Effect of Lactic Acid Fermentation on Phytochemical Content, Antioxidant Capacity, Sensory Acceptability and Microbial Safety of African Black Nightshade and African Spider Plant Vegetables
Abstract
1. Introduction
2. Results and Discussion
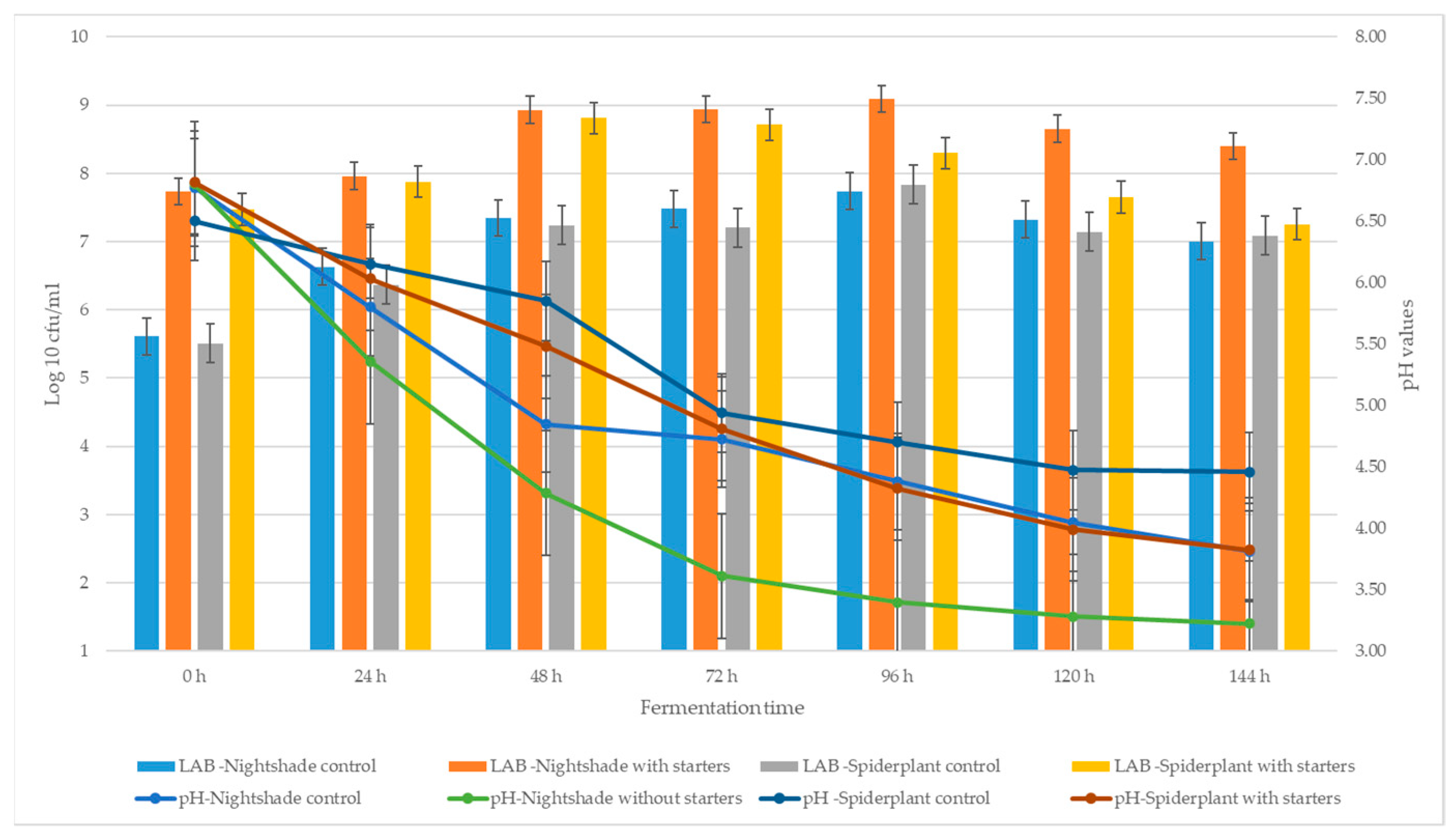
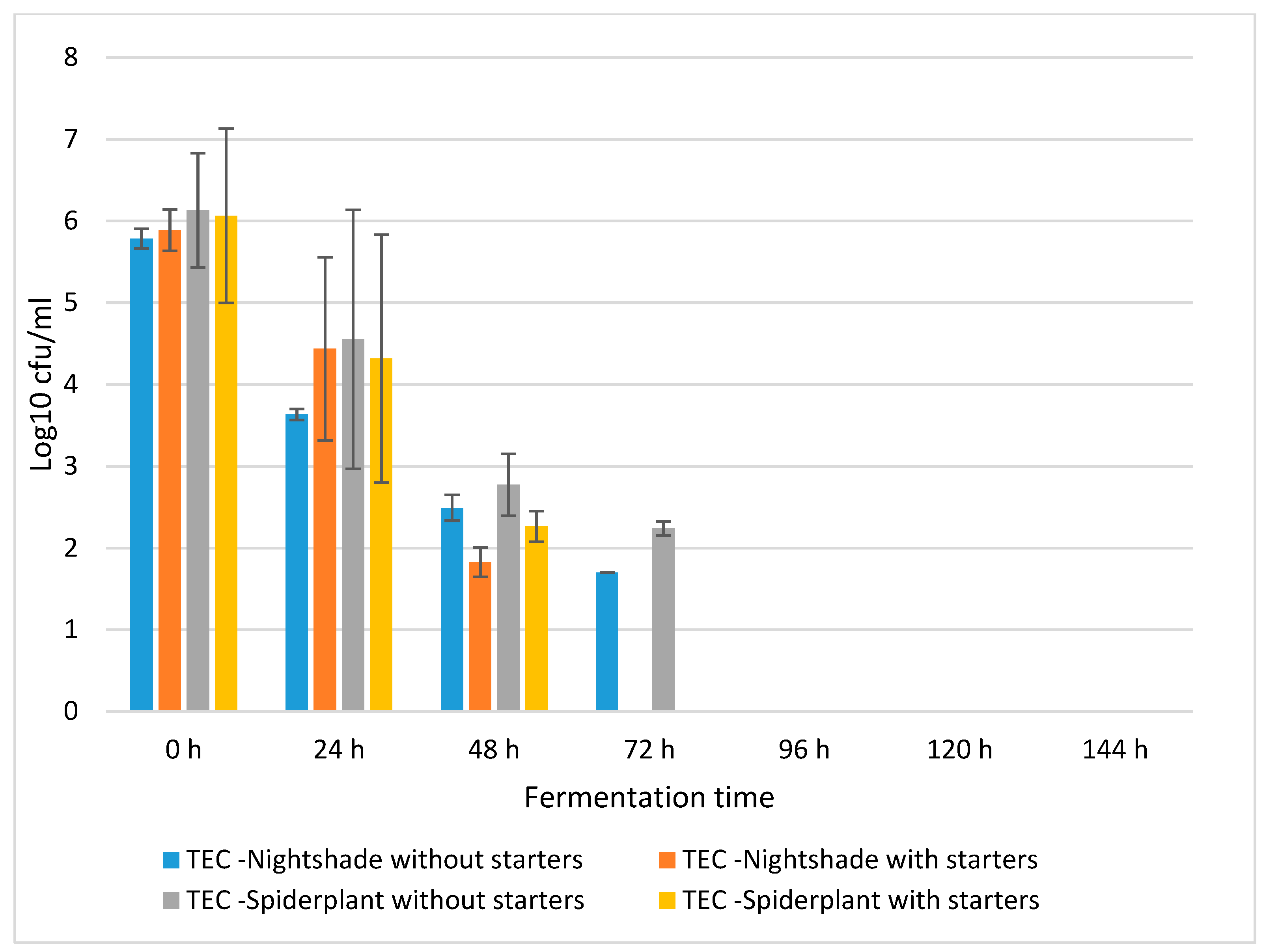
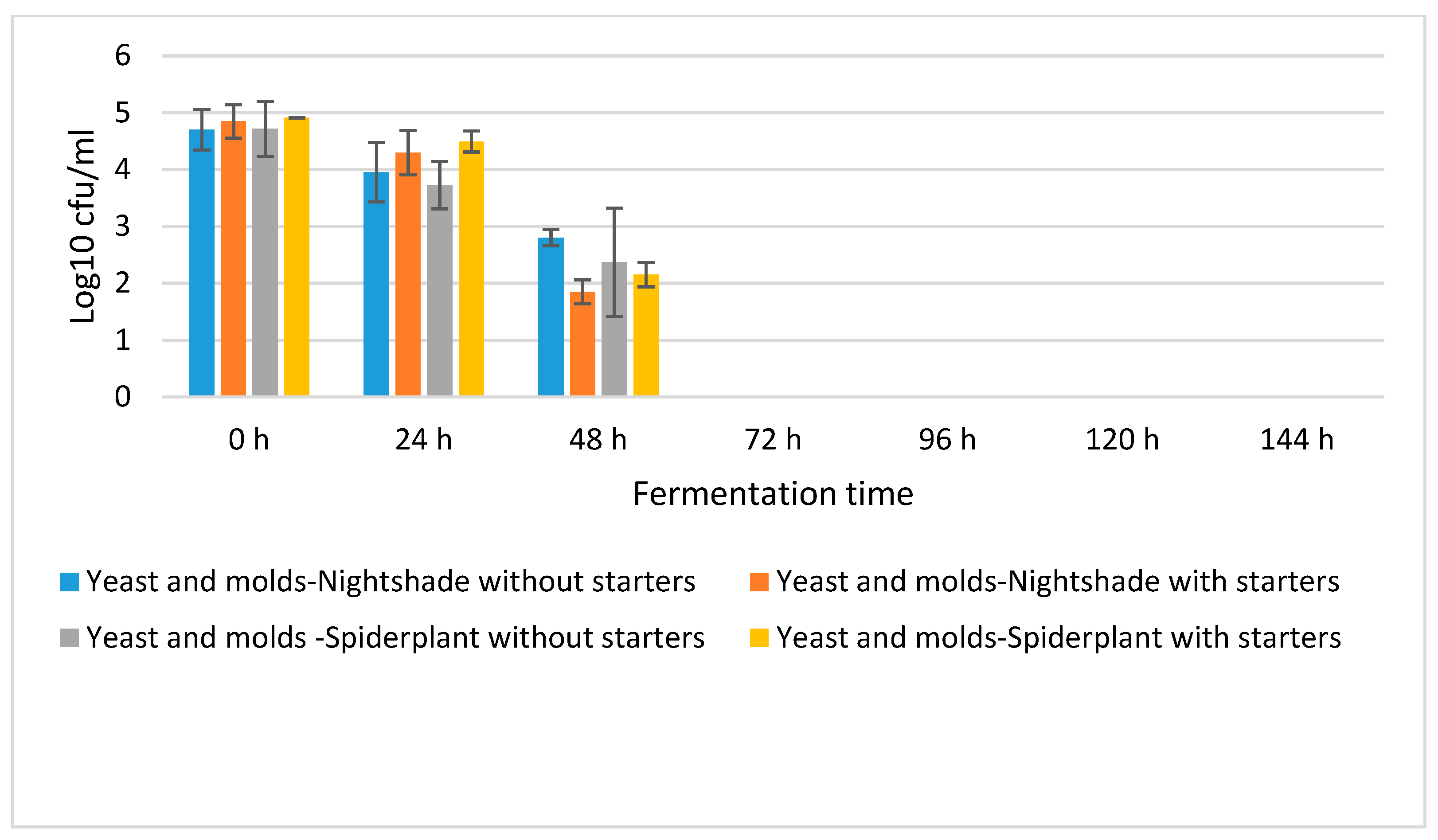
2.1. Fermentation Dynamics
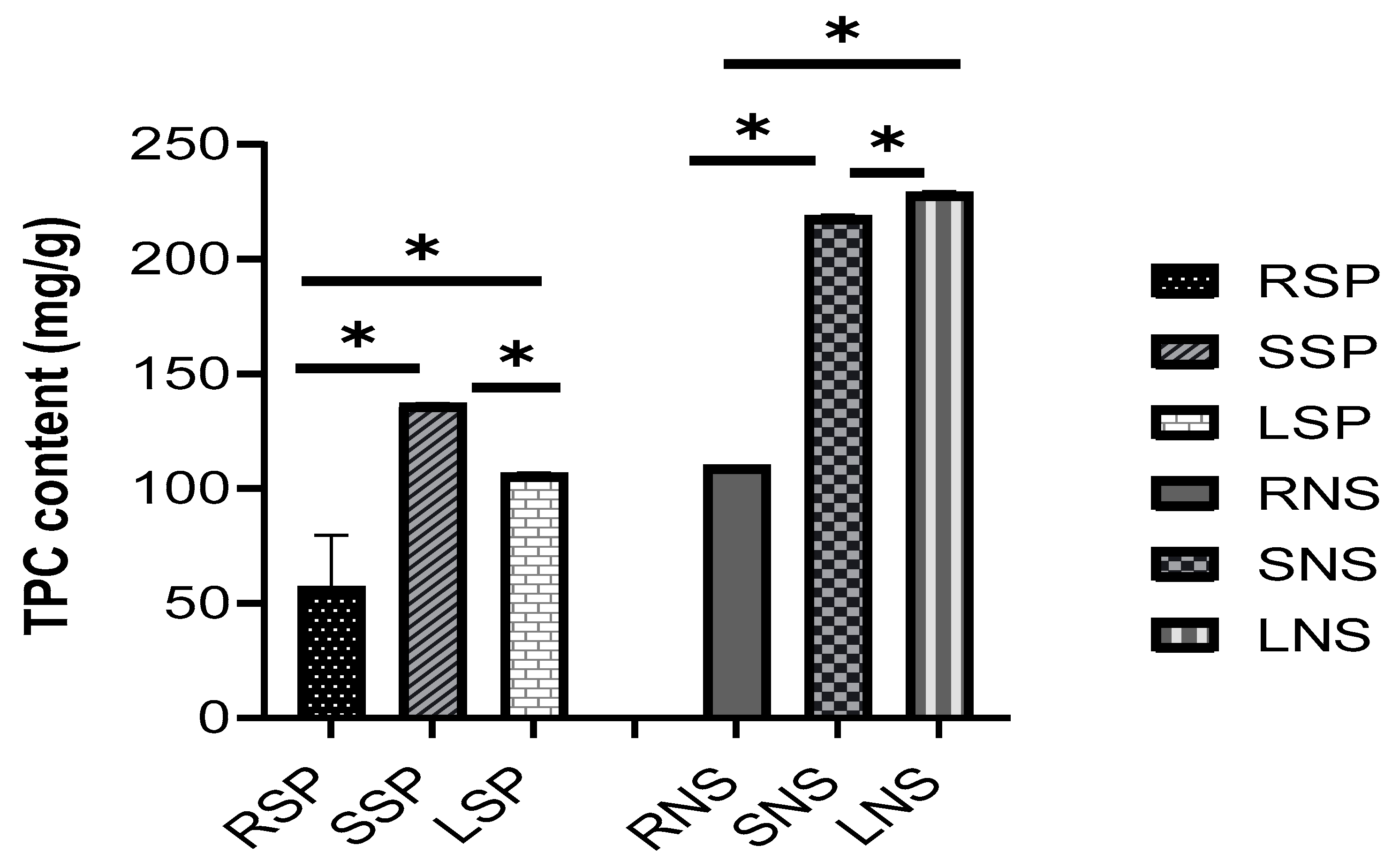
2.2. Phytochemical Content (Total Phenolic Compounds and Flavonoids)
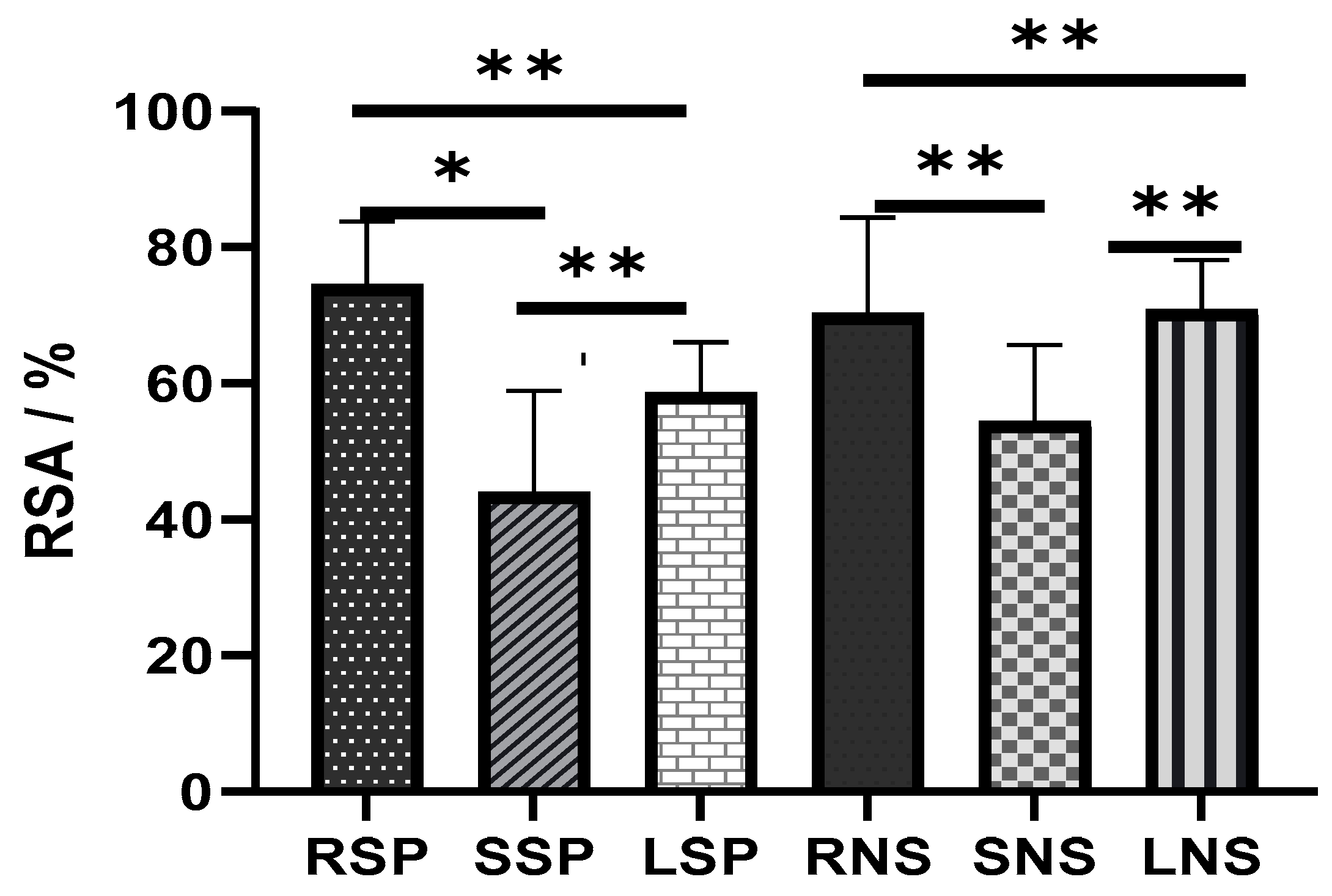
2.3. Antioxidant Capacity
2.4. Evaluation of Sensory Acceptability
3. Materials and Methods
3.1. Preparation of Materials and African Indigenous Leafy Vegetables
3.2. Preparation of Starter Inocula
3.3. Fermentation of African Black Nightshade and Spider Plant Leaves
3.4. Determination of Phytochemical Content
- (a)
- Determination of flavonoid content
- (b)
- Determination of total phenolic compound content
3.5. Determination of Antioxidant Capacity
3.6. Evaluation of Sensory Acceptability
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matenge, S.T.P.; Li, J.; Apau, S.; Tapera, R. Nutritional And Phytochemical Content of Indigenous Leafy Vegetables Consumed In Botswana. Front. Food Nutr. Res. 2017, 3, 1–7. [Google Scholar]
- Mbhenyane, X.G. Indigenous Foods and Their Contribution to Nutrient Requirements. South Afr. J. Clin. Nutr. 2017, 30, 5–7. [Google Scholar]
- FAO. FAO Fruit and Vegetables–Your Dietary Essentials. In The International Year of Fruits and Vegetables; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Nsabimana, A.; Bali Swain, R.; Surry, Y.; Ngabitsinze, J.C. Income and Food Engel Curves in Rwanda: A Household Microdata Analysis. Agric. Food Econ. 2020, 8, 11. [Google Scholar] [CrossRef]
