Unveiling the Hidden Allies in the Fight Against Antimicrobial Resistance—Medicinal Plant Endophytes
Abstract
1. Introduction
2. Endophytic Microbial Communities: Diversity and Ecological Distributions
2.1. Diversity of Endophytes in Plants
2.1.1. Endophytic Bacteria
2.1.2. Endophytic Actinomycetes
| Endophyte Class | Medicinal Plant | Endophyte | Bioactive Metabolite | Test Pathogen | Solvent | Activity | References |
|---|---|---|---|---|---|---|---|
| Bacteria | Cordia dichotoma L. | B. thuringiensis | Eicosane, heneicosane, hexadecane, tetradecane, tetrapentacontane, trichlorooctadecyl, and 2,4-di-tert-butylphenol. | S. aureus, E. coli, Bacillus subtilis, P. aeruginosa, Klebsiella pneumonia, and S. typhi | Ethyl acetate | Antibacterial | [92] |
| Urtica dioica L. | B. cereus | Polyphenol compounds (caffeic acid, chlorogenic acid) and fatty acid esters (hexadecenoic, heptadecanoic, and octadecanoic acids) | P. aeruginosa, E. coli, Mucor racemosus, and Phanerochaete chrysosporium | Hexane | Antimicrobial | [21] | |
| Alectra sessiliflora | Bacillus sp. strain AS_4, Lysinibacillus sp. strain AS_1, and Peribacillus sp. strain AS_2 | Tridecane, hexadecane, tetracosane, and ergotaman-3′,6′,18-trione,9,10-dihydro-12′-hydroxy-2′-methyl-5′-(phenylmethyl)-, benzyl 2-coumaranone, and octacosane | B. cereus, E. coli, K. pneumoniae, K. oxytoca, Mycobacterium smegmatis, P. aeruginosa, S. aureus, S. saprophyticus, S. epidermidis, Veillonella parvula, and Enterococcus faecium | Ethyl acetate | Antimicrobial, anticancer (0.25–16 mg/mL) | [93] | |
| Origanum vulgare L. | Arthrobacter sp. OVS8 | Volatile organic compounds | P. aeruinosa, S. aureus, K. pneumonia, S. epidermidis | - | Antagonistic | [94] | |
| Actinomycetes | Pharmaceutical plants from different sites at Xishuangbanna, tropical rainforest, Yunnan province China | Streptomyces sp. | - | S. aureus, S. epidermidis, E. coli, Klebsiella pneumoniae, and C. albicans | - | Antitumor and antimicrobial | [86] |
| Camella sinensis var. assamica | Brevibacterium celere | - | Staphylococuss epidermidis, Shigella flexneri, E. coli, and Bacillus cereus | Ethyl acetate | Antibacterial and immunomodulatory activity | [87] | |
| Algae | Macroalgae | Colaconema. formosnum | Phycobiliproteins (phycoerythrin, phycocyanin, and allophycocyanin) | - | - | Antibacterial, anticancer, antidiabetes, anti-inflammation, antioxidants, anti-obesity, neuroprotective activity | [95] |
2.1.3. Endophytic Mycoplasma Species
2.1.4. Endophytic Algae
2.1.5. Endophytic Fungi
2.2. Environmental Influences on Endophytic Microbial Communities
2.2.1. Seasonal and Abiotic Stress
2.2.2. Geographic and Environmental Gradients
2.2.3. Salinity and Soil Conditions
2.3. Community Structure and Host Interactions
2.3.1. Host Plant Influence
2.3.2. Functional Traits and Plant Adaptation
3. Pharmacological Significance of Bioactive Compounds Produced by Medicinal Plant Endophytes
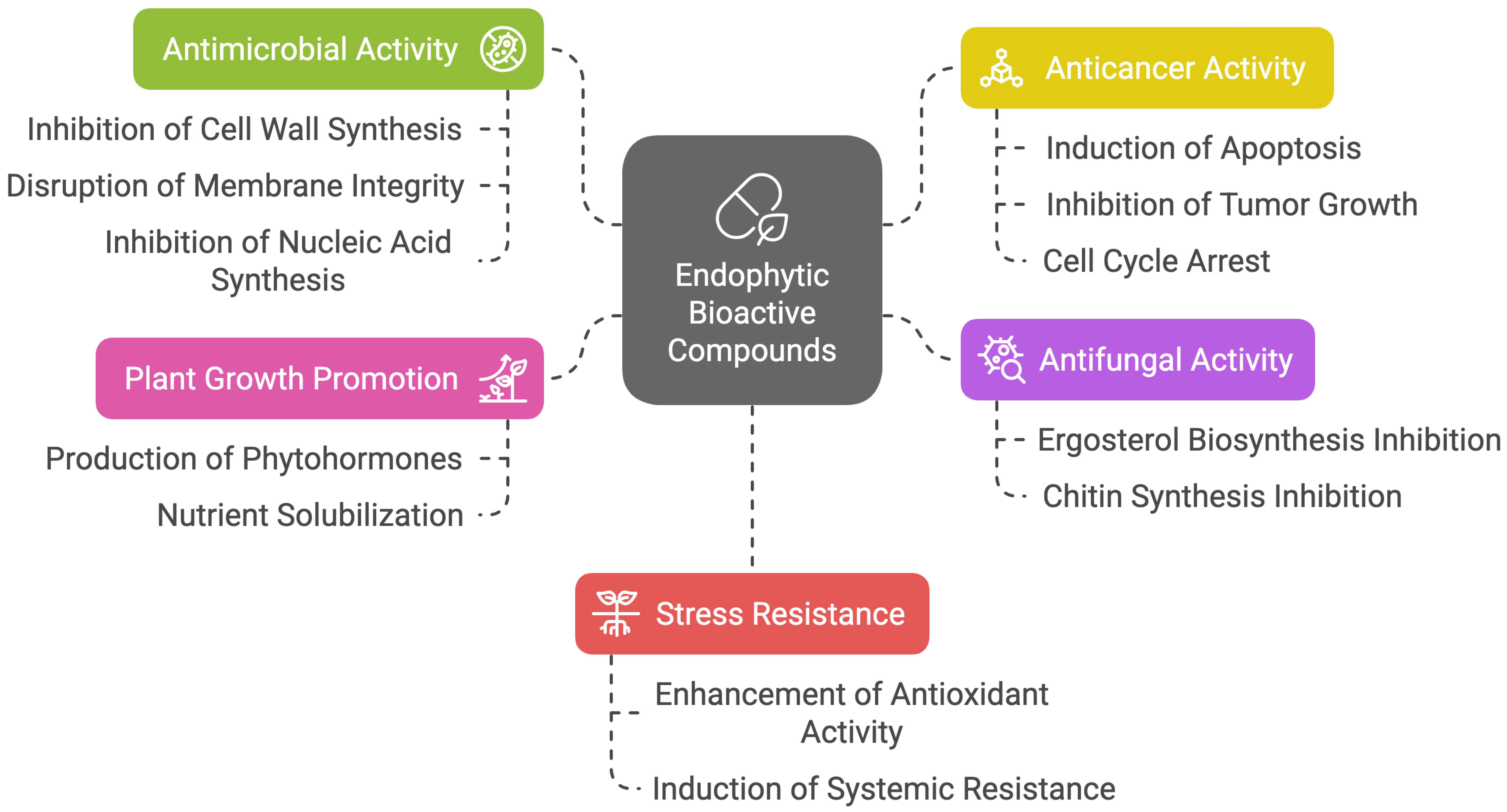
3.1. Mechanism of Actions of Endophytic Bioactive Compounds
3.2. Antibiotic Compounds of Medicinal Plant Endophytes
3.3. Antioxidant Properties
3.4. Anticancer Compounds
3.5. Antidiabetic Compounds
4. Boosting Metabolite Efficacy Through Endophyte-Derived Nanoparticle Synthesis
5. Computational Strategies for Endophytic Metabolite Interactions
6. Strategies Against Drug-Resistant Microorganisms
7. Safety, Efficacy, and Commercial Viability of Endophyte Therapies
8. Economic Challenges of Alternative Therapies
9. One Health Approach
10. Role of Policymakers and Regulatory Bodies
11. Geographical Perspective of Antibiotic Resistance
Endophytes and Antibiotic Resistance in Africa
12. Limitations in the Study of Endophytes
13. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ronco, T.; Kappel, L.H.; Aragao, M.F.; Biagi, N.; Svenningsen, S.; Christensen, J.B.; Permin, A.; Saaby, L.; Holmstrøm, K.; Klitgaard, J.K.; et al. Insight Into the Anti-Staphylococcal Activity of JBC 1847 at Sub-Inhibitory Concentration. Front. Microbiol. 2022, 12, 786173. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical Review of Medicinal Plants’ Usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef]
- Gurib-Fakim, A.; Kasilo, M. Promoting African Medicinal Plants through an African Herbal Pharmacopoeia. Afr. Health Monit. Spec. Issue Decad. Afr. Tradit. Med. 2010, 14, 64–67. [Google Scholar]
- van Wyk, A.S.; Prinsloo, G. Health, Safety and Quality Concerns of Plant-Based Traditional Medicines and Herbal Remedies. S. Afr. J. Bot. 2020, 133, 54–62. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635–642. [Google Scholar] [CrossRef]
- Street, R.A.; Prinsloo, G. Commercially Important Medicinal Plants of South Africa: A Review. J. Chem. 2013, 2013, 205048. [Google Scholar] [CrossRef]
- Arozal, W.; Louisa, M.; Soetikno, V. Selected Indonesian Medicinal Plants for the Management of Metabolic Syndrome: Molecular Basis and Recent Studies. Front. Cardiovasc. Med. 2020, 7, 82. [Google Scholar] [CrossRef]
- Tanvir, R.; Javeed, A.; Bajwa, A.G. Endophyte Bioprospecting in South Asian Medicinal Plants: An Attractive Resource for Biopharmaceuticals. Appl. Microbiol. Biotechnol. 2017, 101, 1831–1844. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Mougel, C.; Jaillard, B.; Hinsinger, P. Plant-Microbe-Soil Interactions in the Rhizosphere: An Evolutionary Perspective. Plant Soil 2009, 83–115. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiong, K.; Wen, W.; Li, L.; Xu, D. Functional Endophytes Regulating Plant Secondary Metabolism: Current Status, Prospects and Applications. Int. J. Mol. Sci. 2023, 24, 1153. [Google Scholar] [CrossRef]
- Akter, Y.; Barua, R.; Uddin, N.; Muhammad Sanaullah, A.F.; Marzan, L.W. Bioactive Potentiality of Secondary Metabolites from Endophytic Bacteria against SARS-COV-2: An in-Silico Approach. PLoS ONE 2022, 17, e0269962. [Google Scholar] [CrossRef]
- Ancheeva, E.; Daletos, G.; Proksch, P. Bioactive Secondary Metabolites from Endophytic Fungi. Curr. Med. Chem. 2019, 27, 1836–1854. [Google Scholar] [CrossRef]
- Digra, S.; Nonzom, S. An Insight into Endophytic Antimicrobial Compounds: An Updated Analysis. Plant Biotechnol. Rep. 2023, 17, 427–457. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, T.T.; Pham, M.N.; Duong, H.N.; Pham, T.T.; Nguyen, T.P.; Nguyen, P.T.; Thi Nguyen, T.U.; Nguyen, H.H.; Nguyen, H.T. Relationships between Endophytic Bacteria and Medicinal Plants on Bioactive Compounds Production. Rhizosphere 2023, 27, 100720. [Google Scholar] [CrossRef]
- Mishra, S.; Priyanka; Sharma, S. Metabolomic Insights Into Endophyte-Derived Bioactive Compounds. Front. Microbiol. 2022, 13, 835931. [Google Scholar] [CrossRef]
- Marchut-Mikołajczyk, O.; Chlebicz, M.; Kawecka, M.; Michalak, A.; Prucnal, F.; Nielipinski, M.; Filipek, J.; Jankowska, M.; Perek, Z.; Drożdżyński, P.; et al. Endophytic Bacteria Isolated from Urtica dioica L.-Preliminary Screening for Enzyme and Polyphenols Production. Microb. Cell Fact. 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Sachin, N.; Manjunatha, B.L.; Kumara, P.M.; Ravikanth, G.; Shweta, S.; Suryanarayanan, T.S.; Ganeshaiah, K.N.; Shaanker, R.U. Do Endophytic Fungi Possess Pathway Genes for Plant Secondary Metabolites? Curr. Sci. 2013, 104, 178. [Google Scholar]
