases (ESBLs) Gene Mutations in Kuwait: How Much Do We Know? Not Much! Bacteria, 4(2), 22. https://doi.org/10.3390/bacteria4020022