- Ekhuya, N.A.; Wesonga, J.M.; Abukutsa-Onyango, M.O. Production, Processing and Storage Techniques of African Nightshade (Solanum Spp.) Seeds and Their Correlations with Farmers’ Characteristics in Western Kenya. Afr. J. Food Agric. Nutr. Dev. 2018, 18, 13338–13351. [Google Scholar] [CrossRef]
- Ontita, E.; Onyango, C.; Onwonga, R.; Nyamongo, D. Indigenous Knowledge on the Uses of African Nightshades (Solanum nigram L.) Species among Three Kenyan Communities. Asian J. Agric. Ext. Econ. Sociol. 2016, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Odongo, G.A.; Schlotz, N.; Baldermann, S.; Neugart, S.; Huyskens-Keil, S.; Ngwene, B.; Trierweiler, B.; Schreiner, M.; Lamy, E. African Nightshade (Solanum scabrum Mill.): Impact of Cultivation and Plant Processing on Its Health Promoting Potential as Determined in a Human Liver Cell Model. Nutrients 2018, 10, 1532. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A.; Fusco, V.; Cho, G.S.; Kabisch, J.; Neve, H.; Bockelmann, W.; Huch, M.; Frommherz, L.; Trierweiler, B.; Becker, B.; et al. Produce from Africa’s Gardens: Potential for Leafy Vegetable and Fruit Fermentations. Front. Microbiol. 2016, 7, 981. [Google Scholar] [CrossRef]
- Irakoze, M.L.; Wafula, E.N.; Owaga, E. Potential Role of African Fermented Indigenous Vegetables in Maternal and Child Nutrition in Sub-Saharan Africa. Int. J. Food Sci. 2021, 2021, 3400329. [Google Scholar] [CrossRef]