- Santra, H.K.; Maity, S.; Banerjee, D. Production of Bioactive Compounds with Broad Spectrum Bactericidal Action, Bio-Film Inhibition and Antilarval Potential by the Secondary Metabolites of the Endophytic Fungus Cochliobolus sp. APS1 Isolated from the Indian Medicinal Herb Andrographis Paniculata. Molecules 2022, 27, 1459. [Google Scholar] [CrossRef]
- Hassane, A.M.A.; Taha, T.M.; Awad, M.F.; Mohamed, H.; Melebari, M. Radical Scavenging Potency, HPLC Profiling and Phylogenetic Analysis of Endophytic Fungi Isolated from Selected Medicinal Plants of Saudi Arabia. Electron. J. Biotechnol. 2022, 58, 37–45. [Google Scholar] [CrossRef]
- Padhi, L.; Laxmipriya Padhi, Y.; Sujogya, K.M.; Panda, K. Endophytic Fungi with Great Promises: A Review. J. Adv. Pharm. Educ. Res. 2013, 3. [Google Scholar]
- Akshatha, V.J.; Nalini, M.S.; D’Souza, C.; Prakash, H.S. Streptomycete Endophytes from Anti-Diabetic Medicinal Plants of the Western Ghats Inhibit Alpha-Amylase and Promote Glucose Uptake. Lett. Appl. Microbiol. 2014, 58, 433–439. [Google Scholar] [CrossRef]
- Shao, Z.; Tian, Y.; Liu, S.; Chu, X.; Mao, W. Anti-Diabetic Activity of a Novel Exopolysaccharide Produced by the Mangrove Endophytic Fungus Penicillium janthinellum N29. Mar. Drugs 2023, 21, 270. [Google Scholar] [CrossRef]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E.; et al. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability. Microb. Ecol. 2023, 86, 1455–1486. [Google Scholar] [CrossRef]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The Potential Application of Endophytes in Management of Stress from Drought and Salinity in Crop Plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef]
- Burragoni, S.G.; Jeon, J. Applications of Endophytic Microbes in Agriculture, Biotechnology, Medicine, and Beyond. Microbiol. Res. 2021, 245, 126691. [Google Scholar] [CrossRef]
- Papik, J.; Folkmanova, M.; Polivkova-Majorova, M.; Suman, J.; Uhlik, O. The Invisible Life inside Plants: Deciphering the Riddles of Endophytic Bacterial Diversity. Biotechnol. Adv. 2020, 44, 107614. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.J.; Palombo, E.A.; Moulton, S.E.; Zaferanloo, B. Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds. Microorganisms 2022, 10, 1990. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Kumar, V.; Singh, D.P.; Saxena, A.K. Beneficial Plant-Microbes Interactions: Biodiversity of Microbes from Diverse Extreme Environments and Its Impact for Crop Improvement. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Berlin/Heidelberg, Germany, 2017; Volume 2, pp. 543–580. [Google Scholar]
- Verma, S.K.; Gond, S.K.; Mishra, A.; Sharma, V.K.; Kumar, J.; Singh, D.K.; Kumar, A.; Kharwar, R.N. Fungal Endophytes Representing Diverse Habitats and Their Role in Plant Protection. In Developments in Fungal Biology and Applied Mycology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 135–157. [Google Scholar]
- Verma, S.K.; Lal, M.; Debnath Das, M. Optimization of Process Parameters for Production of Antimicrobial Metabolites by an Endophytic Fungus Aspergillus sp. CPR5 Isolated from Calotropis Procera Root. Asian J. Pharm. Clin. Res. 2017, 10, 225–230. [Google Scholar] [CrossRef]
- Strobel, G.A. Rainforest Endophytes and Bioactive Products. Crit. Rev. Biotechnol. 2002, 22, 315–333. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.M.; Teuscher, F.; Li, D.L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B.G. Chaetopyranin, a Benzaldehyde Derivative, and Other Related Metabolites from Chaetomium globosum, an Endophytic Fungus Derived from the Marine Red Alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef]
- Lin, Z.; Zhu, T.; Fang, Y.; Gu, Q.; Zhu, W. Polyketides from Penicillium sp. JP-1, an Endophytic Fungus Associated with the Mangrove Plant Aegiceras corniculatum. Phytochemistry 2008, 69, 1273–1278. [Google Scholar] [CrossRef]
- Fisher, P.J.; Graf, F.; Petrini, L.E.; Sutton, B.C.; Wookey, P.A. Fungal Endophytes of Dryas octopetala from a High Arctic Polar Semidesert and from the Swiss Alps. Mycologia 1995, 87, 319–323. [Google Scholar] [CrossRef]
- Rosa, L.H.; Vaz, A.B.M.; Caligiorne, R.B.; Campolina, S.; Rosa, C.A. Endophytic Fungi Associated with the Antarctic Grass Deschampsia antarctica Desv. (Poaceae). Polar Biol. 2009, 32, 161–167. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Wittlinger, S.K.; Faeth, S.H. Endophytic Fungi Associated with Cacti in Arizona. Mycol. Res. 2005, 109, 635–639. [Google Scholar] [CrossRef]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance Generated by Plant/Fungal Symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef]
- Bashyal, B.P.; Kithsiri Wijeratne, E.M.; Faeth, S.H.; Gunatilaka, A.A.L. Globosumones A-C, Cytotoxic Orsellinic Acid Esters from the Sonoran Desert Endophytic Fungus Chaetomium Globosum. J. Nat. Prod. 2005, 68, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Lutzoni, F. Diversity and Host Range of Foliar Fungal Endophytes: Are Tropical Leaves Biodiversity Hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Porras-Alfaro, A.; Bayman, P. Hidden Fungi, Emergent Properties: Endophytes and Microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ashraf, N.; Mohiuddin, T.; Riyaz-Ul-Hassan, S. Plant-Endophyte Symbiosis, an Ecological Perspective. Appl. Microbiol. Biotechnol. 2015, 99, 2955–2965. [Google Scholar] [CrossRef]
- Qadri, M.; Rajput, R.; Abdin, M.Z.; Vishwakarma, R.A.; Riyaz-Ul-Hassan, S. Diversity, Molecular Phylogeny, and Bioactive Potential of Fungal Endophytes Associated with the Himalayan Blue Pine (Pinus wallichiana). Microb. Ecol. 2014, 67, 877–887. [Google Scholar] [CrossRef]
- Yedukondalu, N.; Arora, P.; Wadhwa, B.; Malik, F.A.; Vishwakarma, R.A.; Gupta, V.K.; Riyaz-Ul-Hassan, S.; Ali, A. Diapolic Acid A-B from an Endophytic Fungus, Diaporthe terebinthifolii Depicting Antimicrobial and Cytotoxic Activity. J. Antibiot. 2017, 70, 212–215. [Google Scholar] [CrossRef]
- Mohali, S.; Burgess, T.I.; Wingfield, M.J. Diversity and Host Association of the Tropical Tree Endophyte Lasiodiplodia theobromae Revealed Using Simple Sequence Repeat Markers. For. Pathol. 2005, 35, 385–396. [Google Scholar] [CrossRef]
- Selim, K.A.; Nagia, M.M.S.; Dina, D.E. Endophytic Fungi Are Multifunctional Biosynthesizers: Ecological Role and Chemical Diversity. In Endophytic Fungi: Diversity, Characterization and Biocontrol; Nova Publishers: Hauppauge, NY, USA, 2016; Volume 39. [Google Scholar]
- Yadav, A.N. Extreme Cold Environments: A Suitable Niche for Selection of Novel Psychrotrophic Microbes for Biotechnological Applications. Adv. Biotechnol. Microbiol. 2017, 2, 1–4. [Google Scholar] [CrossRef]
- Pavithra, N.; Sathish, L.; Suneel Kumar, A.; Venkatarathanamma, V.; Pushpalatha, H.; Bhanuprakash Reddy, G. In-vitro Studies on α-Amylase, α-Glucosidase and Aldose Reductase Inhibitors found in Endophytic Fungi Isolated from Ocimum sanctum. Curr. Enzym. Inhib. 2014, 10, 129–136. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A.N.; Tiwari, R.; Prasanna, R.; Saxena, A.K. Evaluating the Diversity of Culturable Thermotolerant Bacteria from Four Hot Springs of India. J. Biodivers. Bioprospecting Dev. 2014, 1, 2. [Google Scholar] [CrossRef]