- Mashamaite, C.V.; Manyevere, A.; Chakauya, E. Cleome Gynandra: A Wonder Climate-Smart Plant for Nutritional Security for Millions in Semi-Arid Areas. Front. Plant Sci. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Imanirampa, L.; Alele, P.E. Antifungal Activity of Cleome gynandra L. Aerial Parts for Topical Treatment of Tinea Capitis: An in Vitro Evaluation. BMC Complement. Altern. Med. 2016, 16, 194. [Google Scholar] [CrossRef]
- Sogbohossou, E.O.D.; Kortekaas, D.; Achigan-Dako, E.G.; Maundu, P.; Stoilova, T.; Van Deynze, A.; de Vos, R.C.H.; Schranz, M.E. Association between Vitamin Content, Plant Morphology and Geographical Origin in a Worldwide Collection of the Orphan Crop Gynandropsis gynandra (Cleomaceae). Planta 2019, 250, 933–947. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, A.; Mishra, A.K. The Chemistry and Pharmacology of Cleome Genus: A Review. Biomed. Pharmacother. 2018, 101, 37–48. [Google Scholar] [CrossRef]
- Seburanga, J.L. Decline of Indigenous Crop Diversity in Colonial and Postcolonial Rwanda. Int. J. Biodivers. 2013, 2013, 401938. [Google Scholar] [CrossRef]
- Moyo, M.; Amoo, S.O.; Aremu, A.O.; Gruz, J.; Šubrtová, M.; Jarošová, M.; Tarkowski, P.; Doležal, K. Determination of Mineral Constituents, Phytochemicals and Antioxidant Qualities of Cleome gynandra, Compared to Brassica oleracea and Beta vulgaris. Front. Chem. 2018, 5, 128. [Google Scholar] [CrossRef]
- Traoré, K.; Parkouda, C.; Savadogo, A.; Ba/Hama, F.; Kamga, R.; Traoré, Y. Effect of Processing Methods on the Nutritional Content of Three Traditional Vegetables Leaves: Amaranth, Black Nightshade and Jute Mallow. Food Sci. Nutr. 2017, 5, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Essack, H.; Odhav, B.; Mellem, J.J. Screening of Traditional South African Leafy Vegetables for Specific Anti-Nutritional Factors before and after Processing. Food Sci. Technol. 2017, 37, 462–471. [Google Scholar] [CrossRef]