- Sahay, H.; Yadav, A.N.; Singh, A.K.; Singh, S.; Kaushik, R.; Saxena, A.K. Hot Springs of Indian Himalayas: Potential Sources of Microbial Diversity and Thermostable Hydrolytic Enzymes. 3 Biotech 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, V.; Dhaliwal, H.S.; Prasad, R.; Saxena, A.K. Microbiome in Crops: Diversity, Distribution, and Potential Role in Crop Improvement. In Crop Improvement Through Microbial Biotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 305–332. [Google Scholar]
- Yadav, G.; Meena, M. Bioprospecting of Endophytes in Medicinal Plants of Thar Desert: An Attractive Resource for Biopharmaceuticals. Biotechnol. Rep. 2021, 30, e00629. [Google Scholar] [CrossRef]
- Singh, D.K.; Sharma, V.K.; Kumar, J.; Mishra, A.; Verma, S.K.; Sieber, T.N.; Kharwar, R.N. Diversity of Endophytic Mycobiota of Tropical Tree Tectona grandis Linn.f.: Spatiotemporal and Tissue Type Effects. Sci. Rep. 2017, 7, 3745. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Venkatesan, G.; Murali, T.S. Endophytic Fungal Communities in Leaves of Tropical Forest Trees: Diversity and Distribution Patterns. Curr. Sci. 2003, 489–493. [Google Scholar]
- Stone, J.K.; Polishook, J.D.; White, J.R. Endophytic Fungi. Biodiversity of Fungi: Inventory and Monitoring Methods. In Biodiversity of Fungi: Inventory and Monitoring Methods; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; pp. 241–270. [Google Scholar]
- Mishra, A.; Gond, S.K.; Kumar, A.; Sharma, V.K.; Verma, S.K.; Kharwar, R.N.; Sieber, T.N. Season and Tissue Type Affect Fungal Endophyte Communities of the Indian Medicinal Plant Tinospora Cordifolia More Strongly than Geographic Location. Microb. Ecol. 2012, 64, 388–398. [Google Scholar] [CrossRef]
- Huang, M.; Chen, L.; Ma, J.; Mo, J.; He, L.; Liang, Q.; Peng, G.; Tan, Z. Biological Functions of Endophytic Bacteria in Robinia Pseudoacacia ‘Hongsen’. Front. Microbiol. 2023, 14, 1128727. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Ding, C.; Zhang, B.; Huang, Q.; Huang, R.; Su, X. Endophytic Communities of Transgenic Poplar Were Determined by the Environment and Niche Rather than by Transgenic Events. Front. Microbiol. 2019, 10, 588. [Google Scholar] [CrossRef]
- Michalska-Smith, M.; Song, Z.; Spawn-Lee, S.A.; Hansen, Z.A.; Johnson, M.; May, G.; Borer, E.T.; Seabloom, E.W.; Kinkel, L.L. Network Structure of Resource Use and Niche Overlap within the Endophytic Microbiome. ISME J. 2022, 16, 435–446. [Google Scholar] [CrossRef]
- Brown, S.P.; Shahrtash, M.; Stokes, C.E.; Baird, S.; Baird, R.; Lu, S. Model-Based Community Analyses Identify Fungal Endophytes That May Modulate Symptom Development of Charcoal Rot Disease in Soybean. PhytoFrontiersTM 2024, 4, 83–88. [Google Scholar] [CrossRef]
- Willing, C.E.; Pellitier, P.T.; Van Nuland, M.E.; Alvarez-Manjarrez, J.; Berrios, L.; Chin, K.N.; Villa, L.M.; Yeam, J.J.; Bourque, S.D.; Tripp, W.; et al. A Risk Assessment Framework for the Future of Forest Microbiomes in a Changing Climate. Nat. Clim. Chang. 2024, 14, 448–461. [Google Scholar] [CrossRef]
- Kelliher, J.M.; Robinson, A.J.; Longley, R.; Johnson, L.Y.D.; Hanson, B.T.; Morales, D.P.; Cailleau, G.; Junier, P.; Bonito, G.; Chain, P.S.G. The Endohyphal Microbiome: Current Progress and Challenges for Scaling down Integrative Multi-Omic Microbiome Research. Microbiome 2023, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arias, C.; Sobrino-Plata, J.; Rodríguez-Calcerrada, J.; Martín, J.A. Mycobiome Shifts in Ulmus Minor Stems After Infection with Beneficial and Pathogenic Fungi. Phytobiomes J. 2023, 7, 464–477. [Google Scholar] [CrossRef]
- Muhammad, M.; Wahab, A.; Waheed, A.; Mohamed, H.I.; Hakeem, K.R.; Li, L.; Li, W.J. Harnessing Bacterial Endophytes for Environmental Resilience and Agricultural Sustainability. J. Environ. Manag. 2024, 368, 122201. [Google Scholar] [CrossRef]
- Sena, L.; Mica, E.; Valè, G.; Vaccino, P.; Pecchioni, N. Exploring the Potential of Endophyte-Plant Interactions for Improving Crop Sustainable Yields in a Changing Climate. Front. Plant Sci. 2024, 15, 1349401. [Google Scholar] [CrossRef]
- Enyi, E.O.; Chigozie, V.U.; Okezie, U.M.; Udeagbala, N.T.; Oko, A.O. A Review of the Pharmaceutical Applications of Endophytic Fungal Secondary Metabolites. Nat. Prod. Res. 2024, 1–17. [Google Scholar] [CrossRef]
- Nazir, A.; Puthuveettil, A.R.; Hussain, F.H.N.; Hamed, K.E.; Munawar, N. Endophytic Fungi: Nature’s Solution for Antimicrobial Resistance and Sustainable Agriculture. Front. Microbiol. 2024, 15, 1461504. [Google Scholar] [CrossRef]
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic Actinobacteria of Medicinal Plants: Diversity and Bioactivity. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 108, 267–289. [Google Scholar] [CrossRef]
- Hui, S.; Yan, H.; Qing, X.; Renyuan, Y.; Yongqiang, T. Isolation, Characterization, and Antimicrobial Activity of Endophytic Bacteria from Polygonum Cuspidatum. Afr. J. Microbiol. Res. 2013, 7, 1496–1504. [Google Scholar] [CrossRef]
- Bérdy, J. Thoughts and Facts about Antibiotics: Where We Are Now and Where We Are Heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef]
- Rustamova, N.; Wubulikasimu, A.; Mukhamedov, N.; Gao, Y.; Egamberdieva, D.; Yili, A. Endophytic Bacteria Associated with Medicinal Plant Vernonia Anthelmintica: Diversity and Characterization. Curr. Microbiol. 2020, 77, 1457–1465. [Google Scholar] [CrossRef]
- Köberl, M.; Ramadan, E.M.; Adam, M.; Cardinale, M.; Hallmann, J.; Heuer, H.; Smalla, K.; Berg, G. Bacillus and Streptomyces Were Selected as Broad-Spectrum Antagonists against Soilborne Pathogens from Arid Areas in Egypt. FEMS Microbiol. Lett. 2013, 342, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Li, L.; Abd-Allah, E.F.; Lindström, K. Microbial Cooperation in the Rhizosphere Improves Liquorice Growth under Salt Stress. Bioengineered 2017, 8, 433–438. [Google Scholar] [CrossRef]
- Pullen, C.; Schmitz, P.; Meurer, K.; Bamberg, D.D.V.; Lohmann, S.; De Castro França, S.; Groth, I.; Schlegel, B.; Möllmann, U.; Gollmick, F.; et al. New and Bioactive Compounds from Streptomyces Strains Residing in the Wood of Celastraceae. Planta 2002, 216, 162–167. [Google Scholar] [CrossRef]
- Castillo, U.; Harper, J.K.; Strobel, G.A.; Sears, J.; Alesi, K.; Ford, E.; Lin, J.; Hunter, M.; Maranta, M.; Ge, H.; et al. Kakadumycins, Novel Antibiotics from Streptomyces Sp. NRRL 30566, an Endophyte of Grevillea Pteridifolia. FEMS Microbiol. Lett. 2003, 224, 183–190. [Google Scholar] [CrossRef]
- Kumaresan, S.; Karthi, V.; Senthilkumar, V.; Balakumar, B.S.; Stephen, A. Biochemical Constituents and Antioxidant Potential of Endophytic Fungi Isolated from the Leaves of Azadirachta Indica A. Juss (Neem) from Chennai, India. J. Acad. Ind. Res. 2015, 3, 355–361. [Google Scholar]
- Zhao, J.; Fu, Y.; Luo, M.; Zu, Y.; Wang, W.; Zhao, C.; Gu, C. Endophytic Fungi from Pigeon Pea [Cajanus cajan (L.) Millsp.] Produce Antioxidant Cajaninstilbene Acid. J. Agric. Food Chem. 2012, 60, 4314–4319. [Google Scholar] [CrossRef]
- Chaudhary, H.S.; Soni, B.; Shrivastava, A.R.; Shrivastava, S. Diversity and Versatility of Actinomycetes and Its Role in Antibiotic Production. J. Appl. Pharm. Sci. 2013, 3, S83–S94. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, P.; Muralikrishnan, V. Isolation and Characterization of Endophytic Actinomycetes from Mangrove Plant for Antimicrobial Activity. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 78–89. [Google Scholar]
- Singh, R.; Dubey, A.K. Endophytic Actinomycetes as Emerging Source for Therapeutic Compounds. Indo Glob. J. Pharm. Sci. 2015, 5, 106–116. [Google Scholar] [CrossRef]
- Li, J.; Zhao, G.Z.; Chen, H.H.; Wang, H.B.; Qin, S.; Zhu, W.Y.; Xu, L.H.; Jiang, C.L.; Li, W.J. Antitumour and Antimicrobial Activities of Endophytic Streptomycetes from Pharmaceutical Plants in Rainforest. Lett. Appl. Microbiol. 2008, 47, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhou, Y.; Chen, F.; Yan, X.; Lai, Y.; Wei, C.; Chen, X.; Xu, J.; Wang, X. Isolation, Diversity, and Antimicrobial and Immunomodulatory Activities of Endophytic Actinobacteria from Tea Cultivars Zijuan and Yunkang-10 (Camellia sinensis Var. assamica). Front. Microbiol. 2018, 9, 1304. [Google Scholar] [CrossRef]
- Gos, F.M.W.R.; Savi, D.C.; Shaaban, K.A.; Thorson, J.S.; Aluizio, R.; Possiede, Y.M.; Rohr, J.; Glienke, C. Antibacterial Activity of Endophytic Actinomycetes Isolated from the Medicinal Plant Vochysia divergens (Pantanal, Brazil). Front. Microbiol. 2017, 8, 1642. [Google Scholar] [CrossRef]
- Singh, K.; Dwivedi, G.R.; Sanket, A.S.; Pati, S. Therapeutic Potential of Endophytic Compounds: A Special Reference to Drug Transporter Inhibitors. Curr. Top. Med. Chem. 2019, 19, 754–783. [Google Scholar] [CrossRef]
- Sanglier, J.J.; Wellington, E.M.H.; Behal, V.; Fiedler, H.P.; Ellouz Ghorbel, R.; Finance, C.; Hacene, M.; Kamoun, A.; Kelly, C.; Mercer, D.K.; et al. Novel Bioactive Compounds from Actinomycetes. Res. Microbiol. 1993, 144, 661–663. [Google Scholar] [CrossRef]
- Muhilan, B.; Chattopadhyay, I. Endophytes and Their Bioactive Metabolite’s Role against Various MDR Microbes Causing Diseases in Humans. In Endophytic Association: What, Why and How; Academic Press: Cambridge, MA, USA, 2022; pp. 135–158. [Google Scholar]
- Sharma, M.; Mallubhotla, S. Diversity, Antimicrobial Activity, and Antibiotic Susceptibility Pattern of Endophytic Bacteria Sourced From Cordia dichotoma L. Front. Microbiol. 2022, 13, 879386. [Google Scholar] [CrossRef]
- Maela, M.P.; van der Walt, H.; Serepa-Dlamini, M.H. The Antibacterial, Antitumor Activities, and Bioactive Constituents’ Identification of Alectra Sessiliflora Bacterial Endophytes. Front. Microbiol. 2022, 13, 870821. [Google Scholar] [CrossRef]
- Semenzato, G.; Del Duca, S.; Vassallo, A.; Bechini, A.; Calonico, C.; Delfino, V.; Berti, F.; Vitali, F.; Mocali, S.; Frascella, A.; et al. Genomic, Molecular, and Phenotypic Characterization of Arthrobacter sp. OVS8, an Endophytic Bacterium Isolated from and Contributing to the Bioactive Compound Content of the Essential Oil of the Medicinal Plant Origanum vulgare L. Int. J. Mol. Sci. 2023, 24, 4845. [Google Scholar] [CrossRef]
- Lee, M.C.; Huang, C.Y.; Lai, C.L.; Yeh, H.Y.; Huang, J.; Lung, W.Q.C.; Lee, P.T.; Nan, F.H. Colaconema formosanum, Sarcodia suae, and Nostoc commune as Fermentation Substrates for Bioactive Substance Production. Fermentation 2022, 8, 343. [Google Scholar] [CrossRef]
- Lanao, A.E.; Chakraborty, R.K.; Pearson-Shaver, A.L. Mycoplasma Infections; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023; ISBN NBK536927. [Google Scholar]
- Tanaka, R.; Ootsubo, M.; Sawabe, T.; Ezura, Y.; Tajima, K. Biodiversity and in Situ Abundance of Gut Microflora of Abalone (Haliotis Discus Hannai) Determined by Culture-Independent Techniques. Aquaculture 2004, 241, 453–463. [Google Scholar] [CrossRef]
- Huang, Z.B.; Guo, F.; Zhao, J.; Li, W.D.; Ke, C.H. Molecular Analysis of the Intestinal Bacterial Flora in Cage-Cultured Adult Small Abalone, Haliotis Diversicolor. Aquac. Res. 2010, 41, e760–e769. [Google Scholar] [CrossRef]
- Hollants, J.; Leroux, O.; Leliaert, F.; Decleyre, H.; de Clerck, O.; Willems, A. Who Is in There? Exploration of Endophytic Bacteria within the Siphonous Green Seaweed Bryopsis (Bryopsidales, Chlorophyta). PLoS ONE 2011, 6, e26458. [Google Scholar] [CrossRef] [PubMed]
- Contreras, N.; Alvíz, A.; Torres, J.; Uribe, S. Bryopsis Spp.: Generalities, Chemical and Biological Activities. Pharmacogn. Rev. 2019, 13, 63–70. [Google Scholar] [CrossRef]
- Hollants, J.; Leliaert, F.; Verbruggen, H.; Willems, A.; De Clerck, O. Permanent Residents or Temporary Lodgers: Characterizing Intracellular Bacterial Communities in the Siphonous Green Alga Bryopsis. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122659. [Google Scholar] [CrossRef]
- Hollants, J.; Decleyre, H.; Leliaert, F.; De Clerck, O.; Willems, A. Life without a Cell Membrane: Challenging the Specificity of Bacterial Endophytes within Bryopsis (Bryopsidales, Chlorophyta). BMC Microbiol. 2011, 11, 1–10. [Google Scholar] [CrossRef]
- Rao, K.V.; Na, M.K.; Cook, J.C.; Peng, J.; Matsumoto, R.; Hamann, M.T. Kahalalides V-Y Isolated from a Hawaiian Collection of the Sacoglossan Mollusk Elysia Rufescens. J. Nat. Prod. 2008, 71, 772–778. [Google Scholar] [CrossRef]
- Shalaby, E.A. Algae as Promising Organisms for Environment and Health. Plant Signal. Behav. 2011, 6, 1338–1350. [Google Scholar] [CrossRef]
- El-Baroty, G.S.; Moussa, M.Y.; Shallan, M.A.; Ali, M.A.; Sabh, A.Z.; Shalaby, E.A. Contribution to the Aroma, Biological Activities, Minerals, Protein, Pigments and Lipid Contents of the Red Alga: Asparagopsis taxiformis (Delile) Trevisan. J. Appl. Sci. Res. 2007, 3, 1825–1834. [Google Scholar]
- Lee, S.; Yeon, S.L.; Sang, H.J.; Sam, S.K.; Kuk, H.S. Anti-Oxidant Activities of Fucosterol from the Marine Algae Pelvetia Siliquosa. Arch. Pharm. Res. 2003, 26, 719–722. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M.M.; El-Fayoumy, E.A. Enteromorpha Compressa Exhibits Potent Antioxidant Activity. J. Biomed. Biotechnol. 2011, 2011, 726405. [Google Scholar] [CrossRef]
- Lee, M.C.; Yeh, H.Y. Molecular and Morphological Characterization of Colaconema formosanum sp. Nov. (Colaconemataceae, Rhodophyta)—A New Endophytic Filamentous Red Algal Species from Taiwan. J. Mar. Sci. Eng. 2021, 9, 809. [Google Scholar] [CrossRef]
- Yusoff, F.M.; Wong, N.L.W.S. Microalgae as Feeds for Bivalves. In Handbook of Food and Feed from Microalgae: Production, Application, Regulation, and Sustainability; Academic Press: Cambridge, MA, USA, 2023; pp. 451–470. [Google Scholar]
- Trémouillaux-Guiller, J.; Rohr, T.; Rohr, R.; Huss, V.A.R. Discovery of an Endophytic Alga in Ginkgo Biloba. Am. J. Bot. 2002, 89, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Correa, J.A.; Nielsen, R.; Grund, D.W. Endophytic algae of Chondrus crispus (rhodohyta). II. Acrochaete heteroclada sp. nov., A. operculata sp. nov., and Phaeophila dendroides (chlorophyta). J. Phycol. 1988, 24, 528–539. [Google Scholar] [CrossRef]
- Tam, C.E.; Cole, K.M.; Garbary, D.J. In Situ and in Vitro Studies on the Endophytic Red Algae Audouinella porphyrae and A. vaga (Acrochaetiales). Can. J. Bot. 1987, 65, 532–538. [Google Scholar] [CrossRef]
- Montoya, V.; Meynard, A.; Contreras-Porcia, L.; Contador, C.B. Molecular Identification, Growth, and Reproduction of Colaconema Daviesii (Rhodophyta; Colaconematales) Endophyte of the Edible Red Seaweed Chondracanthus Chamissoi. J. Appl. Phycol. 2020, 32, 3533–3542. [Google Scholar] [CrossRef]
- Gao, X.; Ogandaga, C.A.M.; Park, S.K.; Oh, J.C.; Choi, H.G. Algal Endophytes of Commercial Chondrus Ocellatus (Gigartinaceae, Rhodophyta) from Different Wild Populations in Korea. J. Appl. Phycol. 2020, 32, 697–703. [Google Scholar] [CrossRef]