- Stoll, D.A.; Wafula, E.N.; Mathara, J.M.; Trierweiler, B.; Kulling, S.E.; Huch, M. Fermentation of African Nightshade Leaves with Lactic Acid Bacterial Starter Cultures. Int. J. Food Microbiol. 2021, 342, 109056. [Google Scholar] [CrossRef]
- Medina, E.; de Castro, A.; Romero, C.; Ramírez, E.M.; Brenes, M. Safety of Fermented Fruits and Vegetables; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128006207. [Google Scholar]
- Gadaga, T.; Lehohla, M.; Ntuli, V. Traditional Fermented Foods of Lesotho. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 2387–2391. [Google Scholar]
- Capozzi, V.; Fragasso, M.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous Food Fermentations and Potential Risks for Human Health. Fermentation 2017, 3, 49. [Google Scholar] [CrossRef]
- Medina, P.E.; Pérez-Díaz, I.M.; Garrido-Fernández, A.; Arroyo-López, F.N. Review of Vegetable Fermentations with Particular Emphasis on Processing Modifications, Microbial Ecology, and Spoilage. In The Microbiological Quality of Food Foodborne Spoilers; Woodhead Publishing: Sawston, UK, 2017; pp. 211–236. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Belguesmia, Y.; Bendali, F.; Spano, G.; Seal, B.S.; Drider, D. Lactobacillus fermentum: A Bacterial Species with Potential for Food Preservation and Biomedical Applications. Crit. Rev. Food Sci. Nutr. 2019, 60, 3387–3399. [Google Scholar] [CrossRef]
- Darby, T.M.; Owens, J.A.; Saeedi, B.J.; Luo, L.; Matthews, J.D.; Robinson, B.S.; Naudin, C.R.; Jones, R.M. Lactococcus Lactis Subsp. Cremoris Is an Efficacious Beneficial Bacterium That Limits Tissue Injury in the Intestine. iScience 2019, 12, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Maalaoui, A.; Trimeche, A.; Marnet, P.G.; Demarigny, Y. Use of Lactococcus lactis subsp. lactis strains to inhibit the development of Pathogens. Food Nutr. Sci. 2020, 11, 98–112. [Google Scholar] [CrossRef]
- Lemi, B.W. Microbiology of Ethiopian Traditionally Fermented. Int. J. Microbiol. 2020, 2020, 1478536. [Google Scholar]
- Tamang, J.p.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Gabaza, M.; Muchuweti, M.; Vandamme, P.; Raes, K. Can Fermentation Be Used as a Sustainable Strategy to Reduce Iron and Zinc Binders in Traditional African Fermented Cereal Porridges or Gruels? Food Rev. Int. 2017, 33, 561–586. [Google Scholar] [CrossRef]
- Ibinabo, T.I.; Wafula, E.N.; Josiah, K.; Julius, M.M. Phenotypic and Genotypic Characterization of Lactic Acid Bacteria Isolated from Spontaneously Fermented Vegetable Amaranth. Afr. J. Food Sci. 2021, 15, 254–261. [Google Scholar] [CrossRef]
- Wafula, E.N.; Brinks, E.; Becker, B.; Huch, M.; Trierweiler, B.; Mathara, J.M.; Oguntoyinbo, F.A.; Cho, G.S.; Franz, C.M.A.P. Draft Genome Sequence of Lactobacillus Fermentum BFE 6620, a Potential Starter Culture for African Vegetable Foods, Isolated from Fermented Cassava. Genome Announc. 2017, 5, 7–8. [Google Scholar] [CrossRef]
- Chen, Z.; Kang, J.; Zhang, Y.; Yi, X.; Pang, X.; Li-Byarlay, H.; Gao, X. Differences in the Bacterial Profiles and Physicochemical between Natural and Inoculated Fermentation of Vegetables from Shanxi Province. Ann. Microbiol. 2020, 70, 60. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A.; Cho, G.S.; Trierweiler, B.; Kabisch, J.; Rösch, N.; Neve, H.; Bockelmann, W.; Frommherz, L.; Nielsen, D.S.; Krych, L.; et al. Fermentation of African Kale (Brassica carinata) Using L. plantarum BFE 5092 and L. fermentum BFE 6620 Starter Strains. Int. J. Food Microbiol. 2016, 238, 103–112. [Google Scholar] [CrossRef]
- Huch (née Kostinek), M.; Hanak, A.; Specht, I.; Dortu, C.M.; Thonart, P.; Mbugua, S.; Holzapfel, W.H.; Hertel, C.; Franz, C.M.A.P. Use of Lactobacillus Strains to Start Cassava Fermentations for Gari Production. Int. J. Food Microbiol. 2008, 128, 258–267. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Lo, R.; Bansal, N.; Turner, M.S. Characterisation of Lactococcus Lactis Isolates from Herbs, Fruits and Vegetables for Use as Biopreservatives against Listeria Monocytogenes in Cheese. Food Control 2018, 85, 472–483. [Google Scholar] [CrossRef]
- Bukvicki, D.; Siroli, L.; D’Alessandro, M.; Cosentino, S.; Fliss, I.; Said, L.B.; Hassan, H.; Lanciotti, R.; Patrignani, F. Unravelling the Potential of Lactococcus Lactis Strains to Be Used in Cheesemaking Production as Biocontrol Agents. Foods 2020, 9, 1815. [Google Scholar] [CrossRef]
- Degrain, A.; Manhivi, V.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of Lactic Acid Fermentation on Color, Phenolic Compounds and Antioxidant Activity in African Nightshade. Microorganisms 2020, 8, 1324. [Google Scholar] [CrossRef] [PubMed]
- García-Díez, J.; Saraiva, C. Use of Starter Cultures in Foods from Animal Origin to Improve Their Safety. Int. J. Environ. Res. Public Health 2021, 18, 2544. [Google Scholar] [CrossRef]