- Hrabovský, J.; Pisera, A.; Gischler, E. A First Account of the Semi-Endophytic Coralline Algae Lithophyllum Cuneatum from the Caribbean Sea and Its Evolutionary and Biogeographic Significance. Geol. Carpathica 2022, 73, 81–93. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Agrawal, P. A review fungal endophytes: As a store house of bioactive compound. World J. Pharm. Pharmaceut. Sci. 2014, 3, 228. [Google Scholar]
- Petrini, O. Fungal Endophytes of Tree Leaves. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Eds.; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health. Forests 2019, 10, 42. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F.; Arnold, A.E.; Redman, R.S. Fungal Endophytes: Diversity and Functional Roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal Endophytes Limit Pathogen Damage in a Tropical Tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress Tolerance in Plants via Habitat-Adapted Symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Verma, V.C.; Kumar, A.; Gond, S.K.; Harper, J.K.; Hess, W.M.; Lobkovosky, E.; Ma, C.; Ren, Y.; Strobel, G.A. Javanicin, an Antibacterial Naphthaquinone from an Endophytic Fungus of Neem, Chloridium sp. Curr. Microbiol. 2009, 58, 233–238. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and Taxane Production by Taxomyces Andreanae, an Endophytic Fungus of Pacific Yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Rana, K.L.; Yadav, N.; Singh, B.; Chauhan, V.S.; Rastegari, A.A.; Hesham, A.E.L.; Gupta, V.K. Metabolic Engineering to Synthetic Biology of Secondary Metabolites Production. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Secondary Metabolites Biochemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 279–320. [Google Scholar]
- Jalgaonwala, R.E.; Mohite, B.V.; Mahajan, R.T. Natural Products from Plant Associated Endophytic Fungi. J. Microbiol. Biotechnol. Res. 2011, 1, 21–32. [Google Scholar]
- Kapoor, N.; Jamwal, V.L.; Gandhi, S.G. Endophytes as a Source of High-Value, Bioactive Metabolites. In Endophytes and Secondary Metabolites; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Khan, S.S.; Rasool, S. Endophytes: A Hunt for Important Bioactive Compounds. In Endophyte Biology; Apple Academic Press: New York, NY, USA, 2022; pp. 185–208. [Google Scholar]
- Bora, P.; Devi, N.N. Exploration of the Chemical Constituents and Its Antioxidant, Antibacterial Activities of Endophytic Fungi Isolated from the Medicinal Plant Dillenia Indica. Arch. Microbiol. 2023, 205, 67. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ghosh, S.; Mandal, N.C. Potential of an Endophytic Fungus Alternaria Tenuissima PE2 Isolated from Psidium guajava L. for the Production of Bioactive Compounds. S. Afr. J. Bot. 2022, 150, 658–667. [Google Scholar] [CrossRef]
- Jamal, H.A.A.; Husaini, A.; Sing, N.N.; Roslan, H.A.; Zulkharnain, A.; Akinkunmi, W.A. Characterization of Bioactive Compounds Produced by Endophytic Fungi Isolated from Gynura Procumbens (Sambung Nyawa). Braz. J. Microbiol. 2022, 53, 1857–1870. [Google Scholar] [CrossRef]
- Niu, L.; Rustamova, N.; Ning, H.; Paerhati, P.; Lu, C.; Yili, A. Diversity and Biological Activities of Endophytic Fungi from the Flowers of the Medicinal Plant Vernonia anthelmintica. Int. J. Mol. Sci. 2022, 23, 11935. [Google Scholar] [CrossRef]
- Kumar, V.; Prasher, I.B. Antimicrobial Potential of Endophytic Fungi Isolated from Dillenia Indica L. and Identification of Bioactive Molecules Produced by Fomitopsis Meliae (Undrew.) Murril. Nat. Prod. Res. 2022, 36, 6064–6068. [Google Scholar] [CrossRef]
- Gagana, S.L.; Kumaraswamy, B.E.; Shivanna, M.B. Diversity, Antibacterial and Antioxidant Activities of the Fungal Endophytes Associated with Schleichera oleosa (Lour.) Merr. S. Afr. J. Bot. 2020, 134, 369–381. [Google Scholar] [CrossRef]
- El-Zawawy, N.A.; Ali, S.S.; Khalil, M.A.; Sun, J.; Nouh, H.S. Exploring the Potential of Benzoic Acid Derived from the Endophytic Fungus Strain Neurospora Crassa SSN01 as a Promising Antimicrobial Agent in Wound Healing. Microbiol. Res. 2022, 262, 127108. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; Stephenson, S.L.; AlNadhari, S.; Yassin, M.A. Isolation, Identification and Bioactivity Analysis of an Endophytic Fungus Isolated from Aloe Vera Collected from Asir Desert, Saudi Arabia. Bioprocess Biosyst. Eng. 2021, 44, 1063–1070. [Google Scholar] [CrossRef]
- Hu, X.; Saravanakumar, K.; Park, S.J.; Han, K.S.; Wang, M.H. Isolation, Characterization, Antioxidant, and Wound Healing Activities of Extracellular Polysaccharide from Endophytic Fungus Talaromyces purpureogenus. Appl. Biochem. Biotechnol. 2023, 195, 3822–3839. [Google Scholar] [CrossRef]
- Nischitha, R.; Shivanna, M.B. Screening of Secondary Metabolites and Antioxidant Potential of Endophytic Fungus Penicillium citrinum and Host Digitaria bicornis by Spectrophotometric and Electrochemical Methods. Arch. Microbiol. 2022, 204, 206. [Google Scholar] [CrossRef]
- Sayed, A.M.; Sherif, N.H.; El-Gendy, A.O.; Shamikh, Y.I.; Ali, A.T.; Attia, E.Z.; El-Katatny, M.H.; Khalifa, B.A.; Hassan, H.M.; Abdelmohsen, U.R. Metabolomic Profiling and Antioxidant Potential of Three Fungal Endophytes Derived from Artemisia Annua and Medicago Sativa. Nat. Prod. Res. 2022, 36, 2404–2408. [Google Scholar] [CrossRef]
- Druzian, S.P.; Pinheiro, L.N.; Susin, N.M.B.; Dal Prá, V.; Mazutti, M.A.; Kuhn, R.C.; Terra, L. de M. Production of Metabolites with Antioxidant Activity by Botryosphaeria Dothidea in Submerged Fermentation. Bioprocess Biosyst. Eng. 2020, 43, 13–20. [Google Scholar] [CrossRef]
- Ujam, N.T.; Ajaghaku, D.L.; Okoye, F.B.C.; Esimone, C.O. Antioxidant and Immunosuppressive Activities of Extracts of Endophytic Fungi Isolated from Psidium Guajava and Newbouldia Laevis. Phytomedicine Plus 2021, 1, 100028. [Google Scholar] [CrossRef]
- Harrison, J.G.; Griffin, E.A. The Diversity and Distribution of Endophytes across Biomes, Plant Phylogeny and Host Tissues: How Far Have We Come and Where Do We Go from Here? Environ. Microbiol. 2020, 22, 2107–2123. [Google Scholar] [CrossRef]
- Gupta, S.; Chaturvedi, P. Foliar Endophytic Diversity of Centella asiatica (L.) Urban in Relation to Different Seasons and Leaf Age. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 468–477. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Shin, K.C.; Lee, J.K. Fungal Community Analyses of Endophytic Fungi from Two Oak Species, Quercus mongolica and Quercus serrata, in Korea. Mycobiology 2021, 49, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, D.; Ji, Z.; Chen, X.; Yu, L. A Comprehensive Assessment of Fungal Communities in Various Habitats from an Ice-Free Area of Maritime Antarctica: Diversity, Distribution, and Ecological Trait. Environ. Microbiomes 2022, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, D.; Yu, L.; Zhang, T. An Integrative Study of Mycobiome in Different Habitats from a High Arctic Region: Diversity, Distribution, and Functional Role. J. Fungi 2023, 9, 437. [Google Scholar] [CrossRef]
- Ji-Won Kim, Y.-J.K.; Eom, A.-H. Diversity and Community Structure of Endophytic Fungi Isolated from the Brown Seaweed Sargassum Thunbergii in Coastal Regions of Korea. Mycobiology 2024, 52, 317–323. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, G.; Kim, D.R.; Kwak, Y.S. Analysis of Endophytic Bacterial Communities and Investigation of Core Taxa in Apple Trees. Plant Pathol. J. 2023, 39, 397. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, X.; Tang, Q.Y.; Zhang, Z.D. Seasonal Dynamics and Persistency of Endophyte Communities in Kalidium Schrenkianum Shifts Under Radiation Stress. Front. Microbiol. 2021, 12, 778327. [Google Scholar] [CrossRef]
- Taniguchi, T.; Isobe, K.; Imada, S.; Eltayeb, M.M.; Akaji, Y.; Nakayama, M.; Allen, M.F.; Aronson, E.L. Root Endophytic Bacterial and Fungal Communities in a Natural Hot Desert Are Differentially Regulated in Dry and Wet Seasons by Stochastic Processes and Functional Traits. Sci. Total Environ. 2023, 899, 165524. [Google Scholar] [CrossRef]
- Pantinople, D.; Conner, R.; Sutton-Dauber, S.; Broussard, K.; Siniscalchi, C.; Engle-Wrye, N.; Jordan, H.; Folk, R. Continental Sampling Reveals Core Bacterial and Environmentally Driven Fungal Leaf Endophytes in Heuchera. Am. J. Bot. 2024, 111, e16428. [Google Scholar] [CrossRef]
- Pantinople, D.J.; Conner, R.; Sutton-dauber, S.; Broussard, K.; Carolina, M.; Engle-wrye, N.J.; Jordan, H.R.; Folk, R.A. Phylogenetic and Spatial Determinants of Leaf Endophyte Microbiomes in the Flowering Plant Genus Heuchera (Saxifragaceae). bioRxiv 2023, 1–49. [Google Scholar] [CrossRef]
- Zimmerman, N.B.; Vitousek, P.M. Fungal Endophyte Communities Reflect Environmental Structuring across a Hawaiian Landscape. Proc. Natl. Acad. Sci. USA 2012, 109, 13022–13027. [Google Scholar] [CrossRef]
- Furtado, B.U.; Gołebiewski, M.; Skorupa, M.; Hulisz, P.; Hrynkiewicz, K. Bacterial and Fungal Endophytic Microbiomes of Salicornia Europaea. Appl. Environ. Microbiol. 2019, 85, e00305-19. [Google Scholar] [CrossRef] [PubMed]
- David, A.S.; Seabloom, E.W.; May, G. Plant Host Species and Geographic Distance Affect the Structure of Aboveground Fungal Symbiont Communities, and Environmental Filtering Affects Belowground Communities in a Coastal Dune Ecosystem. Microb. Ecol. 2016, 71, 912–926. [Google Scholar] [CrossRef]
- Sohaib, H.; Fays, M.; Khatib, A.; Rivière, J.; El Aouad, N.; Desoignies, N. Contribution to the Characterization of the Seed Endophyte Microbiome of Argania Spinosa across Geographical Locations in Central Morocco Using Metagenomic Approaches. Front. Microbiol. 2024, 15, 1310395. [Google Scholar] [CrossRef]
- Babalola, O.O.; Adedayo, A.A. Endosphere Microbial Communities and Plant Nutrient Acquisition toward Sustainable Agriculture. Emerg. Top. Life Sci. 2023, 7, 207–217. [Google Scholar] [CrossRef]
- Firáková, S.; Šturdíková, M.; Múčková, M. Bioactive Secondary Metabolites Produced by Microorganisms Associated with Plants. Biologia (Bratisl). 2007, 62, 251–257. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic Bacteria: A New Source of Bioactive Compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef]
- Raimi, A.; Adeleke, R. Bioprospecting of Endophytic Microorganisms for Bioactive Compounds of Therapeutic Importance. Arch. Microbiol. 2021, 203, 1917–1942. [Google Scholar] [CrossRef]
- Godstime, O.; Felix, E.; Augustina, J.; Christopher, E. Mechanisms of Antimicrobial Actions of Phytochemicals against Enteric Pathogens—A Review. J. Pharm. Chem. Biol. Sci. 2014, 2, 77–85. [Google Scholar]
- Sharma, G.; Agarwal, S.; Verma, K.; Bhardwaj, R.; Mathur, V. Therapeutic Compounds from Medicinal Plant Endophytes: Molecular and Metabolic Adaptations. J. Appl. Microbiol. 2023, 134, lxad074. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Lappe-Oliveras, P.; Villanueva-Silva, R. Disruption of Cell Wall and Membrane Integrity as Antioomycete and Antifungal Mode of Action by Fusaric and 9,10-Dehydrofusaric Acids from Endophytic Fungus Fusarium Lactis Strain SME13-2. J. Appl. Microbiol. 2025, 136, lxae301. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Zheng, K.; Ma, Q.; Wen, M.; Shao, S.; Wu, S. Exploring the Diversity, Bioactivity of Endophytes, and Metabolome in Synsepalum Dulcificum. Front. Microbiol. 2024, 15, 1258208. [Google Scholar] [CrossRef]
- Akshatha, J.V.; SantoshKumar, H.S.; Prakash, H.S.; Nalini, M.S. In Silico Docking Studies of α-Amylase Inhibitors from the Anti-Diabetic Plant Leucas Ciliata Benth. and an Endophyte, Streptomyces Longisporoflavus. 3 Biotech 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.S.; da Silva, C.A.; Hamerski, L. Natural Products from Endophytic Fungi Associated with Rubiaceae Species. J. Fungi 2020, 6, 128. [Google Scholar] [CrossRef]
- Strobel, G.A.; Ford, E.; Li, J.Y.; Sears, J.; Sidhu, R.S.; Hess, W.M. Seimatoantlerium Tepuiense Gen. Nov., a Unique Epiphytic Fungus Producing Taxol from the Venezuelan Guyana. Syst. Appl. Microbiol. 1999, 22, 426–433. [Google Scholar] [CrossRef]
- Sharma, G.; Bhardwaj, R.; Jyoti; Barvkar, V.T.; Godbole, R.C.; Kumar, V.; Mathur, V. Host-Specific Endophytes of Momordica Charantia: A Promising Source for Affordable Lung Cancer Therapeutics. S. Afr. J. Bot. 2024, 170, 181–193. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, P.; Thakur, V.; Chand, D.; Bhatia, R.K.; Kulshrestha, S.; Kumar, P. Paclitaxel Production from Endophytic Mucor Circinelloides Isolated from Taxus Sp. of the Northern Himalayan Region. 3 Biotech 2024, 14, 251. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Kumudini, B.S. Halotolerant Fungi Secreting Phytohormones and Volatile Organic Compounds Enhance Growth and Mineral Content in Finger Millet under Salinity Stress. Plant Stress 2024, 11, 100426. [Google Scholar] [CrossRef]
- Gupte, S. Novel Approaches to Developing New Antibiotics. J. Bacteriol. Mycol. Open Access 2017, 4, 00089. [Google Scholar] [CrossRef]
- Guo, B.; Dai, J.R.; Ng, S.; Huang, Y.; Leong, C.; Ong, W.; Carté, B.K. Cytonic Acids A and B: Novel Tridepside Inhibitors of HCMV Protease from the Endophytic Fungus Cytonaema Species. J. Nat. Prod. 2000, 63, 602–604. [Google Scholar] [CrossRef]
- Inahashi, Y.; Iwatsuki, M.; Ishiyama, A.; Matsumoto, A.; Hirose, T.; Oshita, J.; Sunazuka, T.; Panbangred, W.; Takahashi, Y.; Kaiser, M.; et al. Actinoallolides A-E, New Anti-Trypanosomal Macrolides, Produced by an Endophytic Actinomycete, Actinoallomurus Fulvus MK10-036. Org. Lett. 2015, 17, 864–867. [Google Scholar] [CrossRef]
- Nakashima, T.; Okuyama, R.; Kamiya, Y.; Matsumoto, A.; Iwatsuki, M.; Inahashi, Y.; Yamaji, K.; Takahashi, Y.; Omura, S. Trehangelins A, B and C, Novel Photo-Oxidative Hemolysis Inhibitors Produced by an Endophytic Actinomycete, Polymorphospora Rubra K07-0510. J. Antibiot. 2013, 66, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Omura, S. ChemInform Abstract: Spoxazomicins A-C, Novel Antitrypanosomal Alkaloids Produced by an Endophytic Actinomycete, Streptosporangium Oxazolinicum K07-0460T. ChemInform 2011, 64, 303–307. [Google Scholar] [CrossRef]
- Matsumoto, A.; Takahashi, Y. Endophytic Actinomycetes: Promising Source of Novel Bioactive Compounds. J. Antibiot. 2017, 70, 514–519. [Google Scholar] [CrossRef]
- Hassan, W.; Noreen, H.; Rehman, S.; Gul, S.; Amjad Kamal, M.; Paul Kamdem, J.; Zaman, B.; da Rocha, J.B.T. Oxidative Stress and Antioxidant Potential of One Hundred Medicinal Plants. Curr. Top. Med. Chem. 2017, 17, 1336–1370. [Google Scholar] [CrossRef]
- Cui, X.; Lin, Q.; Liang, Y. Plant-Derived Antioxidants Protect the Nervous System From Aging by Inhibiting Oxidative Stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef]