- Martín, I.; Barbosa, J.; Pereira, S.I.A.; Rodríguez, A.; Córdoba, J.J.; Teixeira, P. Study of Lactic Acid Bacteria Isolated from Traditional Ripened Foods and Partial Characterization of Their Bacteriocins. LWT 2023, 173, 114300. [Google Scholar] [CrossRef]
- Mora-Villalobos, J.A.; Montero-Zamora, J.; Barboza, N.; Rojas-Garbanzo, C.; Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; Pablo López-Gómez, J. Fermentation Multi-Product Lactic Acid Bacteria Fermentations: A Review. Fermentation 2020, 6, 23. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; De Los Angeles Serradell, M.; Ozogul, F.; Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef]
- Adebo, O.A.; Medina-Meza, I.G. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Wijayanti, E.D.; Setiawan, N.C.E.; Christi, J.P. Effect of Lactic Acid Fermentation on Total Phenolic Content and Antioxidant Activity of Fig Fruit Juice (Ficus carica). Atlantis Press. 2017, 2, 282–289. [Google Scholar] [CrossRef]
- Amaral, G.; Bushee, J.; Cordani, U.G.; KAWASHITA, K.; Reynolds, J.H.; ALMEIDA, F.F.M.D.E.; de Almeida, F.F.M.; Hasui, Y.; de Brito Neves, B.B.; Fuck, R.A.; et al. Patterns and Determinants of Fruit and Vegetable Consumption in Sub-Sahara Africa: A Multicountry Comparison. J. Petrol. 2013, 369, 1689–1699. [Google Scholar] [CrossRef]
- Chelule, P.K.; Madiba, S.; Mokgatle, M. Perceptions and Usage of Selected Fermented Foods for Feeding Children Aged 13-60 Months in Tshwane, Gauteng Province. Pan Afr. Med. J. 2020, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Felix, E.A.; Francis, K.A. Effect of Traditional Fermentation Process on the Nutrient and Anti-Nutrient Content of Maize and African Locust Beans. J. Food Sci. Nutr. Res. 2019, 2, 65–75. [Google Scholar] [CrossRef]