- Caicedo, N.H.; Davalos, A.F.; Puente, P.A.; Rodríguez, A.Y.; Caicedo, P.A. Antioxidant Activity of Exo-Metabolites Produced by Fusarium oxysporum: An Endophytic Fungus Isolated from Leaves of Otoba gracilipes. Microbiologyopen 2019, 8, e903. [Google Scholar] [CrossRef]
- Rumidatul, A.; Rahmawati, N.; Sunarya, S. Production of Secondary Metabolites and Its Antibacterial and Antioxidant Activity during the Growth Period of Endophytic Fungi Isolated from Gall Rust Sengon Plants. Pharmacogn. J. 2021, 8, e903. [Google Scholar] [CrossRef]
- Palanichamy, P.; Krishnamoorthy, G.; Kannan, S.; Marudhamuthu, M. Bioactive Potential of Secondary Metabolites Derived from Medicinal Plant Endophytes. Egypt. J. Basic Appl. Sci. 2018, 5, 303–312. [Google Scholar] [CrossRef]
- Rai, N.; Kumari Keshri, P.; Verma, A.; Kamble, S.C.; Mishra, P.; Barik, S.; Kumar Singh, S.; Gautam, V. Plant Associated Fungal Endophytes as a Source of Natural Bioactive Compounds. Mycology 2021, 12, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca Acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Lee, J.C.; Strobel, G.A.; Lobkovsky, E.; Clardy, J. Torreyanic Acid: A Selectively Cytotoxic Quinone Dimer from the Endophytic Fungus Pestalotiopsis microspora. J. Org. Chem. 1996, 61, 3232–3233. [Google Scholar] [CrossRef]
- Pandi, M.; Manikandan, R.; Muthumary, J. Anticancer Activity of Fungal Taxol Derived from Botryodiplodia Theobromae Pat., an Endophytic Fungus, against 7, 12 Dimethyl Benz(a)Anthracene (DMBA)-Induced Mammary Gland Carcinogenesis in Sprague Dawley Rats. Biomed. Pharmacother. 2010, 64, 48–53. [Google Scholar] [CrossRef]
- Pandi, M.; Kumaran, R.S.; Choi, Y.K.; Kim, H.J.; Muthumary, J. Isolation and Detection of Taxol, an Anticancer Drug Produced from Lasiodiplodia Theobromae, an Endophytic Fungus of the Medicinal Plant Morinda Citrifolia. African J. Biotechnol. 2011, 10, 1428–1435. [Google Scholar]
- Suresh, G.; Kokila, D.; Suresh, T.C.; Kumaran, S.; Velmurugan, P.; Vedhanayakisri, K.A.; Sivakumar, S.; Ravi, A.V. Mycosynthesis of Anticancer Drug Taxol by Aspergillus Oryzae, an Endophyte of Tarenna Asiatica, Characterization, and Its Activity against a Human Lung Cancer Cell Line. Biocatal. Agric. Biotechnol. 2020, 24, 101525. [Google Scholar] [CrossRef]
- Mohammed, S.I.; Patil, M.P.; Patil, R.H.; Maheshwari, V.L. Endophytes: Potential Source of Therapeutically Important Secondary Metabolites of Plant Origin. In Endophytes: Crop Productivity and Protection; Springer: Cham, Switzerland, 2017; Volume 16, pp. 213–237. [Google Scholar]
- Biswas, D.; Nazir, R.; Biswas, P.; Kumar, V.; Nandy, S.; Mukherjee, A.; Dey, A.; Pandey, D.K. Endophytic Sources of Diosgenin, a Natural Steroid with Multiple Therapeutic Values. S. Afr. J. Bot. 2020, 134, 119–125. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Swapnil, P.; Harish; Marwal, A.; Yadav, G.; Sonigra, P. Endophytic Nanotechnology: An Approach to Study Scope and Potential Applications. Front. Chem. 2021, 134, 119–125. [Google Scholar] [CrossRef]
- Barupal, T.; Meena, M.; Sharma, K. Inhibitory Effects of Leaf Extract of Lawsonia inermis on Curvularia Lunata and Characterization of Novel Inhibitory Compounds by GC–MS Analysis. Biotechnol. Reports 2019, 23, e00335. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based Nanomaterials: Synthesis and Recent Progress in Electrochemical Sensor Applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef]
- Singh, T.; Jyoti, K.; Patnaik, A.; Singh, A.; Chauhan, R.; Chandel, S.S. Biosynthesis, Characterization and Antibacterial Activity of Silver Nanoparticles Using an Endophytic Fungal Supernatant of Raphanus Sativus. J. Genet. Eng. Biotechnol. 2017, 15, 31–39. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Yin, Y.; Shen, M. Interactions between Engineered Nanoparticles and Dissolved Organic Matter: A Review on Mechanisms and Environmental Effects. J. Environ. Sci. 2018, 63, 198–217. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Hassan, D.; Maaza, M. Sageretia Thea (Osbeck.) Modulated Biosynthesis of NiO Nanoparticles and Their in Vitro Pharmacognostic, Antioxidant and Cytotoxic Potential. Artif. Cells Nanomed. Biotechnol. 2018, 46, 838–852. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, L.; Khalil, A.T.; Ali, N.; Zia, D.; Ali, M.; Shinwari, Z.K. Endophyte-Mediated Synthesis of Silver Nanoparticles and Their Biological Applications. Appl. Microbiol. Biotechnol. 2019, 103, 2551–2569. [Google Scholar] [CrossRef]
- Syed, B.; Rao, H.C.Y.; Nagendra-Prasad, M.N.; Prasad, A.; Harini, B.P.; Azmath, P.; Rakshith, D.; Satish, S. Biomimetic Synthesis of Silver Nanoparticles Using Endosymbiotic Bacterium Inhabiting Euphorbia hirta L. and Their Bactericidal Potential. Scientifica 2016, 2016, 9020239. [Google Scholar] [CrossRef]
- Andrade, F.; Jenipher, C.; Gurav, N.; Nadaf, S.; Khan, M.S.; Mahajan, N.; Bhagwat, D.; Kalaskar, M.; Chikhale, R.; Bhole, R.; et al. Endophytic Fungi-Assisted Biomass Synthesis of Eco-Friendly Formulated Silver Nanoparticles for Enhanced Antibacterial, Antioxidant, and Antidiabetic Activities. J. Drug Deliv. Sci. Technol. 2024, 97, 105749. [Google Scholar] [CrossRef]
- Netala, V.R.; Kotakadi, V.S.; Bobbu, P.; Gaddam, S.A.; Tartte, V. Endophytic Fungal Isolate Mediated Biosynthesis of Silver Nanoparticles and Their Free Radical Scavenging Activity and Anti Microbial Studies. 3 Biotech 2016, 1, p9020239. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A Review on the Classification, Characterisation, Synthesis of Nanoparticles and Their Application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar]
- Messaoudi, O.; Bendahou, M. Biological Synthesis of Nanoparticles Using Endophytic Microorganisms: Current Development. In Nanotechnology and the Environment; IntechOpen: London, UK, 2020; Volume 37. [Google Scholar]
- Roy, K.; Kar, S.; Das, R.N.; Roy, K.; Kar, S.; Das, R.N. Chapter 10—Other Related Techniques. In Understanding the Basics of QSAR for Applications in Pharmaceutical Sciences and Risk Assessment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 357–425. [Google Scholar]
- Garuba, T.; Govender, R.; Isah, H.A.; Sabiu, S. Metabolites Profiling and Molecular Docking Identification of Putative Leads from Endophytic Phyllosticta Capitalensis as Modulators of Key Druggable Structural Targets of Rotavirus A. Trans. R. Soc. S. Afr. 2022, 77, 207–217. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the Pharmaceutical Industry: New Estimates of R&D Costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [CrossRef]
- Stanzione, F.; Giangreco, I.; Cole, J.C. Use of Molecular Docking Computational Tools in Drug Discovery. Prog. Med. Chem. 2021, 60, 273–343. [Google Scholar]
- Kuntz, I.D.; Blaney, J.M.; Oatley, S.J.; Langridge, R.; Ferrin, T.E. A Geometric Approach to Macromolecule-Ligand Interactions. J. Mol. Biol. 1982, 161, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, H.; Hendlich, M.; Klebe, G. Knowledge-Based Scoring Function to Predict Protein-Ligand Interactions. J. Mol. Biol. 2000, 295, 337–356. [Google Scholar] [CrossRef]
- Khamis, M.A.; Gomaa, W.; Ahmed, W.F. Machine Learning in Computational Docking. Artif. Intell. Med. 2015, 63, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Pragya Tiwari, S.T.; Dufossé, L. Antimicrobials from Endophytes as Novel Therapeutics to Counter Drug-Resistant Pathogens. Crit. Rev. Biotechnol. 2025, 45, 164–190. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P. Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance. Antibiotics 2024, 13, 746. [Google Scholar] [CrossRef]
- Martinez-Klimova, E.; Rodríguez-Peña, K.; Sánchez, S. Endophytes as Sources of Antibiotics. Biochem. Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef]
- Alsheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Moiketsi, B.N.; Makale, K.P.P.; Rantong, G.; Rahube, T.O.; Makhzoum, A. Potential of Selected African Medicinal Plants as Alternative Therapeutics against Multi-Drug-Resistant Bacteria. Biomedicines 2023, 11, 2605. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities With Multifunctional Prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Kumar, K.K.; Dara, S.K. Fungal and Bacterial Endophytes as Microbial Control Agents for Plant-Parasitic Nematodes. Int. J. Environ. Res. Public Health 2021, 18, 4269. [Google Scholar] [CrossRef]
- Hashem, A.H.; Attia, M.S.; Kandil, E.K.; Fawzi, M.M.; Abdelrahman, A.S.; Khader, M.S.; Khodaira, M.A.; Emam, A.E.; Goma, M.A.; Abdelaziz, A.M. Bioactive Compounds and Biomedical Applications of Endophytic Fungi: A Recent Review. Microb. Cell Fact. 2023, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Kayode, A.J.; Okoh, A.I. Assessment of Multidrug-Resistant Listeria Monocytogenes in Milk and Milk Product and One Health Perspective. PLoS ONE 2022, 17, e0270993. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Nina, P.B.; JP, D.; Kumar, S.; Singh, B.; Tiwari, R.R. Futuristic Non-Antibiotic Therapies to Combat Antibiotic Resistance: A Review. Front. Microbiol. 2021, 12, 609459. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Anand Kumar, P.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
| Medicinal Plant | Endophyte | Bioactive Metabolite | Test Pathogen | Solvent | Activity | References |
|---|---|---|---|---|---|---|
| Andrographis paniculata (Green Chiretta) | Cochliobolus sp. APS1 | - | Bacillus cereus, B. subtilis, Proteus. mirabilis, P. aeruginosa, Escherichia coli, Shigella flexneri, Vancomycin-Resistant Staphylococcus aureus (VRSA), and Methicillin-Resistant Staphylococcus aureus (MRSA) | Ethyl acetate | Anti-biofilm, antilarval potency, and Cidal antibacterial (MIC and MBC values range: 15.62–125 µg/mL and 62.5–125 µg/mL, respectively). Larvicidal effect against Dengue-vector Aedes aegypti | [23] |
| Dillenia indica | Aspergillus flavus, A. niger, and A. fumigatus | Dodecane, 2-isopropyl-5-methyl-1-heptanol, and 2-ethylhexyl ester, 1-octanol | B. subtilis, E. coli, and S. aureus | Ethyl acetate | 6.0–14.0 µg/mL | [128] |
| Psidium guajava L. (leaves) | Alternaria tenuissima PE2 | Several natural bioactive compounds | Listeria monocytogenes, B. subtilis, S. aureus, S. epidermidis, Salmonella typhimurium | Ethyl acetate | MIC and MBC values (~500 µg/mL ~800 µg/mL, respectively) | [129] |
| Gynura procumbens (Sambung Nyawa) | Colletotrichum gloeosporiodes Macrophomina phaseolina, Mycoleptodiscus indicus, Phomopsis sp., and Diaporthe hongkongensis | A plethora of bioactive metabolites including isoelemicin, terpinel, eucalyptol, oleic acid, β-pinene, γ-terpinine, 4-carene, octadecanoic acid, caryophyllene, aromadendrene, and globulol. | E. coli, P. aeruginosa, S. typhi, S. aureus, and MRSA | Ethyl acetate and methanol | Antibacterial—MIC and MBC (5000 µg/mL) | [130] |
| Vernonia anthelmintica | Ovatospora senegalensis, Chaetomium globusum, A. calidoustus, A. keveii, and A. terreus | 9,12-octadecadienoic acid (Z, Z) | E. coli, S. aureus, and Candida albicans | Ethyl acetate | Antimicrobial anticancer and cytotoxic (MIC: 62.5–250 µg/mL) | [131] |
| Rumex nervous, Pulicaria crispa, and Withania somnifera | Aspergillus flavipes, Fusarium clamydosporum, Penicillium commune, and P. glaucoroseum | Antioxidants Phenols: catechol, cinnamic acid, p-OH benzoic, ferulic, and protocatechulic acids Flavonoids: acacetin, apigenin, chrysin, and epicatectin, luteolin, rutin, quercetin. | B. subtilis, E. coli, C. albicans, C. glabrata, K. pneumonia, and S. aureus | Ethyl acetate | Weak antimicrobial and cytotoxic effects and cancer activity | [24] |
| Dillenia indica L. | Fomitopsis meliae, Colletotrichum gloeosporioides, Nigrospora sphaerica, Chaetomium globosum, Schizophyllum commune, Fomes meliae, Fusarium oxysporum, Xylaria longipes | A plethora of bioactive compounds including benzaldehyde, 4-(1-methylethyl)-, dodecane, ethyl 2-thiopheneacetate, griseofulvin, hexadecane, octadecane, tetradecane | E. coli, P. aeruginosa, B. subtilis, and S. aureus | Ethyl acetate and methanol | Antibacterial (zones of inhibition: 0–29 mm) | [132] |
| Scheleichera oleosa (Lour.) Merr | Arcopilus cupreus | Caffeic acid, citric acid, isofraxidin, and quercetin | E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, P. syringae, S. enterica, S. typhi, Enterococcus faecalis, S. aureus, and Xanthomonas campestris | Ethyl acetate | Antimicrobial and antioxidant activities | [133] |
| Lycium shawii | Neurospora crassa | - | P. aeruginosa, E. coli, Klebsiella pneumonia, S. aureus, Aspergillus niger, and Candida albicans | Ethyl acetate | Antimicrobial and wound healing | [134] |
| Aloe vera | Preussia africana | - | - | - | Antioxidant | [135] |
| Talaromyces purpureogenus | Polysaccharides TEPS1 and TEPS2 | - | - | Antioxidant and wound healing | [136] | |
| Digitaria bicornis | Penicillium citrinum | benzophenone, caffeic acid, cannabidol, ergosterol, α-eleostearic acid, oleamide, sclerotiorin, and solanine | - | Ethyl acetate | Antioxidant | [137] |
| Artemisia annua | Aspergillus terreus | Coumarins, phenolics, and polyketide | - | Ethyl acetate | Antioxidant | [138] |
| Plants from Brazilians pampa and Atlantic Forest biomes | Botryosphaeria dothidea | bis(2-methylpropyl) ester, Hexahydropyrrolizin-3-one, and 6-bis(2-methylpropyl)-2.5-piperazinedione | - | Ethyl acetate | Antioxidant | [139] |
| Psidium guajava and Newbouldia leavis | Fusarium sp. and Cladosporium sp. | Citrinin, citreohybridinol, cyclopenin, p-hydrobenzoic acid, nakijinol, nidulanin, and protocatechuic acid | - | Ethyl acetate | Antioxidant and immunomodulatory | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayode, A.J.; Igwaran, A.; Banji-Onisile, F.; Akwu, N.A.; Unuofin, J.O.; Osunla, A.C.; Egbewale, S.O.; Purnobasuki, H. Unveiling the Hidden Allies in the Fight Against Antimicrobial Resistance—Medicinal Plant Endophytes. Bacteria 2025, 4, 26. https://doi.org/10.3390/bacteria4020026
Kayode AJ, Igwaran A, Banji-Onisile F, Akwu NA, Unuofin JO, Osunla AC, Egbewale SO, Purnobasuki H. Unveiling the Hidden Allies in the Fight Against Antimicrobial Resistance—Medicinal Plant Endophytes. Bacteria. 2025; 4(2):26. https://doi.org/10.3390/bacteria4020026
Chicago/Turabian StyleKayode, Adeoye J., Aboi Igwaran, Folasade Banji-Onisile, Nneka A. Akwu, John O. Unuofin, Ayodeji C. Osunla, Samson O. Egbewale, and Hery Purnobasuki. 2025. "Unveiling the Hidden Allies in the Fight Against Antimicrobial Resistance—Medicinal Plant Endophytes" Bacteria 4, no. 2: 26. https://doi.org/10.3390/bacteria4020026
APA StyleKayode, A. J., Igwaran, A., Banji-Onisile, F., Akwu, N. A., Unuofin, J. O., Osunla, A. C., Egbewale, S. O., & Purnobasuki, H. (2025). Unveiling the Hidden Allies in the Fight Against Antimicrobial Resistance—Medicinal Plant Endophytes. Bacteria, 4(2), 26. https://doi.org/10.3390/bacteria4020026