- Wafula, E.N.; Kuja, J.O.; Wekesa, T.B. Isolation and Identification of Autochthonous Lactic Acid Bacteria from Commonly Consumed African Indigenous Leafy Vegetables in Kenya. Bacteria 2023, 2, 1–20. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In Vitro Antioxidant and Free Radical Scavenging Activity of Different Parts of Tabebuia Pallida Growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irakoze, M.L.; Wafula, E.N.; Owaga, E.E. Effect of Lactic Acid Fermentation on Phytochemical Content, Antioxidant Capacity, Sensory Acceptability and Microbial Safety of African Black Nightshade and African Spider Plant Vegetables. Bacteria 2023, 2, 48-59. https://doi.org/10.3390/bacteria2010004
Irakoze ML, Wafula EN, Owaga EE. Effect of Lactic Acid Fermentation on Phytochemical Content, Antioxidant Capacity, Sensory Acceptability and Microbial Safety of African Black Nightshade and African Spider Plant Vegetables. Bacteria. 2023; 2(1):48-59. https://doi.org/10.3390/bacteria2010004
Chicago/Turabian StyleIrakoze, Marie Lys, Eliud Nalianya Wafula, and Eddy Elkana Owaga. 2023. "Effect of Lactic Acid Fermentation on Phytochemical Content, Antioxidant Capacity, Sensory Acceptability and Microbial Safety of African Black Nightshade and African Spider Plant Vegetables" Bacteria 2, no. 1: 48-59. https://doi.org/10.3390/bacteria2010004
APA StyleIrakoze, M. L., Wafula, E. N., & Owaga, E. E. (2023). Effect of Lactic Acid Fermentation on Phytochemical Content, Antioxidant Capacity, Sensory Acceptability and Microbial Safety of African Black Nightshade and African Spider Plant Vegetables. Bacteria, 2(1), 48-59. https://doi.org/10.3390/bacteria2010004





